Long Period Grating Mach–Zehnder Interferometer Based Immunosensor with Temperature and Bulk Refractive Index Compensation
Abstract
:1. Introduction
2. Methodology
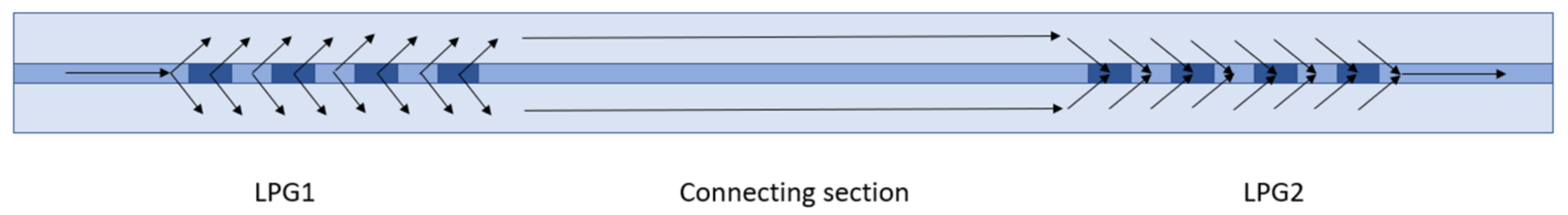
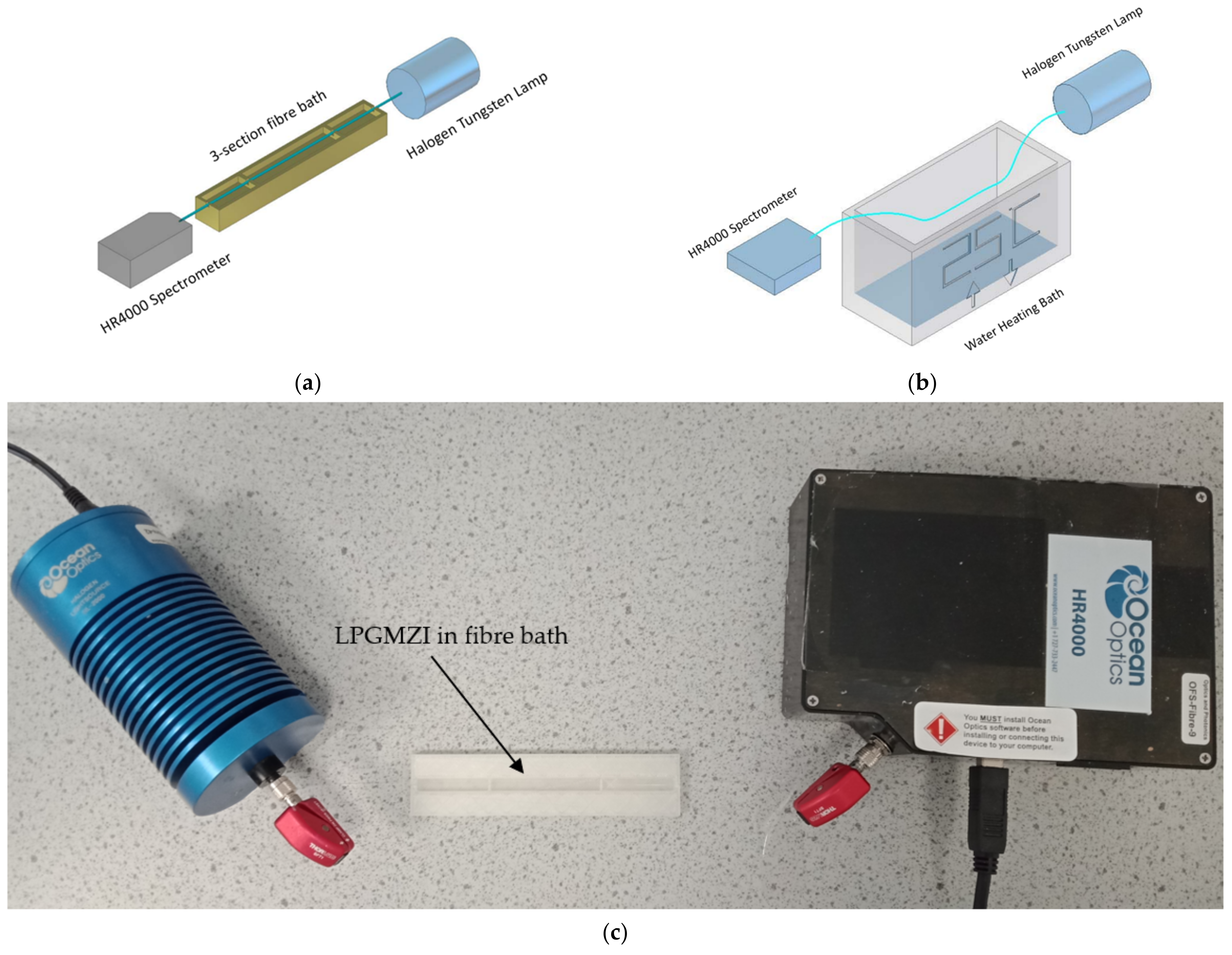
2.1. Experimental Set-Ups and Signal Processing
2.2. Surface Functionalisation
2.2.1. Materials
2.2.2. Preparation Scheme
3. Results
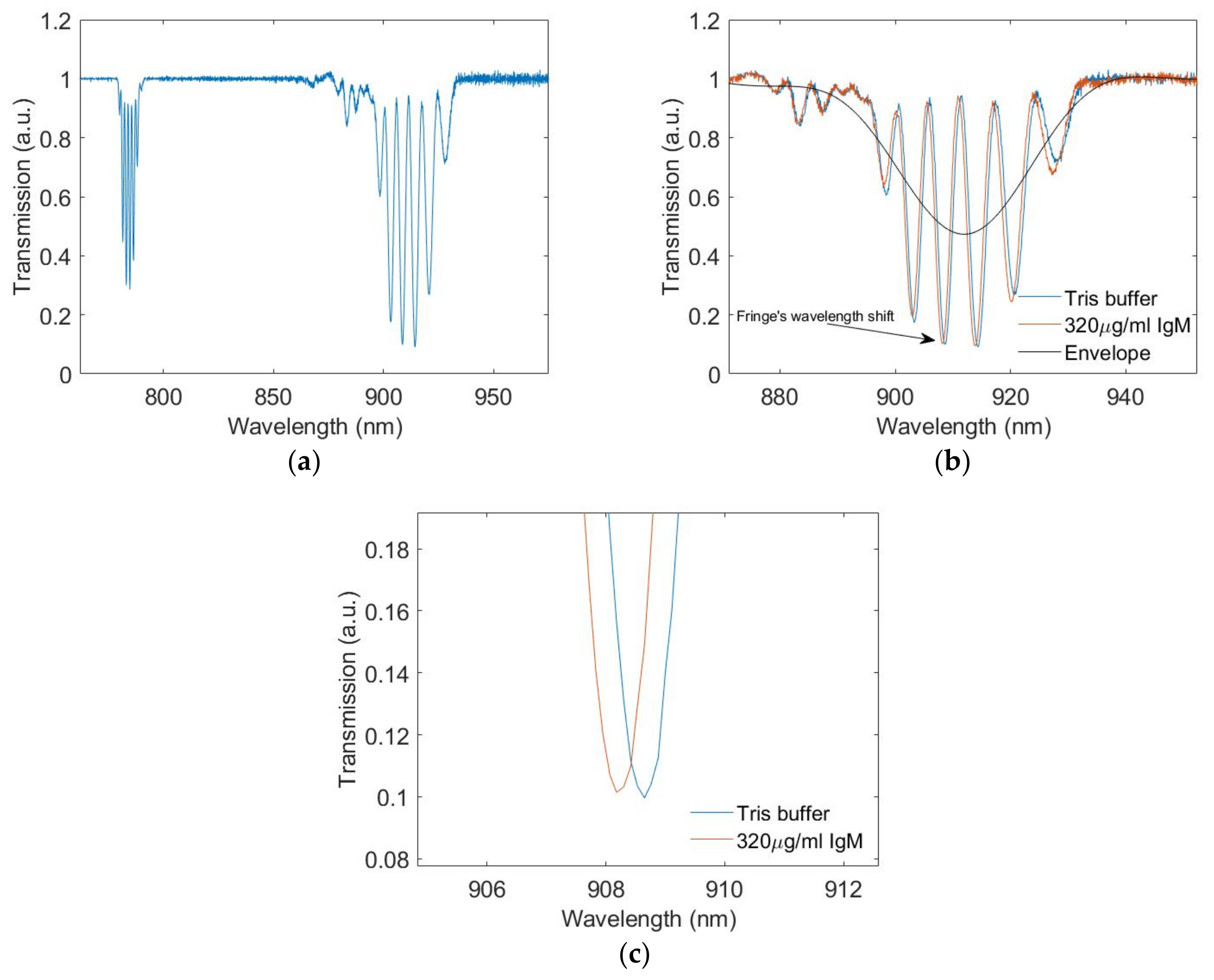
3.1. Transmission Spectra
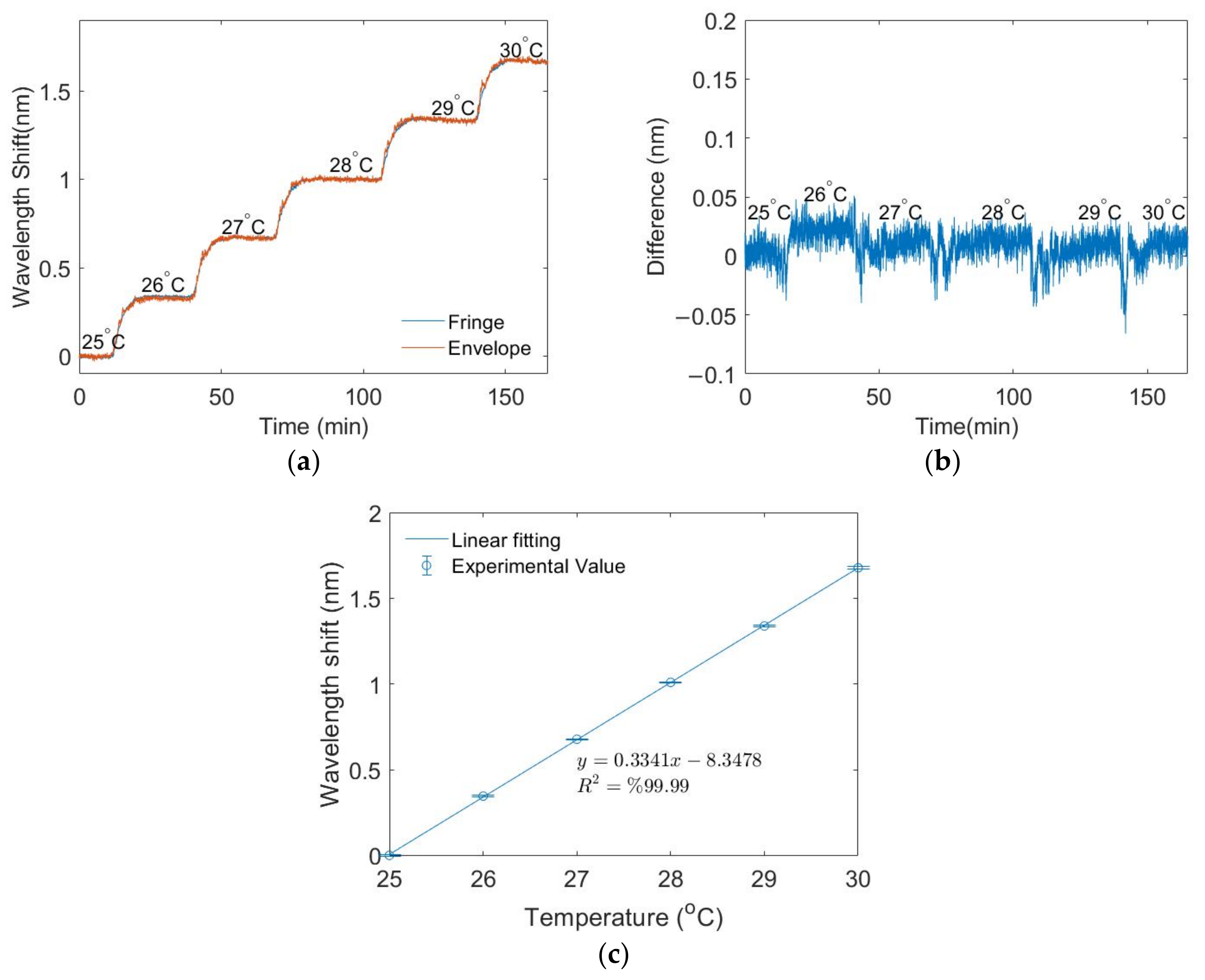
3.2. Temperature Measurements
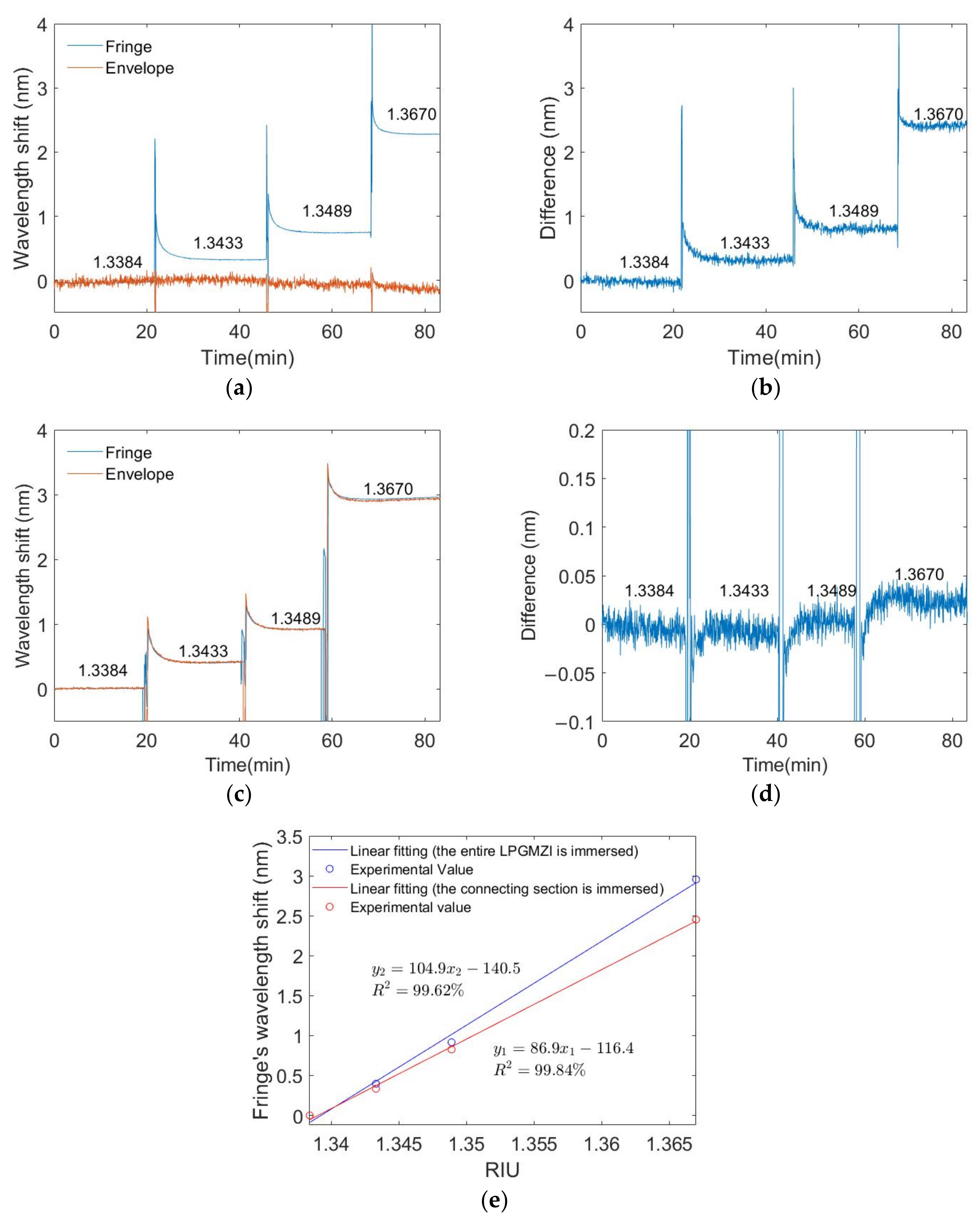
3.3. External Refractive Index Measurements
3.4. IgM Measuremnts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esposito, F.; Srivastava, A.; Sansone, L.; Giordano, M.; Campopiano, S.; Iadicicco, A. Label-Free Biosensors Based on Long Period Fiber Gratings: A Review. IEEE Sensors J. 2020, 21, 12692–12705. [Google Scholar] [CrossRef]
- Esposito, F. (INVITED) Chemical sensors based on long period fiber gratings: A review. Results Opt. 2021, 5, 100196. [Google Scholar] [CrossRef]
- Liu, L.; Marques, L.; Correia, R.; Morgan, S.P.; Lee, S.-W.; Tighe, P.; Fairclough, L.; Korposh, S. Highly sensitive label-free antibody detection using a long period fibre grating sensor. Sens. Actuators B: Chem. 2018, 271, 24–32. [Google Scholar] [CrossRef]
- Chiavaioli, F.; Trono, C.; Giannetti, A.; Brenci, M.; Baldini, F. Characterisation of a label-free biosensor based on long period grating. J. Biophotonics 2012, 7, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Trono, C.; Baldini, F.; Brenci, M.; Chiavaioli, F.; Mugnaini, M. Flow cell for strain- and temperature-compensated refractive index measurements by means of cascaded optical fibre long period and Bragg gratings. Meas. Sci. Technol. 2011, 22, 075204. [Google Scholar] [CrossRef]
- Liu, C.; Cai, Q.; Xu, B.; Zhu, W.; Zhang, L.; Zhao, J.; Chen, X. Graphene oxide functionalized long period grating for ultrasensitive label-free immunosensing. Biosens. Bioelectron. 2017, 94, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Zhang, X.; Li, K.; Guo, T.; Ianoul, A.; Albert, J. Discrimination of Bulk and Surface Refractive Index Change in Plasmonic Sensors with Narrow Bandwidth Resonance Combs. ACS Sensors 2021, 6, 3013–3023. [Google Scholar] [CrossRef]
- Janik, M.; Koba, M.; Król, K.; Mikulic, P.; Bock, W.J.; Śmietana, M. Combined long-period fiber grating and microcavity inline Mach–Zehnder interferometer for refractive index measurements with limited cross-sensitivity. Sensors 2020, 20, 2431. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Meng, H.; Wang, W.; Xue, H.; Xiong, R.; Huang, B.; Tan, C.; Huang, X. Simultaneous measurement of refractive index and temperature based on a core-offset Mach–Zehnder interferometer combined with a fiber Bragg grating. Sensors Actuators A Phys. 2014, 209, 73–77. [Google Scholar] [CrossRef]
- Enríquez, D.A.C.; da Cruz, A.R.; Giraldi, M.T.M.R. Hybrid FBG–LPG sensor for surrounding refractive index and temperature simultaneous discrimination. Opt. Laser Technol. 2012, 44, 981–986. [Google Scholar] [CrossRef]
- Hu, X.; Si, H.; Shen, H. Spectral characteristics and space division multiplexing scheme of long period fiber grating pair. Optik 2020, 227, 166117. [Google Scholar] [CrossRef]
- James, S.W.; Korposh, S.; Lee, S.-W.; Tatam, R.P. A long period grating-based chemical sensor insensitive to the influence of interfering parameters. Opt. Express 2014, 22, 8012–8023. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, H.C.A.S.G.; de Almeida, J.M.M.M.; Saraiva, C.M.T.; Jorge, P.A.D.S.; Coelho, L.C.C. Mach–Zehnder Interferometers Based on Long Period Fiber Grating Coated with Titanium Dioxide for Refractive Index Sensing. J. Light. Technol. 2019, 37, 4584–4589. [Google Scholar] [CrossRef]
- Hao, T.; Chiang, K.S. Graphene-Based Ammonia-Gas Sensor Using In-Fiber Mach-Zehnder Interferometer. IEEE Photon- Technol. Lett. 2017, 29, 2035–2038. [Google Scholar] [CrossRef]
- Wu, J.; Tang, C.; Zhang, W.; Ma, X.; Qu, S.; Chen, K.; Hao, T.; Chiang, K.S. Lab on optical fiber: Surface nano-functionalization for real-time monitoring of VOC adsorption/desorption in metal-organic frameworks. Nanophotonics 2021, 10, 2705–2716. [Google Scholar] [CrossRef]
- Kaushik, S.; Tiwari, U.; Nilima Prashar, S.; Das, B.; Sinha, R.K. Label-free detection of E scherichia coli bacteria by cascaded chirped long period gratings immunosensor. Rev. Sci. Instrum. 2019, 90, 025003. [Google Scholar] [CrossRef]
- Liu, Y.; Williams, J.; Zhang, L.; Bennion, I. Phase shifted and cascaded long-period fiber gratings. Opt. Commun. 1999, 164, 27–31. [Google Scholar] [CrossRef]
- Greenspan, N.S.; Dacek, D.A.; Cooper, L.J. Fc region-dependence of IgG3 anti-streptococcal group A carbohydrate antibody functional affinity. I. The effect of temperature. J. Immunol. 1988, 141, 4276–4282. [Google Scholar]
- Tonezzer, M.; Izidoro, S.; Moraes, J.P.A.; Le Dang, T.T. Improved Gas Selectivity Based on Carbon Modified SnO2 Nanowires. Front. Mater. 2019, 6, 277. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Preininger, C.; Mencaglia, A.; Baldini, F. Polymer-coated optical fibres for application in a direct evanescent wave immunoassay. Anal. Chim. Acta 2000, 403, 67–76. [Google Scholar] [CrossRef]
- Lichtenberg, J.Y.; Ling, Y.; Kim, S. Non-Specific Adsorption Reduction Methods in Biosensing. Sensors 2019, 19, 2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.; Liu, L.; Morgan, S.P.; Correia, R.; Korposh, S. (INVITED) Label-Free Detection of Antibodies Using Functionalised Long Period Grating Optical Fibre Sensors. Results Opt. 2021, 5, 100172. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Hsieh, H.-Y.; Kuo, W.-C.; Wei, P.-K.; Sheen, H.-J.; Tahara, H.; Chiu, T.-W.; Fan, Y.-J. Nanoplasmonic Structure of a Polycarbonate Substrate Integrated with Parallel Microchannels for Label-Free Multiplex Detection. Polymers 2021, 13, 3294. [Google Scholar] [CrossRef]
- Yue, Y.; Ding, H.; Chen, C. Label-free optical antibody testing kit based on a self-assembled whispering-gallery-mode microsphere. J. Biophotonics 2021, 14, e202000338. [Google Scholar] [CrossRef]
- Hermanson, G. Silane coupling agents. In Bioconjugate Techniques; Academic Press: Cambridge, MA, USA, 2013; Volume 2. [Google Scholar]
- Kong, F.; Hu, Y.F. Biomolecule immobilization techniques for bioactive paper fabrication. Anal. Bioanal. Chem. 2012, 403, 7–13. [Google Scholar] [CrossRef]




| Platform | Measurand | IgM Concentration Range | Signal Change at the Highest Concentration | Limit of Detection | Selectivity | Reference |
|---|---|---|---|---|---|---|
| Long period grating | Wavelength shift | 15.6 μg/mL–1 mg/mL | ~12 nm | 15 pg/mm2 | Not stated | [3] |
| Long period grating | Wavelength shift | 10 μg/mL–320 μ/mL | 4.96 nm | Not Stated | 9.4 | [23] |
| LSPR on substrate | Wavelength shift | 1 μg/mL–100 μg/mL | ~1.6 nm | 1 μg/mL | ~16 | [24] |
| WGM microsphere | Wavelength shift | 1 μg/mL–100 μg/mL | ~1.7 nm | 2 μg/mL | ~14.2 | [25] |
| LPGMZI | Wavelength shift | 20 μg/mL-320 μg/mL | 0.39 nm | 13 μg/mL | 17.9 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.; Liu, L.; Morgan, S.P.; Correia, R.; Korposh, S. Long Period Grating Mach–Zehnder Interferometer Based Immunosensor with Temperature and Bulk Refractive Index Compensation. Biosensors 2022, 12, 1099. https://doi.org/10.3390/bios12121099
Wu P, Liu L, Morgan SP, Correia R, Korposh S. Long Period Grating Mach–Zehnder Interferometer Based Immunosensor with Temperature and Bulk Refractive Index Compensation. Biosensors. 2022; 12(12):1099. https://doi.org/10.3390/bios12121099
Chicago/Turabian StyleWu, Peizhou, Liangliang Liu, Stephen P. Morgan, Ricardo Correia, and Serhiy Korposh. 2022. "Long Period Grating Mach–Zehnder Interferometer Based Immunosensor with Temperature and Bulk Refractive Index Compensation" Biosensors 12, no. 12: 1099. https://doi.org/10.3390/bios12121099






