Bioadhesive Gauze Embedded with Chitosan-Butein Bioconjugate: A Redox-Active pH Sensor Platform
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
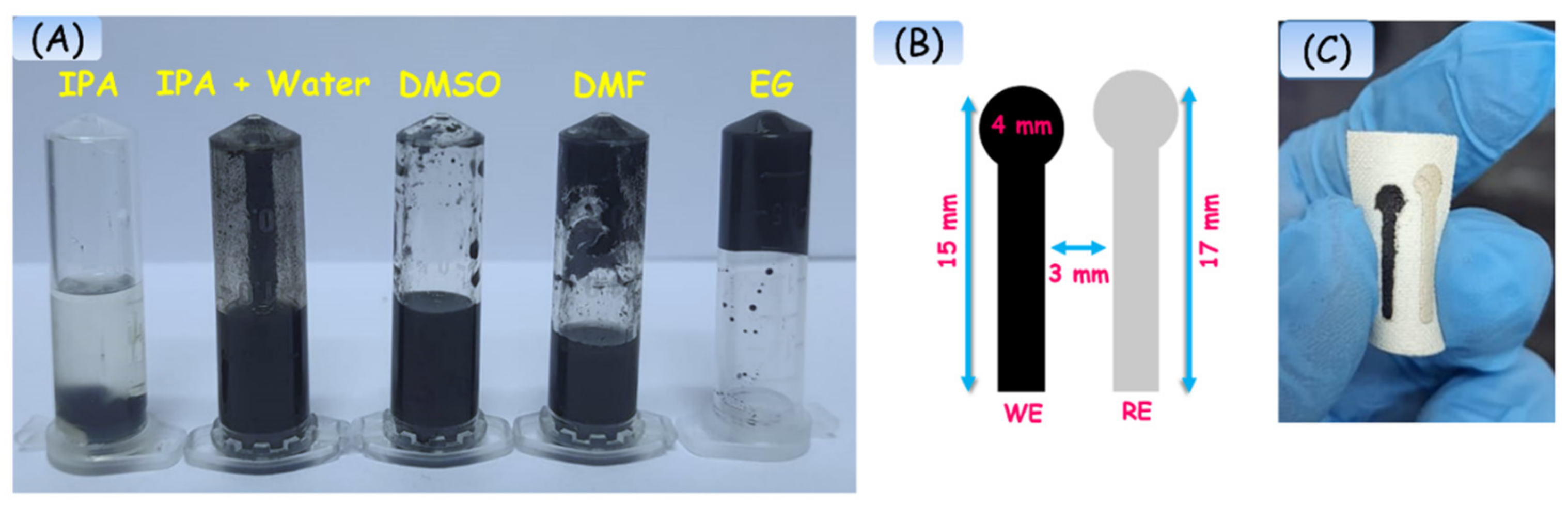
2.2. Preparation of Electrode Ink
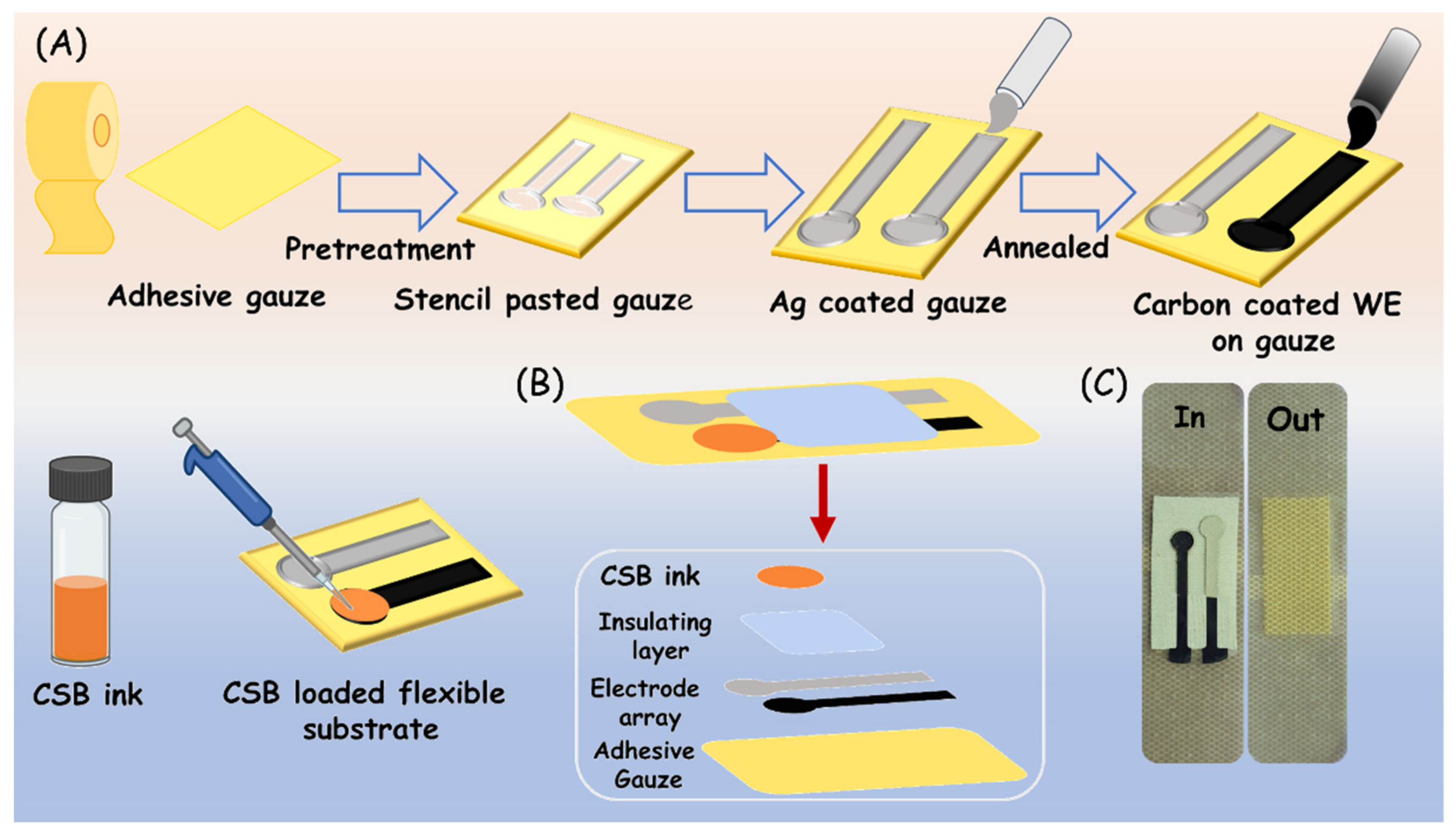
2.3. Design and Fabrication of Flexible Electrode
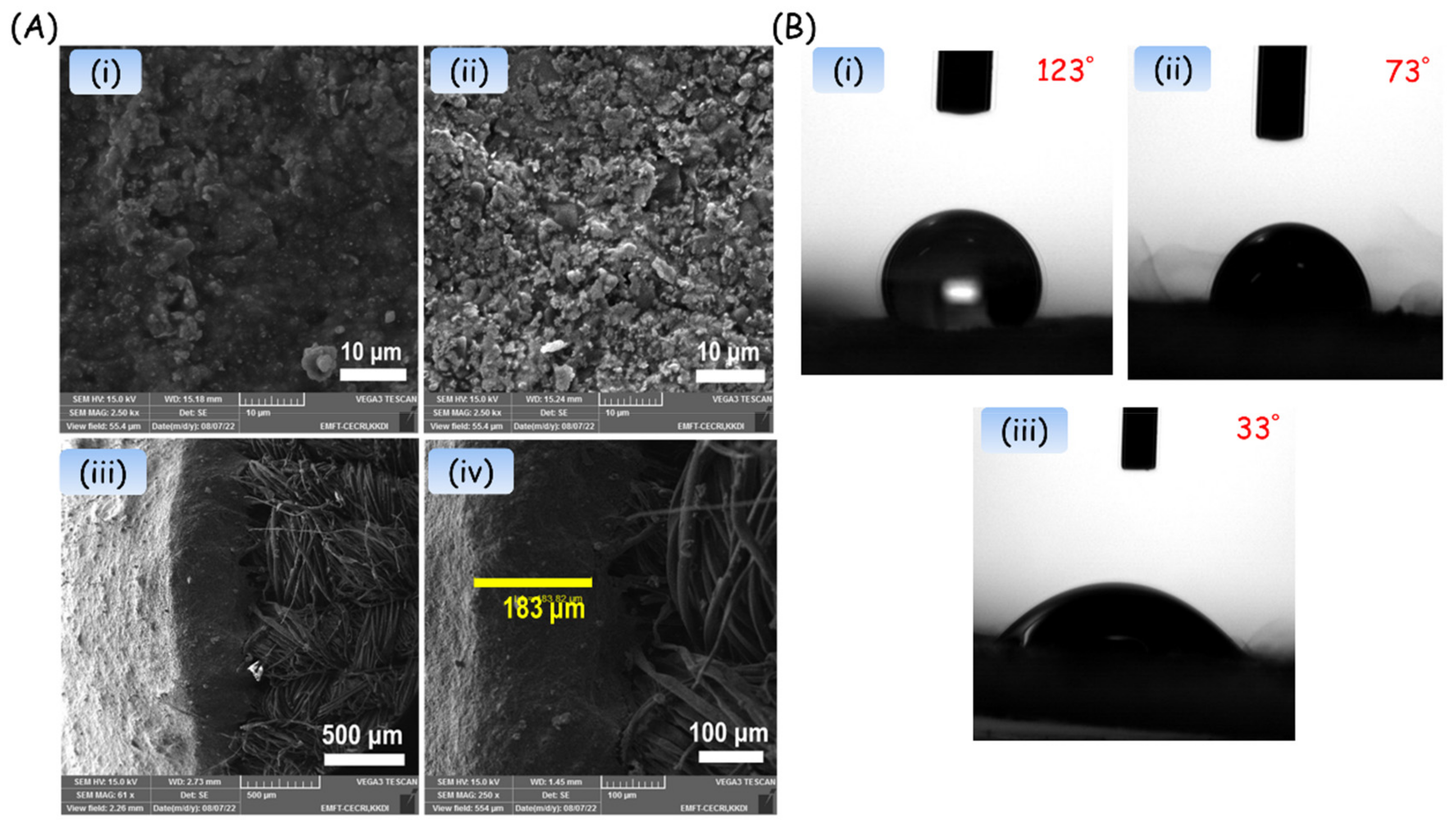
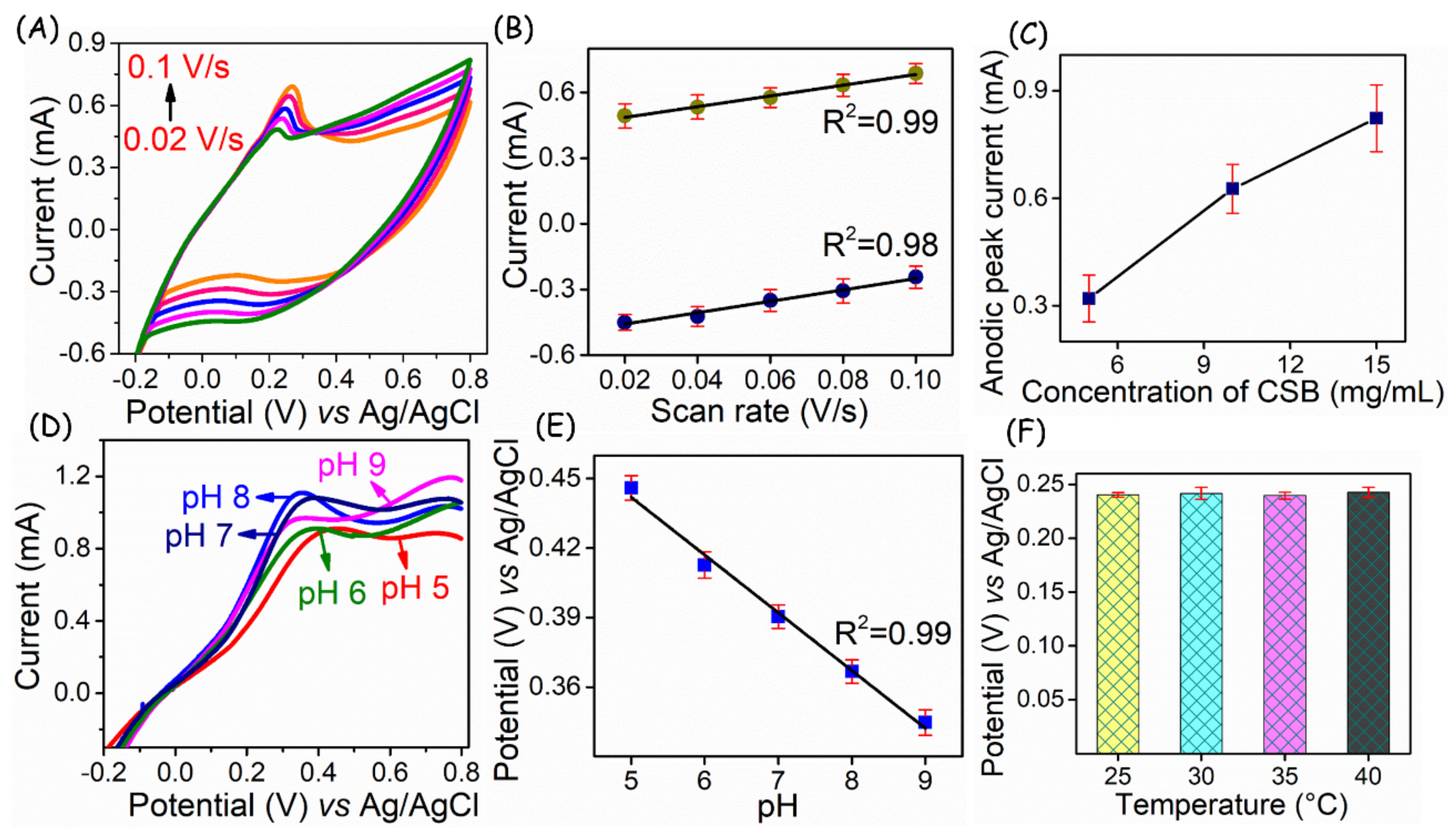
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, X.; Li, H.; Dou, J.; Wang, Q.; He, W.; Wang, C.; Li, D.; Lin, J.-M.; Zhang, Y. Stable and Biocompatible Carbon Nanotube Ink Mediated by Silk Protein for Printed Electronics. Adv. Mater. 2020, 32, 2000165. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cai, Z.; Lee, H.S.; Choi, G.S.; Lee, D.H.; Jo, C. Preparation and characterization of a Bacterial cellulose/Chitosan composite for potential biomedical application. J. Polym. Res. 2011, 18, 739–744. [Google Scholar] [CrossRef]
- Huang, Y.; Song, Y.; Gou, L.; Zou, Y.A.-O. A Novel Wearable Flexible Dry Electrode Based on Cowhide for ECG Measurement. Biosensors 2021, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Thangavel, G.; Li, Y.; Xiong, J.; Gao, D.; Ciou, J.; Tan, M.W.M.; Aziz, I.; Chen, S.; Chen, J.; et al. Printable elastomeric electrodes with sweat-enhanced conductivity for wearables. Sci. Adv. 2021, 7, eabg8433. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Nguyen, D.T.; Yeo, T.; Lim, S.B.; Tan, W.X.; Madden, L.E.; Jin, L.; Long, J.Y.K.; Aloweni, F.A.B.; Liew, Y.J.A.; et al. A flexible multiplexed immunosensor for point-of-care in situ wound monitoring. Sci. Adv. 2021, 7, eabg9614. [Google Scholar] [CrossRef]
- Lagoumintzis, G.; Zagoriti, Z.; Jensen, M.S.; Argyrakos, T.; Koutsojannis, C.; Poulas, K. Wireless Direct Microampere Current in Wound Healing: Clinical and Immunohistological Data from Two Single Case Reports. Biosensors 2019, 9, 107. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Wang, C. Graphene and the related conductive inks for flexible electronics. J. Mater. Chem. C 2016, 4, 7193–7207. [Google Scholar] [CrossRef]
- Jafarpour, M.; Nüesch, F.; Heier, J.; Abdolhosseinzadeh, S. Functional Ink Formulation for Printing and Coating of Graphene and Other 2D Materials: Challenges and Solutions. Small Sci. 2022, 2, 2200040. [Google Scholar] [CrossRef]
- Ditta, N.A.; Yaqub, M.; Nadeem, S.; Jamil, S.; Hassan, S.U.; Iqbal, S.; Javed, M.; Elkaeed, E.B.; Alshammari, F.H.; Alwadai, N.; et al. Electrochemical Studies of LbL Films With Dawson Type Heteropolyanion Glassy Carbon Electrode Sensor Modified for Methyl Parathion Detection. Front. Mater. 2022, 9, 877683. [Google Scholar] [CrossRef]
- Khalilpour, H.; Shafiee, P.; Darbandi, A.; Yusuf, M.; Mahmoudi, S.; Moazzami Goudarzi, Z.; Mirzamohammadi, S. Application of Polyoxometalate-based composites for sensor systems: A review. J. Compos. Compd. 2021, 3, 129–139. [Google Scholar] [CrossRef]
- Zaumseil, J. Single-walled carbon nanotube networks for flexible and printed electronics. Semicond. Sci. Technol. 2015, 30, 74001. [Google Scholar] [CrossRef]
- Menon, H.; Aiswarya, R.; Surendran, K.P. Screen printable MWCNT inks for printed electronics. RSC Adv. 2017, 7, 44076–44081. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Jia, M.; Kim, J.; Nguyen, T.; Duong, T.; Rolandi, M. Natural biopolymers as proton conductors in bioelectronics. Biopolymers 2021, 112, e23433. [Google Scholar] [CrossRef]
- Ho, C.M.B.; Mishra, A.; Lin, P.T.P.; Ng, S.H.; Yeong, W.Y.; Kim, Y.-J.; Yoon, Y.-J. 3D Printed Polycaprolactone Carbon Nanotube Composite Scaffolds for Cardiac Tissue Engineering. Macromol. Biosci. 2017, 17, 1600250. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.; Zhu, M.; Zhu, Y.; Zhang, Y.; Liu, Z.; Zhang, C. 3D-printed magnetic Fe3O4/MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. J. Mater. Chem. B 2014, 2, 7583–7595. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Yang, Z.; Liu, L.; Wang, H. Flexible Electrically Conductive Nanocomposite Membrane Based on Bacterial Cellulose and Polyaniline. J. Phys. Chem. B 2011, 115, 8453–8457. [Google Scholar] [CrossRef]
- Kai, D.; Prabhakaran, M.P.; Jin, G.; Ramakrishna, S. Polypyrrole-contained electrospun conductive nanofibrous membranes for cardiac tissue engineering. J. Biomed. Mater. Res. Part A 2011, 99A, 376–385. [Google Scholar] [CrossRef]
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Xiong, H.; Yang, Z. The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode. Open Chem. 2021, 19, 961–973. [Google Scholar] [CrossRef]
- Kanagavalli, P.; Radhakrishnan, S.; Pandey, G.; Ravichandiran, V.; Perumal Pazhani, G.; Veerapandian, M.; Hegde, G. Electrochemical Tracing of Butein Using Carbon Nanoparticles Interfaced Electrode Processed from Biowaste. Electroanalysis 2020, 32, 1220–1225. [Google Scholar] [CrossRef]
- Shan, Q.; Tian, J.; Ding, Q.; Wu, W. Electrochemical sensor based on metal-free materials composed of graphene and graphene oxide for sensitive detection of cadmium ions in water. Mater. Chem. Phys. 2022, 284, 126064. [Google Scholar] [CrossRef]
- Gandhi, M.; Chen, S.-S.; Ray, S.S.; Jaiswal, N.K.; Ranjan, S. Phyto-Nanosensors: Advancement of Phytochemicals as an Electrochemical Platform for Various Biomedical Applications. In Environmental Nanotechnology Volume 5; Dasgupta, N., Ranjan, S., Lichtfouse, E., Mishra, B.N., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 311–338. [Google Scholar]
- Darshani, P.; Gumpu, M.B.; Thumpati, P.; Rayappan, J.B.B.; Ravichandiran, V.; Pazhani, G.P.; Veerapandian, M. Chemically synthesized butein and butin: Optical, structure and electrochemical redox functionality at electrode interface. J. Photochem. Photobiol. B: Biol. 2018, 182, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Pandey, G.R.; Babu, K.A.; Paramasivam, S.; Kumar, S.S.; Balasubramanian, S.; Ravichandiran, V.; Pazhani, G.P.; Veerapandian, M. Chitosan grafted butein: A metal-free transducer for electrochemical genosensing of exosomal CD24. Carbohydr. Polym. 2021, 269, 118333. [Google Scholar] [CrossRef]
- Brown, M.S.; Ashley, B.; Koh, A. Wearable Technology for Chronic Wound Monitoring: Current Dressings, Advancements, and Future Prospects. Front. Bioeng. Biotechnol. 2018, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Bates, M. The Future of Wound Care. IEEE Pulse 2020, 11, 22–25. [Google Scholar] [CrossRef]
- Kamel, S.; Khattab, T.A. Recent Advances in Cellulose-Based Biosensors for Medical Diagnosis. Biosensors 2020, 10, 67. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.-M.; Karrer, S.; Heinlin, J.; Landthaler, M.; Babilas, P. The impact of the pH value on skin integrity and cutaneous wound healing. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 373–378. [Google Scholar] [CrossRef]
- Drinkwater, S.L.; Smith, A.; Burnand, K.G. What Can Wound Fluids Tell Us About the Venous Ulcer Microenvironment? Int. J. Lower Extrem. Wounds 2002, 1, 184–190. [Google Scholar] [CrossRef]
- RoyChoudhury, S.; Umasankar, Y.; Jaller, J.; Herskovitz, I.; Mervis, J.; Darwin, E.; Hirt, P.A.; Borda, L.J.; Lev-Tov, H.A.; Kirsner, R.; et al. Continuous Monitoring of Wound Healing Using a Wearable Enzymatic Uric Acid Biosensor. J. Electrochem. Soc. 2018, 165, b3168–b3175. [Google Scholar] [CrossRef]
- Bennison, L.; Miller, C.; Summers, R.H.; Minnis, A.; Sussman, G.; McGuiness, W. The pH of wounds during healing and infection: A descriptive literature review. Wound Pract. Res. 2017, 25, 63. [Google Scholar]
- Mathew, M.; Radhakrishnan, S.; Vaidyanathan, A.; Chakraborty, B.; Rout, C.S. Flexible and wearable electrochemical biosensors based on two-dimensional materials: Recent developments. Anal. Bioanal. Chem. 2021, 413, 727–762. [Google Scholar] [CrossRef]
- Sardini, E.A.-O.; Serpelloni, M.A.-O.; Tonello, S.A.-O. Printed Electrochemical Biosensors: Opportunities and Metrological Challenges. Biosensors 2020, 10, 166. [Google Scholar] [CrossRef]
- Andryukov, B.G.; Besednova, N.N.; Romashko, R.V.; Zaporozhets, T.S.; Efimov, T.A. Label-Free Biosensors for Laboratory-Based Diagnostics of Infections: Current Achievements and New Trends. Biosensors 2020, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Anjum, S.; Arora, A.; Alam, M.S.; Gupta, B. Development of antimicrobial and scar preventive chitosan hydrogel wound dressings. Int. J. Pharm. 2016, 508, 92–101. [Google Scholar] [CrossRef]
- Tan, G.; Liao, J.; Ning, C.; Zhang, L. Preparation, characterization, and drug-release properties of PEG-DA-based copolymer hydrogel microspheres. J. Appl. Polym. Sci. 2012, 125, 3509–3516. [Google Scholar] [CrossRef]
- Zhao, S.; Li, J.; Cao, D.; Zhang, G.; Li, J.; Li, K.; Yang, Y.; Wang, W.; Jin, Y.; Sun, R.; et al. Recent Advancements in Flexible and Stretchable Electrodes for Electromechanical Sensors: Strategies, Materials, and Features. ACS Appl. Mater. Interfaces 2017, 9, 12147–12164. [Google Scholar] [CrossRef]
- Naftaly, M.; Das, S.; Gallop, J.; Pan, K.; Alkhalil, F.; Kariyapperuma, D.; Constant, S.; Ramsdale, C.; Hao, L. Sheet Resistance Measurements of Conductive Thin Films: A Comparison of Techniques. Electronics 2021, 10, 960. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnan, V.; Ananth, V.; Velayutham, J.; Manickam, P.; Veerapandian, M. Bioadhesive Gauze Embedded with Chitosan-Butein Bioconjugate: A Redox-Active pH Sensor Platform. Biosensors 2023, 13, 6. https://doi.org/10.3390/bios13010006
Krishnan V, Ananth V, Velayutham J, Manickam P, Veerapandian M. Bioadhesive Gauze Embedded with Chitosan-Butein Bioconjugate: A Redox-Active pH Sensor Platform. Biosensors. 2023; 13(1):6. https://doi.org/10.3390/bios13010006
Chicago/Turabian StyleKrishnan, Vinoth, Venkatachalam Ananth, Jayasudha Velayutham, Pandiaraj Manickam, and Murugan Veerapandian. 2023. "Bioadhesive Gauze Embedded with Chitosan-Butein Bioconjugate: A Redox-Active pH Sensor Platform" Biosensors 13, no. 1: 6. https://doi.org/10.3390/bios13010006




