A Review from a Clinical Perspective: Recent Advances in Biosensors for the Detection of L-Amino Acids
Abstract
:1. Typical Concentrations of Total L-Amino Acids in Human Blood
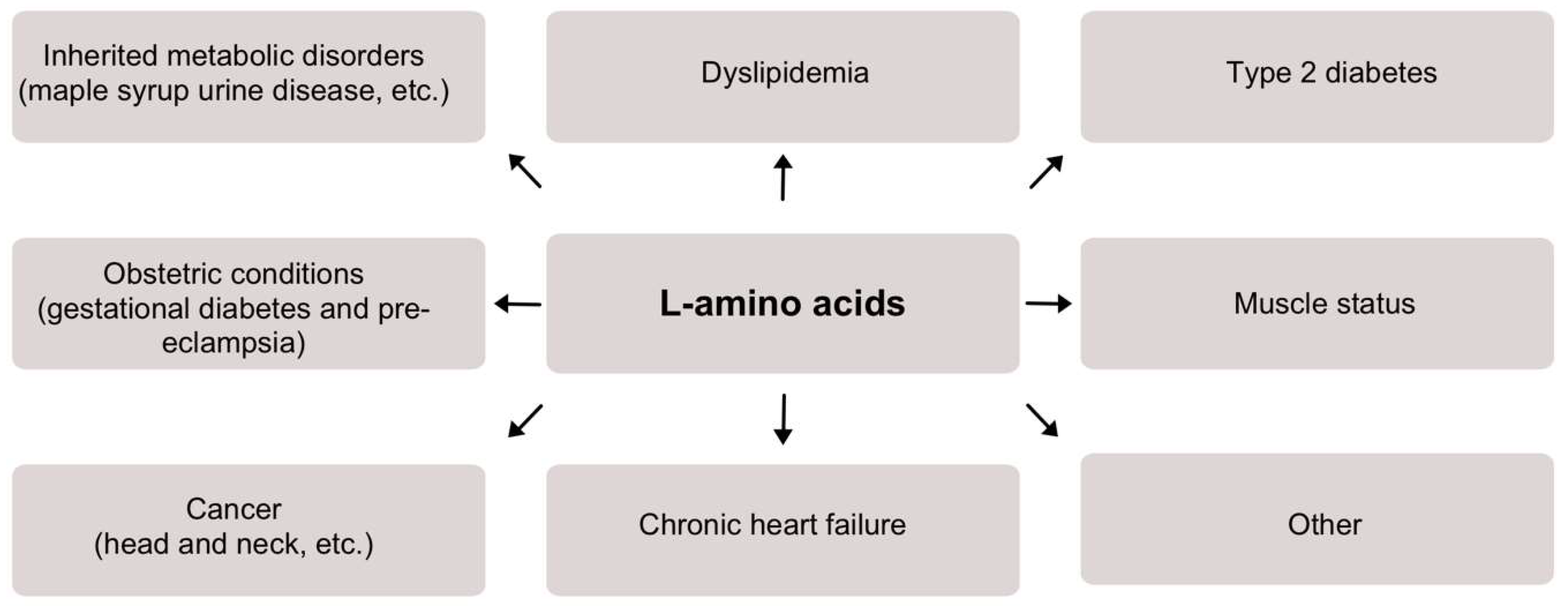
2. Overview of L-Amino Acids in Clinical Conditions
2.1. Inherited Metabolic Disorders
2.2. Dyslipidemia
2.3. Type 2 Diabetes
2.4. Obstetric Conditions
2.5. Muscle Status
2.6. Cancer
2.7. Chronic Heart Failure
2.8. Other
3. Biosensors for Measurement of Total L-Amino Acid Concentration
3.1. General Overview of Enzymatic Biosensors
3.2. Overview of Biosensors for L-Amino Acid Detection
3.3. Current Challenges Limiting the Applicability of L-Amino Acid Biosensors for Clinical Applications and Possible Solutions
4. Concluding Remarks and Future Perspectives of L-Amino Acid Detection
Author Contributions
Funding
Conflicts of Interest
References
- Stein, W.H.; Moore, S. The free amino acids of human blood plasma. J. Biol. Chem. 1954, 211, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the EPIC-Oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Miškinis, J.; Ramonas, E.; Gurevičienė, V.; Razumienė, J.; Dagys, M.; Ratautas, D. Capacitance-Based Biosensor for the Measurement of Total Loss of L-Amino Acids in Human Serum during Hemodialysis. ACS Sens. 2022, 7, 3352–3359. [Google Scholar] [CrossRef] [PubMed]
- Aliu, E.; Kanungo, S.; Arnold, G.L. Amino acid disorders. Ann. Transl. Med. 2018, 6, 471. [Google Scholar] [CrossRef] [PubMed]
- Nunes, V.; Niinikoski, H. Lysinuric Protein Intolerance. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, M.G., Pagon, R.A., Wallace, S.E., Bean, J.H.L., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Blackburn, P.R.; Gass, J.M.; e Vairo, F.P.; Farnham, K.M.; Atwal, H.K.; Macklin, S.; Klee, E.W.; Atwal, P.S. Maple syrup urine disease: Mechanisms and management. Appl. Clin. Genet. 2017, 10, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, S.; Yang, H.J.; Shin, P.K.; Hur, H.J.; Park, S.J.; Lee, K.H.; Hong, M.; Kim, J.H.; Choi, S.W.; et al. Alleviation of Dyslipidemia via a Traditional Balanced Korean Diet Represented by a Low Glycemic and Low Cholesterol Diet in Obese Women in a Randomized Controlled Trial. Nutrients 2022, 14, 235. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, A.K.; Samocha-Bonet, D.; Jernerén, F.; Turner, C.; Refsum, H.; Heilbronn, L.K. Food Overconsumption in Healthy Adults Triggers Early and Sustained Increases in Serum Branched-Chain Amino Acids and Changes in Cysteine Linked to Fat Gain. J. Nutr. 2018, 148, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, T.M.; Salonen, M.K.; Kajantie, E.; Kautiainen, H.; Eriksson, J.G. Associations of Fat and Lean Body Mass with Circulating Amino Acids in Older Men and Women. J. Gerontol. Ser. A 2020, 75, 885–891. [Google Scholar] [CrossRef]
- Bi, X.; Tey, S.L.; Loo, Y.T.; Henry, C.J. Central adiposity-induced plasma-free amino acid alterations are associated with increased insulin resistance in healthy Singaporean adults. Eur. J. Clin. Nutr. 2017, 71, 1080–1087. [Google Scholar] [CrossRef]
- Wiklund, P.; Zhang, X.; Tan, X.; Keinänen-Kiukaanniemi, S.; Alen, M.; Cheng, S. Serum Amino Acid Profiles in Childhood Predict Triglyceride Level in Adulthood: A 7-Year Longitudinal Study in Girls. J. Clin. Endocrinol. Metab. 2016, 101, 2047–2055. [Google Scholar] [CrossRef]
- Mook-Kanamori, D.O.; Römisch-Margl, W.; Kastenmüller, G.; Prehn, C.; Petersen, A.K.; Illig, T.; Gieger, C.; Wang-Sattler, R.; Meisinger, C.; Peters, A.; et al. Increased amino acids levels and the risk of developing of hypertriglyceridemia in a 7-year follow-up. J. Endocrinol. Investig. 2014, 37, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.; Krssak, M.; Bernroider, E.; Anderwald, C.; Brehm, A.; Meyerspeer, M.; Nowotny, P.; Roth, E.; Waldhäusl, W.; Roden, M. Mechanism of Amino Acid-Induced Skeletal Muscle Insulin Resistance in Humans. Diabetes 2002, 51, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.H. Emerging Perspectives on Essential Amino Acid Metabolism in Obesity and the Insulin-Resistant State. Adv. Nutr. 2011, 2, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Tillin, T.; Hughes, A.; Wang, Q.; Würtz, P.; Ala-Korpela, M.; Sattar, N.; Forouhi, N.; Godsland, I.F.; Eastwood, S.; McKeigue, P.M.; et al. Diabetes risk and amino acid profiles: Cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall and Brent REvisited) Study. Diabetologia 2015, 58, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Karusheva, Y.; Koessler, T.; Strassburger, K.; Markgraf, D.; Mastrototaro, L.; Jelenik, T.; Simon, M.-C.; Pesta, D.; Zaharia, O.-P.; Bódis, K.; et al. Short-term dietary reduction of branched-chain amino acids reduces meal-induced insulin secretion and modifies microbiome composition in type 2 diabetes: A randomized controlled crossover trial. Am. J. Clin. Nutr. 2019, 110, 1098–1107. [Google Scholar] [CrossRef]
- Wang-Sattler, R.; Yu, Z.; Herder, C.; Messias, A.C.; Floegel, A.; He, Y.; Heim, K.; Campillos, M.; Holzapfel, C.; Thorand, B.; et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol. 2012, 8, 615. [Google Scholar] [CrossRef]
- Floegel, A.; Stefan, N.; Yu, Z.; Mühlenbruch, K.; Drogan, D.; Joost, H.-G.; Fritsche, A.; Häring, H.-U.; De Angelis, M.H.; Peters, A.; et al. Identification of Serum Metabolites Associated with Risk of Type 2 Diabetes Using a Targeted Metabolomic Approach. Diabetes 2013, 62, 639–648. [Google Scholar] [CrossRef]
- Nevalainen, J.; Sairanen, M.; Appelblom, H.; Gissler, M.; Timonen, S.; Ryynänen, M. First-Trimester Maternal Serum Amino Acids and Acylcarnitines Are Significant Predictors of Gestational Diabetes. Rev. Diabet. Stud. 2016, 13, 236–245. [Google Scholar] [CrossRef]
- Evans, R.W.; Powers, R.W.; Ness, R.B.; Cropcho, L.J.; Daftary, A.R.; Harger, G.F.; Vergona, R.; Finegold, D.N. Maternal and fetal amino acid concentrations and fetal outcomes during pre-eclampsia. Reproduction 2003, 125, 785–790. [Google Scholar] [CrossRef]
- Lehmann, M.; Huonker, M.; Dimeo, F.; Heinz, N.; Gastmann, U.; Treis, N.; Steinacker, J.M.; Keul, J.; Kajewski, R.; Häussinger, D. Serum Amino Acid Concentrations in Nine Athletes Before and After the 1993 Colmar Ultra Triathlon. Int. J. Sports Med. 1995, 16, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.S.; McNaughton, L.R. Effects of branched-chain amino acid supplementation on serum creatine kinase and lactate dehydrogenase after prolonged exercise. J. Sports Med. Phys. Fit. 2000, 40, 240–246. [Google Scholar]
- Yoshikawa, N.; Yamamoto, M.; Kuribara-Souta, A.; Uehara, M.; Yamazaki, H.; Tanaka, H. Amino Acid Profile in 18 Patients with Rheumatic Diseases Treated with Glucocorticoids and BCAAs. J. Nutr. Sci. Vitaminol. 2021, 67, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Pavlickova Aimova, P.; Hronek, M.; Zadak, Z. The Importance and Dosage of Amino Acids in Nutritional Support of Various Pathological Conditions in ICU Patients; Biomedical Papers of the Medical Faculty of the Palacky University: Olomouc, Czech Republic, 2014; Volume 158, pp. 346–355. [Google Scholar]
- Vicka, V.; Vickiene, A.; Miskinyte, S.; Bartuseviciene, I.; Lisauskiene, I.; Serpytis, M.; Ringaitiene, D.; Sipylaite, J. Role of Fat-Free Mass Index on Amino Acid Loss during CRRT in Critically Ill Patients. Medicina 2023, 59, 389. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, M.; Haji, S.; Amagai, T. High Serum Essential Amino Acids as a Predictor of Skeletal Muscle Depletion in Patients with Cachexia and Advanced Gastrointestinal Cancers. Nutr. Clin. Pract. 2017, 32, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Cadoni, G.; Giraldi, L.; Chiarla, C.; Gervasoni, J.; Persichilli, S.; Primiano, A.; Settimi, S.; Galli, J.; Paludetti, G.; Arzani, D.; et al. Prognostic Role of Serum Amino Acids in Head and Neck Cancer. Dis. Markers 2020, 2020, 2291759. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-S. Dietary restriction of amino acids for Cancer therapy. Nutr. Metab. 2020, 17, 20. [Google Scholar] [CrossRef]
- Hakuno, D.; Hamba, Y.; Toya, T.; Adachi, T. Plasma Amino Acid Profiling Identifies Specific Amino Acid Associations with Cardiovascular Function in Patients with Systolic Heart Failure. PLoS ONE 2015, 10, e0117325. [Google Scholar] [CrossRef]
- Tsuji, S.; Koyama, S.; Taniguchi, R.; Fujiwara, T.; Fujiwara, H.; Sato, Y. Nutritional status of outpatients with chronic stable heart failure based on serum amino acid concentration. J. Cardiol. 2018, 72, 458–465. [Google Scholar] [CrossRef]
- Puskarich, M.A.; McHugh, C.; Flott, T.L.; Karnovsky, A.; Jones, A.E.; Stringer, K.A. Serum Levels of Branched Chain Amino Acids Predict Duration of Cardiovascular Organ Failure in Septic Shock. Shock 2021, 56, 65–72. [Google Scholar] [CrossRef]
- Su, L.; Li, H.; Xie, A.; Liu, D.; Rao, W.; Lan, L.; Li, X.; Li, F.; Xiao, K.; Wang, H.; et al. Dynamic Changes in Amino Acid Concentration Profiles in Patients with Sepsis. PLoS ONE 2015, 10, e0121933. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Tanaka, M.; Nozaki, S.; Yamaguti, K.; Mizuma, H.; Sasabe, T.; Sugino, T.; Shirai, T.; Kataoka, Y.; Kajimoto, Y.; et al. Mental fatigue-induced decrease in levels of several plasma amino acids. J. Neural Transm. 2007, 114, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Qian-Qian, L.; Cong, Y.; Xiao-Bing, Z.; Hong-Zhu, D. Reduction of essential amino acid levels and sex-specific alterations in serum amino acid concentration profiles in children with autism spectrum disorder. Psychiatry Res. 2021, 297, 113675. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Skalnaya, M.G.; Skalny, A.V. Serum trace element and amino acid profile in children with cerebral palsy. J. Trace Elem. Med. Biol. 2021, 64, 126685. [Google Scholar] [CrossRef] [PubMed]
- Figura, M.; Kuśmierska, K.; Bucior, E.; Szlufik, S.; Koziorowski, D.; Jamrozik, Z.; Janik, P. Serum amino acid profile in patients with Parkinson’s disease. PLoS ONE 2018, 13, e0191670. [Google Scholar] [CrossRef] [PubMed]
- Ooi, P.H.; Gilmour, S.M.; Yap, J.; Mager, D.R. Effects of branched chain amino acid supplementation on patient care outcomes in adults and children with liver cirrhosis: A systematic review. Clin. Nutr. ESPEN 2018, 28, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Sato, S.; Watanabe, A.; Moriwaki, H.; Suzuki, K.; Kato, A.; Kato, M.; Nakamura, T.; Higuchi, K.; Nishiguchi, S.; et al. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol. Res. 2006, 35, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Duranton, F.; Lundin, U.; Gayrard, N.; Mischak, H.; Aparicio, M.; Mourad, G.; Daurès, J.-P.; Weinberger, K.M.; Argilés, A. Plasma and Urinary Amino Acid Metabolomic Profiling in Patients with Different Levels of Kidney Function. Clin. J. Am. Soc. Nephrol. 2014, 9, 37–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Yang, R.; Hu, C.; Huang, Z.; Zheng, C.; Liang, Q.; Gong, R.; Zhu, X.; Gong, H.; et al. Serum branched-chain amino acids are associated with leukocyte telomere length and frailty based on residents from Guangxi longevity county. Sci. Rep. 2020, 10, 10252. [Google Scholar] [CrossRef]
- Mirzaei, H.; Suarez, J.A.; Longo, V.D. Protein and amino acid restriction, aging and disease: From yeast to humans. Trends Endocrinol. Metab. 2014, 25, 558–566. [Google Scholar] [CrossRef]
- Dato, S.; Hoxha, E.; Crocco, P.; Iannone, F.; Passarino, G.; Rose, G. Amino acids and amino acid sensing: Implication for aging and diseases. Biogerontology 2019, 20, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P.F. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.-H.; Lee, S.-Y. Glucose Biosensors: An Overview of Use in Clinical Practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Updike, S.J.; Hicks, G.P. The Enzyme Electrode. Nature 1967, 214, 986–988. [Google Scholar] [CrossRef]
- Adachi, T.; Kitazumi, Y.; Shirai, O.; Kano, K. Development Perspective of Bioelectrocatalysis-Based Biosensors. Sensors 2020, 20, 4826. [Google Scholar] [CrossRef]
- Razumiene, J.; Leo, D.; Gureviciene, V.; Ratautas, D.; Gaidukevic, J.; Sakinyte-Urbikiene, I. L-Glutamate Biosensor for In Vitro Investigations: Application in Brain Extracts. Chemosensors 2023, 11, 418. [Google Scholar] [CrossRef]
- McMahon, C.P.; Rocchitta, G.; Serra, P.A.; Kirwan, S.M.; Lowry, J.P.; O’Neill, R.D. Control of the Oxygen Dependence of an Implantable Polymer/Enzyme Composite Biosensor for Glutamate. Anal. Chem. 2006, 78, 2352–2359. [Google Scholar] [CrossRef]
- Chinnadayyala, S.R.; Santhosh, M.; Singh, N.K.; Goswami, P. Alcohol oxidase protein mediated in-situ synthesized and stabilized gold nanoparticles for developing amperometric alcohol biosensor. Biosens. Bioelectron. 2015, 69, 155–161. [Google Scholar] [CrossRef]
- Rocchitta, G.; Secchi, O.; Alvau, M.D.; Farina, D.; Bazzu, G.; Calia, G.; Migheli, R.; Desole, M.S.; O’neill, R.D.; Serra, P.A. Simultaneous Telemetric Monitoring of Brain Glucose and Lactate and Motion in Freely Moving Rats. Anal. Chem. 2013, 85, 10282–10288. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wen, Y.; Xu, J.; He, H.; Li, D.; Yue, R.; Liu, G. An Amperometric Biosensor Based on Ascorbate Oxidase Immobilized in Poly(3,4-ethylenedioxythiophene)/Multi-Walled Carbon Nanotubes Composite Films for the Determination of l-Ascorbic Acid. Anal. Sci. 2011, 27, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Malik, J.; Prashant, A.; Jaiwal, P.K.; Pundir, C.S. Amperometric determination of serum total cholesterol with nanoparticles of cholesterol esterase and cholesterol oxidase. Anal. Biochem. 2016, 500, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Asiri, A.M. Selective choline biosensors based on choline oxidase co-immobilized into self-assembled monolayers on micro-chips at low potential. Anal. Methods 2015, 7, 9426–9434. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V.; et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Scheller, F.W.; Schubert, F.; Neumann, B.; Pfeiffer, D.; Hintsche, R.; Dransfeld, I.; Wollenberger, U.; Renneberg, R.; Warsinke, A.; Johansson, G.; et al. Second generation biosensors. Biosens. Bioelectron. 1991, 6, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Anzai, J. Recent Progress in Ferrocene-Modified Thin Films and Nanoparticles for Biosensors. Materials 2013, 6, 5742–5762. [Google Scholar] [CrossRef]
- Haccoun, J.; Piro, B.; Noël, V.; Pham, M.C. The development of a reagentless lactate biosensor based on a novel conducting polymer. Bioelectrochemistry 2006, 68, 218–226. [Google Scholar] [CrossRef]
- Ramonas, E.; Ratautas, D.; Dagys, M.; Meškys, R.; Kulys, J. Highly sensitive amperometric biosensor based on alcohol dehydrogenase for determination of glycerol in human urine. Talanta 2019, 200, 333–339. [Google Scholar] [CrossRef]
- Habermüller, K.; Ramanavicius, A.; Laurinavicius, V.; Schuhmann, W. An Oxygen-Insensitive Reagentless Glucose Biosensor Based on Osmium-Complex Modified Polypyrrole. Electroanalysis 2000, 12, 1383–1389. [Google Scholar] [CrossRef]
- Dhand, C.; Das, M.; Datta, M.; Malhotra, B.D. Recent advances in polyaniline based biosensors. Biosens. Bioelectron. 2011, 26, 2811–2821. [Google Scholar] [CrossRef] [PubMed]
- Rohaizad, N.; Mayorga-Martinez, C.C.; Sofer, Z.; Pumera, M. 1T-Phase Transition Metal Dichalcogenides (MoS2, MoSe2, WS2, and WSe2) with Fast Heterogeneous Electron Transfer: Application on Second-Generation Enzyme-Based Biosensor. ACS Appl. Mater. Interfaces 2017, 9, 40697–40706. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, C.; Madasamy, T.; Sethy, N.K. Enzymatic Biosensors. In Biosensors and Bioelectronics; Elsevier: Amsterdam, The Netherlands, 2015; pp. 133–204. [Google Scholar] [CrossRef]
- Das, P.; Das, M.; Chinnadayyala, S.R.; Singha, I.M.; Goswami, P. Recent advances on developing 3rd generation enzyme electrode for biosensor applications. Biosens. Bioelectron. 2016, 79, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Ratautas, D.; Laurynėnas, A.; Dagys, M.; Marcinkevičienė, L.; Meškys, R.; Kulys, J. High current, low redox potential mediatorless bioanode based on gold nanoparticles and glucose dehydrogenase from Ewingella americana. Electrochim. Acta 2016, 199, 254–260. [Google Scholar] [CrossRef]
- Gineitytė, J.; Meškys, R.; Dagys, M.; Ratautas, D. Highly efficient direct electron transfer bioanode containing glucose dehydrogenase operating in human blood. J. Power Sources 2019, 441, 227163. [Google Scholar] [CrossRef]
- Ratautas, D.; Tetianec, L.; Marcinkevičienė, L.; Meškys, R.; Kulys, J. Bioanode with alcohol dehydrogenase undergoing a direct electron transfer on functionalized gold nanoparticles for an application in biofuel cells for glycerol conversion. Biosens. Bioelectron. 2017, 98, 215–221. [Google Scholar] [CrossRef]
- Ratautas, D.; Ramonas, E.; Marcinkevičienė, L.; Meškys, R.; Kulys, J. Wiring Gold Nanoparticles and Redox Enzymes: A Self-Sufficient Nanocatalyst for the Direct Oxidation of Carbohydrates with Molecular Oxygen. ChemCatChem 2018, 10, 971–974. [Google Scholar] [CrossRef]
- Dagys, M.; Laurynėnas, A.; Ratautas, D.; Kulys, J.; Vidžiūnaitė, R.; Talaikis, M.; Niaura, G.; Marcinkevičienė, L.; Meškys, R.; Shleev, S. Oxygen electroreduction catalysed by laccase wired to gold nanoparticles via the trinuclear copper cluster. Energy Environ. Sci. 2017, 10, 498–502. [Google Scholar] [CrossRef]
- Teišerskytė, V.; Urbonavičius, J.; Ratautas, D. A direct electron transfer formaldehyde dehydrogenase biosensor for the determination of formaldehyde in river water. Talanta 2021, 234, 122657. [Google Scholar] [CrossRef]
- Shafaat, A.; Žalnėravičius, R.; Ratautas, D.; Dagys, M.; Meškys, R.; Rutkienė, R.; Gonzalez-Martinez, J.F.; Neilands, J.; Björklund, S.; Sotres, J.; et al. Glucose-to-Resistor Transduction Integrated into a Radio-Frequency Antenna for Chip-less and Battery-less Wireless Sensing. ACS Sens. 2022, 7, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Ratautas, D.; Dagys, M. Nanocatalysts Containing Direct Electron Transfer-Capable Oxidoreductases: Recent Advances and Applications. Catalysts 2019, 10, 9. [Google Scholar] [CrossRef]
- Bedendi, G.; Torquato, L.D.D.M.; Webb, S.; Cadoux, C.; Kulkarni, A.; Sahin, S.; Maroni, P.; Milton, R.D.; Grattieri, M. Enzymatic and Microbial Electrochemistry: Approaches and Methods. ACS Meas. Sci. Au 2022, 2, 517–541. [Google Scholar] [CrossRef] [PubMed]
- Nanjo, M.; Guilbault, G. Enzyme electrode for l-amino acids and glucose. Anal. Chim. Acta 1974, 73, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Adanyi, N.; Szabo, E.E.; Trummer, N. Determination of the ratio of D- and L-amino acids in brewing by an immobilised amino acid oxidase enzyme reactor coupled to amperometric detection. Biosens. Bioelectron. 1999, 14, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Tothill, I.E.; Setford, S.J.; Turner, A.P.F. Screen-printed amperometric biosensors for the rapid measurement of L- and D-amino acids. Analyst 1999, 124, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Lata, S.; Pundir, C.S. L-amino acid biosensor based on L-amino acid oxidase immobilized onto NiHCNFe/c-MWCNT/PPy/GC electrode. Int. J. Biol. Macromol. 2013, 54, 250–257. [Google Scholar] [CrossRef]
- Domínguez, R.; Serra, B.; Reviejo, A.J.; Pingarrón, J.M. Chiral Analysis of Amino Acids Using Electrochemical Composite Bienzyme Biosensors. Anal. Biochem. 2001, 298, 275–282. [Google Scholar] [CrossRef]
- Odewunmi, N.A.; Kawde, A.-N.; Ibrahim, M. In-situ single-step electrochemical AgO modified graphite pencil electrode for trace determination of DL-methionine in human serum sample. Sens. Actuators B Chem. 2019, 281, 765–773. [Google Scholar] [CrossRef]
- García-Carmona, L.; Moreno-Guzmán, M.; Sierra, T.; González, M.C.; Escarpa, A. Filtered carbon nanotubes-based electrodes for rapid sensing and monitoring of L-tyrosine in plasma and whole blood samples. Sens. Actuators B Chem. 2018, 259, 762–767. [Google Scholar] [CrossRef]
- Hua, Y.; Lv, X.; Cai, Y.; Liu, H.; Li, S.; Wan, Y.; Wang, H. Highly selective and reproducible electroanalysis for histidine in blood with turn-on responses at a potential approaching zero using tetrahedral copper metal organic frameworks. Chem. Commun. 2019, 55, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Pundir, C.S.; Lata, S.; Narwal, V. Biosensors for determination of D and L- amino acids: A review. Biosens. Bioelectron. 2018, 117, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.; Kimura, T. Use of plasma-free amino acids as biomarkers for detecting and predicting disease risk. Nutr. Rev. 2020, 78, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Izidoro, L.F.M.; Sobrinho, J.C.; Mendes, M.M.; Costa, T.R.; Grabner, A.N.; Rodrigues, V.M.; da Silva, S.L.; Zanchi, F.B.; Zuliani, J.P.; Fernandes, C.F.C.; et al. Snake Venom L-Amino Acid Oxidases: Trends in Pharmacology and Biochemistry. BioMed Res. Int. 2014, 2014, 196754. [Google Scholar] [CrossRef]
- Hahn, K.; Neumeister, K.; Mix, A.; Kottke, T.; Gröger, H.; von Mollard, G.F. Recombinant expression and characterization of a l-amino acid oxidase from the fungus Rhizoctonia solani. Appl. Microbiol. Biotechnol. 2017, 101, 2853–2864. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Wang, Y.; Xu, R.; Sun, Z.; Jie, Z. An innovative reactor-type biosensor for BOD rapid measurement. Biosens. Bioelectron. 2010, 25, 1705–1709. [Google Scholar] [CrossRef]
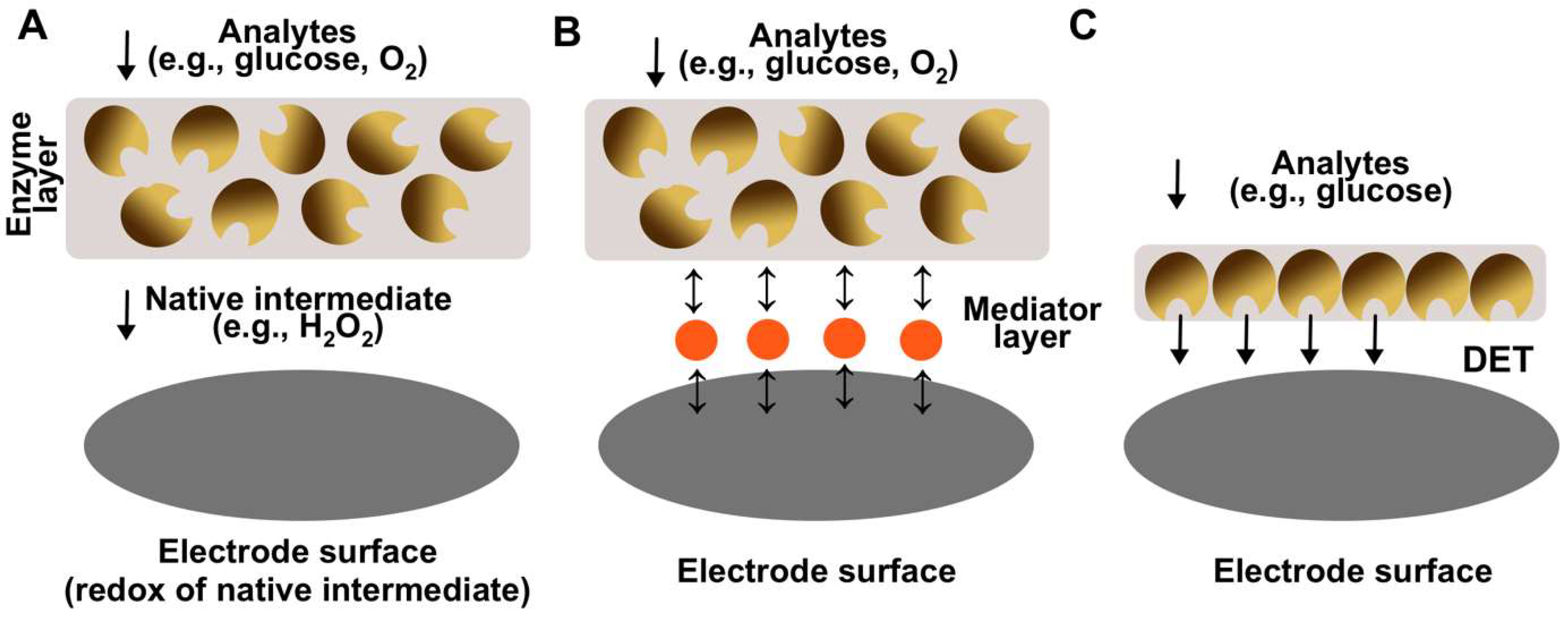



| Generation | Key Advantages | Key Disadvantages |
|---|---|---|
| First (electrons are being transferred via native mediator, e.g., H2O2) | Simple to design Native mediator is constantly being produced by the enzyme, thus cannot be depleted The sensing layer can be separated from the electrode surface The sensing layer can be made using excessive amount of enzyme to thus increase stability | Depended on the concentration of the dissolved oxygen in a solution Lack of sensitivity High operational potential (e.g., +0.6 V vs. Ag/AgCl) |
| Second (electrons are being transferred via synthetic mediator, e.g., tetrathiafulvalene) | Usually do not depend on the concentration of the dissolved oxygen in a solution Have better sensitivities in comparisonto first generation The sensing layer can be made using excessive amount of enzyme to thus increase stability Low operational potential (e.g., +0.2 V vs. Ag/AgCl) | Complex design Synthetic mediator needs to be constantly added to the solution Desorption of mediator from the surfacecan occur |
| Third (electrons are being transferred directly) | Usually do not depend on the concentration of the dissolved oxygen in a solution Highest sensitivities in comparison to the first and second generations Lowest operational potentials (e.g., 0 V vs. Ag/AgCl) No mediator (native or synthetic) is needed for the electrode operation | Most complex design Can be implemented for a limited number of selective enzymes Low operational stability in comparison to other generations |
| Biosensor | Enzyme, Nanomaterials Used | Analysis Method | Analytical Parameters (as Reported) | Real Sample |
|---|---|---|---|---|
| Nanjo and Guilbault [77] | Enzyme: L-amino acid oxidase (Crotalus adamanteus) Nanomaterials: N/A | Chronoamperometry | Sensitivity: N/A LOD: 10−6–10−5 M Linear range: N/A Stability: stable for at least 4 months | N/A |
| Varadi et al. [78] | Enzyme: L-amino acid oxidase (Crotalus adamanteus), D-amino acid oxidase (porcine kidney) Nanomaterials: N/A | Flow-through amperometry | Sensitivity: N/A LOD: N/A Linear range: 0.1–3 mM Stability: 900–1000 measurements | Brewing process samples (ginger and brown beer) |
| Sarkar et al. [79] | Enzyme: L-amino acid oxidase (Crotalus adamanteus), D-amino acid oxidase (porcine kidney) Nanomaterials: N/A | Chronoamperometry | Sensitivity: N/A LOD: 0.15–0.47 mM Linear range: 0.47–2.5 mM Stability: 40% activity loss after 56 days | Milk, fruit juice, urine |
| Lata and Pundir [80] | Enzyme: L-amino acid oxidase (goat kidney) Nanomaterials: MWCNT | Linear square voltammetry | Sensitivity: N/A LOD: 0.5 µM Linear range: 0.5 µM–100 mM Stability: 70% left after 140 days | Fruit juices, alcoholic beverages |
| Dominguez et al. [81] | Enzyme: L-amino acid oxidase (Gratelis Adamate) *, D-amino acid oxidase (porcine kidney) Nanomaterials: N/A | Chronoamperometry | Sensitivity: 10–480 µA M−1 LOD: 1.1–160 µM Linear range: 10−6–10−4 M Stability: around 10 days with no need to regenerate the electrode surface | Grapes |
| Odewunmi et al. [82] | Enzyme: enzyme-free Nanomaterials: AgO nanoparticles | Chronoamperometry | Sensitivity: 4230 µA mM−1 cm−2 LOD: 0.42 µM Linear range: 60–500 µM Stability: N/A | Human serum |
| Garcia-Carmona et al. [83] | Enzyme: enzyme-free Nanomaterials: MWCNT | Differential pulse voltammograms | Sensitivity: 0.012 µA µM−1 LOD: 8 µM Linear range: 25–750 µM Stability: N/A | Human serum |
| Hua et al. [84] | Enzyme: enzyme-free Nanomaterials: tetrahedral copper metal–organic frameworks | Linear sweep voltammetry | Sensitivity: N/A LOD: 25 nM Linear range: N/A Stability: no significant change after 6-month storage | Human blood |
| Miškinis et al. [3] | Enzyme: L-amino acid oxidase (Crotalus adamanteus) Nanomaterials: gold nanoparticles | Chronocoulometry | Sensitivity: 0.73 µC/µM LOD: 5.5 µM Linear range: 5.5–100 µM Stability: 50% of the initial activity after 10 days of storage | Human serum and blood |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratautė, K.; Ratautas, D. A Review from a Clinical Perspective: Recent Advances in Biosensors for the Detection of L-Amino Acids. Biosensors 2024, 14, 5. https://doi.org/10.3390/bios14010005
Ratautė K, Ratautas D. A Review from a Clinical Perspective: Recent Advances in Biosensors for the Detection of L-Amino Acids. Biosensors. 2024; 14(1):5. https://doi.org/10.3390/bios14010005
Chicago/Turabian StyleRatautė, Kristina, and Dalius Ratautas. 2024. "A Review from a Clinical Perspective: Recent Advances in Biosensors for the Detection of L-Amino Acids" Biosensors 14, no. 1: 5. https://doi.org/10.3390/bios14010005





