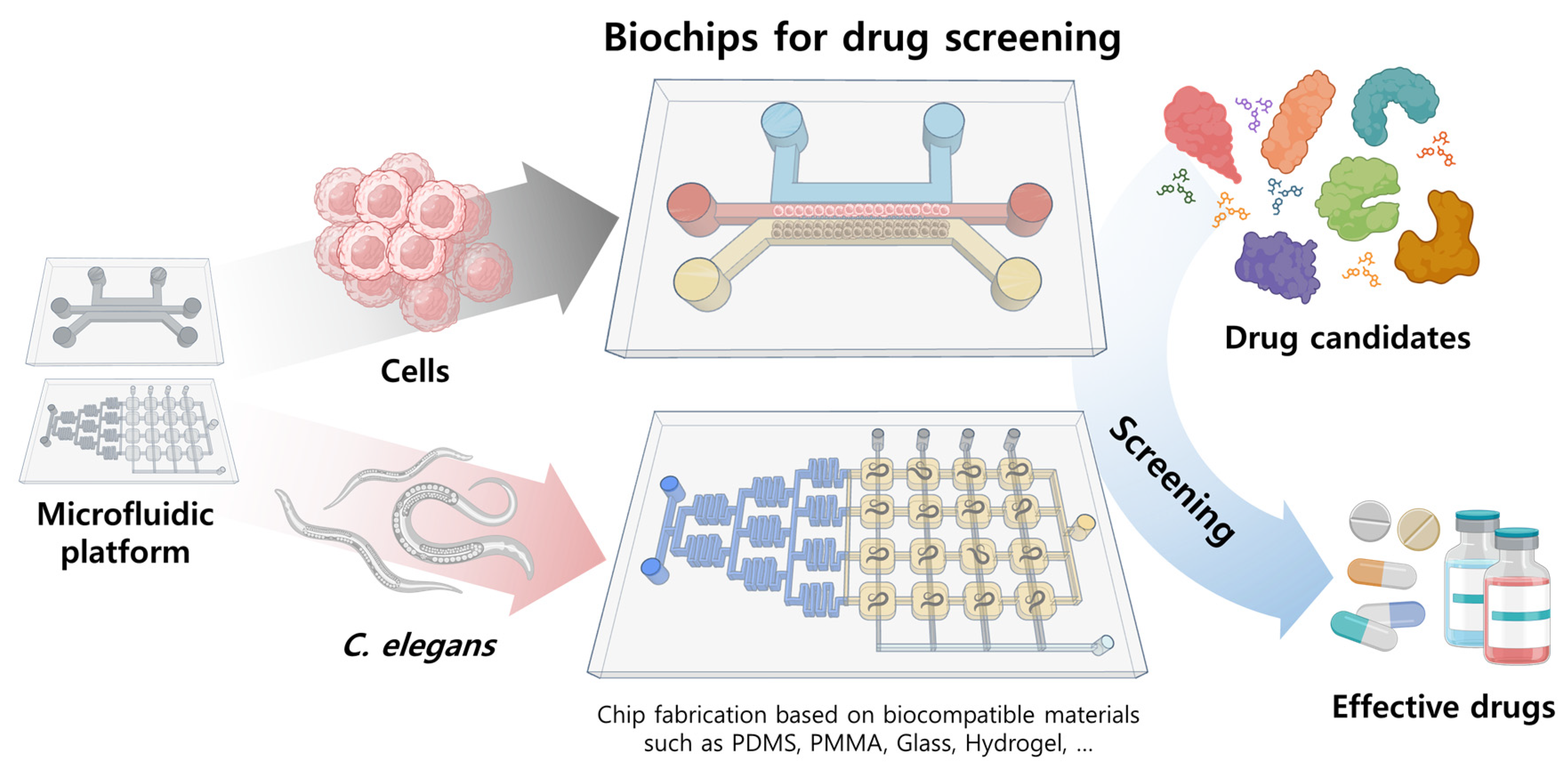
Microfluidics in High-Throughput Drug Screening: Organ-on-a-Chip and C. elegans-Based Innovations
Abstract
:1. Introduction
2. Overview of Microfluidic Systems Utilized as Drug Screening Systems and How They Are Fabricated
3. Drug Screening Applications of Various Cell/Organ and Model Organism-Based Biochips
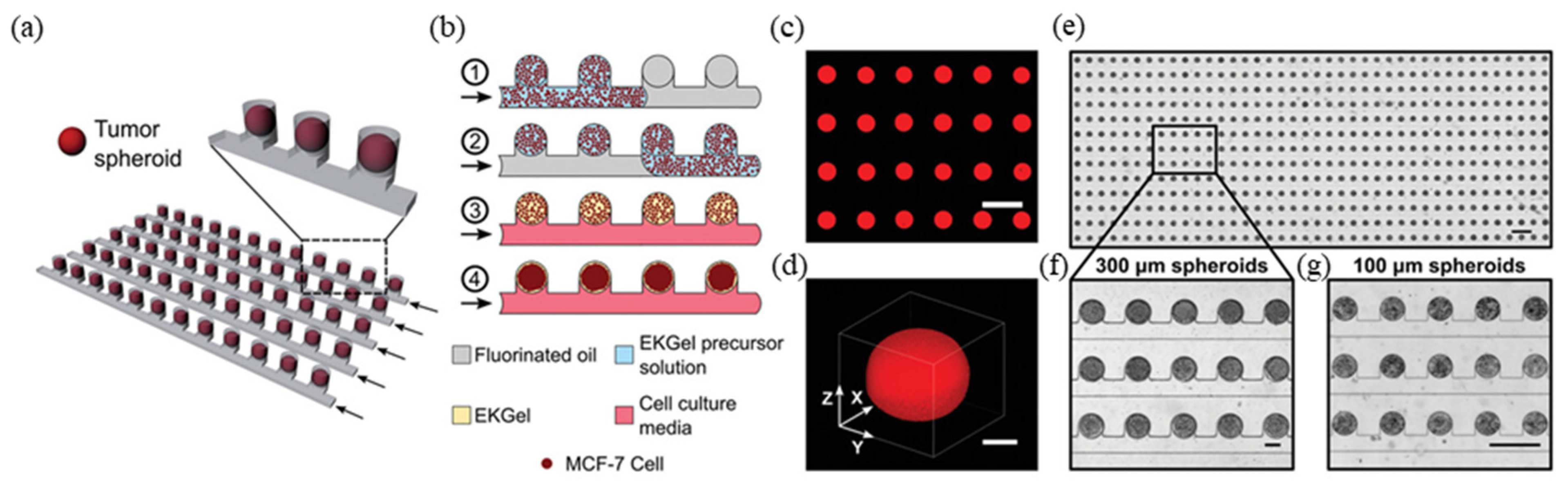
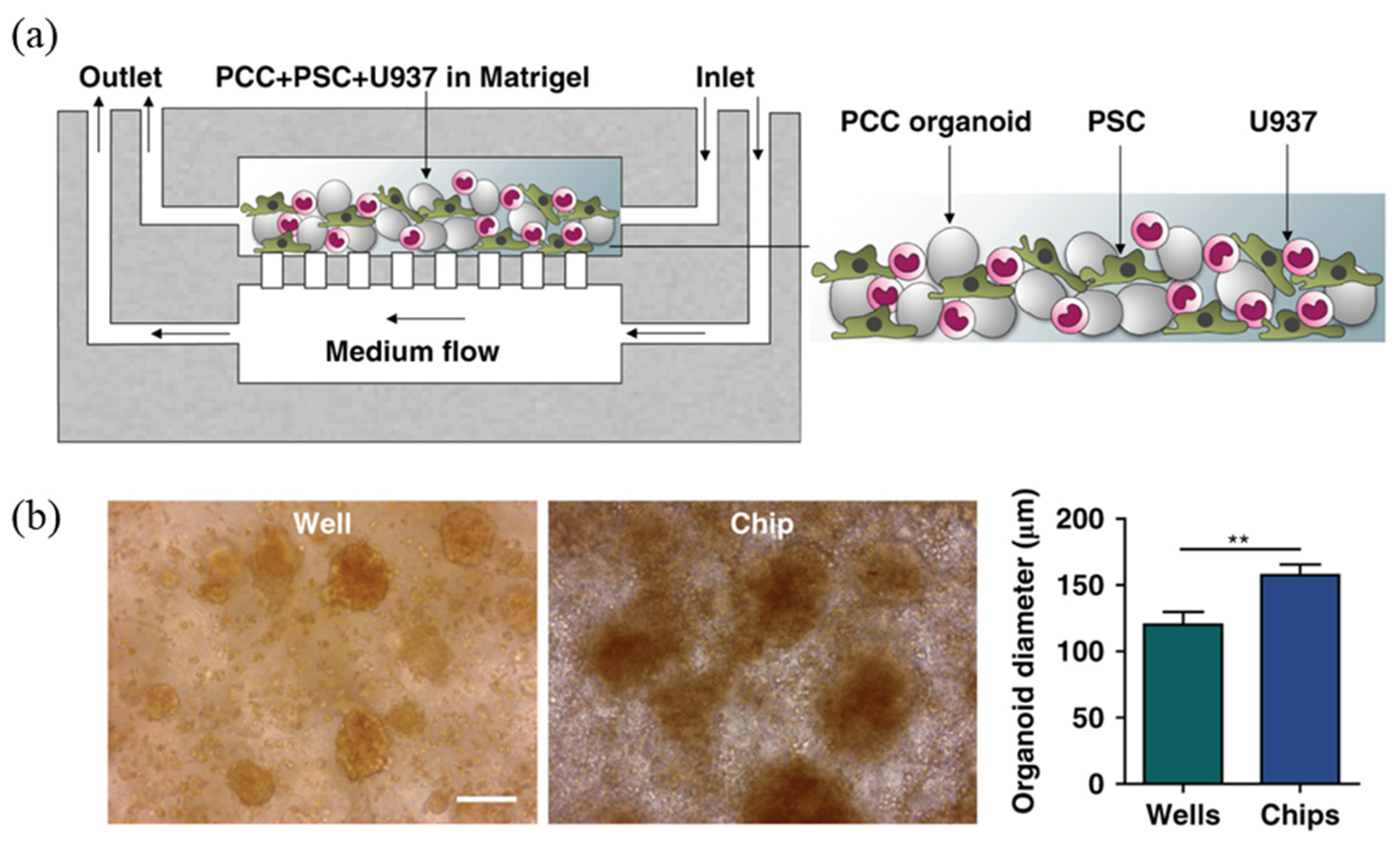
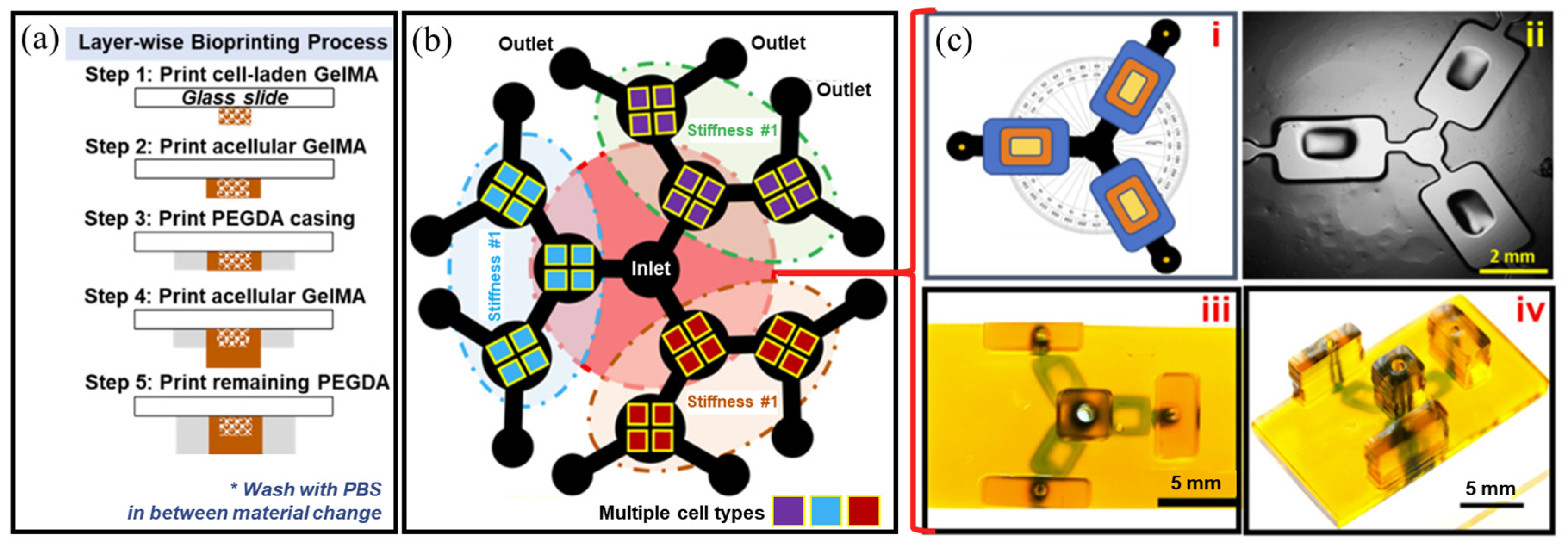
3.1. Cell/Organ Chips for Drug Screening
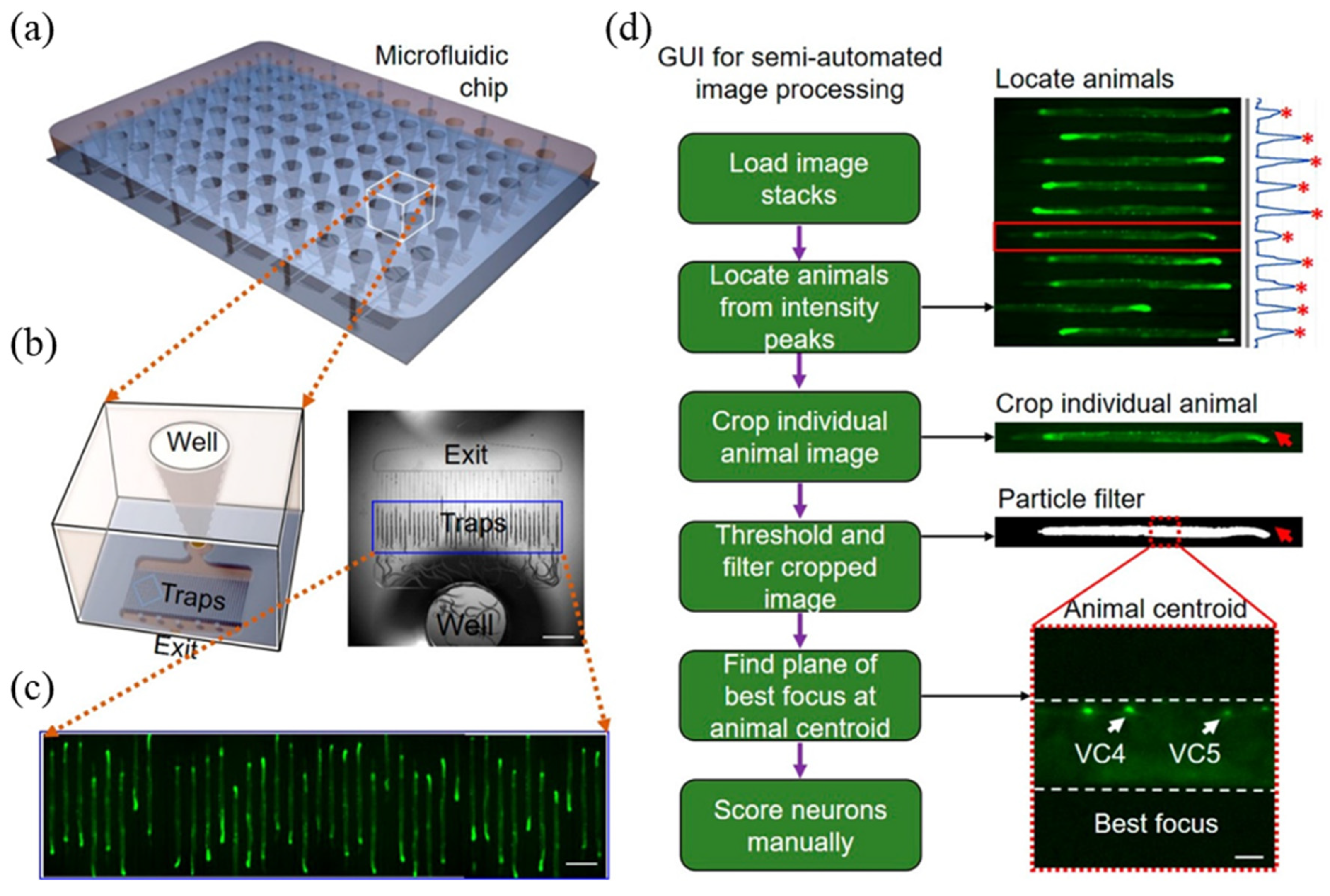
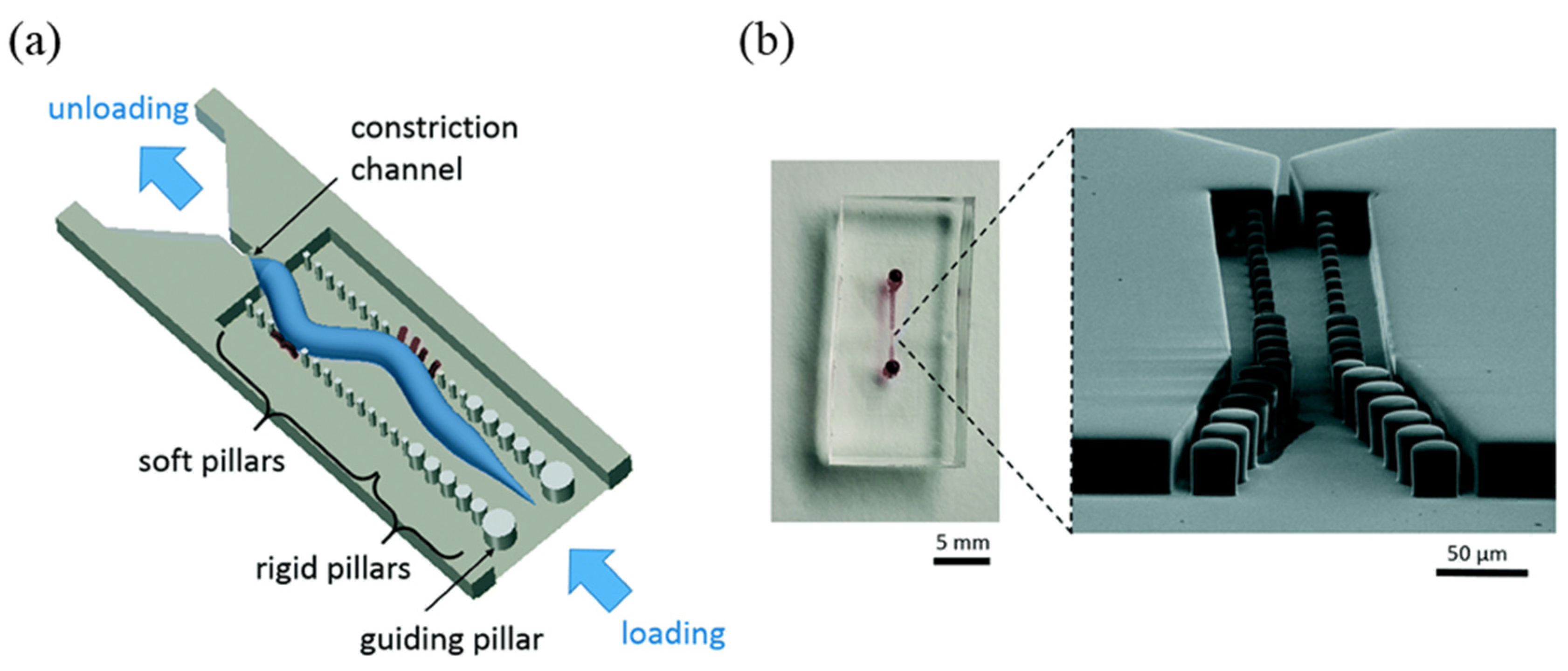
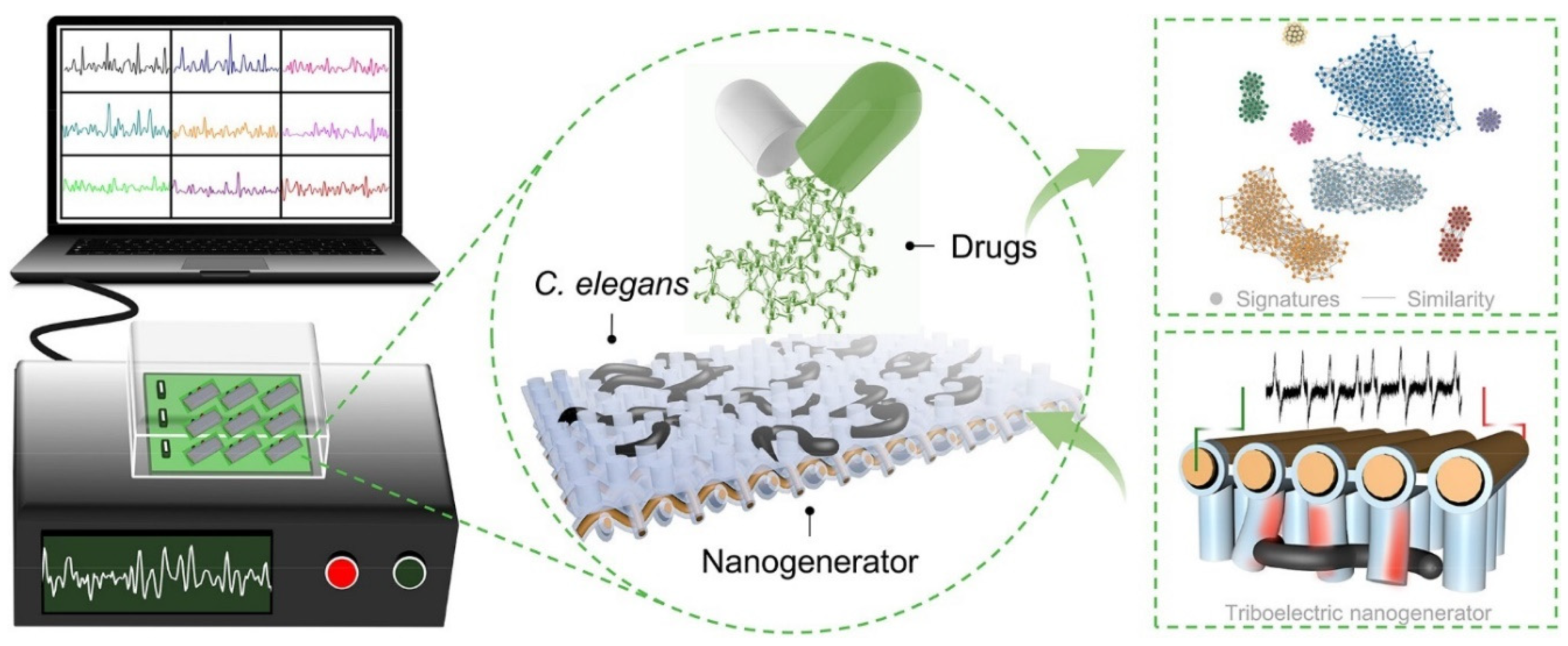
3.2. C. elegans Chips for Drug Screening
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irwin, S. Drug Screening and Evaluative Procedures. Science 1962, 136, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.P.; Rees, S.S.; Kalindjian, S.B.; Philpott, K.L. Principles of Early Drug Discovery. Br. J. Pharmacol. 2011, 162, 1239. [Google Scholar] [CrossRef]
- Wu, G.; Doberstein, S.K. HTS Technologies in Biopharmaceutical Discovery. Drug Discov. Today 2006, 11, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Iversen, P.W.; Beck, B.; Chen, Y.-F.; Dere, W.; Devanarayan, V.; Eastwood, B.J.; Farmen, M.W.; Iturria, S.J.; Montrose, C.; Moore, R.A.; et al. HTS Assay Validation. In Assay Guidance Manual; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2012; pp. 1–20. [Google Scholar]
- Murphy, B.; Yin, H.; Maris, J.M.; Kolb, E.A.; Gorlick, R.; Reynolds, C.P.; Kang, M.H.; Keir, S.T.; Kurmasheva, R.T.; Dvorchik, I.; et al. Evaluation of Alternative in Vivo Drug Screening Methodology: A Single Mouse Analysis. Cancer Res. 2016, 76, 5798–5809. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Lin, C.Y.; Tsai, H.J. Zebrafish, an In Vivo Platform to Screen Drugs and Proteins for Biomedical Use. Pharmaceuticals 2021, 14, 500. [Google Scholar] [CrossRef] [PubMed]
- Dreiman, G.H.S.; Bictash, M.; Fish, P.V.; Griffin, L.; Svensson, F. Changing the HTS Paradigm: AI-Driven Iterative Screening for Hit Finding. SLAS Discov. 2021, 26, 257. [Google Scholar] [CrossRef] [PubMed]
- Szymański, P.; Markowicz, M.; Mikiciuk-Olasik, E. Adaptation of High-Throughput Screening in Drug Discovery—Toxicological Screening Tests. Int. J. Mol. Sci. 2012, 13, 427. [Google Scholar] [CrossRef]
- Butkiewicz, M.; Wang, Y.; Bryant, S.H.; Lowe, E.W., Jr.; Weaver, D.C.; Meiler, J. High-Throughput Screening Assay Datasets from the PubChem Database. Chem. Inform. 2017, 3, 1. [Google Scholar] [CrossRef]
- Szabo, M.; Akusjärvi, S.S.; Saxena, A.; Liu, J.; Chandrasekar, G.; Kitambi, S.S. Cell and Small Animal Models for Phenotypic Drug Discovery. Drug Des. Dev. Ther. 2017, 11, 1957–1967. [Google Scholar] [CrossRef]
- Fursov, N.; Cong, M.; Federici, M.; Platchek, M.; Haytko, P.; Tacke, R.; Livelli, T.; Zhong, Z. Improving Consistency of Cell-Based Assays by Using Division-Arrested Cells. Assay Drug Dev. Technol. 2005, 3, 7–15. [Google Scholar] [CrossRef]
- Tannenbaum, J.; Bennett, B.T. Russell and Burch’s 3Rs Then and Now: The Need for Clarity in Definition and Purpose. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 120. [Google Scholar] [PubMed]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is It Time to Rethink Our Current Approach? JACC Basic Transl. Sci. 2019, 4, 845. [Google Scholar] [CrossRef] [PubMed]
- Beebe, D.J.; Mensing, G.A.; Walker, G.M. Physics and Applications of Microfluidics in Biology. Annu. Rev. Biomed. Eng. 2002, 4, 261–286. [Google Scholar] [CrossRef]
- Masuda, S.; Washizu, M.; Nanba, T. Novel Method of Cell Fusion In Field Constriction Area In Fluid Integrated Circuit. IEEE Trans. Ind. Appl. 1989, 25, 732–737. [Google Scholar] [CrossRef]
- Du, G.; Fang, Q.; den Toonder, J.M.J. Microfluidics for Cell-Based High Throughput Screening Platforms—A Review. Anal. Chim. Acta 2016, 903, 36–50. [Google Scholar] [CrossRef]
- Chen, Y.; Chan, H.N.; Michael, S.A.; Shen, Y.; Chen, Y.; Tian, Q.; Huang, L.; Wu, H. A Microfluidic Circulatory System Integrated with Capillary-Assisted Pressure Sensors. Lab Chip 2017, 17, 653–662. [Google Scholar] [CrossRef]
- Tan, K.; Keegan, P.; Rogers, M.; Lu, M.; Gosset, J.R.; Charest, J.; Bale, S.S. A High-Throughput Microfluidic Microphysiological System (PREDICT-96) to Recapitulate Hepatocyte Function in Dynamic, Re-Circulating Flow Conditions. Lab Chip 2019, 19, 1556–1566. [Google Scholar] [CrossRef]
- Chramiec, A.; Teles, D.; Yeager, K.; Marturano-Kruik, A.; Pak, J.; Chen, T.; Hao, L.; Wang, M.; Lock, R.; Tavakol, D.N.; et al. Integrated Human Organ-on-a-Chip Model for Predictive Studies of Anti-Tumor Drug Efficacy and Cardiac Safety. Lab Chip 2020, 20, 4357–4372. [Google Scholar] [CrossRef]
- Kaletta, T.; Hengartner, M.O. Finding Function in Novel Targets: C. elegans as a Model Organism. Nat. Rev. Drug Discov. 2006, 5, 387–398. [Google Scholar] [CrossRef]
- Brenner, S. The Genetics of Caenorhabditis Elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and Specific Genetic Interference by Double-Stranded RNA in Caenorhabditis Elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Sönnichsen, B.; Koski, L.B.; Walsh, A.; Marschall, P.; Neumann, B.; Brehm, M.; Alleaume, A.M.; Artelt, J.; Bettencourt, P.; Cassin, E.; et al. Full-Genome RNAi Profiling of Early Embryogenesis in Caenorhabditis Elegans. Nature 2005, 434, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Girard, L.R.; Fiedler, T.J.; Harris, T.W.; Carvalho, F.; Antoshechkin, I.; Han, M.; Sternberg, P.W.; Stein, L.D.; Chalfie, M. WormBook: The Online Review of Caenorhabditis Elegans Biology. Nucleic Acids Res. 2007, 35, D472–D475. [Google Scholar] [CrossRef] [PubMed]
- C. elegans Sequencing Consortium. Genome Sequence of the Nematode C. elegans: A Platform for Investigating Biology. Science 1998, 282, 2012–2018. [Google Scholar] [CrossRef]
- Lockery, S. Channeling the Worm: Microfluidic Devices for Nematode Neurobiology. Nat. Methods 2007, 4, 691–692. [Google Scholar] [CrossRef] [PubMed]
- Hulme, S.E.; Shevkoplyas, S.S.; Samuel, A. Microfluidics: Streamlining Discovery in Worm Biology. Nat. Methods 2008, 5, 589–590. [Google Scholar] [CrossRef]
- Hwang, H.; Lu, H. Microfluidic Tools for Developmental Studies of Small Model Organisms—Nematodes, Fruit Flies, and Zebrafish. Biotechnol. J. 2013, 8, 192–205. [Google Scholar] [CrossRef]
- Mondal, S.; Hegarty, E.; Sahn, J.J.; Scott, L.L.; Gökçe, S.K.; Martin, C.; Ghorashian, N.; Satarasinghe, P.N.; Iyer, S.; Sae-Lee, W.; et al. High-Content Microfluidic Screening Platform Used to Identify Σ2R/Tmem97 Binding Ligands That Reduce Age-Dependent Neurodegeneration in C. elegans SC-APP Model. ACS Chem. Neurosci. 2018, 9, 1014–1026. [Google Scholar] [CrossRef]
- Yang, A.; Lin, X.; Liu, Z.; Duan, X.; Yuan, Y.; Zhang, J.; Liang, Q.; Ji, X.; Sun, N.; Yu, H.; et al. Worm Generator: A System for High-Throughput in Vivo Screening. Nano Lett. 2023, 23, 1280–1288. [Google Scholar] [CrossRef]
- Dong, L.; Jankele, R.; Cornaglia, M.; Lehnert, T.; Gönczy, P.; Gijs, M.A.M. Integrated Microfluidic Device for Drug Studies of Early C. elegans Embryogenesis. Adv. Sci. 2018, 5, 1700751. [Google Scholar] [CrossRef]
- Sofela, S.; Sahloul, S.; Song, Y.A. Biophysical Analysis of Drug Efficacy on C. Elegans Models for Neurodegenerative and Neuromuscular Diseases. PLoS ONE 2021, 16, e0246496. [Google Scholar] [CrossRef] [PubMed]
- Weeks, J.C.; Robinson, K.J.; Lockery, S.R.; Roberts, W.M. Anthelmintic Drug Actions in Resistant and Susceptible C. elegans Revealed by Electrophysiological Recordings in a Multichannel Microfluidic Device. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Whitesides, G.M. The Origins and the Future of Microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A New Paradigm for Drug Development. Trends Pharmacol. Sci. 2021, 42, 119. [Google Scholar] [CrossRef]
- Carr, J.A.; Parashar, A.; Gibson, R.; Robertson, A.P.; Martin, R.J.; Pandey, S. A Microfluidic Platform for High-Sensitivity, Real-Time Drug Screening on C. elegans and Parasitic Nematodes. Lab Chip 2011, 11, 2385–2396. [Google Scholar] [CrossRef] [PubMed]
- Prince, E.; Kheiri, S.; Wang, Y.; Xu, F.; Cruickshank, J.; Topolskaia, V.; Tao, H.; Young, E.W.K.; McGuigan, A.P.; Cescon, D.W.; et al. Microfluidic Arrays of Breast Tumor Spheroids for Drug Screening and Personalized Cancer Therapies. Adv. Healthc. Mater. 2022, 11, 2101085. [Google Scholar] [CrossRef]
- Sun, Q.; Tan, S.H.; Chen, Q.; Ran, R.; Hui, Y.; Chen, D.; Zhao, C.X. Microfluidic Formation of Coculture Tumor Spheroids with Stromal Cells As a Novel 3D Tumor Model for Drug Testing. ACS Biomater. Sci. Eng. 2018, 4, 4425–4433. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, M.; Yang, F.; Zhang, X.; Yin, W.; Liu, X.; Zhang, G.J.; Chen, Z. Pseudomonas Aeruginosa Infected Nematode-on-a-Chip Model Array for Antibacterials Screening. Sens. Actuators B Chem. 2018, 275, 373–381. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Wang, H.; An, G.; Wu, H.; Huang, W. A High-Throughput, Open-Space and Reusable Microfluidic Chip for Combinational Drug Screening on Tumor Spheroids. Lab Chip 2021, 21, 3924–3932. [Google Scholar] [CrossRef]
- Tobias, F.; McIntosh, J.C.; Labonia, G.J.; Boyce, M.W.; Lockett, M.R.; Hummon, A.B. Developing a Drug Screening Platform: MALDI-Mass Spectrometry Imaging of Paper-Based Cultures. Anal. Chem. 2019, 91, 15370–15376. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ko, J.; Lee, S.R.; Park, D.; Park, S.; Jeon, N.L. Anchor-IMPACT: A Standardized Microfluidic Platform for High-Throughput Antiangiogenic Drug Screening. Biotechnol. Bioeng. 2021, 118, 2524–2535. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Ahn, G.N.; Na, G.S.; Cha, H.J.; Kim, D.P. A Perfluoropolyether Microfluidic Device for Cell-Based Drug Screening with Accurate Quantitative Analysis. ACS Biomater. Sci. Eng. 2022, 8, 4577–4585. [Google Scholar] [CrossRef] [PubMed]
- Kulthong, K.; Duivenvoorde, L.; Sun, H.; Confederat, S.; Wu, J.; Spenkelink, B.; de Haan, L.; Marin, V.; van der Zande, M.; Bouwmeester, H. Microfluidic Chip for Culturing Intestinal Epithelial Cell Layers: Characterization and Comparison of Drug Transport between Dynamic and Static Models. Toxicol. In Vitr. 2020, 65, 104815. [Google Scholar] [CrossRef]
- Bhusal, A.; Dogan, E.; Nieto, D.; Mousavi Shaegh, S.A.; Cecen, B.; Miri, A.K. 3D Bioprinted Hydrogel Microfluidic Devices for Parallel Drug Screening. ACS Appl. Bio. Mater. 2022, 2022, 4492. [Google Scholar] [CrossRef]
- Markov, D.A.; Lillie, E.M.; Garbett, S.P.; McCawley, L.J. Variation in Diffusion of Gases through PDMS Due to Plasma Surface Treatment and Storage Conditions. Biomed. Microdevices 2014, 16, 91. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D.E. Soft Lithography in Biology and Biochemistry. Annu. Rev. Biomed. Eng. 2003, 3, 335–373. [Google Scholar] [CrossRef]
- Abgrall, P.; Conedera, V.; Camon, H.; Gue, A.M.; Nguyen, N.T. SU-8 as a Structural Material for Labs-on-Chips and Microelectromechanical Systems. Electrophoresis 2007, 28, 4539–4551. [Google Scholar] [CrossRef]
- Wu, J.; Gao, Y.; Xi, J.; You, X.; Zhang, X.; Zhang, X.; Cao, Y.; Liu, P.; Chen, X.; Luan, Y. A High-Throughput Microplate Toxicity Screening Platform Based on Caenorhabditis Elegans. Ecotoxicol. Environ. Saf. 2022, 245, 114089. [Google Scholar] [CrossRef]
- Shirure, V.S.; George, S.C. Design Considerations to Minimize the Impact of Drug Absorption in Polymer-Based Organ-on-a-Chip Platforms. Lab Chip 2017, 17, 681–690. [Google Scholar] [CrossRef]
- van Meer, B.J.; de Vries, H.; Firth, K.S.A.; van Weerd, J.; Tertoolen, L.G.J.; Karperien, H.B.J.; Jonkheijm, P.; Denning, C.; IJzerman, A.P.; Mummery, C.L. Small Molecule Absorption by PDMS in the Context of Drug Response Bioassays. Biochem. Biophys. Res. Commun. 2017, 482, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Sofela, S.; Sahloul, S.; Stubbs, C.; Orozaliev, A.; Refai, F.S.; Esmaeel, A.M.; Fahs, H.; Abdelgawad, M.O.; Gunsalus, K.C.; Song, Y.A. Phenotyping of the Thrashing Forces Exerted by Partially Immobilized C. elegans Using Elastomeric Micropillar Arrays. Lab Chip 2019, 19, 3685–3696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhuang, L.; Sun, D.; Li, Y.; Chen, Z. An Integrated Microfluidics for Assessing the Anti-Aging Effect of Caffeic Acid Phenethylester in Caenorhabditis Elegans. Electrophoresis 2021, 42, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, S.; Zhao, X.; Chen, L.; Tian, Z.; Chen, X.; Qin, S. A Novel Wide-Range Microfluidic Dilution Device for Drug Screening. Biomicrofluidics 2019, 13, 024105. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, F.; Hakala, T.; Jokinen, V. Long-Term Hydrophilization of Polydimethylsiloxane (PDMS) for Capillary Filling Microfluidic Chips. Microfluid. Nanofluid. 2020, 24, 2. [Google Scholar] [CrossRef]
- Matellan, C.; Del Río Hernández, A.E. Cost-Effective Rapid Prototyping and Assembly of Poly(Methyl Methacrylate) Microfluidic Devices. Sci. Rep. 2018, 8, 6971. [Google Scholar] [CrossRef]
- Du, L.; Chang, H.; Song, M.; Liu, C. The Effect of Injection Molding PMMA Microfluidic Chips Thickness Uniformity on the Thermal Bonding Ratio of Chips. Microsyst. Technol. 2012, 18, 815–822. [Google Scholar] [CrossRef]
- Tweedie, M.; Maguire, P.D. Microfluidic Ratio Metering Devices Fabricated in PMMA by CO2 Laser. Microsyst. Technol. 2021, 27, 47–58. [Google Scholar] [CrossRef]
- Mecomber, J.S.; Stalcup, A.M.; Hurd, D.; Halsall, H.B.; Heineman, W.R.; Seliskar, C.J.; Wehmeyer, K.R.; Limbach, P.A. Analytical Performance of Polymer-Based Microfluidic Devices Fabricated by Computer Numerical Controlled Machining. Anal. Chem. 2006, 78, 936–941. [Google Scholar] [CrossRef]
- Balaji, V.; Castro, K.; Folch, A. A Laser-Engraving Technique for Portable Micropneumatic Oscillators. Micromachines 2018, 9, 426. [Google Scholar] [CrossRef]
- Riester, O.; Laufer, S.; Deigner, H.P. Direct 3D Printed Biocompatible Microfluidics: Assessment of Human Mesenchymal Stem Cell Differentiation and Cytotoxic Drug Screening in a Dynamic Culture System. J. Nanobiotechnol. 2022, 20, 540. [Google Scholar] [CrossRef] [PubMed]
- Schuster, B.; Junkin, M.; Kashaf, S.S.; Romero-Calvo, I.; Kirby, K.; Matthews, J.; Weber, C.R.; Rzhetsky, A.; White, K.P.; Tay, S. Automated Microfluidic Platform for Dynamic and Combinatorial Drug Screening of Tumor Organoids. Nat. Commun. 2020, 11, 5271. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.K.; Hu, X.J.; Zhu, J.M.; Luo, Z.Y.; Liang, L.; Yang, D.Y.; Chen, Y.L.; Chen, L.F.; Zheng, Y.J.; Hu, Q.H.; et al. On-Chip Rapid Drug Screening of Leukemia Cells by Acoustic Streaming. Lab Chip 2021, 21, 4005–4015. [Google Scholar] [CrossRef] [PubMed]
- Eilenberger, C.; Rothbauer, M.; Selinger, F.; Gerhartl, A.; Jordan, C.; Harasek, M.; Schädl, B.; Grillari, J.; Weghuber, J.; Neuhaus, W.; et al. A Microfluidic Multisize Spheroid Array for Multiparametric Screening of Anticancer Drugs and Blood–Brain Barrier Transport Properties. Adv. Sci. 2021, 8, 2004856. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Wu, D.; Chen, Q.; Zhou, Z.; Zhang, R.; Peng, X.; Su, Y.C.; Sun, D. 3D Printed Microfluidic Chip for Multiple Anticancer Drug Combinations. Sens. Actuators B Chem. 2018, 276, 507–516. [Google Scholar] [CrossRef]
- Jaberi, A.; Monemian Esfahani, A.; Aghabaglou, F.; Park, J.S.; Ndao, S.; Tamayol, A.; Yang, R. Microfluidic Systems with Embedded Cell Culture Chambers for High-Throughput Biological Assays. ACS Appl. Bio. Mater. 2020, 3, 6661–6671. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Liu, Z.; Du, Z.; Yi, X.; Sun, W. Three-Dimensional Microfluidic Tumor–Macrophage System for Breast Cancer Cell Invasion. Biotechnol. Bioeng. 2019, 116, 1731–1741. [Google Scholar] [CrossRef]
- Haque, M.R.; Wessel, C.R.; Leary, D.D.; Wang, C.; Bhushan, A.; Bishehsari, F. Patient-Derived Pancreatic Cancer-on-a-Chip Recapitulates the Tumor Microenvironment. Microsyst. Nanoeng. 2022, 8, 36. [Google Scholar] [CrossRef]
- Yin, L.; Du, G.; Zhang, B.; Zhang, H.; Yin, R.; Zhang, W.; Yang, S.M. Efficient Drug Screening and Nephrotoxicity Assessment on Co-Culture Microfluidic Kidney Chip. Sci. Rep. 2020, 10, 6568. [Google Scholar] [CrossRef]
- Fantuzzo, J.A.; Robles, D.A.; Mirabella, V.R.; Hart, R.P.; Pang, Z.P.; Zahn, J.D. Development of a High-Throughput Arrayed Neural Circuitry Platform Using Human Induced Neurons for Drug Screening Applications. Lab Chip 2020, 20, 1140–1152. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, D.; Wang, Y.; Lin, S.; Jiang, Y. A Novel 3D Breast-Cancer-on-Chip Platform for Therapeutic Evaluation of Drug Delivery Systems. Anal. Chim. Acta 2018, 1036, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Mitxelena-Iribarren, O.; Zabalo, J.; Arana, S.; Mujika, M. Improved Microfluidic Platform for Simultaneous Multiple Drug Screening towards Personalized Treatment. Biosens. Bioelectron. 2019, 123, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chintalaramulu, N.; Vadivelu, R.; An, H.; Yuan, D.; Jin, J.; Ooi, C.H.; Cock, I.E.; Li, W.; Nguyen, N.T. Inertial Microfluidic Purification of Floating Cancer Cells for Drug Screening and Three-Dimensional Tumor Models. Anal. Chem. 2020, 92, 11558–11564. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Li, P.; Feng, J.; Lin, Z.; Chen, X.; Yang, N.; Wang, L.; Liu, D. Screening Therapeutic Agents Specific to Breast Cancer Stem Cells Using a Microfluidic Single-Cell Clone-Forming Inhibition Assay. Small 2020, 16, 1901001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiao, L.; Li, Q.; Qi, X.; Zhou, A. Microfluidic Chip for Non-Invasive Analysis of Tumor Cells Interaction with Anti-Cancer Drug Doxorubicin by AFM and Raman Spectroscopy. Biomicrofluidics 2018, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.S.; Yoo, C.E.; Kim, S.; Lee, J.E.; Park, W.Y. Application of an Open-Chamber Multi-Channel Microfluidic Device to Test Chemotherapy Drugs. Sci. Rep. 2020, 10, 20343. [Google Scholar] [CrossRef]
- Chang, S.; Wen, J.; Su, Y.; Ma, H. Microfluidic Platform for Studying the Anti-Cancer Effect of Ursolic Acid on Tumor Spheroid. Electrophoresis 2022, 43, 1466–1475. [Google Scholar] [CrossRef]
- Jang, S.D.; Song, J.; Kim, H.A.; Im, C.N.; Khawar, I.A.; Park, J.K.; Kuh, H.J. Anti-Cancer Activity Profiling of Chemotherapeutic Agents in 3D Co-Cultures of Pancreatic Tumor Spheroids with Cancer- Associated Fibroblasts and Macrophages. Cancers 2021, 13, 5955. [Google Scholar] [CrossRef]
- Sun, Y.J.; Hsu, C.H.; Ling, T.Y.; Liu, L.; Lin, T.C.; Jakfar, S.; Young, I.C.; Lin, F.H. The Preparation of Cell-Containing Microbubble Scaffolds to Mimic Alveoli Structure as a 3D Drug-Screening System for Lung Cancer. Biofabrication 2020, 12, 025031. [Google Scholar] [CrossRef]
- Sankar, S.; Mehta, V.; Ravi, S.; Sharma, C.S.; Rath, S.N. A Novel Design of Microfluidic Platform for Metronomic Combinatorial Chemotherapy Drug Screening Based on 3D Tumor Spheroid Model. Biomed. Microdevices 2021, 23, 50. [Google Scholar] [CrossRef]
- Khot, M.I.; Levenstein, M.A.; de Boer, G.N.; Armstrong, G.; Maisey, T.; Svavarsdottir, H.S.; Andrew, H.; Perry, S.L.; Kapur, N.; Jayne, D.G. Characterising a PDMS Based 3D Cell Culturing Microfluidic Platform for Screening Chemotherapeutic Drug Cytotoxic Activity. Sci. Rep. 2020, 10, 15915. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.P.; Deng, X.; Chen, D.Y.; Zhu, D.; Tong, J.L.; Zhao, T.; Ma, J.H.; Liu, Y.Q. A Microfluidic Chip-Based Co-Culture of Fibroblast-like Synoviocytes with Osteoblasts and Osteoclasts to Test Bone Erosion and Drug Evaluation. R. Soc. Open Sci. 2018, 5, 180528. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; He, X.; Wang, H.; Dai, J.; Fang, J.; He, Y.; Chen, X.; Hong, Z.; Chai, Y. Construction of a Novel Blood Brain Barrier-Glioma Microfluidic Chip Model: Applications in the Evaluation of Permeability and Anti-Glioma Activity of Traditional Chinese Medicine Components. Talanta 2023, 253, 123971. [Google Scholar] [CrossRef] [PubMed]
- Clancy, A.; Chen, D.; Bruns, J.; Nadella, J.; Stealey, S.; Zhang, Y.; Timperman, A.; Zustiak, S.P. Hydrogel-Based Microfluidic Device with Multiplexed 3D in Vitro Cell Culture. Sci. Rep. 2022, 12, 17781. [Google Scholar] [CrossRef]
- Ming, L.; Zhipeng, Y.; Fei, Y.; Feng, R.; Jian, W.; Baoguo, J.; Yongqiang, W.; Peixun, Z. Microfluidic-Based Screening of Resveratrol and Drug-Loading PLA/Gelatine Nano-Scaffold for the Repair of Cartilage Defect. Artif. Cells Nanomed. Biotechnol. 2018, 46, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, T.; McAllister, M.; Patek, S.; Flint, D.; Underwood, M.; Sim, A.; Edwards, J.; Zagnoni, M. Drug Screening of Biopsy-Derived Spheroids Using a Self-Generated Microfluidic Concentration Gradient. Sci. Rep. 2018, 8, 14672. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kim, J.; Lee, J.S.; Min, S.; Kim, S.; Ahn, D.H.; Kim, Y.G.; Cho, S.W. Vascularized Liver Organoids Generated Using Induced Hepatic Tissue and Dynamic Liver-Specific Microenvironment as a Drug Testing Platform. Adv. Funct. Mater. 2018, 28, 1801954. [Google Scholar] [CrossRef]
- Mo, S.J.; Lee, J.H.; Kye, H.G.; Lee, J.M.; Kim, E.J.; Geum, D.; Sun, W.; Chung, B.G. A Microfluidic Gradient Device for Drug Screening with Human IPSC-Derived Motoneurons. Analyst 2020, 145, 3081–3089. [Google Scholar] [CrossRef]
- Tang, Q.; Li, X.; Lai, C.; Li, L.; Wu, H.; Wang, Y.; Shi, X. Fabrication of a Hydroxyapatite-PDMS Microfluidic Chip for Bone-Related Cell Culture and Drug Screening. Bioact. Mater. 2021, 6, 169–178. [Google Scholar] [CrossRef]
- Ko, J.; Ahn, J.; Kim, S.; Lee, Y.; Lee, J.; Park, D.; Jeon, N.L. Tumor Spheroid-on-a-Chip: A Standardized Microfluidic Culture Platform for Investigating Tumor Angiogenesis. Lab Chip 2019, 19, 2822–2833. [Google Scholar] [CrossRef]
- Zhai, J.; Li, C.; Li, H.; Yi, S.; Yang, N.; Miao, K.; Deng, C.; Jia, Y.; Mak, P.I.; Martins, R.P. Cancer Drug Screening with an On-Chip Multi-Drug Dispenser in Digital Microfluidics. Lab Chip 2021, 21, 4749–4759. [Google Scholar] [CrossRef]
- Akay, M.; Hite, J.; Avci, N.G.; Fan, Y.; Akay, Y.; Lu, G.; Zhu, J.J. Drug Screening of Human GBM Spheroids in Brain Cancer Chip. Sci. Rep. 2018, 8, 15423. [Google Scholar] [CrossRef] [PubMed]
- Atakan, H.B.; Xiang, R.; Cornaglia, M.; Mouchiroud, L.; Katsyuba, E.; Auwerx, J.; Gijs, M.A.M. Automated Platform for Long-Term Culture and High-Content Phenotyping of Single C. elegans Worms. Sci. Rep. 2019, 9, 14340. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Manning, C.A.; Lee, G.H.; Majeed, M.; Lu, H. Microswimmer Combing: Controlling Interfacial Dynamics for Open-Surface Multifunctional Screening of Small Animals. Adv. Healthc. Mater. 2021, 10, 2001887. [Google Scholar] [CrossRef] [PubMed]
- Letizia, M.C.; Cornaglia, M.; Tranchida, G.; Trouillon, R.; Gijs, M.A.M. A Design of Experiment Approach for Efficient Multi-Parametric Drug Testing Using a Caenorhabditis Elegans Model. Integr. Biol. 2018, 10, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Banse, S.A.; Blue, B.W.; Robinson, K.J.; Jarrett, C.M.; Phillips, P.C. The Stress-Chip: A Microfluidic Platform for Stress Analysis in Caenorhabditis Elegans. PLoS ONE 2019, 14, e0216283. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ge, A.; Wang, X.; Wang, S.; Yue, X.; Wang, J.; Feng, X.; Du, W.; Liu, B.F. Real-Time Monitoring of Immune Responses under Pathogen Invasion and Drug Interference by Integrated Microfluidic Device Coupled with Worm-Based Biosensor. Biosens. Bioelectron. 2018, 110, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Atakan, H.B.; Hof, K.S.; Cornaglia, M.; Auwerx, J.; Gijs, M.A.M. The Detection of Early Epigenetic Inheritance of Mitochondrial Stress in C. elegans with a Microfluidic Phenotyping Platform. Sci. Rep. 2019, 9, 19315. [Google Scholar] [CrossRef]
- Rezai, P.; Siddiqui, A.; Selvaganapathy, P.R.; Gupta, B.P. Electrotaxis of Caenorhabditis Elegans in a Microfluidic Environment. Lab Chip 2010, 10, 220–226. [Google Scholar] [CrossRef]
- Dwyer, N.; Adler, E.C.; Crump, G.J.; L’Etoile, D.N.; Bargmann, I.C. Polarized Dendritic Transport and the AP-1 Μ1 Clathrin Adaptor UNC-101 Localize Odorant Receptors to Olfactory Cilia. Neuron 2001, 31, 277–287. [Google Scholar] [CrossRef]
- Fang-Yen, C.; Gabel, C.V.; Samuel, A.D.; Bargmann, C.I.; Avery, L. Laser Microsurgery in Caenorhabditis Elegans. Methods Cell Biol. 2012, 107, 177–206. [Google Scholar] [PubMed]
- Kerr, R.; Lev-Ram, V.; Baird, G.; Vincent, P.; Tsien, R.Y.; Schafer, W.R. Optical Imaging of Calcium Transients in Neurons and Pharyngeal Muscle of C. elegans. Neuron 2000, 26, 583–594. [Google Scholar] [CrossRef]
- Goodman, M.B.; Hall, D.H.; Avery, L.; Lockery, S.R. Active Currents Regulate Sensitivity and Dynamic Range in C. elegans Neurons. Neuron 1998, 20, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Ahlawat, S.; Koushika, S.P. Simple Microfluidic Devices for in Vivo Imaging of C. elegans, Drosophila and Zebrafish. J. Vis. Exp. 2012, 67, e3780. [Google Scholar]
- Cooksey, G.A.; Atencia, J. Pneumatic Valves in Folded 2D and 3D Fluidic Devices Made from Plastic Films and Tapes. Lab Chip 2014, 14, 1665–1668. [Google Scholar] [CrossRef] [PubMed]
- Krajniak, J.; Lu, H. Long-Term High-Resolution Imaging and Culture of C. elegans in Chip-Gel Hybrid Microfludic Device for Developmental Studies. Lab Chip 2010, 10, 1862. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Ah Lee, S.; Lian Chew, Y.; Broderick, K.; Schafer, W.R.; Lu, H.; Cho, Y.; Lee, S.A.; Broderick, K.; Lu, H.; et al. Multimodal Stimulation in a Microfluidic Device Facilitates Studies of Interneurons in Sensory Integration in C. elegans. Small 2020, 16, 1905852. [Google Scholar] [CrossRef]
- Migliozzi, D.; Cornaglia, M.; Mouchiroud, L.; Uhlmann, V.; Unser, M.A.; Auwerx, J.; Gijs, M.A.M. Multimodal Imaging and High-Throughput Image-Processing for Drug Screening on Living Organisms on-Chip. J. Biomed. Opt. 2019, 24, 021205. [Google Scholar] [CrossRef]
- Chen, Z.; Deng, J.; Zhang, X.; Luo, Y.; Lu, Y.; Wu, Z.; Lin, B. A Novel Micro-Injection Droplet Microfluidic System for Studying Locomotive Behavior Responses to Cu2+ Induced Neurotoxin in Individual C. elegans. Anal. Chim. Acta 2020, 1106, 61–70. [Google Scholar] [CrossRef]
- Letizia, M.C.; Cornaglia, M.; Trouillon, R.; Sorrentino, V.; Mouchiroud, L.; Bou Sleiman, M.S.; Auwerx, J.; Gijs, M.A.M. Microfluidics-Enabled Phenotyping of a Whole Population of C. elegans Worms over Their Embryonic and Post-Embryonic Development at Single-Organism Resolution. Microsyst. Nanoeng. 2018, 4, 6. [Google Scholar] [CrossRef]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial Intelligence in Drug Discovery and Development. Drug Discov. Today 2021, 26, 80. [Google Scholar] [CrossRef] [PubMed]




| Chip Sturucture and Examples | Characteristics of Structure | Features | Representative Ref. | |
|---|---|---|---|---|
| Horizontal | 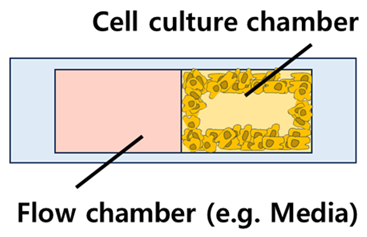 | - Mono-layer - Separation method: Post structure (most popular) | - Relatively simple fabrication process - Extra-cellular matrix is required to separate the cells and others effectively | [38,68] |
| Vertical |  | - Multi-layer - Separation method: Porous membrane (most popular) | - Space-efficient, reducing the size of the chip - Finer flow control is possible independently | [69,70] |
| Chip Materials | Cell Line | Test Drugs | Structures | Ref. |
|---|---|---|---|---|
| PDMS | BT549, T47D | Doxorubicin | Horizontal | [72] |
| U-2 OS | Methotrexate | Horizontal | [73] | |
| MDA-MB-231 | Paclitaxel | Horizontal | [68] | |
| 5-fluorouracil, Cisplatin, Docetaxel, Gemcitabine, Irinotecan, Oxaliplatin, Paclitaxel | Vertical | [63] | ||
| Doxorubicin | Horizontal | [74] | ||
| MDA-MB-231, MCF-7, T47D | Doxorubicin, Paclitaxel, Salinomycin, Thiostrepton | Vertical | [75] | |
| MCF-7 | Doxorubicin | Horizontal | [76] | |
| Horizontal | [38] | |||
| Curcumin, Paclitaxel | Horizontal | [39] | ||
| Cisplatin, Cyclophosphamide, Doxorubicin, Paclitaxel | Vertical | [77] | ||
| Ursolic acid | Vertical | [78] | ||
| THP-1 | Cytarabine | Horizontal | [64] | |
| aPSCs, M2, PANC-1, THP-1, | 5-fluorouracil, Gemcitabine, Oxaliplatin, Paclitaxel | Horizontal | [79] | |
| A431 | Doxorubicin | Horizontal | [67] | |
| A549 | Gemcitabine | Horizontal | [80] | |
| Cisplatin, Doxorubicin | Horizontal | [65] | ||
| Etoposide, Paclitaxel, Vinorelbine | Vertical | [81] | ||
| HT29 | 5-Fluorouracil | Vertical | [82] | |
| SW982 | Celastrol | Horizontal | [83] | |
| U251 | Docetaxel, Temozolomide | Horizontal | [84] | |
| U87 | Carmustine, Temozolomide | Vertical | [85] | |
| U937, Human pancreatic stellate cells (PSCs) | ATRA, Clodrosome, Gemcitabine | Vertical | [69] | |
| Chondrocytes | Resveratrol | Horizontal | [86] | |
| Renal proximal tubular epithelial cell (RPTEC), peritubular capillary endothelial cells (PCEC) | Cisplatin, Cyclosporin A, Gentamycin | Vertical | [70] | |
| Primary human prostate cancer cells | Cisplatin | Horizontal | [87] | |
| Induced hepatic (iHep) cells | Acetaminophen (APAP) | Horizontal | [88] | |
| Human induced pluripotent stem cell (hiPSC)-derived motoneurons | Riluzole | Horizontal | [89] | |
| Human stem cell-derived neurons | Clozapine, Clozapine-N-oxide (CNO) | Vertical | [71] | |
| Hydroxyapatite-PDMS | UMR-106 | Doxorubicin | Horizontal | [90] |
| Polystyrene (PS) | HCT116, SW480 | Axitinib, Bevacizumab, Sunitinib | Horizontal | [43] |
| HUVEC (human umbilical vein endothelial cell) | Bevacizumab, Cetuximab | Horizontal | [91] | |
| Polysulfone | ES bone tumor cell lines | Linsitinib | Horizontal | [19] |
| PMMA | F9 cell line, HeLa cell line, HeLa-LC3 reporter cell line | Cisplatin | Vertical | [41] |
| Perfluoro- polyether | NIH3T3 | Doxorubicin | Horizontal | [44] |
| Glass | HTB-37 | Amoxicillin, Antipyrine, Digoxin, Ketoprofen | Vertical | [45] |
| MDA-MB-231, MCF-10A | Cisplatin, Epirubicin | Horizontal | [92] | |
| Hydrogel | HT-1080 | Doxorubicin | Horizontal/ Vertical | [46] |
| Patient-derived primary glioblastoma multiforme (GBM) cells | Bevacizumab, Temozolomide | Horizontal | [93] | |
| 3D printing resin | A549 | 5-Fluorouracil, Celecoxib, Cyclophosphamide, Doxorubicin | Vertical | [66] |
| Materials | Strains | Test Drugs | Key Points | Ref. |
|---|---|---|---|---|
| PDMS | AU133 agls17(irg-1::gfp), ERT012 zip-2(tm4067), TJ356 zls356(daf-16::gfp,rol-6) | Erythromycin, Gentamicin | Long-term monitoring of the immune responses and evaluating the antibiotic effect of antibiotics | [98] |
| N2, glp-4(bn2ts), sek-1(km4) | Baicalin, Cefepime hydrochloride, Ciprofloxacin, Coptisine, Gypenoside, Meropenem | Automated worm dispensation based micro-sampler | [40] | |
| N2, SJ4100 zcIs13[hsp-6::GFP] | Doxycycline, Tetramisole | High-throughput imaging and analysis for antibiotics test | [109] | |
| N2 | Doxycycline | Observation of the development of life stages during drug testing | [96] | |
| Hydrogen peroxide | Using the priming valve, the flows were controlled | [55] | ||
| Cu2+ | Locomotive behavior analysis on neurotoxicity using a micro-injection droplet microfluidic system | [110] | ||
| N2, DA1316 avr-14(ad1305); avr-15(vu227); glc-1(pk54) VC2937 unc-38(ok2896) CB407 unc-49(e407) CB6147 bus-8(e2882) | Ivermectin, Levamisole, Piperazine | Pharyngeal pumping was analyzed by microfluidic electropharyngeogram (EPG) to confirm the effect of anthelmintic drugs | [33] | |
| LS587 (dys-1(cx18)I; hlh-1(cc561)II), AM725 (rmIs290[unc-54p::Hsa-sod-1(127X)::YFP]), NL5901([unc54p::alphasynuclein::YFP+ unc-119(+)]) | Doxycycline, Levodopa, Melatonin, Pramipexole, Prednisone, Riluzole | Thrashing and muscle morphology was analyzed in the micropillar platform | [32] | |
| N2, GZ1326 (expressing mCherry::H2B to mark chromatin and GFP::PH to mark cell membranes) | Cytochalasin-D | A fully integrated microfluidic approach for studies of C. elegans early embryogenesis | [31] | |
| LX959 vsIs13 [lin-11::pes-10::GFP + lin-15(+)] IV lin-15B(n765) X, JPS67 vxSi38 [Prab-3::huAPP695::unc-54UTR, Cb unc-119(+)] II unc-119(ed3) III vsIs13IV, JPS449 vxSi38 II; unc119(ed3) III; vsIs13 IV; lin15b(n765), vem-1(gk220) X, JPS607 vxSi38 II; unc-119(ed3) III; vxIs13 IV;lin15b(n765) vem-1(ok1058) X | Bexarotene, Norbenzomorphan | 40 trap microchannels at the bottom of each well, enabling the researchers to test more than 3000 worms in a single 96-well platform with high-throughput | [29] | |
| hsp-6::gfp | Doxycycline | Microfluidic platform to observe mother-to-progeny heritable transmission | [99] | |
| N2, CB4108 fog-2(q71)V, AU166 daf-16(mgDf47) I; fog-2(q71) V | Hydrogen peroxide, Sodium chloride | The platform with two 50 arena arrays per chip and an imaging capacity of 600 animals per scanning device | [97] | |
| N2, CL2166 (dvIs19) | CdCl2 | Automated and integrated platform based on C. elegans relieving manual operations on worm dispensing, maintenance, imaging, and endpoint analyses | [50] | |
| PDMS-Glass | SJ4100 (zcIs13[hsp-6p::GFP]) | Doxycycline | Tracked different phenotypic traits of individual C. elegans nematodes throughout their full life cycle | [111] |
| N2, CF1038 (daf-16(mu86)I.), TJ356 (daf-16(zIs356)IV), CF1553 (sod-3(muIs84)), CL2070 (hsp-16.2(dvIs70)) | Caffeic acid phenethylester (propolis) | The combination use of multiple functional units, including micro-pillar, worm responder, a branching network of distribution channels, and microchambers | [54] | |
| N2, RB1169 [oga-1(ok1207)], RB653 [ogt-1(ok430)] | Metformin | A loss of thrashing force following the introduction of glucose into a wild-type worm culture that could be reversed upon treatment with the type 2 diabetes drug metformin | [53] | |
| PDMS-Copper | N2 | Total 37 test drugs including Apigenin, Allomatrine, Baicalin, Epicateching, etc. | In vivo screening strategy combining hierarchically structured biohybrid triboelectric nanogenerators (HB-TENGs) arrays | [30] |
| PDMS-Hydrogel | N2 | Tetramisole | A simple and easy-to-use microfluidic system for automated long-term culturing and phenotyping of C. elegans at single-organism resolution | [94] |
| Hydrogel | N2, CB211 (lev-1(e211)), JR667 (wIs51[SCMp::GFP]; unc-119(e2948)), OH15089 (otIs657[klp-6p::mCherry + flp-3p::mCherry + klp-6p::NLG1::spGFP1-10 + flp-3p::NLG1::spGFP11]) | Tetramisole | “Microswimmer combing” rapidly isolated live small animals on an open-surface array | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.; Kilicarslan You, D.; Jeong, U.; Lee, M.; Kim, E.; Jeon, T.-J.; Kim, S.M. Microfluidics in High-Throughput Drug Screening: Organ-on-a-Chip and C. elegans-Based Innovations. Biosensors 2024, 14, 55. https://doi.org/10.3390/bios14010055
Yoon S, Kilicarslan You D, Jeong U, Lee M, Kim E, Jeon T-J, Kim SM. Microfluidics in High-Throughput Drug Screening: Organ-on-a-Chip and C. elegans-Based Innovations. Biosensors. 2024; 14(1):55. https://doi.org/10.3390/bios14010055
Chicago/Turabian StyleYoon, Sunhee, Dilara Kilicarslan You, Uiechan Jeong, Mina Lee, Eunhye Kim, Tae-Joon Jeon, and Sun Min Kim. 2024. "Microfluidics in High-Throughput Drug Screening: Organ-on-a-Chip and C. elegans-Based Innovations" Biosensors 14, no. 1: 55. https://doi.org/10.3390/bios14010055





