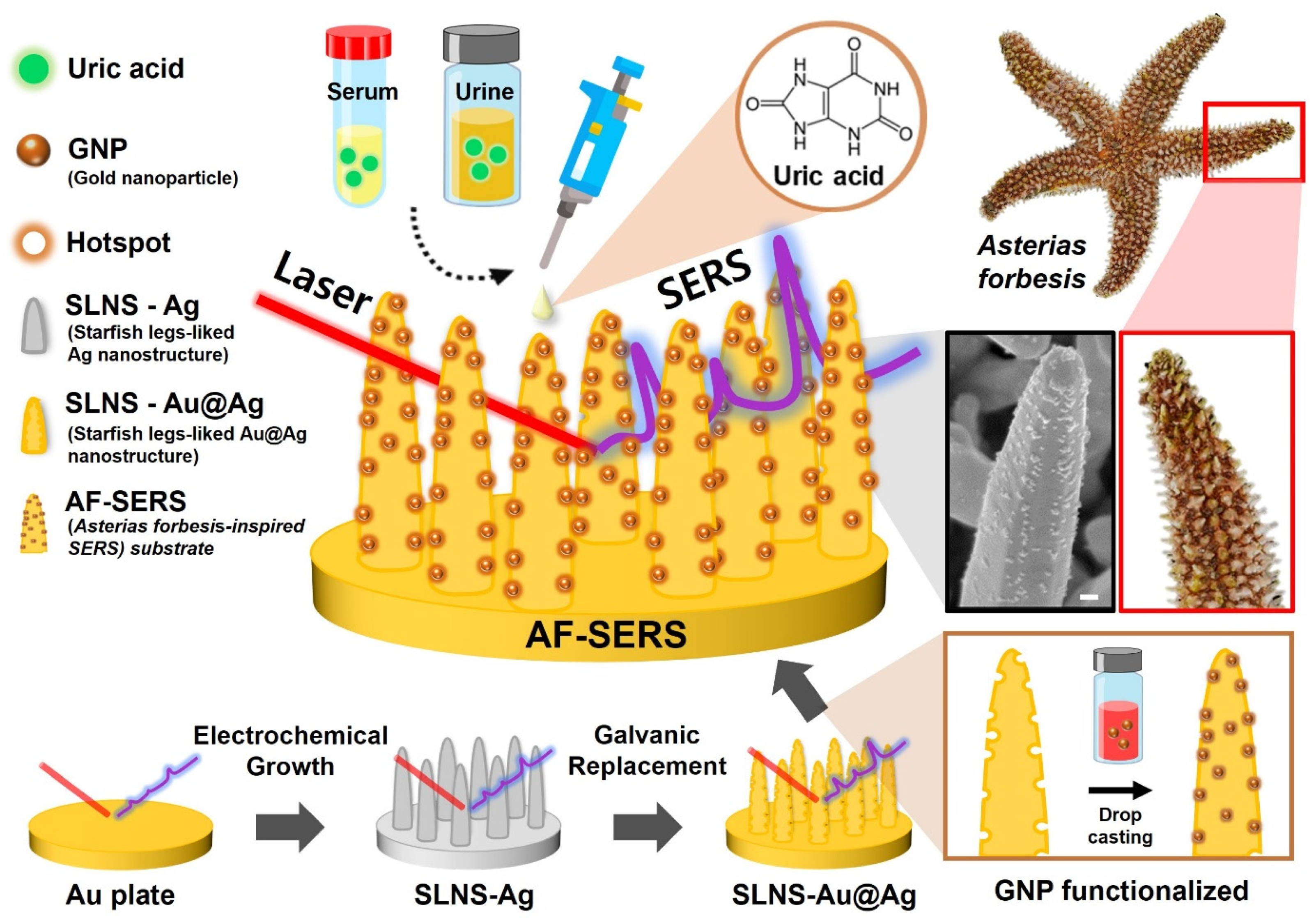
Asterias forbesi-Inspired SERS Substrates for Wide-Range Detection of Uric Acid
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemical Agents
2.2. Uric Acid Detection Strategy Using AF-SERS Substrate
2.3. Synthesis and Optimization of AF-SERS Substrate
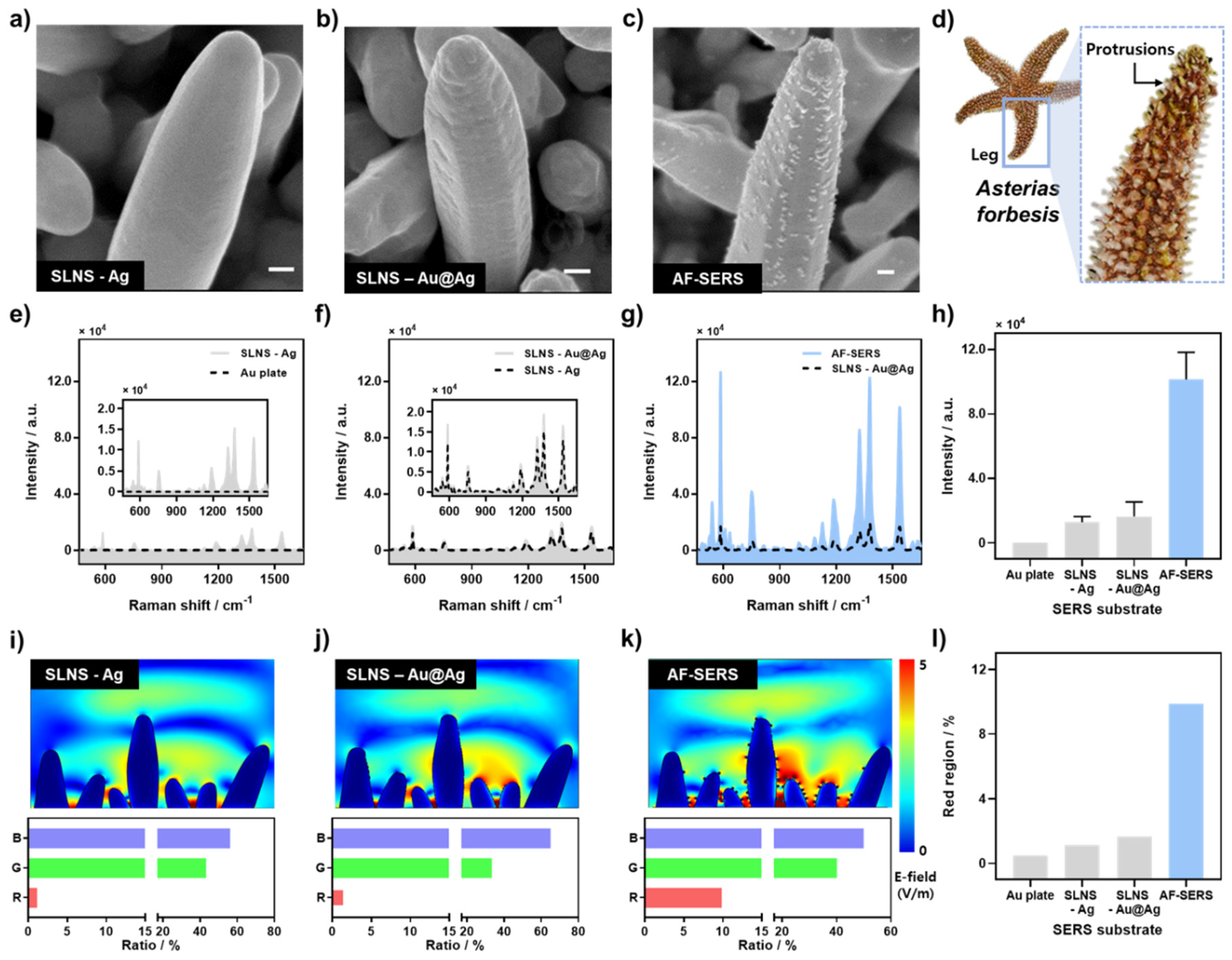
2.4. SEM- and FEM-Based Simulation Data
2.5. SERS Analysis
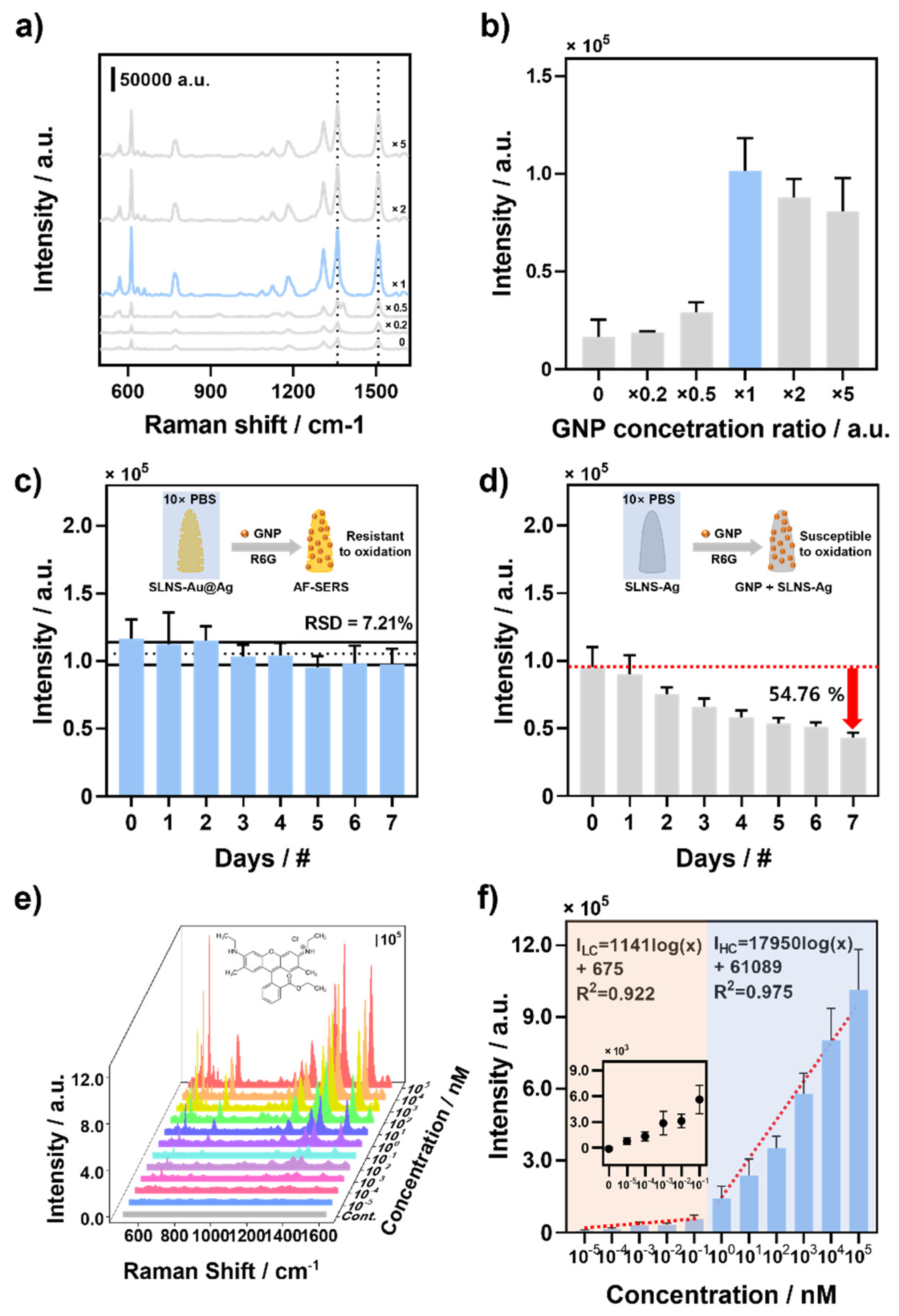
2.6. Optimization of SERS Substrate
2.7. Uric Acid Detection with AF-SERS SERS Sensors
3. Results and Discussion
3.1. Characterization of AF-SERS Substrate
3.2. Optimization and Sensor Performance of AF-SERS Substrate
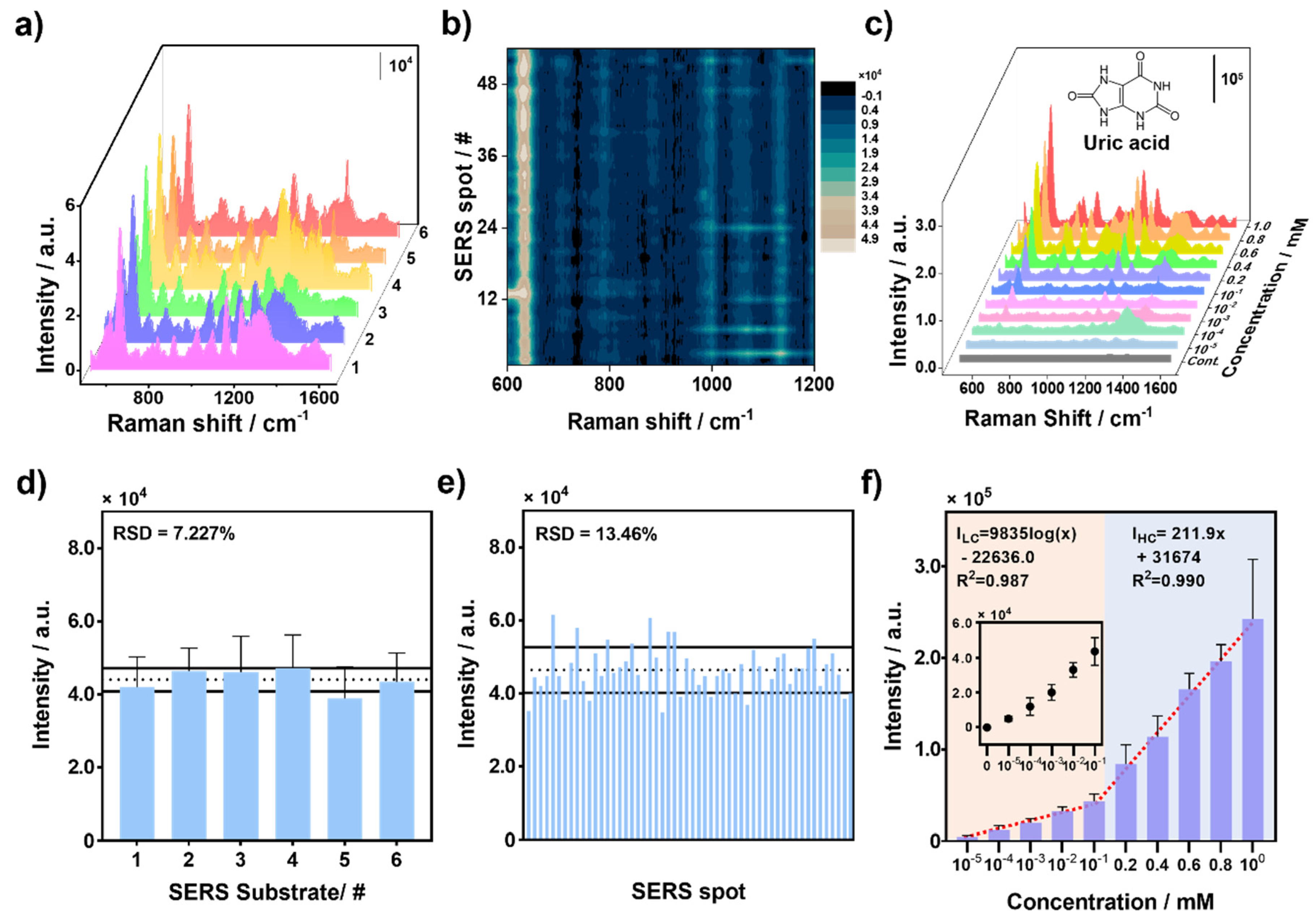
3.3. Performance Evaluation of AF-SERS Substrate with UA
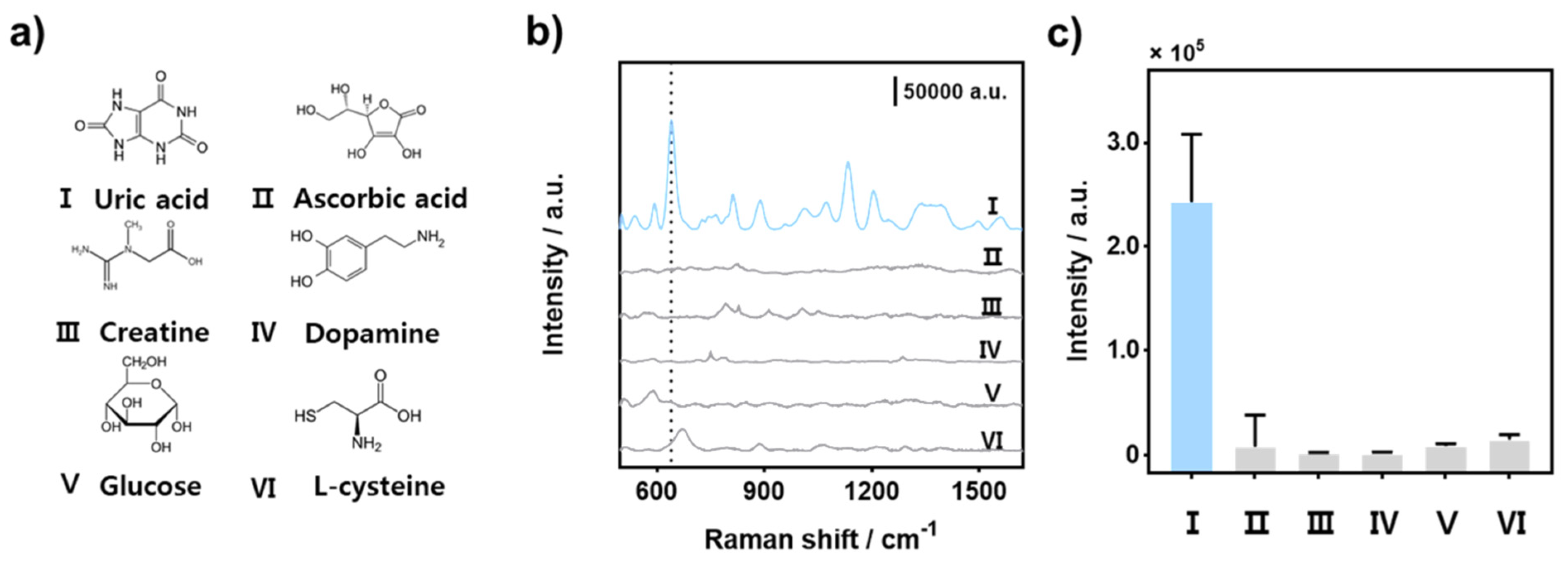
3.4. Detection Selectivity of UA Sensor with AF-SERS
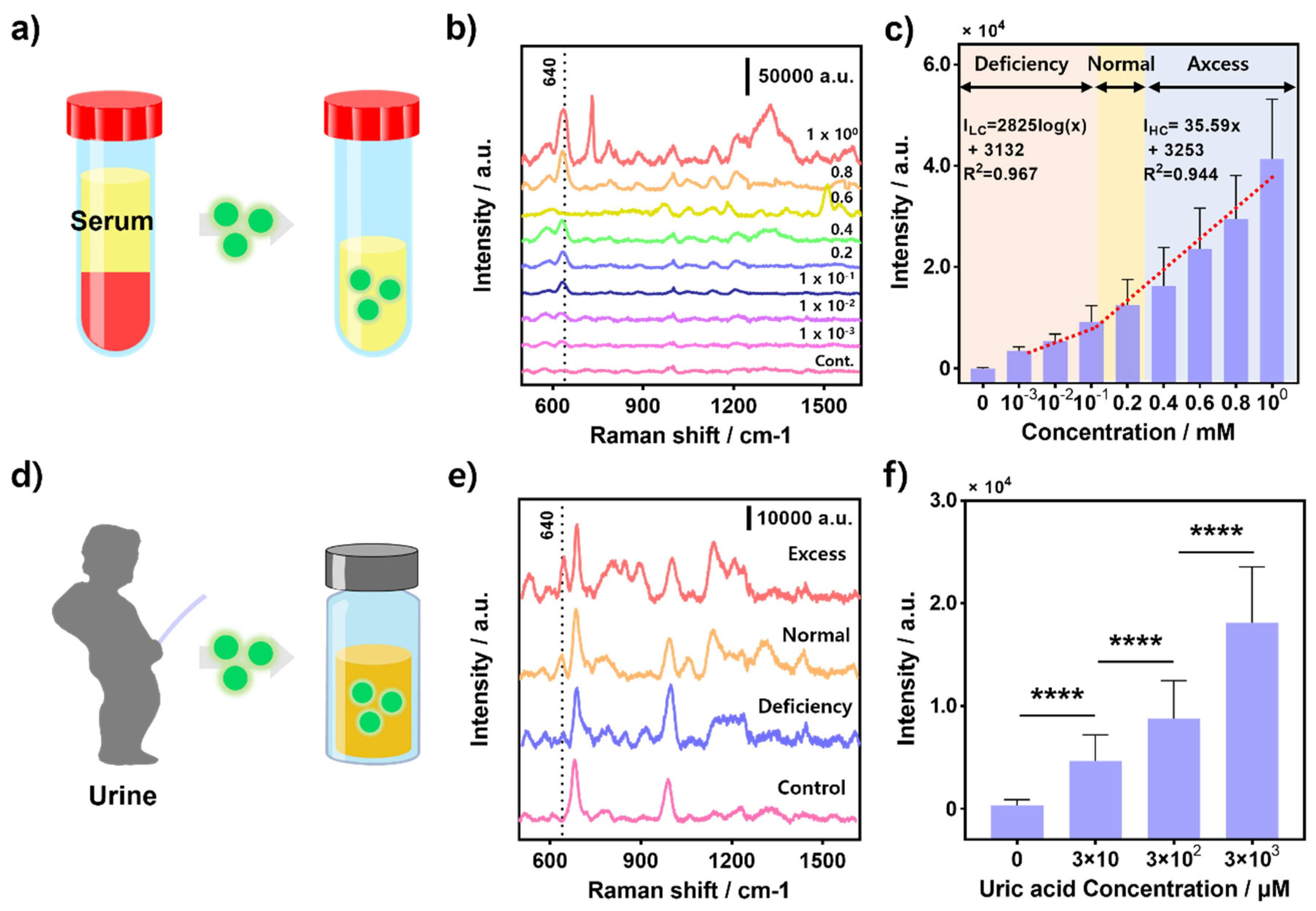
3.5. UA Detection in Real Sample: Human Serum and Urine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of Uric Acid Metabolism and Excretion. Int. J. Cardiol. 2015, 213, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Lytvyn, Y.; Perkins, B.A.; Cherney, D.Z.I. Uric Acid as a Biomarker and a Therapeutic Target in Diabetes. Can. J. Diabetes 2015, 39, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Azmi, N.E.; Ramli, I.; Abdullah, J.; Azmi, M.; Hamid, A.; Sidek, H.; Rahman, S.A.; Ariffin, N.; Yusof, A. A Simple and Sensitive Fluorescence Based Biosensor for the Determination of Uric Acid Using H2O2-Sensitive Quantum Dots/Dual Enzymes. Biosens. Bioelectron. 2014, 67, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Gill, K.D. To Determine the Uric Acid Concentration in Serum and Urine. In Basic Concepts in Clinical Biochemistry: A Practical Guide; Springer: Berlin/Heidelberg, Germany, 2018; pp. 81–84. [Google Scholar] [CrossRef]
- Miake, J.; Hisatome, I.; Tomita, K.; Isoyama, T.; Sugihara, S.; Kuwabara, M.; Ogino, K.; Ninomiya, H. Impact of Hyper- and Hypo-Uricemia on Kidney Function. Biomedicines 2023, 11, 1258. [Google Scholar] [CrossRef] [PubMed]
- Mccarty, D.J.; Hollander, J.L. Identification of Urate Crystals in Gouty Synovial Fluid. Ann. Intern. Med. 1961, 54, 452–460. [Google Scholar] [CrossRef]
- Merriman, T.R.; Dalbeth, N. The Genetic Basis of Hyperuricaemia and Gout. Jt. Bone Spine 2011, 78, 35–40. [Google Scholar] [CrossRef]
- Saito, Y.; Tanaka, A.; Node, K.; Kobayashi, Y. Uric Acid and Cardiovascular Disease: A Clinical Review. J. Cardiol. 2021, 78, 51–57. [Google Scholar] [CrossRef]
- Kanbay, M.; Jensen, T.; Solak, Y.; Le, M.; Roncal-Jimenez, C.; Rivard, C.; Lanaspa, M.A.; Nakagawa, T.; Johnson, R.J. Uric Acid in Metabolic Syndrome: From an Innocent Bystander to a Central Player. Eur. J. Intern. Med. 2016, 29, 3–8. [Google Scholar] [CrossRef]
- Bainbridge, S.A.; Roberts, J.M. Uric Acid as a Pathogenic Factor in Preeclampsia. Placenta 2008, 29, 67–72. [Google Scholar] [CrossRef]
- Grassi, D.; Ferri, L.; Desideri, G.; Di Giosia, P.; Cheli, P.; Del Pinto, R.; Properzi, G.; Ferri, C. Chronic Hyperuricemia, Uric Acid Deposit and Cardiovascular Risk. Curr. Pharm. Des. 2013, 19, 2432–2438. [Google Scholar] [CrossRef]
- Mazzara, F.; Patella, B.; Aiello, G.; O’Riordan, A.; Torino, C.; Vilasi, A.; Inguanta, R. Electrochemical Detection of Uric Acid and Ascorbic Acid Using R-GO/NPs Based Sensors. Electrochim. Acta 2021, 388, 138652. [Google Scholar] [CrossRef]
- Martinez-Pérez, D.; Ferrer, M.L.; Mateo, C.R. A Reagent Less Fluorescent Sol–Gel Biosensor for Uric Acid Detection in Biological Fluids. Anal. Biochem. 2003, 322, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Norazmi, N.; Rasad, Z.R.A.; Mohamad, M.; Manap, H. Uric Acid Detection Using Uv-Vis Spectrometer. IOP Conf. Ser. Mater. Sci. Eng. 2017, 257, 012031. [Google Scholar] [CrossRef]
- Chen, J.C.; Chung, H.H.; Hsu, C.T.; Tsai, D.M.; Kumar, A.S.; Zen, J.M. A Disposable Single-Use Electrochemical Sensor for the Detection of Uric Acid in Human Whole Blood. Sens. Actuators B Chem. 2005, 110, 364–369. [Google Scholar] [CrossRef]
- Omar, M.N.; Salleh, A.B.; Lim, H.N.; Ahmad Tajudin, A. Electrochemical Detection of Uric Acid via Uricase-Immobilized Graphene Oxide. Anal. Biochem. 2016, 509, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Xiong, L.; Yu, L.; Wu, D.; Yang, C.; Xiao, Y. An Enzyme-Free Fluorescent Sensing Platform for the Detection of Uric Acid in Human Urine. J. Lumin. 2021, 236, 118076. [Google Scholar] [CrossRef]
- Das, S.C.; Pandey, R.R.; Devkota, T.; Chusuei, C.C. Raman Spectroscopy as an Assay to Disentangle Zinc Oxide Carbon Nanotube Composites for Optimized Uric Acid Detection. Chemosensors 2018, 6, 65. [Google Scholar] [CrossRef]
- Pucetaite, M.; Velicka, M.; Pilipavicius, J.; Beganskiene, A.; Ceponkus, J.; Sablinskas, V. Uric Acid Detection by Means of SERS Spectroscopy on Dried Ag Colloidal Drops. J. Raman Spectrosc. 2016, 47, 681–686. [Google Scholar] [CrossRef]
- Ansah, I.B.; Lee, W.C.; Mun, C.W.; Rha, J.J.; Jung, H.S.; Kang, M.; Park, S.G.; Kim, D.H. In Situ Electrochemical Surface Modification of Au Electrodes for Simultaneous Label-Free SERS Detection of Ascorbic Acid, Dopamine and Uric Acid. Sens. Actuators B Chem. 2022, 353, 131196. [Google Scholar] [CrossRef]
- Manbohi, A.; Ahmadi, S.H. Sensitive and Selective Detection of Dopamine Using Electrochemical Microfluidic Paper-Based Analytical Nanosensor. Sens. Biosens. Res. 2019, 23, 100270. [Google Scholar] [CrossRef]
- Murugan, N.; Jerome, R.; Preethika, M.; Sundaramurthy, A.; Sundramoorthy, A.K. 2D-Titanium Carbide (MXene) Based Selective Electrochemical Sensor for Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. J. Mater. Sci. Technol. 2021, 72, 122–131. [Google Scholar] [CrossRef]
- Tavakkoli Yaraki, M.; Tukova, A.; Wang, Y. Emerging SERS Biosensors for the Analysis of Cells and Extracellular Vesicles. Nanoscale 2022, 14, 15242–15268. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Lin, D.; Lai, S.; Tao, H.; Chen, T.; Peng, M.; Qiu, S.; Feng, S. Highly Sensitive and Reliable Detection of MicroRNA for Clinically Disease Surveillance Using SERS Biosensor Integrated with Catalytic Hairpin Assembly Amplification Technology. Biosens. Bioelectron. 2022, 208, 114236. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Cai, R.; Liao, Y.; You, R.; Lu, Y. Two-Dimensional Glass/p-ATP/Ag NPs as Multifunctional SERS Substrates for Label-Free Quantification of Uric Acid in Sweat. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 296, 122631. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, J.; Lee, G.; Kim, W.; Park, J. Detection of Chlorpyrifos Using Bio-Inspired Silver Nanograss. Materials 2022, 15, 3454. [Google Scholar] [CrossRef] [PubMed]
- Bang, D.; Chang, Y.W.; Park, J.; Lee, T.; Park, J.; Yeo, J.S.; Kim, E.K.; Yoo, K.H.; Huh, Y.M.; Haam, S. One-Step Electrochemical Fabrication of Vertically Self-Organized Silver Nanograss. J. Mater. Chem. A Mater. 2013, 1, 4851–4857. [Google Scholar] [CrossRef]
- Matikainen, A.; Nuutinen, T.; Itkonen, T.; Heinilehto, S.; Puustinen, J.; Hiltunen, J.; Lappalainen, J.; Karioja, P.; Vahimaa, P. Atmospheric Oxidation and Carbon Contamination of Silver and Its Effect on Surface-Enhanced Raman Spectroscopy (SERS). Sci. Rep. 2016, 6, 37192. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Lai, F.J.; Chou, H.L.; Lu, W.T.; Hwang, B.J.; Brolo, A.G. Surface-Enhanced Raman Scattering (SERS) from Au:Ag Bimetallic Nanoparticles: The Effect of the Molecular Probe. Chem. Sci. 2012, 4, 509–515. [Google Scholar] [CrossRef]
- Kim, W.; Park, J.; Kim, W.; Jo, S.; Kim, M.; Kim, C.; Park, H.; Bang, D.; Lee, W.; Park, J. Bio-Inspired Ag Nanovilli-Based Sandwich-Type SERS Aptasensor for Ultrasensitive and Selective Detection of 25-Hydroxy Vitamin D3. Biosens. Bioelectron. 2021, 188, 113341. [Google Scholar] [CrossRef]
- Kim, W.; Lee, W.; Park, H.; Park, J.; Kim, W.; Kang, B.; Choi, E.; Kim, C.S.; Park, J.O.; Lee, G.; et al. Biomimetic Nano-Pine-Pollen Structure-Based Surface-Enhanced Raman Spectroscopy Sensing Platform for the Hypersensitive Detection of Toxicants: Cadmium and Amyloid. ACS Sustain. Chem. Eng. 2022, 10, 3180–3190. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Seo, M.; Ha, J.W. Effective Surface-Enhanced Raman Scattering of Randomly Branched Gold Nano-Urchins with Rhodamine 6G as Raman Reporters. Microchem. J. 2018, 140, 47–51. [Google Scholar] [CrossRef]
- Ujihara, M.; Dang, N.M.; Imae, T. Surface-Enhanced Resonance Raman Scattering of Rhodamine 6G in Dispersions and on Films of Confeito-Like Au Nanoparticles. Sensors 2017, 17, 2563. [Google Scholar] [CrossRef] [PubMed]
- Gualerzi, A.; Picciolini, S.; Meloni, M.; Lax, A.; Silani, V.; Bedoni, M. Human Salivary Raman Fingerprint as Biomarker for the Diagnosis of Amyotrophic Lateral Sclerosis. Sci. Rep. 2020, 10, 10175. [Google Scholar] [CrossRef]
- Cheng, Z.Q.; Li, Z.W.; Yao, R.; Xiong, K.W.; Cheng, G.L.; Zhou, Y.H.; Luo, X.; Liu, Z.M. Improved SERS Performance and Catalytic Activity of Dendritic Au/Ag Bimetallic Nanostructures Based on Ag Dendrites. Nanoscale Res. Lett. 2020, 15, 117. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, L.J.; Guo, H.Y.X.; Li, R.; Feng, J. Preparation of 3D Nano Silver Trees/Sea Urchin-like Gold and SERS Detection of Uric Acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 305, 123464. [Google Scholar] [CrossRef]
- Jia, F.; Barber, E.; Turasan, H.; Seo, S.; Dai, R.; Liu, L.; Li, X.; Bhunia, A.K.; Kokini, J.L. Detection of Pyocyanin Using a New Biodegradable SERS Biosensor Fabricated Using Gold Coated Zein Nanostructures Further Decorated with Gold Nanoparticles. J. Agric. Food Chem. 2019, 67, 4603–4610. [Google Scholar] [CrossRef] [PubMed]
- Atta, S.; Vo-Dinh, T. Ultra-Trace SERS Detection of Cocaine and Heroin Using Bimetallic Gold–Silver Nanostars (BGNS-Ag). Anal. Chim. Acta 2023, 1251, 340956. [Google Scholar] [CrossRef]
- Lu, H.; Jin, M.; Ma, Q.; Yan, Z.; Liu, Z.; Wang, X.; Akinoglu, E.M.; van den Berg, A.; Zhou, G.; Shui, L. Ag Nano-Assemblies on Si Surface via CTAB-Assisted Galvanic Reaction for Sensitive and Reliable Surface-Enhanced Raman Scattering Detection. Sens. Actuators B Chem. 2020, 304, 127224. [Google Scholar] [CrossRef]
- Wen, H.; Inose, T.; Hirai, K.; Akashi, T.; Sugioka, S.; Li, J.; Peeters, W.; Fron, E.; Fortuni, B.; Nakata, Y.; et al. Gold-Coated Silver Nanowires for Long Lifetime AFM-TERS Probes. Nanoscale 2022, 14, 5439–5446. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoint, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Yan, Q.; Zhi, N.; Yang, L.; Xu, G.; Feng, Q.; Zhang, Q.; Sun, S. A Highly Sensitive Uric Acid Electrochemical Biosensor Based on a Nano-Cube Cuprous Oxide/Ferrocene/Uricase Modified Glassy Carbon Electrode. Sci. Rep. 2020, 10, 10607. [Google Scholar] [CrossRef]
- Tan, Y.; Qi, M.; Jiang, H.; Wang, B.; Zhang, X. Determination of Uric Acid in Serum by SERS System Based on VO-MnCo2O4/Ag Nanozyme. Anal. Chim. Acta 2023, 1274, 341584. [Google Scholar] [CrossRef]
- Baqi, O.; Shatery, A.; Omer, K.M. Selectivity Enhancement for Uric Acid Detection via In Situ Preparation of Blue Emissive Carbon Dots Entrapped in Chromium Metal—Organic Frameworks. ACS Omega 2022, 7, 16576–16583. [Google Scholar] [CrossRef]
- Putra, B.R.; Nisa, U.; Heryanto, R.; Khalil, M.; Khoerunnisa, F.; Ridhova, A.; Thaha, Y.N.; Marken, F.; Wahyuni, W.T. Selective Non-Enzymatic Uric Acid Sensing in the Presence of Dopamine: Electropolymerized Poly-Pyrrole Modified with a Reduced Graphene Oxide/PEDOT:PSS Composite. Analyst 2022, 147, 5334–5346. [Google Scholar] [CrossRef]
- Pang, X.; Yan, R.; Li, L.; Wang, P.; Zhang, Y.; Liu, Y.; Liu, P.; Dong, W.; Miao, P.; Mei, Q. Non-Doped and Non-Modified Carbon Dots with High Quantum Yield for the Chemosensing of Uric Acid and Living Cell Imaging. Anal. Chim. Acta 2022, 1199, 339571. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Y.; Xiao, C.; Xiao, S.; Wang, C.; Nie, Q.; Xu, P.; Chen, J.; You, R.; Zhang, G.; et al. Flexible SERS Wearable Sensor Based on Nanocomposite Hydrogel for Detection of Metabolites and PH in Sweat. Chem. Eng. J. 2023, 474, 145953. [Google Scholar] [CrossRef]
- Ruggiero, C.; Cherubini, A.; Ble, A.; Bos, A.J.G.; Maggio, M.; Dixit, V.D.; Lauretani, F.; Bandinelli, S.; Senin, U.; Ferrucci, L. Uric Acid and Inflammatory Markers. Eur. Heart J. 2006, 27, 1174–1181. [Google Scholar] [CrossRef]
- Huang, C.Y.; Hsiao, H.C. Integrated EC-SERS Chip with Uniform Nanostructured EC-SERS Active Working Electrode for Rapid Detection of Uric Acid. Sensors 2020, 20, 7066. [Google Scholar] [CrossRef]
- Bhattacharjee, G.; Majumder, S.; Senapati, D.; Banerjee, S.; Satpati, B. Core-Shell Gold @silver Hollow Nanocubes for Higher SERS Enhancement and Non-Enzymatic Biosensor. Mater. Chem. Phys. 2020, 239, 122113. [Google Scholar] [CrossRef]
- Li, J.; Cui, X.; Yang, X.; Qiu, Y.; Li, Y.; Cao, H.; Wang, D.; He, W.; Feng, Y.; Yang, Z. Quantification of Uric Acid Concentration in Tears by Using PDMS Inverse Opal Structure Surface-Enhanced Raman Scattering Substrates: Application in Hyperuricemia. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 278, 121326. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.; Li, Y.; Zhang, Y. Top-down Produced CdSe Quantum Dots as an Ultrasensitive SERS Platform for the Detection of Uric Acid. Mater. Chem. Front. 2023, 7, 1624–1632. [Google Scholar] [CrossRef]
- Westley, C.; Xu, Y.; Carnell, A.J.; Turner, N.J.; Goodacre, R. Label-Free Surface Enhanced Raman Scattering Approach for High-Throughput Screening of Biocatalysts. Anal Chem. 2016, 88, 5898–5903. [Google Scholar] [CrossRef]
- Goodall, B.L.; Robinson, A.M.; Brosseau, C.L. Electrochemical- Surface Enhanced Raman Spectroscopy (E-SERS) of Uric Acid: A Potential Rapid Diagnostic Method for Early Preeclampsia Detection. Phys. Chem. Chem. Phys. 2013, 15, 1382–1388. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Wang, T.J.; Kumar, E.A.; Duraisamy, N. An-Ting Lee An Electrochemical Platform Based on Yttrium Oxide/Boron Nitride Nanocomposite for the Detection of Dopamine. Sens. Actuators B Chem. 2021, 349, 130787. [Google Scholar] [CrossRef]
- Zhu, X.; Xuan, L.; Gong, J.; Liu, J.; Wang, X.; Xi, F.; Chen, J. Three-Dimensional Macroscopic Graphene Supported Vertically-Ordered Mesoporous Silica-Nanochannel Film for Direct and Ultrasensitive Detection of Uric Acid in Serum. Talanta 2022, 238, 123027. [Google Scholar] [CrossRef]
- Wang, H.; Xie, A.; Li, S.; Wang, J.; Chen, K.; Su, Z.; Song, N.; Luo, S. Three-Dimensional g-C3N4/MWNTs/GO Hybrid Electrode as Electrochemical Sensor for Simultaneous Determination of Ascorbic Acid, Dopamine and Uric Acid. Anal Chim. Acta 2022, 1211, 339907. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Yang, Q.S.; Wang, J.; Fu, D.L.; Jiang, D.K. A Novel 3D Coordination Polymer Constructed by Dual-Ligand for Highly Sensitive Detection of Purine Metabolite Uric Acid. Spectrochim. Acta A Mol Biomol. Spectrosc. 2021, 262, 120065. [Google Scholar] [CrossRef]
- Li, F.; Chen, J.; Wen, J.; Peng, Y.; Tang, X.; Qiu, P. Ratiometric Fluorescence and Colorimetric Detection for Uric Acid Using Bifunctional Carbon Dots. Sens. Actuators B Chem. 2022, 369, 132381. [Google Scholar] [CrossRef]
- Sumalatha, V.; Anujya, C.; Balchander, V.; Dhanalaxmi, B.; Pradeep Kumar, M.; Ayodhya, D. Hydrothermal Fabrication of N-CeO2/p-CuS Heterojunction Nanocomposite for Enhanced Photodegradation of Pharmaceutical Drugs in Wastewater under Visible-Light and Fluorometric Sensor for Detection of Uric Acid. Inorg. Chem. Commun. 2023, 155, 110962. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, L.; Zhan, D.S.; Jiang, W.R.; Fodjo, E.K.; Hafez, M.E.; Zhang, Y.M.; Zhao, H.; Qian, R.C.; Li, D.W. Electrochemically Renewable SERS Sensor: A New Platform for the Detection of Metabolites Involved in Peroxide Production. Biosens. Bioelectron. 2021, 175, 112918. [Google Scholar] [CrossRef] [PubMed]
- Durai, L.; Badhulika, S. A Wearable PVA Film Supported TiO2 Nanoparticles Decorated NaNbO3 Nanoflakes-Based SERS Sensor for Simultaneous Detection of Metabolites and Biomolecules in Human Sweat Samples. Adv. Mater. Interfaces 2022, 9, 2200146. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Chai, K.; Kim, W.; Park, J.; Lee, W.; Park, J. Asterias forbesi-Inspired SERS Substrates for Wide-Range Detection of Uric Acid. Biosensors 2024, 14, 8. https://doi.org/10.3390/bios14010008
Park H, Chai K, Kim W, Park J, Lee W, Park J. Asterias forbesi-Inspired SERS Substrates for Wide-Range Detection of Uric Acid. Biosensors. 2024; 14(1):8. https://doi.org/10.3390/bios14010008
Chicago/Turabian StylePark, Hyunjun, Kyunghwan Chai, Woochang Kim, Joohyung Park, Wonseok Lee, and Jinsung Park. 2024. "Asterias forbesi-Inspired SERS Substrates for Wide-Range Detection of Uric Acid" Biosensors 14, no. 1: 8. https://doi.org/10.3390/bios14010008




