Sensing Magnetic Directions in Birds: Radical Pair Processes Involving Cryptochrome
Abstract
:1. Introduction
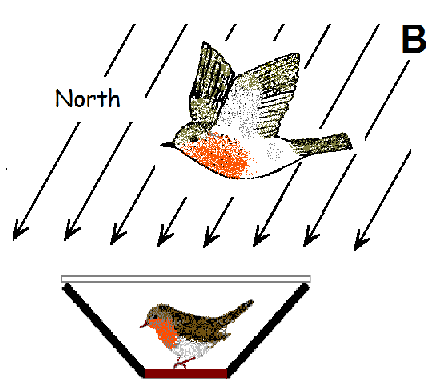
2. Demonstrating Magnetic Compass Orientation in Birds

3. Characteristics of the Avian Magnetic Compass
3.1. The Functional Window

3.2. The Inclination Compass

3.3. Wavelength Dependency of Magnetic Orientation

4. Magnetoreception Based on Spin-Chemical Processes
4.1. The Radical Pair Model

4.2. Testing the Model

4.3. Further Analysis of the Radical Pair Mechanism

5. The Receptor Molecule
5.1. Localization of Cryptochrome 1a

5.2. Light-Activation of Cryptochrome 1a

5.3. The Flavin Cycle and the Radical Pairs

6. Processing Magnetic Directional Information
Acknowledgments
Conflicts of Interest
References
- Wiltschko, W. Über den Einfluß statischer Magnetfelder auf die Zugorientierung der Rotkehlchen (Erithacus rubecula). Z. Tierpsychol. 1968, 25, 537–558. [Google Scholar] [CrossRef]
- Wiltschko, W.; Wiltschko, R. Magnetoreception in birds: Two receptors for two different tasks. J. Ornithol. 2007, 148 (Suppl. 1), S61–S76. [Google Scholar] [CrossRef]
- Keeton, W.T. Magnets interfere with pigeon homing. Proc. Natl. Acad. Sci. USA 1971, 68, 102–106. [Google Scholar] [CrossRef]
- Walcott, C.; Green, R.F. Orientation of homing pigeons altered by a change in the direction of an applied magnetic field. Science 1974, 184, 180–182. [Google Scholar]
- Gudmundson, G.A.; Sandberg, R. Sanderlings (Calidris alba) have a magnetic compass: Orientation experiments during spring migration in Iceland. J. Exp. Biol. 2000, 203, 3137–3144. [Google Scholar]
- Freire, R.; Munro, U.H.; Rogers, L.J.; Wiltschko, R.; Wiltschko, W. Chicken orient using a magnetic compass. Curr. Biol. 2005, 15, R620–R621. [Google Scholar] [CrossRef]
- Wiltschko, R.; Wiltschko, W. Magnetic Orientation in Animals; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Thalau, P.; Ritz, T.; Burda, H.; Wegner, R.E.; Wiltschko, R. The magnetic compass mechanisms of birds and rodents are based on different physical principles. J. R. Soc. Interface 2006, 3, 583–587. [Google Scholar] [CrossRef]
- Wiltschko, W.; Wiltschko, R. Magnetic orientation and magnetoreception in birds and other animals. J. Comp. Physiol. A 2005, 191, 675–693. [Google Scholar] [CrossRef]
- Begall, S.B.; Burda, H.; Malkemper, E.O. Magnetoreception in mammals. Adv. Study Behav. 2014, 46, 45–88. [Google Scholar] [CrossRef]
- Emlen, S.T.; Emlen, J.T. A technique for recording migratory orientation of captive birds. Auk 1966, 84, 361–367. [Google Scholar] [CrossRef]
- Voss, J.; Keary, N.; Bischof, H.J. The use of the geomagnetic field for short distance orientation in zebra finches. Behaviour 2007, 18, 1053–1057. [Google Scholar]
- Wiltschko, W.; Gesson, M.; Wiltschko, R. Magnetic compass orientation of European Robins under 565 nm Green light. Naturwissenschaften 2001, 88, 387–390. [Google Scholar] [CrossRef]
- Batschelet, E. Circular Statistics in Biology; Academic Press: London, UK, 1981. [Google Scholar]
- Wiltschko, W. Further analysis of the magnetic compass of migratory birds. In Animal Migration, Navigation, and Homing; Schmidt-Koenig, K., Keeton, W.T., Eds.; Springer Verlag: Berlin/Heidelberg, Germany, 1978; pp. 302–310. [Google Scholar]
- Wiltschko, W.; Freire, R.; Munro, U.; Ritz, T.; Rogers, L.; Thalau, P.; Wiltschko, R. The magnetic compass of domestic chickens, Gallus gallus. J. Exp. Biol. 2007, 210, 2300–2310. [Google Scholar] [CrossRef]
- Wiltschko, W.; Stapput, K.; Thalau, P.; Wiltschko, R. Avian magnetic compass: Fast adjustment to intensities outside the normal functional window. Naturwissenschaften 2006, 93, 300–304. [Google Scholar] [CrossRef]
- Winklhofer, M.; Dylda, E.; Thalau, P.; Wiltschko, W.; Wiltschko, R. Avian magnetic compass can be tuned to anomalously low magnetic intensities. Proc. R. Soc. B 2013, 280. [Google Scholar] [CrossRef]
- Wiltschko, W.; Wiltschko, R. Magnetic compass of European Robins. Science 1972, 176, 62–64. [Google Scholar]
- Wiltschko, R.; Wiltschko, W. Evidence for the use of magnetic outward-journey information in homing pigeons. Naturwissenschaften 1978, 65, 112–113. [Google Scholar] [CrossRef]
- Wiltschko, W.; Wiltschko, R. Disorientation of inexperienced young pigeons after transportation in total darkness. Nature 1981, 291, 433–435. [Google Scholar] [CrossRef]
- Stapput, K.; Thalau, P.; Wiltschko, R.; Wiltschko, W. Orientation of birds in total darkness. Curr. Biol. 2008, 18, 602–606. [Google Scholar] [CrossRef]
- Wiltschko, R.; Stapput, K.; Thalau, P.; Wiltschko, W. Directional orientation of birds by the magnetic field under different light conditions. J. R. Soc. Interface 2010, 7 (Suppl. 2), S163–S178. [Google Scholar] [CrossRef]
- Wiltschko, W.; Munro, U.; Ford, U.; Wiltschko, R. Red light disrupts magnetic orientation of migratory birds. Nature 1993, 364, 525–527. [Google Scholar] [CrossRef]
- Wiltschko, W.; Wiltschko, R. Migratory orientation of European robins is affected by the wavelength of light as well as by a magnetic pulse. J. Comp. Physiol. A 1995, 177, 363–369. [Google Scholar]
- Wiltschko, W.; Wiltschko, R. The effect of yellow and blue light on magnetic compass orientation in European Robins. Erithacus rubecula. J. Comp. Physiol. A 1999, 184, 295–299. [Google Scholar] [CrossRef]
- Rappl, R.; Wiltschko, R.; Weindler, P.; Berthold, P.; Wiltschko, W. Orientation of Garden Warblers, Sylvia borin, under monochromatic light of various wavelengths. Auk 2000, 117, 256–260. [Google Scholar] [CrossRef]
- Muheim, R.; Bäckman, J.; Åkesson, S. Magnetic compass orientation in European Robins is dependent on both wavelengths and intensity of light. J. Exp. Biol. 2002, 205, 3845–3856. [Google Scholar]
- Wiltschko, R.; Denzau, S.; Gehring, D.; Thalau, P.; Wiltschko, W. Magnetic orientation of migratory robins, Erithacus rubecula, under long-wavelength light. J. Exp. Biol. 2011, 214, 3096–3101. [Google Scholar] [CrossRef]
- Wiltschko, R.; Munro, U.; Ford, H.; Stapput, K.; Thalau, P.; Wiltschko, W. Orientation of migratory birds under ultraviolet light. J. Comp. Physiol. A 2014, 200, 399–407. [Google Scholar] [CrossRef]
- Wiltschko, R; Wiltschko, W. Pigeon homing: Effect of various wavelength of light during displacement. Naturwissenschaften 1998, 85, 164–167. [Google Scholar] [CrossRef]
- Wiltschko, R.; Stapput, K.; Bischof, H.J.; Wiltschko, W. Light-dependent magnetoreception in birds: Increasing intensity of monochromatic light changes the nature of the response. Front. Zool. 2007, 4. [Google Scholar] [CrossRef]
- Schulten, K.; Swenberg, C.; Weller, A. A biomagnetic sensory mechanism based on magnetic field modulated coherent electron spin motion. Z. Phys. Chem. NF 1978, 111, 1–5. [Google Scholar]
- Schulten, K.; Windemuth, A. Model for a Physiological Magnetic Compass. In Biophysical Effects of Steady Magnetic Fields; Maret, G., Boccara, N., Kiepenheuer, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1986; Volume 11, pp. 99–106. [Google Scholar]
- Ritz, T.; Adem, S.; Schulten, K. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 2000, 78, 797–718. [Google Scholar]
- Maeda, K.; Henbest, K.B.; Cintolesi, F.; Kuprov, I.; Rodgers, C.T.; Liddell, P.A.; Gust, D.; Timmel, C.R.; Hore, P.J. Chemical compass model of avian magnetoreception. Nature 2008, 453, 387–390. [Google Scholar] [CrossRef]
- Efimova, O.; Hore, P.J. Role of exchange and dipolar interactions in the radical pair model of the avian magnetic compass. Biophys. J. 2008, 94, 1565–1574. [Google Scholar] [CrossRef]
- Rodgers, C.T.; Hore, P.J. Chemical magnetoreception in birds: The radical pair mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 353–360. [Google Scholar] [CrossRef]
- Ritz, T. Quantum effects in biology: Bird navigation. Proced. Chem. 2011, 3, 262–275. [Google Scholar] [CrossRef]
- Maeda, K.; Robinson, A.J.; Henbest, K.B.; Hogben, H.J.; Biskup, T.; Ahmad, M.; Schleicher, E.; Weber, S.; Timmel, C.R.; Hore, P.J. Magnetically sensitive light-induced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor. Proc. Natl. Acad. Sci. USA 2012, 109, 4774–4779. [Google Scholar] [CrossRef]
- Ritz, T. Disrupting magnetic compass orientation with radio frequency oscillating fields. In Orientation & Navigation—Birds, Humans & other Animals. Proceedings of the 4th International Conference on Animal Navigation 2001; St. Anne’s College: Oxford, UK, 2001; paper 4. [Google Scholar]
- Henbest, K.B.; Kukura, P.; Rodgers, C.T.; Hore, J.P.; Timmel, C.R. Radio frequency magnetic field effects on a radical recombination reaction: A diagnostic test for the radical pair mechanism. J. Am. Chem. Soc. 2004, 126, 8102–8103. [Google Scholar] [CrossRef]
- Canfield, J.M.; Belford, R.L.; Debrunner, P.G.; Schulten, K.J. A perturbation theory treatment of oscillation magnetic fields in the radical pair mechanism. Chem. Phys. 1994, 182, 1–18. [Google Scholar] [CrossRef]
- Ritz, T.; Thalau, P.; Phillips, J.B.; Wiltschko, R.; Wiltschko, W. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature 2004, 429, 177–180. [Google Scholar] [CrossRef]
- Thalau, P.; Ritz, T.; Stapput, K.; Wiltschko, R.; Wiltschko, W. Magnetic compass orientation of migratory birds in the presence of a 1.315 MHz oscillating field. Naturwissenschaften 2005, 92, 86–90. [Google Scholar] [CrossRef]
- Keary, N.; Ruploh, T.; Voss, J.; Thalau, P.; Wiltschko, R.; Wiltschko, W.; Bischof, H.J. Oscillating magnetic field disrupts magnetic orientation in Zebra finches, Taeniopygia guttata. Front. Zool. 2009, 6. [Google Scholar] [CrossRef]
- Engels, S.; Schneider, N.-L.; Lefled, N.; Hein, C.M.; Zapka, M.; Michalik, A.; Elbers, D.; Kittel, A.; Hore, P.J.; Mouritsen, H. Anthropogenic electromagnetic noise disrupts magnetic compass orientation in a migratory birds. Nature 2014, 509, 353–356. [Google Scholar] [CrossRef]
- Ritz, T.; Wiltschko, R.; Hore, P.J.; Rodgers, C.T.; Stapput, K.; Thalau, P.; Timmel, C.R; Wiltschko, W. Magnetic compass of birds is based on a molecule with optimal directional sensitivity. Biophys. J. 2009, 96, 3451–3457. [Google Scholar] [CrossRef]
- Ritz, T.; Ahmad., M.; Mouritsen, H.; Wiltschko, R.; Wiltschko, W. Photoreceptor-based magnetoreception: Optimal design of receptor molecules, cells, and neural processing. J. R. Soc. Interface 2010, 7 (Suppl. 2), S135–S146. [Google Scholar] [CrossRef]
- Lee, A.A; Lau, J.C.S.; Hodgen, H.J.; Biskup, T.; Kattnig, D.R.; Hore, P.J. Alternative radical pairs for cryptochrome-based magnetoreception. J. R. Soc. Interface 2014, 11. [Google Scholar] [CrossRef]
- Giovani, B.; Byrdin, M.; Ahmad, M.; Brettel, K. Light-induced electron transfer in a cryptochrome blue-light photoreceptor. Nature Struct. Biol. 2003, 6, 489–490. [Google Scholar]
- Ahmad, M.; Cashmore, A.R. Hy4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 1993, 366, 162–166. [Google Scholar] [CrossRef]
- Lin, C.; Todo, T. The cryptochromes. Genome Biol. 2005, 6. [Google Scholar] [CrossRef] [Green Version]
- Chaves, I.; Pokorny, R.; Byrdin, M.; Hoang, N.; Ritz, T.; Brettel, K.; Essen, L.-O.; van der Horst, G.T.J.; Batschauer, A.; Ahmad, M. The cryptochromes: Blue light photoreceptors in plants and animals. Annu. Rev. Plant. Biol. 2011, 62, 335–364. [Google Scholar] [CrossRef]
- Öztürk, N.; Song, S.-H.; Öztürk, S.; Selby, C.P.; Morrison, L.; Partch, C.; Zhong, D.; Sancar, A. Structure and function of animal cryptochromes. Cold Spring Harbor Symp. Quant. Biol. 2007, 72, 119–129. [Google Scholar] [CrossRef]
- Haque, R.; Chaurasia, S.S.; Wessel, J.H.; Iovome, P.M. Dual regulation of cryptochrome 1 mRNA expression in chicken retina by light and circadian oscillators. Neuro Report 2002, 13, 2247–2251. [Google Scholar]
- Bailey, M.J.; Chong, N.W.; Xiong, J.; Cassone, V.M. Chickens’ Cry2: Molecular analysis of an avian cryptochrome in retinal and pineal photoreceptors. FEBS Lett. 2002, 513, 169–174. [Google Scholar] [CrossRef]
- Fu, Z.; Inaba, M.; Noguchi, T.M; Kato, H. Molecular cloning and circadian regulation of cryptochrome genes in Japanese quail. J. Biol. Rhythms 2002, 17, 14–27. [Google Scholar] [CrossRef]
- Nießner, C.; Denzau, S.; Gross, J.C.; Peichl, L.; Bischof, H.J.; Fleissner, G.; Wiltschko, W.; Wiltschko, R. Avian ultraviolet/violet cones identified as probable magneto-receptors. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Watari, R.; Yamaguchi, C.; Zemba, W.; Kubo, Y.; Okano, K.; Okano, T. Light-dependent structural change of chicken retinal cryptochrome4. J. Biol. Chem. 2012, 287, 42634–42641. [Google Scholar]
- Möller, A.; Sagasser, S.; Wiltschko, W.; Schierwater, B. Retinal cryptochrome in a migratory passerine bird: A possible transducer for the avian magnetic compass. Naturwissenschaften 2004, 91, 585–588. [Google Scholar] [CrossRef]
- Mouritsen, H.; Janssen.Bienhold, U.; Liedvogel, M.; Feenders, G.; Stalleicken, J.; Dirks, P.; Weiler, R. Cryptochrome and activity markers co-localize in bird retina during magnetic orientation. Proc. Natl. Acad. Sci. USA 2004, 101, 14294–14299. [Google Scholar] [CrossRef]
- Liedvogel, M.; Mouritsen, H. Cryptochromes—A potential magnetoreceptor: What do we know and what do we want to know? J. R. Soc. Interface 2010, 7, S147–S162. [Google Scholar] [CrossRef]
- Fusani, L.; Bertolucci, C.; Frigato, E.; Foà, A. Cryptochrome expression in the eye of migratory birds depends on their migratory status. J. Exp. Biol. 2014, 217, 918–923. [Google Scholar] [CrossRef]
- Wilkie, S.E.; Vissers, P.M.A.M.; Das, D.; Degrip, W.J.; Bowmaker, J.K. The molecular basis for UV vision in birds: Spectral characteristics, cDNA sequence and retinal localization of the UV-sensitive visual pigment of the budgerigar (Melopsittacus undulatus). Biochem. J. 1998, 330, 541–547. [Google Scholar]
- Kram, Y.A; Mantey, S.; Corbo, J.C. Avian cone photoreceptors tile the retina as five independent, self-organizing mosaics. PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Bischof, H.J.; Nießner, C.; Peichl, L.; Wiltschko, R.; Wiltschko, W. Avian UV/violet cones as magnetoreceptors. Communic. Integrat. Biol. 2012, 4, 713–716. [Google Scholar]
- Maier, E.J.; Bowmaker, J.K. Colour vision in the passeriform bird, Leiothrix lutea: Correlation of visual pigment absorbance and oil droplet transmission with spectral sensitivity. J. Comp. Physol. A 1993, 172, 295–301. [Google Scholar] [CrossRef]
- Das, D.; Wilkie, S.E.; Hunt, D.M.; Bowmaker, J.K. Visual pigments and oil droplets in the retina of a passerine birds, the canary Serinus canaria: Microspectrophotometry and opsin sequences. Vis. Res. 1999, 39, 2801–2815. [Google Scholar] [CrossRef]
- Lau, J.C.S.; Wagner-Rundell, N.; Rodgers, C.T.; Green, N.J.B.; Hoe, P.J. Effects of disorder and motion in a radical pair mechanism. J. R. Soc. Interface 2010, 7 (Suppl. 2), S257–S264. [Google Scholar] [CrossRef]
- Hill, E.; Ritz, T. Can disordered radical pair systems provide a basis for a magnetic compass in animals? J. R. Soc. Interface 2010, 7 (Suppl. 2), S265–S272. [Google Scholar] [CrossRef]
- Solov’yov, I.A.; Mouritsen, H.; Schulten, K. Acuity of a cryptochrome and vision based magnetoreception system in birds. Biophys. J. 2010, 99, 40–49. [Google Scholar] [CrossRef]
- Stoneham, A.M.; Gauger, E.M.; Porfyrakis, K.; Benjamin, S.C.; Lovett., B.W. A new type of radical-pair-based model for magnetoreception. Biophys. J. 2012, 102, 961–968. [Google Scholar]
- Lambert, N.; DeLiberato, S.; Emary, C.; Nori, F. Radical-pair model of magnetoreception with spin-orbit coupling. New J. Phys. 2013, 15. [Google Scholar] [CrossRef]
- Hogben, H.J.; Biskup, T.; Hore, P.J. Entanglement and sources of magnetic anisotropy in radical pair-based avian magentoreceptors. Phys. Rev. Lett. 2012, 109. [Google Scholar] [CrossRef]
- Dobson, C.A.; Hore, P.J.; Wallace, M.I. A radical sense of direction: Signaling and mechanism in cryptochrome magnetoreception. Trends Biochem. Sci. 2013, 38, 435–446. [Google Scholar] [CrossRef]
- Nießner, C.; Denzau, S.; Stapput, K.; Ahmad, M.; Peichl, L.; Wiltschko, W.; Wiltschko, R. Magnetoreception: Activated cryptochrome 1a concurs with magnetic orientation in birds. J. R. Soc. Interface 2013. [Google Scholar] [CrossRef]
- Wiltschko, R.; Dehe, L.; Gehring, D.; Thalau, P.; Wiltschko, W. Interaction between the visual and the magnetoreception system: Different effects of bichromatic light regimes on the directional behavior of migratory birds. J. Physiol. (Paris) 2013, 107, 137–146. [Google Scholar] [CrossRef]
- Müller, P.; Ahmad, M. Light-activated cryptochrome reacts with molecular oxygen to form a flavin-superoxide radical pair consistent with magnetoreception. J. Biol. Chem. 2011, 286, 21033–21040. [Google Scholar] [CrossRef]
- Bouly, J.P.; Schleicher, E.; Dionisio-Sese, M.; Vandenbussche, F.; van der Streaten, D.; Bakrim, M.; Meier, S.; Batschauer, A.; Galland, P.; Bittl, R.; Ahmad, M. Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J. Biol. Chem. 2007, 282, 9383–9391. [Google Scholar] [CrossRef]
- Berndt, A.; Kottke, T.; Breitkreuz, H.; Dvorsky, R.; Hennig, S.; Alexander, M.; Wolf, E. A novel photoreaction mechanism for the circadian blue light photoreceptor Drosophila cryptochrome. J. Biol. Chem. 2007, 282, 13011–13021. [Google Scholar]
- Banerjee, R.; Schleicher, E.; Meier, S.; Viana, R.M.; Pokorny, R.; Ahmad, M.; Bittl, R.; Batschauer, A. The signaling state of Arabidopsis cryptochrome 2 contains flavin semiquinone. J. Biol. Chem. 2007, 282, 14916–14922. [Google Scholar] [CrossRef]
- Solov’yov, I.A.; Schulten, K. Magnetoreception through cryptochrome may involve superoxide. Biophys. J. 2009, 96, 4804–4813. [Google Scholar] [CrossRef]
- Hogben, H.J.; Efimova, O.J.; Wagner-Rundell, N.C.R.T.; Hore, P.J. Possible involvement of superoxide and dioxygen with cryptochrome in avian magnetoreception: Origin of Zeeman resonances observed by in vivo EPR spectroscopy. Chem. Phys. Lett. 2009, 480, 116–122. [Google Scholar]
- Cuthill, I.C.; Partridge, J.C.; Bennett, A.Z.D.; Church, S.C.; Hart, N.S.; Hunt, S. Ultraviolet vision in birds. Adv. Stud. Behav. 2000, 29, 159–214. [Google Scholar] [CrossRef]
- Semm, P.; Nohr, D.; Demaine, C.; Wiltschko, W. Neural basis of the magnetic compass: Interactions of visual, magnetic and vestibular inputs in the pigeon's brain. J. Comp. Physiol. 1984, 155, 283–288. [Google Scholar] [CrossRef]
- Semm, P.; Demaine, C. Neurophysiological properties of magnetic cells in the pigeon’s visual system. J. Comp. Physiol. A 1986, 159, 619–625. [Google Scholar]
- Heyers, D.; Manns, M.; Luksch, H.; Güntürkün, O.; Mouritsen, H. A visual pathway links brain structures active during magnetic compass orientation in migratory birds. PLoS ONE 2007, 9. [Google Scholar] [CrossRef]
- Mouritsen, H.; Feenders, G.; Liedvogel, M.; Wada, K; Jarvis, E.D. Night-vision brain area in migratory songbirds. Proc. Natl. Acad. Sci. USA 2005, 102, 8339–8344. [Google Scholar] [CrossRef]
- Zapka, M.; Heyers, D.; Hein, C.M.; Engels, S.; Schneider, L.; Hans, J.; Weiler, S.; Dreyer, D.; Kishkinev, D.; Wild, J.M.; Mouritsen, H. Visual but not trigeminal mediation of magnetic compass information in a migratory bird. Nature 2009, 461, 1274–1277. [Google Scholar] [CrossRef]
- Zapka, M.; Heyers, D.; Liedvogel, M.; Jarvis, E.D.; Mouritsen, H. Night-time neuronal activation of Cluster N in a day- and night-migrating songbird. Eurp. J. Neurosci. 2010, 32, 619–624. [Google Scholar] [CrossRef] [Green Version]
- Vargas, J.P.; Siegel, J.J.; Bingman, V.P. The effect of a changing ambient magnetic field on single-unit activity in the homing pigeon hippocampus. Brain Res. Bull. 2006, 70, 158–164. [Google Scholar] [CrossRef]
- Keary, N.; Bischof, H.J. Activation changes in zebra finch (Taeniopygia guttata) brain areas evoked by alterations of the earth magnetic field. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wiltschko, R.; Wiltschko, W. Sensing Magnetic Directions in Birds: Radical Pair Processes Involving Cryptochrome. Biosensors 2014, 4, 221-242. https://doi.org/10.3390/bios4030221
Wiltschko R, Wiltschko W. Sensing Magnetic Directions in Birds: Radical Pair Processes Involving Cryptochrome. Biosensors. 2014; 4(3):221-242. https://doi.org/10.3390/bios4030221
Chicago/Turabian StyleWiltschko, Roswitha, and Wolfgang Wiltschko. 2014. "Sensing Magnetic Directions in Birds: Radical Pair Processes Involving Cryptochrome" Biosensors 4, no. 3: 221-242. https://doi.org/10.3390/bios4030221
APA StyleWiltschko, R., & Wiltschko, W. (2014). Sensing Magnetic Directions in Birds: Radical Pair Processes Involving Cryptochrome. Biosensors, 4(3), 221-242. https://doi.org/10.3390/bios4030221