Enterococcus spp. as a Producer and Target of Bacteriocins: A Double-Edged Sword in the Antimicrobial Resistance Crisis Context
Abstract
:1. Introduction
2. Diversity of Bacteriocins Produced by Enterococci
2.1. Classification and Origin of Known Enterocins
2.1.1. Class I—Lantibiotics
2.1.2. Class II—Non-Lantibiotics
Class IIa—The Pediocin-Like Bacteriocins
Class IIb—Two-Peptide Bacteriocins
Class IIc—Circular Bacteriocins
Class IId—Leaderless Bacteriocins
2.1.3. Class II—Other Bacteriocins
2.1.4. Class III—Bacteriolysins
3. Diversity of Enterocins in Clinical Enterococci
4. Use of Bacteriocins to Fight against VRE Human Infections
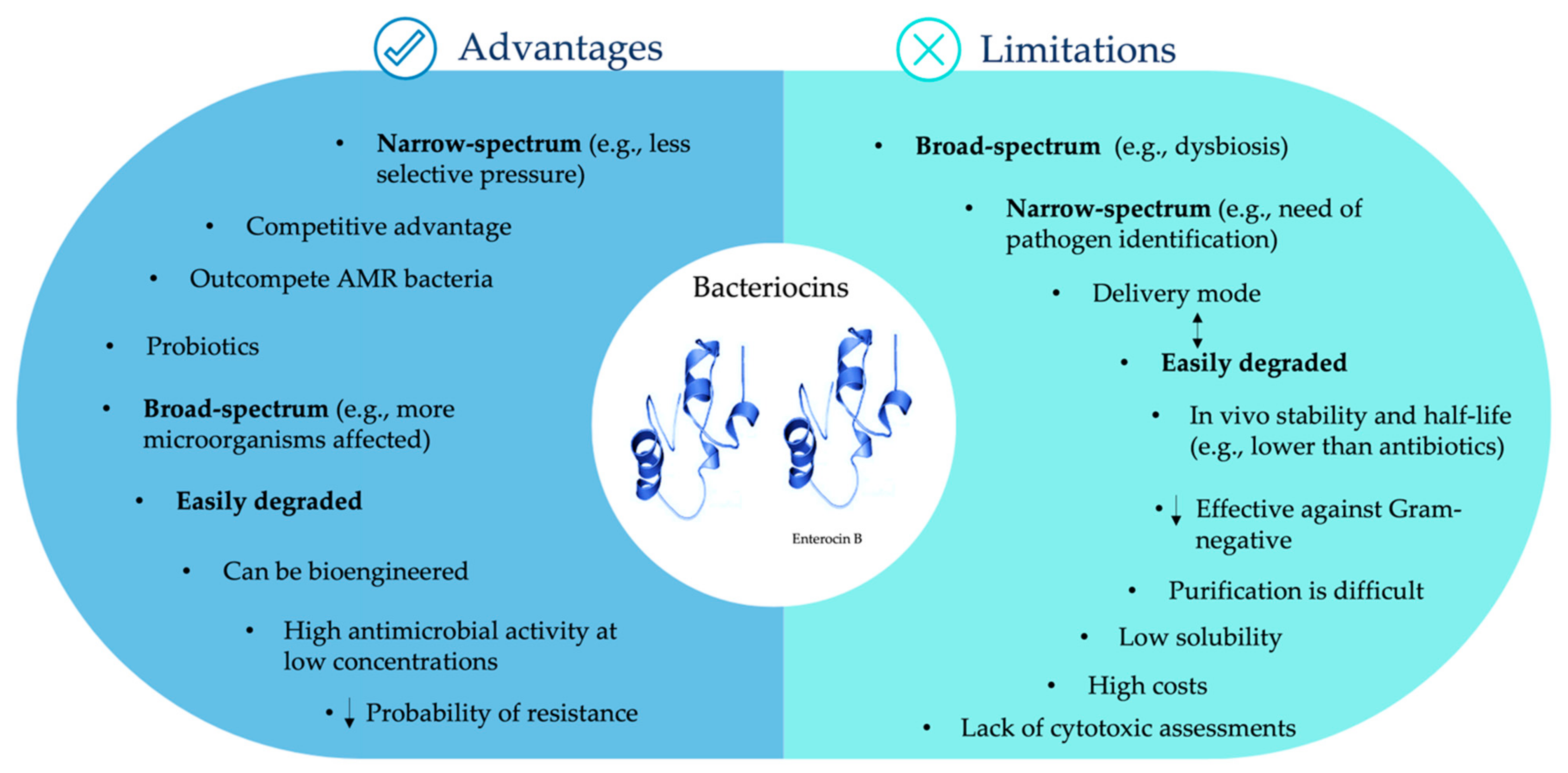
5. Challenges of Bacteriocin Use
6. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMPs | Antimicrobial peptides |
| AMR | Antimicrobial resistance |
| Bac | Bacteriocin |
| ECDC | European Centre for Disease Prevention and Control |
| EEA | European Economic Area |
| EFSA | European Food Safety Authority |
| EMA | European Medicines Agency |
| Ent | Enterocin |
| EU | European Union |
| FDA | Food and Drug Administration |
| GRAS | Generally recognized as safe |
| LAB | Lactic Acid Bacteria |
| Man-PTS | Mannose phosphotransferase system |
| MDRE | Multidrug-resistant enterococci |
| PTS | Phosphoenolpyruvate Carbohydrate Phosphotransferase System |
| QPS | Qualified Presumption of Safety |
| QS | Quorum sensing |
| US | United States |
| VRE | Vancomycin-resistant enterococci |
| VREfm | Vancomycin-resistant E. faecium |
| VREfs | Vancomycin-resistant E. faecalis |
| VSE | Vancomycin-susceptible enterococci |
| VSEfm | Vancomycin-susceptible E. faecium |
| WHO | World Health Organization |
References
- Interagency Coordination Group on Antimicrobial Resistance. No Time to Wait: Securing the Future from Drug-Resistant Infections. 2019. Available online: https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_final_report_EN.pdf?ua=1 (accessed on 25 May 2021).
- O’Neill, J. The Review on Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; The Review on Antimicrobial Resistance; Wellcome Trust: London, UK, 2016. [Google Scholar]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Klare, I.; Fleige, C.; Geringer, U.; Thürmer, A.; Bender, J.; Mutters, N.T.; Mischnik, A.; Werner, G. Increased frequency of linezolid resistance among clinical Enterococcus faecium isolates from German hospital patients. J. Glob. Antimicrob. Resist. 2015, 3, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Li, Y.; Li, Z.; Gao, R.; Zhang, H.; Wen, R.; Gao, G.F.; Hu, Q.; Feng, Y. Diversified mcr-1-Harbouring Plasmid Reservoirs Confer Resistance to Colistin in Human Gut Microbiota. mBio 2016, 7, e00177. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Czaplewski, L.; Bax, R.; Clokie, M.; Dawson, M.; Fairhead, H.; Fischetti, V.A.; Foster, S.; Gilmore, B.F.; Hancock, R.; Harper, D.; et al. Alternatives to antibiotics-a pipeline portfolio review. Lancet Infect. Dis. 2016, 16, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Melander, R.J.; Zurawski, D.V.; Melander, C. Narrow-Spectrum Antibacterial Agents. MedChemComm 2018, 9, 12–21. [Google Scholar] [CrossRef]
- Pircalabioru, G.G.; Popa, L.; Marutescu, L.; Gheorghe, I.; Popa, M.; Barbu, I.C.; Cristescu, R.; Chifiriuc, M.-C. Bacteriocins in the Era of Antibiotic Resistance: Rising to the Challenge. Pharmaceutics 2021, 13, 196. [Google Scholar] [CrossRef]
- Egan, K.; Ross, R.P.; Hill, C. Bacteriocins: Antibiotics in the age of the microbiome. Emerg. Top. Life Sci. 2017, 1, 55–63. [Google Scholar]
- Lopetuso, L.R.; Giorgio, M.E.; Saviano, A.; Scaldaferri, F.; Gasbarrini, A.; Cammarota, G. Bacteriocins and Bacteriophages: Therapeutic Weapons for Gastrointestinal Diseases? Int. J. Mol. Sci. 2019, 20, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heilbronner, S.; Krismer, B.; Brotz-Oesterhelt, H.; Peschel, A. The microbiome-shaping roles of bacteriocins. Nat. Rev. Microbiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bybee, S.N.; Scorza, A.V.; Lappin, M.R. Effect of the probiotic Enterococcus faecium SF68 on presence of diarrhea in cats and dogs housed in an animal shelter. J. Vet. Intern. Med. 2011, 25, 856–860. [Google Scholar] [CrossRef]
- Han, K.J.; Lee, N.K.; Park, H.; Paik, H.D. Anticancer and Anti-Inflammatory Activity of Probiotic Lactococcus lactis NK34. J. Microbiol. Biotechnol. 2015, 25, 1697–1701. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Tabassum, N.; Kim, Y.M. A strategy to control colonization of pathogens: Embedding of lactic acid bacteria on the surface of urinary catheter. Appl. Microbiol. Biotechnol. 2020, 104, 9053–9066. [Google Scholar] [CrossRef]
- Hegarty, J.W.; Guinane, C.M.; Ross, R.P.; Hill, C.; Cotter, P.D. Bacteriocin production: A relatively unharnessed probiotic trait? F1000Research 2016, 5, 2587. [Google Scholar] [CrossRef]
- Ness, I.F.; Diep, D.B.; Ike, Y. Enterococcal Bacteriocins and Antimicrobial Proteins that Contribute to Niche Control. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Miller, W.R.; Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance in enterococci. Expert Rev. Anti. Infect. Ther. 2014, 12, 1221–1236. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Somarajan, S.R.; Murray, B.E. Could a phosphotransferase system provide the means to control outbreaks of Enterococcus faecium infection? J. Infect. Dis. 2013, 207, 1633–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fimland, G.; Eijsink, V.G.H.; Nissen-Meyer, J. Comparative studies of immunity proteins of pediocin-like bacteriocins. Microbiology 2002, 148 Pt 11, 3661–3670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daw, M.A.; Falkiner, F.R. Bacteriocins: Nature, function and structure. Micron 1996, 27, 467–479. [Google Scholar] [CrossRef]
- Tagg, J.R.; Dajani, A.S.; Wannamaker, L.W. Bacteriocins of gram-positive bacteria. Bacteriol. Rev. 1976, 40, 722–756. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Novel bacteriocins from lactic acid bacteria (LAB): Various structures and applications. Microb. Cell Factories 2014, 13 (Suppl. S1), S3. [Google Scholar] [CrossRef] [Green Version]
- Reinseth, I.S.; Ovchinnikov, K.V.; Tonnesen, H.H.; Carlsen, H.; Diep, D.B. The Increasing Issue of Vancomycin-Resistant Enterococci and the Bacteriocin Solution. Probiotics Antimicrob. Proteins 2020, 12, 1203–1217. [Google Scholar] [CrossRef]
- Kjems, E. Studies on streptococcal bacteriophages. I. Technique of isolating phage-producing strains. Acta Pathol. Microbiol. Scand. 1955, 36, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Brandis, H.; Brandis, U. Appearance and Behavior of Spontaneous Mutants of Enterococcus Strains with Resistance to Enterocin. Pathol. Microbiol. 1963, 26, 688–695. [Google Scholar]
- Strompfova, V.; Laukova, A.; Simonova, M.; Marcinakova, M. Occurrence of the structural enterocin A, P, B, L50B genes in enterococci of different origin. Vet. Microbiol. 2008, 132, 293–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontinen, A.K.; Top, J.; Arredondo-Alonso, S.; Tonkin-Hill, G.; Freitas, A.R.; Novais, C.; Gladstone, R.A.; Pesonen, M.; Meneses, R.; Pesonen, H. Apparent nosocomial adaptation of Enterococcus faecalis predates the modern hospital era. Nat. Commun. 2021, 12, 1523. [Google Scholar] [CrossRef]
- Poeta, P.; Costa, D.; Rodrigues, J.; Torres, C. Detection of genes encoding virulence factors and bacteriocins in fecal enterococci of poultry in Portugal. Avian Dis. 2006, 50, 64–68. [Google Scholar] [CrossRef]
- Franz, C.M.; van Belkum, M.J.; Holzapfel, W.H.; Abriouel, H.; Galvez, A. Diversity of enterococcal bacteriocins and their grouping in a new classification scheme. FEMS Microbiol. Rev. 2007, 31, 293–310. [Google Scholar] [CrossRef]
- Lauková, A.; Styková, E.; Kubašová, I.; Gancarčíková, S.; Plachá, I.; Mudroňová, D.; Kandričáková, A.; Miltko, R.; Bełżecki, G.; Valocký, I.; et al. Enterocin M and its Beneficial Effects in Horses-a Pilot Experiment. Probiotics Antimicrob. Proteins 2018, 10, 420–426. [Google Scholar] [CrossRef]
- Todorov, S.D.; Kang, H.J.; Ivanova, I.V.; Holzapfel, W.H. Bacteriocins from LAB and Other Alternative Approaches for the Control of Clostridium and Clostridiodes Related Gastrointestinal Colitis. Front. Bioeng. Biotechnol. 2020, 8, 581778. [Google Scholar] [CrossRef]
- Kommineni, S.; Bretl, D.J.; Lam, V.; Chakraborty, R.; Hayward, M.; Simpson, P.M.; Cao, Y.; Bousounis, P.; Kristich, C.J.; Salzman, N.H. Bacteriocin production augments niche competition by enterococci in the mammalian gastrointestinal tract. Nature 2015, 526, 719–722. [Google Scholar] [CrossRef] [Green Version]
- Phumisantiphong, U.; Siripanichgon, K.; Reamtong, O.; Diraphat, P. A novel bacteriocin from Enterococcus faecalis 478 exhibits a potent activity against vancomycin-resistant enterococci. PLoS ONE 2017, 12, e0186415. [Google Scholar] [CrossRef] [Green Version]
- Ennahar, S.; Deschamps, N. Anti-Listeria effect of enterocin A, produced by cheese-isolated Enterococcus faecium EFM01, relative to other bacteriocins from lactic acid bacteria. J. Appl. Microbiol. 2000, 88, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Hanchi, H.; Mottawea, W.; Sebei, K.; Hammami, R. The Genus Enterococcus: Between Probiotic Potential and Safety Concerns—An Update. Front. Microbiol. 2018, 9, 1791. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Klaenhammer, T.R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 39–85. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Kjos, M.; Borrero, J.; Opsata, M.; Birri, D.J.; Holo, H.; Cintas, L.M.; Snipen, L.; Hernandez, P.E.; Nes, I.F.; Diep, D.B. Target recognition, resistance, immunity and genome mining of class II bacteriocins from Gram-positive bacteria. Microbiology 2011, 157 Pt 12, 3256–3267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Clewell, D.B. Properties of Enterococcus faecalis plasmid pAD1, a member of a widely disseminated family of pheromone-responding, conjugative, virulence elements encoding cytolysin. Plasmid 2007, 58, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Coburn, P.S.; Pillar, C.M.; Jett, B.D.; Haas, W.; Gilmore, M.S. Enterococcus faecalis senses target cells and in response expresses cytolysin. Science 2004, 306, 2270–2272. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.W.; Thal, L.A.; Perri, M.B.; Vazquez, J.A.; Donabedian, S.M.; Clewell, D.B.; Zervos, M.J. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob. Agents Chemother. 1993, 37, 2474–2477. [Google Scholar] [CrossRef] [Green Version]
- Jett, B.D.; Jensen, H.G.; Nordquist, R.E.; Gilmore, M.S. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect. Immun. 1992, 60, 2445–2452. [Google Scholar] [CrossRef] [Green Version]
- Ike, Y.; Hashimoto, H.; Clewell, D.B. High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infections. J. Clin. Microbiol. 1987, 25, 1524–1528. [Google Scholar] [CrossRef] [Green Version]
- Sawa, N.; Wilaipun, P.; Kinoshita, S.; Zendo, T.; Leelawatcharamas, V.; Nakayama, J.; Sonomoto, K. Isolation and characterization of enterocin W, a novel two-peptide lantibiotic produced by Enterococcus faecalis NKR-4-1. Appl. Environ. Microbiol. 2012, 78, 900–903. [Google Scholar] [CrossRef] [Green Version]
- Lohans, C.T.; Vederas, J.C. Development of Class IIa Bacteriocins as Therapeutic Agents. Int. J. Microbiol. 2012, 2012, 386410. [Google Scholar] [CrossRef] [Green Version]
- Eijsink, V.G.; Axelsson, L.; Diep, D.B.; Havarstein, L.S.; Holo, H.; Nes, I.F. Production of class II bacteriocins by lactic acid bacteria; an example of biological warfare and communication. Antonie Van Leeuwenhoek 2002, 81, 639–654. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Martinez, M.I.; Kok, J. Pediocin PA-1, a wide-spectrum bacteriocin from lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2002, 42, 91–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eijsink, V.G.; Skeie, M.; Middelhoven, P.H.; Brurberg, M.B.; Nes, I.F. Comparative studies of class IIa bacteriocins of lactic acid bacteria. Appl. Environ. Microbiol. 1998, 64, 3275–3281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fimland, G.; Johnsen, L.; Axelsson, L.; Brurberg, M.B.; Nes, I.F.; Eijsink, V.G.H.; Nissen-Meyer, J. A C-terminal disulfide bridge in pediocin-like bacteriocins renders bacteriocin activity less temperature dependent and is a major determinant of the antimicrobial spectrum. J. Bacteriol. 2000, 182, 2643–2648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aymerich, T.; Holo, H.; Havarstein, L.S.; Hugas, M.; Garriga, M.; Nes, I.F. Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl. Environ. Microbiol. 1996, 62, 1676–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, A.; Tedim, A.P.; Francia, M.V.; Jensen, L.B.; Novais, C.; Peixe, L.; Sánchez-Valenzuela, A.; Sundsfjord, A.; Hegstad, K.; Werner, G.; et al. Multilevel population genetic analysis of vanA and vanB Enterococcus faecium causing nosocomial outbreaks in 27 countries (1986–2012). J. Antimicrob. Chemother. 2016, 71, 3351–3366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strateva, T.; Dimov, S.G.; Atanasova, D.; Petkova, V.; Savov, E.; Mitov, I. Molecular genetic study of potentially bacteriocinogenic clinical and dairy Enterococcus spp. isolates from Bulgaria. Ann. Microbiol. 2015, 66, 381–387. [Google Scholar] [CrossRef]
- de Maat, V.; Arredondo-Alonso, S.; Willems, R.J.L.; van Schaik, W. Conditionally essential genes for survival during starvation in Enterococcus faecium E745. BMC Genom. 2020, 21, 568. [Google Scholar] [CrossRef] [PubMed]
- Casaus, P.; Nilsen, T.; Cintas, L.M.; Nes, I.F.; Hernandez, P.E.; Holo, H. Enterocin B, a new bacteriocin from Enterococcus faecium T136 which can act synergistically with enterocin A. Microbiology 1997, 143 Pt 7, 2287–2294. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Galvez, A.; Dubois-Dauphin, R.; Campos, D.; Thonart, P. Genetic determination and localization of multiple bacteriocins produced by Enterococcus faecium CWBI-B1430 and Enterococcus mundtii CWBI-B1431. Food Sci. Biotechnol. 2011, 20, 289–296. [Google Scholar] [CrossRef]
- Cintas, L.M.; Casaus, P.; Havarstein, L.S.; Hernandez, P.E.; Nes, I.F. Biochemical and genetic characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl. Environ. Microbiol. 1997, 63, 4321–4330. [Google Scholar] [CrossRef] [Green Version]
- Cintas, L.M.; Casaus, P.; Herranz, C.; Håvarstein, L.S.; Holo, H.; Hernández, P.E.; Nes, I.F. Biochemical and genetic evidence that Enterococcus faecium L50 produces enterocins L50A and L50B, the sec-dependent enterocin P, and a novel bacteriocin secreted without an N-terminal extension termed enterocin Q. J. Bacteriol. 2000, 182, 6806–6814. [Google Scholar] [CrossRef] [Green Version]
- de Farias, F.M.; Francisco, M.S.; Santos, I.N.D.S.; Marques-Bastos, S.L.S.; Miguel, M.A.L.; Albano, R.M.; Bastos, M.D.C.D.F. Draft genome sequence of Enterococcus faecium E86, a strain producing broad-spectrum antimicrobial peptides: Description of a novel bacteriocin immunity protein and a novel sequence type. J. Glob. Antimicrob. Resist. 2019, 17, 195–197. [Google Scholar] [CrossRef]
- Todokoro, D.; Tomita, H.; Inoue, T.; Ike, Y. Genetic analysis of bacteriocin 43 of vancomycin-resistant Enterococcus faecium. Appl. Environ. Microbiol. 2006, 72, 6955–6964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Campo, R.; Tenorio, C.; Jiménez-Díaz, R.; Rubio, C.; Gómez-Lus, R.; Baquero, F.; Torres, C. Bacteriocin production in vancomycin-resistant and vancomycin-susceptible Enterococcus isolates of different origins. Antimicrob. Agents Chemother. 2001, 45, 905–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popović, N.; Dinic, M.; Tolinački, M.; Mihajlović, S.; Terzić-Vidojević, A.; Bojić, S.; Djokic, J.; Golić, N.; Veljović, K. New Insight into Biofilm Formation Ability, the Presence of Virulence Genes and Probiotic Potential of Enterococcus sp. Dairy Isolates. Front. Microbiol. 2018, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Tomita, H.; Fujimoto, S.; Tanimoto, K.; Ike, Y. Cloning and genetic organization of the bacteriocin 31 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pYI17. J. Bacteriol. 1996, 178, 3585–3593. [Google Scholar] [CrossRef] [Green Version]
- Ennahar, S.; Sashihara, T.; Sonomoto, K.; Ishizaki, A. Class IIa bacteriocins: Biosynthesis, structure and activity. FEMS Microbiol. Rev. 2000, 24, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Barragan, A.; Caballero-Guerrero, B.; Jimenez, E.; Jimenez-Diaz, R.; Ruiz-Barba, J.L.; Rodriguez, J.M. Enterocin C, a class IIb bacteriocin produced by E. faecalis C901, a strain isolated from human colostrum. Int. J. Food Microbiol. 2009, 133, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balla, E.; Dicks, L.M.; Du Toit, M.; Van Der Merwe, M.J.; Holzapfel, W.H. Characterization and cloning of the genes encoding enterocin 1071A and enterocin 1071B, two antimicrobial peptides produced by Enterococcus faecalis BFE 1071. Appl. Environ. Microbiol. 2000, 66, 1298–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franz, C.M.A.P.; Grube, A.; Herrmann, A.; Abriouel, H.; Stärke, J.; Lombardi, A.; Tauscher, B.; Holzapfel, W.H. Biochemical and genetic characterization of the two-peptide bacteriocin enterocin 1071 produced by Enterococcus faecalis FAIR-E 309. Appl. Environ. Microbiol. 2002, 68, 2550–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.B.; Malaphan, W.; Zendo, T.; Nakayama, J.; Sonomoto, K. Enterocin X, a novel two-peptide bacteriocin from Enterococcus faecium KU-B5, has an antibacterial spectrum entirely different from those of its component peptides. Appl. Environ. Microbiol. 2010, 76, 4542–4545. [Google Scholar] [CrossRef] [Green Version]
- Galvez, A.; Maqueda, M.; Valdivia, E.; Quesada, A.; Montoya, E. Characterization and partial purification of a broad spectrum antibiotic AS-48 produced by Streptococcus faecalis. Can. J. Microbiol. 1986, 32, 765–771. [Google Scholar] [CrossRef]
- Martinez-Bueno, M.; Galvez, A.; Valdivia, E.; Maqueda, M. A transferable plasmid associated with AS-48 production in Enterococcus faecalis. J. Bacteriol. 1990, 172, 2817–2818. [Google Scholar] [CrossRef] [Green Version]
- Tomita, H.; Fujimoto, S.; Tanimoto, K.; Ike, Y. Cloning and genetic and sequence analyses of the bacteriocin 21 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pPD1. J. Bacteriol. 1997, 179, 7843–7855. [Google Scholar] [CrossRef] [Green Version]
- Quirantes, R.; Valdivia, E.; Martín, I.; Bueno, M.M.; Maqueda, M.; Gálvez, A.; Méndez, E. Purification of sex pheromones specific for pMB1 and pMB2 plasmids of Enterococcus faecalis S-48. Can. J. Microbiol. 1995, 41, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Clewell, D.B.; Weaver, K.E.; Dunny, G.M.; Coque, T.M.; Francia, M.V.; Hayes, F. Extrachromosomal and Mobile Elements in Enterococci: Transmission, Maintenance, and Epidemiology. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Joosten, H.M.; Nunez, M.; Devreese, B.; Van Beeumen, J.; Marugg, J.D. Purification and characterization of enterocin 4, a bacteriocin produced by Enterococcus faecalis INIA 4. Appl. Environ. Microbiol. 1996, 62, 4220–4223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floriano, B.; Ruiz-Barba, J.L.; Jimenez-Diaz, R. Purification and genetic characterization of enterocin I from Enterococcus faecium 6T1a, a novel antilisterial plasmid-encoded bacteriocin which does not belong to the pediocin family of bacteriocins. Appl. Environ. Microbiol. 1998, 64, 4883–4890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, Y.; Togawa, Y.; Shimosaka, M.; Okazaki, M. Purification and characterization of a novel bacteriocin produced by Enterococcus faecalis strain RJ-11. Appl. Environ. Microbiol. 2003, 69, 5746–5753. [Google Scholar] [CrossRef]
- Inoue, T.; Tomita, H.; Ike, Y. Bac 32, a novel bacteriocin widely disseminated among clinical isolates of Enterococcus faecium. Antimicrob. Agents Chemother. 2006, 50, 1202–1212. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, H.; Tomita, H.; Inoue, T.; Ike, Y. Genetic organization and mode of action of a novel bacteriocin, bacteriocin 51: Determinant of VanA-type vancomycin-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 2011, 55, 4352–4360. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, E.; Wagner, C.; Marchioni, E.; Aoude-Werner, D.; Ennahar, S. Enterocin 96, a novel class II bacteriocin produced by Enterococcus faecalis WHE 96, isolated from Munster cheese. Appl. Environ. Microbiol. 2009, 75, 4273–4276. [Google Scholar] [CrossRef] [Green Version]
- Hanchi, H.; Hammami, R.; Gingras, H.; Kourda, R.; Bergeron, M.G.; Ben Hamida, J.; Ouellette, M.; Fliss, I. Inhibition of MRSA and of Clostridium difficile by durancin 61A: Synergy with bacteriocins and antibiotics. Future Microbiol. 2017, 12, 205–212. [Google Scholar] [CrossRef]
- Maky, M.A.; Ishibashi, N.; Zendo, T.; Perez, R.H.; Doud, J.R.; Karmi, M.; Sonomoto, K. Enterocin F4-9, a Novel O-Linked Glycosylated Bacteriocin. Appl. Environ. Microbiol. 2015, 81, 4819–4826. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, E.; Bednarczyk, A.; Schaeffer, C.; Cai, Y.; Marchioni, E.; Van Dorsselaer, A.; Ennahar, S. Production of enterocins L50A, L50B, and IT, a new enterocin, by Enterococcus faecium IT62, a strain isolated from Italian ryegrass in Japan. Antimicrob. Agents Chemother. 2008, 52, 1917–1923. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.S.; Seo, J.-G.; Lee, G.-S.; Kim, J.-H.; Kim, S.Y.; Han, Y.W.; Kang, H.; Kim, H.O.; Rhee, J.H.; Chung, M.-J.; et al. Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J. Microbiol. 2009, 47, 101–109. [Google Scholar] [CrossRef]
- Nilsen, T.; Nes, I.F.; Holo, H. Enterolysin A, a cell wall-degrading bacteriocin from Enterococcus faecalis LMG 2333. Appl. Environ. Microbiol. 2003, 69, 2975–2984. [Google Scholar] [CrossRef] [Green Version]
- Hickey, R.M.; Twomey, D.P.; Ross, R.P.; Hill, C. Production of enterolysin A by a raw milk enterococcal isolate exhibiting multiple virulence factors. Microbiology 2003, 149 Pt 3, 655–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, B.; Tomita, H.; Inoue, T.; Ike, Y. Isolation of VanB-type Enterococcus faecalis strains from nosocomial infections: First report of the isolation and identification of the pheromone-responsive plasmids pMG2200, Encoding VanB-type vancomycin resistance and a Bac41-type bacteriocin, and pMG2201, encoding erythromycin resistance and cytolysin (Hly/Bac). Antimicrob. Agents Chemother. 2009, 53, 735–747. [Google Scholar] [PubMed] [Green Version]
- Kurushima, J.; Ike, Y.; Tomita, H. Partial Diversity Generates Effector Immunity Specificity of the Bac41-Like Bacteriocins of Enterococcus faecalis Clinical Strains. J. Bacteriol. 2016, 198, 2379–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubin, K.; Pamer, E.G. Enterococci and Their Interactions with the Intestinal Microbiome. Microbiol. Spectr. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Ravi, A.; Halstead, F.D.; Bamford, A.; Casey, A.; Thomson, N.M.; van Schaik, W.; Snelson, C.; Goulden, R.; Foster-Nyarko, E.; Savva, G.M.; et al. Loss of microbial diversity and pathogen domination of the gut microbiota in critically ill patients. Microb. Genom. 2019, 5, e000293. [Google Scholar] [CrossRef]
- Freitas, A.R.; Pereira, A.P.; Novais, C.; Peixe, L. Multidrug-resistant high-risk Enterococcus faecium clones: Can we really define them? Int. J. Antimicrob. Agents 2021, 57, 106227. [Google Scholar] [CrossRef]
- Raven, K.E.; Reuter, S.; Reynolds, R.; Brodrick, H.J.; Russell, J.E.; Török, M.E.; Parkhill, J.; Peacock, S.J. A decade of genomic history for healthcare-associated Enterococcus faecium in the United Kingdom and Ireland. Genome Res. 2016, 26, 1388–1396. [Google Scholar] [CrossRef] [Green Version]
- Arredondo-Alonso, S.; Top, J.; McNally, A.; Puranen, S.; Pesonen, M.; Pensar, J.; Marttinen, P.; Braat, J.C.; Rogers, M.R.C.; van Schaik, W.; et al. Plasmids Shaped the Recent Emergence of the Major Nosocomial Pathogen Enterococcus faecium. mBio 2020, 11, e03284-19. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Chlebowicz, M.A.A.; Bathoorn, E.; Rosema, S.; Couto, N.; Lokate, M.; Arends, J.P.; Friedrich, A.W.; Rossen, J.W.A. Elucidating vancomycin-resistant Enterococcus faecium outbreaks: The role of clonal spread and movement of mobile genetic elements. J. Antimicrob. Chemother. 2018, 73, 3259–3267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Ubeda, C.; Taur, Y.; Jenq, R.R.; Equinda, M.J.; Son, T.; Samstein, M.; Viale, A.; Socci, N.D.; Brink, M.R.V.D.; Kamboj, M.; et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J. Clin. Investig. 2010, 120, 4332–4341. [Google Scholar] [CrossRef] [PubMed]
- Puchter, L.; Chaberny, I.F.; Schwab, F.; Vonberg, R.P.; Bange, F.C.; Ebadi, E. Economic burden of nosocomial infections caused by vancomycin-resistant enterococci. Antimicrob. Resist. Infect. Control 2018, 7, 1. [Google Scholar] [CrossRef]
- Werner, G.; Neumann, B.; Weber, R.E.; Kresken, M.; Wendt, C.; Bender, J.K.; Becker, K.; Borgmann, S.; Diefenbach, A.; Hamprecht, A.; et al. Thirty years of VRE in Germany—“Expect the unexpected”: The view from the National Reference Centre for Staphylococci and Enterococci. Drug Resist. Updates 2020, 53, 100732. [Google Scholar] [CrossRef]
- Isenman, H.; Michaels, J.; Fisher, D. Global variances in infection control practices for vancomycin resistant Enterococcus—Results of an electronic survey. Antimicrob. Resist. Infect. Control 2016, 5, 41. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Central Asian and Eastern European Surveillance of Antimicrobial Resistance (CAESAR); Anual Report 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats. 2019. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 25 May 2021).
- Farias, F.M.; Teixeira, L.M.; Vallim, D.C.; Bastos, M.; Miguel, M.A.L.; Bonelli, R.R. Characterization of Enterococcus faecium E86 bacteriocins and their inhibition properties against Listeria monocytogenes and vancomycin-resistant Enterococcus. Braz. J. Microbiol. 2021, 52, 1513–1522. [Google Scholar] [CrossRef]
- Fugaban, J.I.I.; Bucheli, J.E.V.; Holzapfel, W.H.; Todorov, S.D. Characterization of Partially Purified Bacteriocins Produced by Enterococcus faecium Strains Isolated from Soybean Paste Active against Listeria spp. and Vancomycin-Resistant Enterococci. Microorganisms 2021, 9, 1085. [Google Scholar] [CrossRef]
- Severina, E.; Severin, A.; Tomasz, A. Antibacterial efficacy of nisin against multidrug-resistant Gram-positive pathogens. J. Antimicrob. Chemother. 1998, 41, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Piper, C.; Draper, L.A.; Cotter, P.D.; Ross, R.P.; Hill, C. A comparison of the activities of lacticin 3147 and nisin against drug-resistant Staphylococcus aureus and Enterococcus species. J. Antimicrob. Chemother. 2009, 64, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Aunpad, R.; Na-Bangchang, K. Pumilicin 4, a novel bacteriocin with anti-MRSA and anti-VRE activity produced by newly isolated bacteria Bacillus pumilus strain WAPB4. Curr. Microbiol. 2007, 55, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Reinseth, I.; Tonnesen, H.H.; Carlsen, H.; Diep, D.B. Exploring the Therapeutic Potenital of the Leaderless Enterocins K1 and EJ97 in the Treatment of Vancomycin-Resistant Enterococcal Infection. Front. Microbiol. 2021, 12, 649339. [Google Scholar] [CrossRef] [PubMed]
- Millette, M.; Cornut, G.; Dupont, C.; Shareck, F.; Archambault, D.; Lacroix, M. Capacity of human nisin- and pediocin-producing lactic Acid bacteria to reduce intestinal colonization by vancomycin-resistant enterococci. Appl. Environ. Microbiol. 2008, 74, 1997–2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabes, D.; Brunati, C.; Candiani, G.; Riva, S.; Romano, G.; Donadio, S. Efficacy of the new lantibiotic NAI-107 in experimental infections induced by multidrug-resistant Gram-positive pathogens. Antimicrob. Agents Chemother. 2011, 55, 1671–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.G.; Becattini, S.; Moody, T.U.; Shliaha, P.V.; Littmann, E.R.; Seok, R.; Gjonbalaj, M.; Eaton, V.; Fontana, E.; Amoretti, L.; et al. Microbiota-derived lantibiotic restores resistance against vancomycin-resistant Enterococcus. Nature 2019, 572, 665–669. [Google Scholar] [CrossRef]
- Ubeda, C.; Bucci, V.; Caballero, S.; Djukovic, A.; Toussaint, N.; Equinda, M.; Lipuma, L.; Ling, L.; Gobourne, A.; No, D.; et al. Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infect. Immun. 2013, 81, 965–973. [Google Scholar] [CrossRef] [Green Version]
- Crouzet, L.; Rigottier-Gois, L.; Serror, P. Potential use of probiotic and commensal bacteria as non-antibiotic strategies against vancomycin-resistant enterococci. FEMS Microbiol. Lett. 2015, 362, fnv012. [Google Scholar] [CrossRef]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Ben Said, L.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a new generation of antimicrobials: Toxicity aspects and regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef]
- Montalbán-López, M.; Cebrián, R.; Galera, R.; Mingorance, L.; Martín-Platero, A.; Valdivia, E.; Martínez-Bueno, M.; Maqueda, M. Synergy of the Bacteriocin AS-48 and Antibiotics against Uropathogenic Enterococci. Antibiotics 2020, 9, 567. [Google Scholar] [CrossRef]
- Dreyer, L.; Smith, C.; Deane, S.M.; Dicks, L.M.T.; van Staden, A.D. Migration of Bacteriocins across Gastrointestinal Epithelial and Vascular Endothelial Cells, as Determined Using In Vitro Simulations. Sci. Rep. 2019, 9, 11481. [Google Scholar] [CrossRef]
- Zacharof, M.P.; Lovitt, R.W. Bacteriocins Produced by Lactic Acid Bacteria a Review Article. APCBEE Procedia 2012, 2, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Rea, M.; Dobson, A.; O’Sullivan, O.; Crispie, F.; Fouhy, F.; Cotter, P.; Shanahan, F.; Kiely, B.; Hill, C.; Ross, R. Effect of broad- and narrow-spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4639–4644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balloux, F.; Brynildsrud, O.; van Dorp, L.; Shaw, L.; Chen, H.; Harris, K.; Wang, H.; Eldholm, V. From Theory to Practice: Translating Whole-Genome Sequencing (WGS) into the Clinic. Trends Microbiol. 2018, 26, 1035–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novais, A.; Freitas, A.R.; Rodrigues, C.; Peixe, L. Fourier transform infrared spectroscopy: Unlocking fundamentals and prospects for bacterial strain typing. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 427–448. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D. An ‘Upp’-turn in bacteriocin receptor identification. Mol. Microbiol. 2014, 92, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Do de Bastos, M.C.; Coelho, M.L.; Santos, O.C. Resistance to bacteriocins produced by Gram-positive bacteria. Microbiology 2015, 161 Pt 4, 683–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draper, L.A.; Grainger, K.; Deegan, L.H.; Cotter, P.D.; Hill, C.; Ross, R.P. Cross-immunity and immune mimicry as mechanisms of resistance to the lantibiotic lacticin 3147. Mol. Microbiol. 2009, 71, 1043–1054. [Google Scholar] [CrossRef]
- Sedgley, C.M.; Clewell, D.B.; Flannagan, S.E. Plasmid pAMS1-encoded, bacteriocin-related “Siblicide” in Enterococcus faecalis. J. Bacteriol. 2009, 191, 3183–3188. [Google Scholar] [CrossRef] [Green Version]
- Opsata, M.; Nes, I.F.; Holo, H. Class IIa bacteriocin resistance in Enterococcus faecalis V583: The mannose PTS operon mediates global transcriptional responses. BMC Microbiol. 2010, 10, 224. [Google Scholar] [CrossRef] [Green Version]
- Kumariya, R.; Sood, S.K.; Rajput, Y.S.; Garsa, A.K. Gradual pediocin PA-1 resistance in Enterococcus faecalis confers cross-protection to diverse pore-forming cationic antimicrobial peptides displaying changes in cell wall and mannose PTS expression. Ann. Microbiol. 2014, 65, 721–732. [Google Scholar] [CrossRef]
- Sakayori, Y.; Muramatsu, M.; Hanada, S.; Kamagata, Y.; Kawamoto, S.; Shima, J. Characterization of Enterococcus faecium mutants resistant to mundticin KS, a class IIa bacteriocin. Microbiology 2003, 149 Pt 10, 2901–2908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurushima, J.; Tomita, H. Inactivation of GalU Leads to a Cell Wall-Associated Polysaccharide Defect That Reduces the Susceptibility of Enterococcus faecalis to Bacteriolytic Agents. Appl. Environ. Microbiol. 2021, 87, e02875-20. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, T.P.; Malik, R.K.; Bhardwaj, A.; De, S. Antibacterial efficacy of nisin, pediocin 34 and enterocin FH99 against L. monocytogenes, E. faecium and E. faecalis and bacteriocin cross resistance and antibiotic susceptibility of their bacteriocin resistant variants. J. Food Sci. Technol. 2014, 51, 233–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rea, M.C.; Alemayehu, D.; Casey, P.G.; O’Connor, P.M.; Lawlor, P.G.; Walsh, M.; Shanahan, F.; Kiely, B.; Ross, R.; Hill, C. Bioavailability of the anti-clostridial bacteriocin thuricin CD in gastrointestinal tract. Microbiology 2014, 160 Pt 2, 439–445. [Google Scholar] [CrossRef]
- Jaumaux, F.; De Cadiñanos, L.P.G.; Gabant, P. In the Age of Synthetic Biology, Will Antimicrobial Peptides be the Next Generation of Antibiotics? Antibiotics 2020, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Ongey, E.L.; Neubauer, P. Lanthipeptides: Chemical synthesis versus in vivo biosynthesis as tools for pharmaceutical production. Microb. Cell Factories 2016, 15, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Heel, A.J.; de Jong, A.; Montalban-Lopez, M.; Kok, J.; Kuipers, O.P. BAGEL3: Automated identification of genes encoding bacteriocins and (non-)bactericidal posttranslationally modified peptides. Nucleic Acids Res. 2013, 41, W448–W453. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Hammami, R.; Zouhir, A.; Ben Hamida, J.; Fliss, I. BACTIBASE: A new web-accessible database for bacteriocin characterization. BMC Microbiol. 2007, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Al Kassaa, I.; Rafei, R.; Moukhtar, M.; Zaylaa, M.; Gharsallaoui, A.; Asehraou, A.; El Omari, K.; Shahin, A.; Hamze, M.; Chihib, N.-E. LABiocin database: A new database designed specifically for Lactic Acid Bacteria bacteriocins. Int. J. Antimicrob. Agents 2019, 54, 771–779. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida-Santos, A.C.; Novais, C.; Peixe, L.; Freitas, A.R. Enterococcus spp. as a Producer and Target of Bacteriocins: A Double-Edged Sword in the Antimicrobial Resistance Crisis Context. Antibiotics 2021, 10, 1215. https://doi.org/10.3390/antibiotics10101215
Almeida-Santos AC, Novais C, Peixe L, Freitas AR. Enterococcus spp. as a Producer and Target of Bacteriocins: A Double-Edged Sword in the Antimicrobial Resistance Crisis Context. Antibiotics. 2021; 10(10):1215. https://doi.org/10.3390/antibiotics10101215
Chicago/Turabian StyleAlmeida-Santos, Ana C., Carla Novais, Luísa Peixe, and Ana R. Freitas. 2021. "Enterococcus spp. as a Producer and Target of Bacteriocins: A Double-Edged Sword in the Antimicrobial Resistance Crisis Context" Antibiotics 10, no. 10: 1215. https://doi.org/10.3390/antibiotics10101215






