Investigation of the Prevalence of Antibiotic Resistance Genes According to the Wastewater Treatment Scale Using Metagenomic Analysis
Abstract
:1. Introduction
2. Materials and Method
2.1. Study Area and Sampling
2.2. DNA Extraction and High-Throughput Shot-Gun Sequencing
2.3. Bioinformatics Analysis
3. Results and Discussion
3.1. Diversity and Occurrence of ARGs from Metagenomic Analysis
3.2. Bacterial Community Difference between the Large and Small WWTPs
3.3. Variation of the Abundance of MGE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARG | antibiotic resistance gene |
| ARB | antibiotic resistant bacteria |
| AS | activated sludge |
| DNR | Daewoo nutrient removal |
| HGT | horizontal gene transfer |
| KSBNR | Kist Shinwon biological nutrient removal |
| MGEs | mobile genetic elements |
| MLE | modified Ludzack–Ettinger |
| MLS | macrolide–lincosamide–streptogramin |
| NMDS | nonmetric multidimensional scaling |
| qPCR | quantitative real time PCR |
| WWTP | wastewater treatment plant |
References
- World Health Organization 2017 WHO, Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2016–2017 (Geneva, 2017). Available online: www.who.int/glass/resources/publications/early-implementation-report/en/ (accessed on 21 October 2020).
- Ma, X.; Zhang, Q.; Zhu, Q.; Liu, W.; Chen, Y.; Qiu, R.; Wang, B.; Yang, Z.; Li, H.; Lin, Y.; et al. A Robust CRISPR/Cas9 System for Convenient, High-Efficiency Multiplex genome editing in Monocot and Dicot Plants. Mol. Plant 2015, 8, 1274–1284. [Google Scholar] [CrossRef]
- Tiedje, J.M.; Fang, W.; Manaia, C.M.; Virta, M.; Sheng, H.; Liping, M.A.; Tong, Z.; Edward, T. Antibiotic resistance genes in the Human-Impacted environment: A One Health Perspective. Pedosphere 2019, 29, 273–282. [Google Scholar] [CrossRef]
- Marti, E.; Variatza, E.; Balcazar, J.L. The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol. 2014, 22, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Z.; Song, W.; Du, L.; Ye, C.; Zhao, B.; Liu, W.; Deng, D.; Pan, Y.; Lin, H.; et al. Metagenomic insights into the abundance and composition of resistance genes in aquatic environments: Influence of stratification and geography. Environ. Int. 2019, 127, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.; Yoo, H.; Lee, J.; Choi, E.; Park, J. Exploring the antibiotic resistome in activated sludge and anaerobic digestion sludge in an urban wastewater treatment plant via metagenomic analysis. J. Microbiol. 2020, 58, 123–130. [Google Scholar] [CrossRef]
- Fresia, P.; Antelo, V.; Salazar, C.; Giménez, M.; D’Alessandro, B.; Afshinnekoo, E.; Mason, C.; Gonnet, G.H.; Iraola, G. Urban metagenomics uncover antibiotic resistance reservoirs in coastal beach and sewage waters. Microbiome 2019, 7, 35. [Google Scholar] [CrossRef]
- Guo, J.; Li, J.; Chen, H.; Bond, P.L.; Yuan, Z. Metagenomic analysis reveals wastewater treatment plants as hotspots of antibiotic resistance genes and mobile genetic elements. Water Res. 2017, 123, 468–478. [Google Scholar] [CrossRef]
- Gupta, S.K.; Shin, H.; Han, D.; Hur, H.G.; Unno, T. Metagenomic analysis reveals the prevalence and persistence of antibiotic-and heavy metal-resistance genes in wastewater treatment plant. J. Microbiol. 2018, 56, 408–415. [Google Scholar]
- Kim, Y.K.; Yoo, K.; Kim, M.S.; Han, I.; Lee, M.; Kang, B.R.; Lee, T.K.; Park, J. The capacity of wastewater treatment plants drives bacterial community structure and its assembly. Sci. Rep. 2019, 9, 14809. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [Green Version]
- Czekalski, N.; Imminger, S.; Salhi, E.; Veljkovic, M.; Kleffel, K.; Drissner, D.; Hammes, F.; Bürgmann, H.; Gunten, U. Inactivation of antibiotic resistant bacteria and resistance genes by ozone: From laboratory experiments to full-scale wastewater treatment. Environ. Sci. Technol. 2016, 50, 11862–11871. [Google Scholar] [CrossRef]
- Hembach, N.; Alexander, J.; Hiller, C.; Wieland, A.; Schwartz, T. Dissemination prevention of antibiotic resistant and facultative pathogenic bacteria by ultrafiltration and ozone treatment at an urban wastewater treatment plant. Sci. Rep. 2019, 9, 12843. [Google Scholar] [CrossRef] [Green Version]
- Pärnänen, K.; Narciso-da-Rocha, C.; Kneis, D.; Berendonk, T.U.; Cacace, D.; Do, T.T.; Elpers, C.; Fatta-Kassinos, D.; Henriques, I.; Jaeger, T.; et al. Antibiotic resistance in European wastewater treatment plants mirrors the pattern of clinical antibiotic resistance prevalence. Sci. Adv. 2019, 5, eaau9124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Port, J.A.; Cullen, A.C.; Wallace, J.C.; Smith, M.N.; Faustman, E.M. Metagenomic frameworks for monitoring antibiotic resistance in aquatic environments. Environ. Health Perspect. 2014, 122, 222–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, F.; Li, B.; Ma, L.; Wang, Y.; Huang, D.; Zhang, T. Antibiotic resistance genes and human bacterial pathogens: Co-occurrence, removal, and enrichment in municipal sewage sludge digesters. Water Res. 2016, 91, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.; Lee, T.K.; Choi, E.J.; Yang, J.; Shukla, S.K.; Hwang, S.; Park, J. Molecular approaches for the detection and monitoring of microbial communities in bioaerosols: A review. J. Environ. Sci. 2017, 51, 234–247. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, X.; Jiang, X.T.; Chai, B.; Li, L.; Yang, Y.; Cole, J.R.; Tiedje, J.M.; Zhang, T. ARGs-OAP v2.0 with an expanded SARG database and Hidden Markov Models for enhancement characterization and quantification of antibiotic resistance genes in environmental metagenomes. Bioinformatics 2018, 34, 2263–2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Yang, Y.; Ma, L.; Ju, F.; Guo, F.; Tiedje, J.M.; Zhang, T. Metagenomic and network analysis reveal wide distribution and co-occurrence of environmental antibiotic resistance genes. ISME J. 2015, 9, 2490–2502. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.R.; Kassen, R. The effects of competition and predation on diversification in a model adaptive radiation. Nature 2007, 446, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.; Soares, M.; Pereira, C.; Leitão, N.; Henriques, I.; Correia, A. INTEGRALL: A database and search engine for integrons, integrases and gene cassettes. Bioinformatics 2009, 25, 1096–1098. [Google Scholar]
- Kwietniewska, E.; Tys, J. Process characteristics, inhibition factors and methane yields of anaerobic digestion process, with particular focus on microalgal biomass fermentation. Renew. Sustain. Energy Rev. 2014, 34, 491–500. [Google Scholar] [CrossRef]
- Shi, P.; Jia, S.; Zhang, X.X.; Zhang, T.; Cheng, S.; Li, A. Metagenomic insights into chlorination effects on microbial antibiotic resistance in drinking water. Water Res. 2013, 47, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Butaye, P.; Cloeckaert, A.; Schwarz, S. Mobile genes coding for efflux-mediated antimicrobial resistance in Gram-positive and Gram-negative bacteria. Int. J. Antimicrob. Agents 2003, 22, 205–210. [Google Scholar] [CrossRef]
- Abegglen, C.; Joss, A.; McArdell, C.S.; Fink, G.; Schlüsener, M.P.; Ternes, T.A.; Siegrist, H. The fate of selected micropollutants in a single-house MBR. Water Res. 2009, 43, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Dorival-Garcia, N.; Zafra-Gomez, A.; Navalon, A.; Gonzalez-Lopez, J.; Hontoria, E.; Vilchez, J.L. Removal and degradation characteristics of quinolone antibiotics in laboratory-scale activated sludge reactors under aerobic, nitrifying and anoxic conditions. J. Environ. Manag. 2013, 120, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Le-Minh, N.; Khan, S.J.; Drewes, J.E.; Stuetz, R.M. Fate of antibiotics during municipal water recycling treatment processes. Water Res. 2010, 44, 4295–4323. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Huang, K.; Miao, Y.; Shi, P.; Liu, B.; Long, C.; Li, A. Metagenomic Profiling of Antibiotic Resistance Genes and Mobile Genetic Elements in a Tannery Wastewater Treatment Plant. PLoS ONE 2013, 8, e76079. [Google Scholar] [CrossRef]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.-N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T. Mass flows and removal of antibiotics in two municipal wastewater treatment plants. Chemosphere 2011, 83, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geng, J.; Ma, H.; Ren, H.; Xu, K.; Ding, L. Characterization of microbial community and antibiotic resistance genes in activated sludge under tetracycline and sulfamethoxazole selection pressure. Sci. Total Environ. 2016, 571, 479–486. [Google Scholar] [CrossRef]
- Roberts, A.P.; Mullany, P. A modular master on the move: The Tn916 family of mobile genetic elements. Trend Microbiol. 2009, 17, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [PubMed] [Green Version]
- Kazimierczak, K.A.; Flint, H.J.; Scott, K.P. Comparative Analysis of Sequence Flanking tet(W) Resistance Genes in Multiple Species of Gut Bacteria. Antimicrob. Agents Chemother. 2006, 50, 2632–2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolz, A.C.; Ong, S.K.; Moorman, T.B. Sorption of tylosin onto swine manure. Chemosphere 2005, 60, 284–289. [Google Scholar] [CrossRef]
- Roberts, M.C. Resistance to macrolide, lincosamide, streptogramin, ketolide, and oxazolidinone antibiotics. Mol. Biotechnol. 2004, 28, 47. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Yang, M.; Tian, Z.; Ren, L.; Zhang, S. Abundance and distribution of tetracycline resistance genes and mobile elements in an oxytetracycline production wastewater treatment system. Environ. Sci. Technol. 2012, 46, 7551–7557. [Google Scholar] [PubMed]
- Harnisz, M.; Kiedrzyńska, E.; Kiedrzyński, M.; Korzeniewska, E.; Czatzkowska, M.; Koniuszewska, I.; Jóźwik, A.; Szklarek, S.; Niestępski, S.; Zalewski, M. The impact of WWTP size and sampling season on the prevalence of antibiotic resistance genes in wastewater and the river system. Sci. Total Environ. 2020, 741, 140466. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Z.; Xing, S.; Liao, X. The correlation between antibiotic resistance gene abundance and microbial community resistance in pig farm wastewater and surrounding rivers. Ecotoxicol. Environ. Saf. 2019, 182, 109452. [Google Scholar] [CrossRef]
- Laht, M.; Karkman, A.; Voolaid, V.; Ritz, C.; Tenson, T.; Virta, M.; Kisand, V. Abundances of Tetracycline, Sulphonamide and beta-lactam antibiotic resistance genes in conventional wastewater treatment plants (WWTPs) with different waste load. PLoS ONE 2014, 9, e013705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabri, N.A.; Schmitt, H.; Van der Zaan, B.; Gerritsen, H.W.; Zuidema, T.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Prevalence of antibiotics and antibiotic resistance genes in a wastewater effluent-receiving river in the Netherlands. J. Environ. Chem. Eng. 2018, 8, 102245. [Google Scholar] [CrossRef]
- Wagner, M.; Loy, A. Bacterial community composition and function in sewage treatment systems. Curr. Opin. Biotechnol. 2002, 13, 218–227. [Google Scholar] [CrossRef]
- Yang, Y.; Li, B.; Zou, S.; Fang, H.; Zhang, T. Fate of antibiotic resistance genes in sewage treatment plant revealed by metagenomic approach. Water Res. 2014, 62, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Seviour, R.J.; Kragelund, C.; Kong, Y.; Eales, K.; Nielsen, J.L.; Nielsen, P.H. Ecophysiology of the Actinobacteria in activated sludge systems. Antonie Leeuwenhoek. 2008, 94, 21–33. [Google Scholar] [PubMed]
- Schmid, M.; Thill, A.; Purkhold, U.; Walcher, M.; Bottero, J.Y.; Ginestet, P.; Nielsen, P.H.; Wuertz, S.; Wagner, M. Characterization of activated sludge flocs by confocal laser scanning microscopy and image analysis. Water Res. 2003, 37, 2043–2052. [Google Scholar] [CrossRef]
- Nielsen, P.H.; Saunders, A.M.; Hansen, A.A.; Larsen, P.; Nielsen, J.L. Microbial communities involved in enhanced biological phosphorus removal from wastewater—a model system in environmental biotechnology. Curr. Opin. Biotechnol. 2012, 23, 452–459. [Google Scholar]
- Guo, F.; Zhang, T.; Li, B.; Wang, Z.; Ju, F.; Liang, Y. Mycobacterial species and their contribution to cholesterol degradation in wastewater treatment plants. Sci. Rep. 2019, 9, 836. [Google Scholar] [CrossRef] [Green Version]
- Chiellini, C.; Munz, G.; Petroni, G.; Lubello, C.; Mori, G.; Verni, F.; Vannini, C. Characterization and comparison of bacterial communities selected in conventional activated sludge and membrane bioreactor pilot plants: A focus on Nitrospira and Planctomycetes bacterial Phyla. Curr. Microbiol. 2013, 67, 77–90. [Google Scholar] [CrossRef]
- Gilbert, E.M.; Agrawal, S.; Brunner, F.; Schwartz, T.; Horn, H.; Lackner, S. Response of different nitrospira species to anoxic periods depends on operational DO. Environ. Sci. Technol. 2014, 48, 2934–2941. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.D.; Chakraborty, R.; Lack, J.G.; O’Connor, S.M.; Cole, K.A.; Bender, K.S.; Achenbach, L.A. Anaerobic benzene oxidation coupled to nitrate reduction in pure culture by two strains of Dechloromonas. Nature 2001, 411, 1039–1043. [Google Scholar] [CrossRef]
- Mao, Y.; Xia, Y.; Zhang, T. Characterization of Thauera-dominated hydrogen-oxidizing autotrophic denitrifying microbial communities by using high-throughput sequencing. Bioresour. Technol. 2013, 128, 703–710. [Google Scholar] [CrossRef]
- Salinero, K.K.; Keller, K.; Feil, W.S.; Feil, H.; Trong, S.; Bartolo, G.D.; Lapidus, A. Metabolic analysis of the soil microbe Dechloromonas aromatica str. RCB: Indications of a surprisingly complex life-style and cryptic anaerobic pathways for aromatic degradation. BMC Genom. 2009, 10, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibarbalz, F.M.; Figuerola, E.; Erijman, L. Industrial activated sludge exhibit unique bacterial community composition at high taxonomic ranks. Water Res. 2013, 47, 3854–3864. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.M.; Albertsen, M.; Vollertsen, J.; Nielsen, P.H. The activated sludge ecosystem contains a core community of abundant organisms. ISME J. 2015, 10, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Gillings, M.R.; Gaze, W.H.; Pruden, A.; Smalla, K.; Tiedje, J.M.; Zhu, Y.G. Using the class 1 integron-integrase gene as a proxy for anthropogenic pollution. ISME J. 2015, 9, 1269–1279. [Google Scholar] [PubMed]
- Kristiansson, E.; Fick, J.; Janzon, A.; Grabic, R.; Rutgersson, C.; Weijdegård, B.; Söderström, H.; Larsson, D.G.J. Pyrosequencing of antibiotic-contaminated river sediments reveals high levels of resistance and gene transfer elements. PLoS ONE 2011, 6, e17038. [Google Scholar] [CrossRef]
- Su, J.Q.; Wei, B.; Ou-Yang, W.Y.; Huang, F.Y.; Zhao, Y.; Xu, H.J.; Zhu, Y.G. Antibiotic Resistome and Its Association with Bacterial Communities during Sewage Sludge Composting. Environ. Sci. Technol. 2015, 49, 7356–7363. [Google Scholar]
- Christgen, B.; Scott, K.; Dolfing, J.; Head, I.M.; Curtis, T.P. An evaluation of the performance and economics of membranes and separators in single chamber microbial fuel cells treating domestic wastewater. PLoS ONE 2015, 10, e0136108. [Google Scholar] [CrossRef] [PubMed]
- Agersø, Y.; Petersen, A. The tetracycline resistance determinant Tet 39 and the sulphonamide resistance gene sulII are common among resistant Acinetobacter spp. isolated from integrated fish farms in Thailand. J. Antimicrob. Chemother. 2007, 59, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Pruden, A.; Pei, R.; Storteboom, H.; Carlson, K.H. Antibiotic resistance genes as emerging contaminants: Studies in Northern Colorado. Environ. Sci. Technol. 2006, 40, 7445–7450. [Google Scholar] [CrossRef]
- Chen, Q.; An, X.; Li, H.; Su, J.; Ma, Y.; Zho, Y.G. Long-term field application of sewage sludge increases the abundance of antibiotic resistance genes in soil. Environ. Int. 2016, 92–93, 1–10. [Google Scholar] [CrossRef] [PubMed]
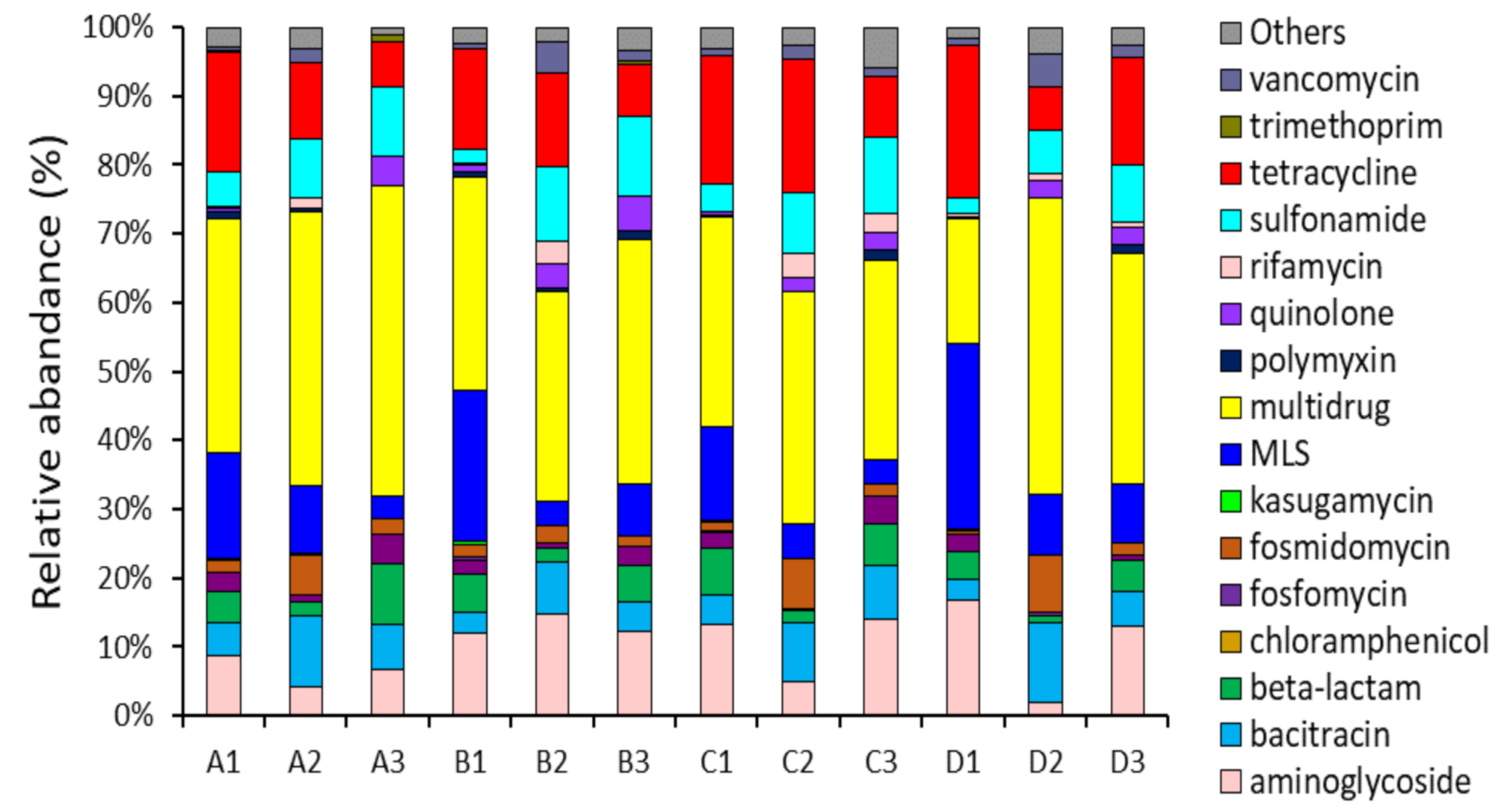

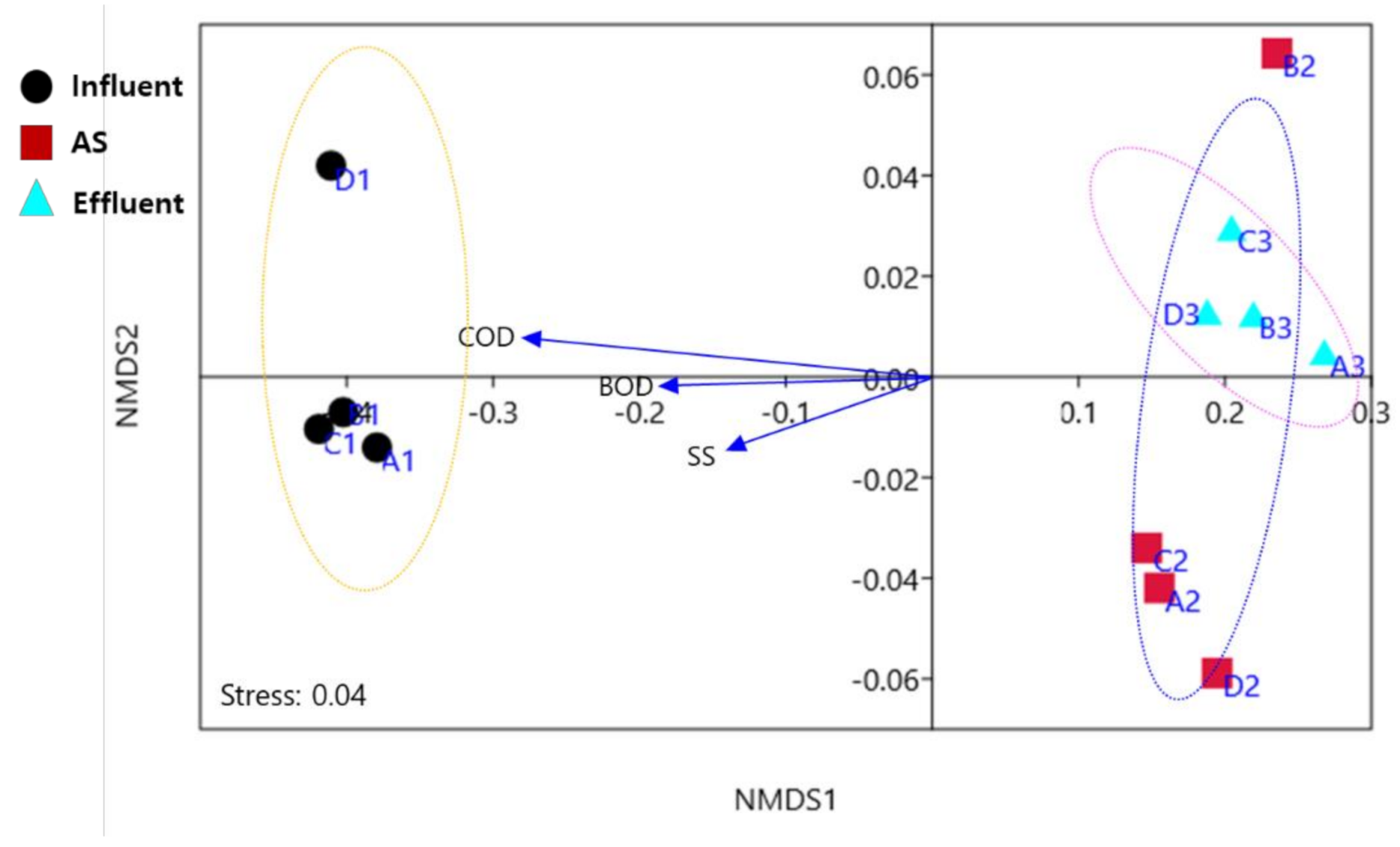

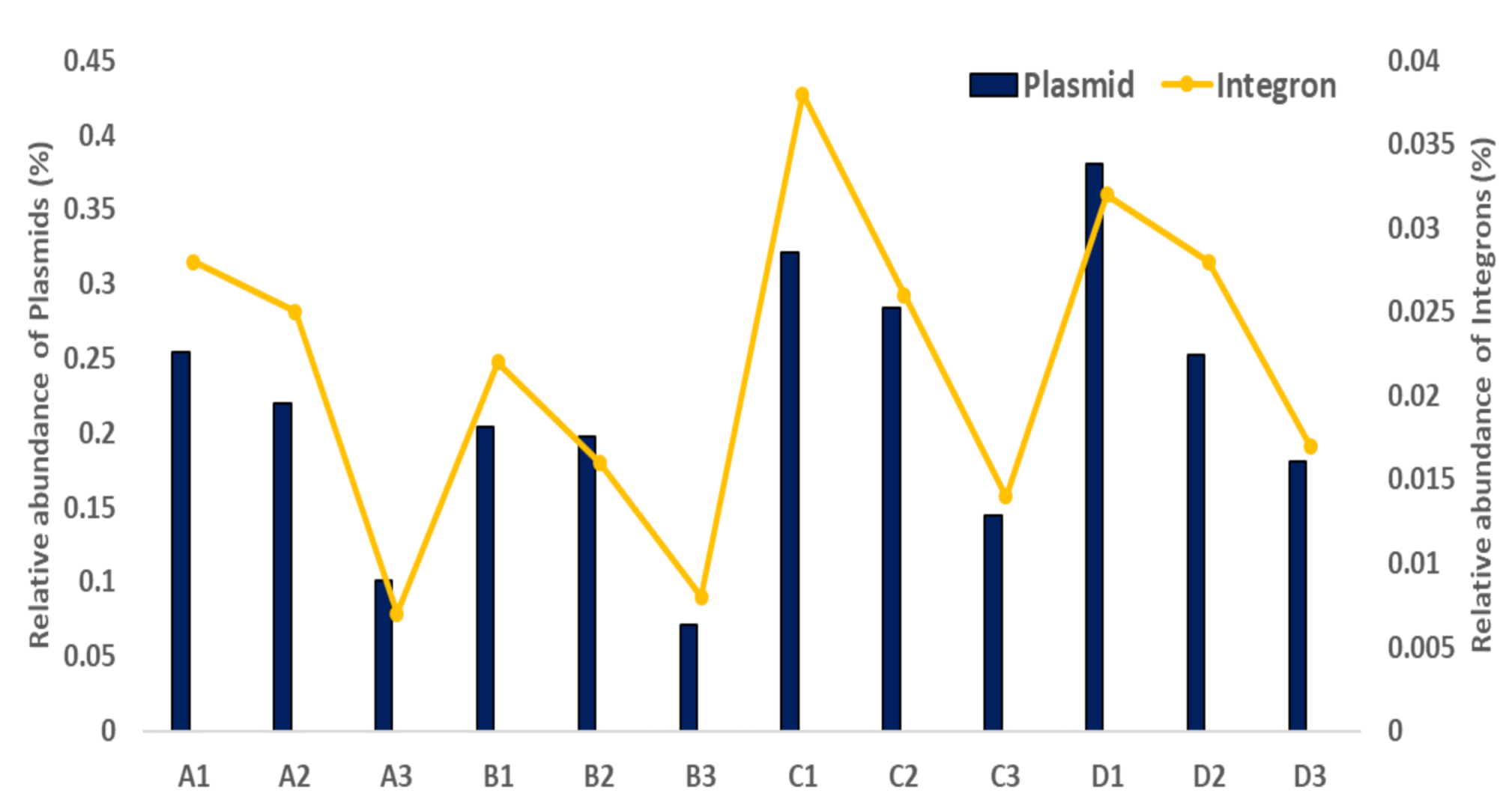
| Category | A | B | C | D | |
|---|---|---|---|---|---|
| Influent type | Domestic sewage and pretreated industrial wastewater | ||||
| Processing Capacity (m3/day) | 9.5 × 104 | 4.0 × 104 | 34.0 × 104 | 45.2 × 104 | |
| Reactor process | KSBNR | DNR | MLE | MLE | |
| Influent wastewater quality | Flow rate (m3/days) | 42,203 | 27,124 | 333,427 | 357,523 |
| BOD (mg/L) | 63.2 ± 5.35 | 131.8 ± 4.38 | 68.8 ± 2.37 | 84.9 ± 5.37 | |
| COD (mg/L) | 130.3 ± 5.75 | 184.1 ± 25.0 | 140.8 ± 12.8 | 178.3 ± 23.1 | |
| SS (mg/L) | 126.9 ± 6.41 | 227.8 ± 29.96 | 183.6 ± 6.74 | 182.1 ± 5.77 | |
| T-N (mg/L) | 32.0 ± 2.62 | 57.1 ± 3.39 | 36.4 ± 1.07 | 45.5 ± 2.41 | |
| T-P (mg/L) | 3.6 ± 0.24 | 5.8 ± 0.46 | 3.8 ± 0.064 | 4.6 ± 0.23 | |
| pH | 7.3 | 7.0 | 7.3 | 7.3 | |
| Effluent wastewater quality | Flow rate (m3/days) | 41,111 | 23,199 | 303,596 | 284,540 |
| BOD (mg/L) | 2.9 ± 1.4 | 6.6 ± 3.2 | 2.7 ± 0.5 | 6.5 ± 0.6 | |
| COD (mg/L) | 7.9 ± 4.0 | 16.9 ± 6.3 | 9.7 ± 3.2 | 11.2 ± 2.4 | |
| SS (mg/L) | 3.2 ± 1.8 | 4.9 ± 2.1 | 2.0 ± 0.3 | 2.8 ± 0.5 | |
| T-N (mg/L) | 8.642 ± 2.145 | 3.948 ± 2.136 | 9.611 ± 0.526 | 7.496 ± 2.048 | |
| T-P (mg/L) | 1.064 ± 0.037 | 0.169 ± 0.016 | 0.419 ± 0.16 | 0.712 ± 0.026 | |
| pH | 6.5 | 6.7 | 6.6 | 7.0 | |
| MLSS (mg/L) | 2200 | 1975 | 2600 | 2220 | |
| HRT (hours) | 11 | 28 | 14 | 14.4 | |
| SRT (days) | 17 | 7.9 | 10–15 | 18.6 | |
| MGEs | Integrons | Plasmids | ||||
|---|---|---|---|---|---|---|
| Relative Abundance 1 | Diversity 2 | Relative Abundance 1 | Diversity 2 | |||
| Large scale WWTP | ARGs | Relative Abundance 1 | 0.90 ** | 0.86 ** | 0.84 * | 0.78 * |
| Diversity 2 | 0.72 * | 0.82 * | 0.72 * | 0.72 * | ||
| Small scale WWTP | ARGs | Relative Abundance 1 | 0.86 ** | 0.82 ** | 0.76 * | 0.72 * |
| Diversity 2 | 0.76 * | 0.78 * | 0.66 * | 0.68 * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, K.; Lee, G. Investigation of the Prevalence of Antibiotic Resistance Genes According to the Wastewater Treatment Scale Using Metagenomic Analysis. Antibiotics 2021, 10, 188. https://doi.org/10.3390/antibiotics10020188
Yoo K, Lee G. Investigation of the Prevalence of Antibiotic Resistance Genes According to the Wastewater Treatment Scale Using Metagenomic Analysis. Antibiotics. 2021; 10(2):188. https://doi.org/10.3390/antibiotics10020188
Chicago/Turabian StyleYoo, Keunje, and Gihan Lee. 2021. "Investigation of the Prevalence of Antibiotic Resistance Genes According to the Wastewater Treatment Scale Using Metagenomic Analysis" Antibiotics 10, no. 2: 188. https://doi.org/10.3390/antibiotics10020188






