Lyophilized Human Bone Allograft as an Antibiotic Carrier: An In Vitro and In Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Bone Allografts
2.2. Antibiotic Substances
2.3. Antibiotic Impregnation of the Bone Allografts
2.4. In Vitro Analysis
2.4.1. Antibiotic Release Kinetic Measurements
2.4.2. Susceptibility Tests
2.5. In Vivo Analysis
2.5.1. Animal Model
2.5.2. Determination of the Residual Antibiotic Concentration in Bone Allograft Blocks In Vivo
2.5.3. Histopathological and Histomorphometrical Evaluation of Bone Regeneration
2.6. Statistical Analysis
3. Results
3.1. In Vitro Antibiotic Release Kinetics
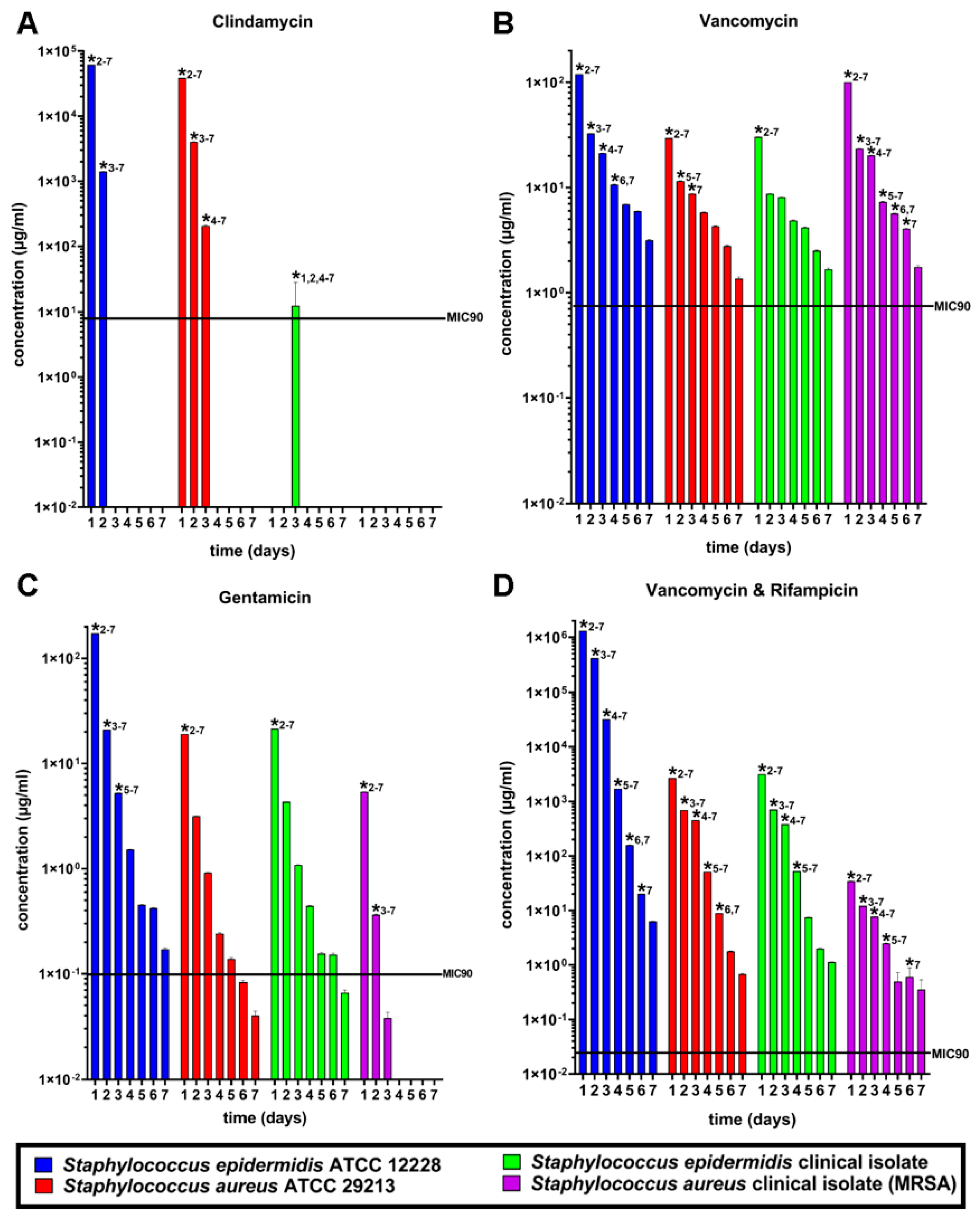
3.2. Susceptibility Tests
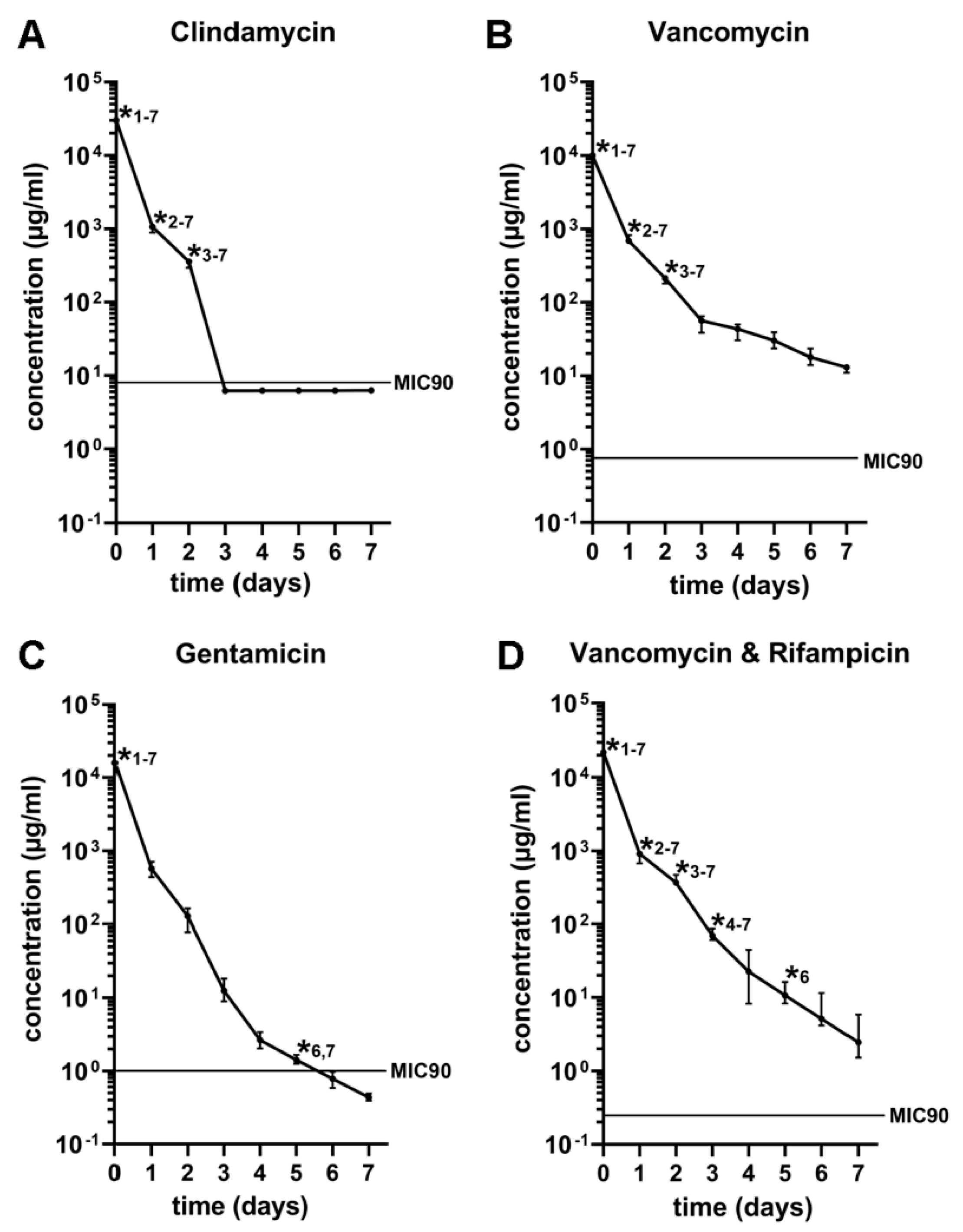
3.3. In Vivo Antibiotic Release Kinetics
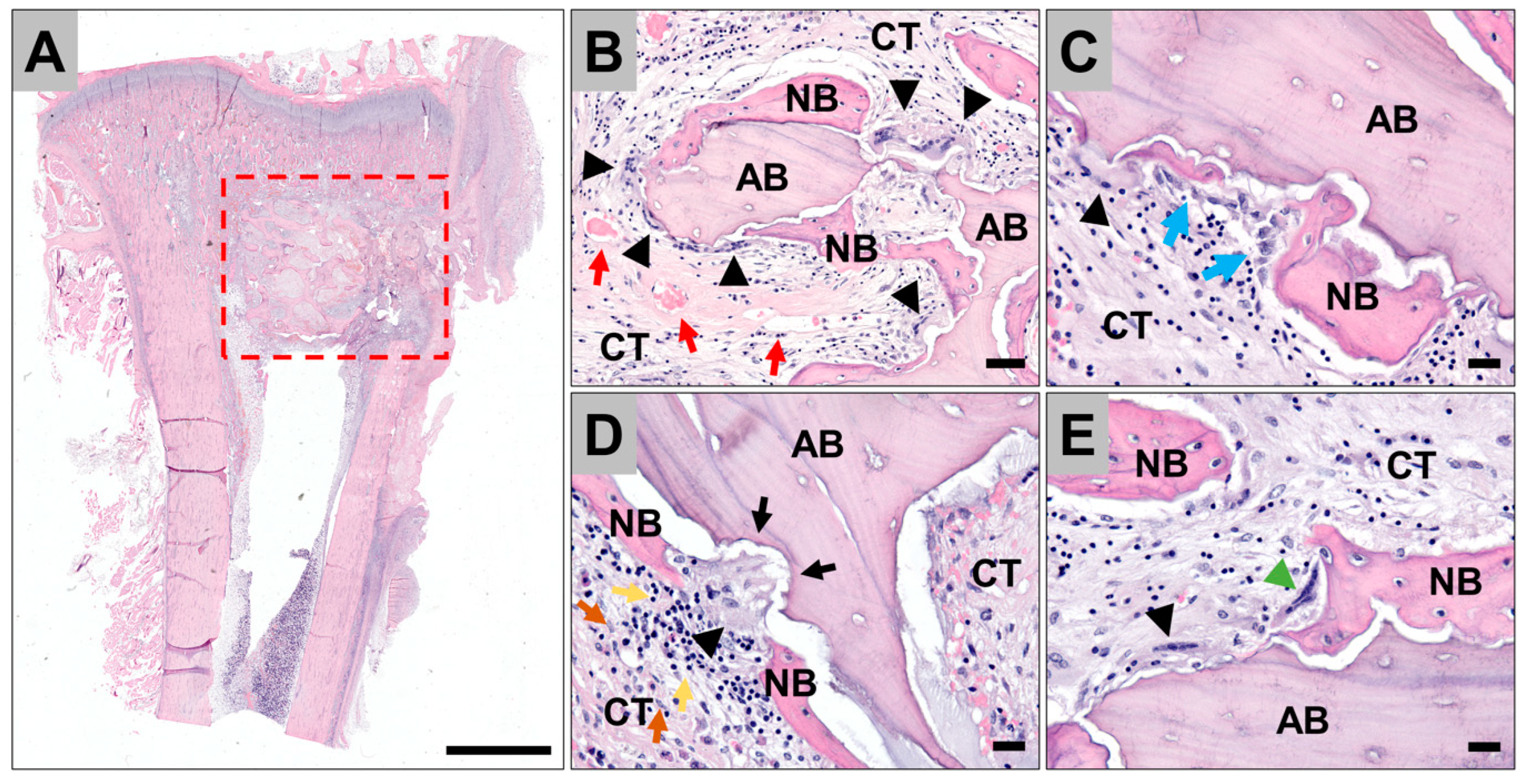
3.4. Histopathological Evaluation of the Tissue Reactions to the Allografts
3.5. Histomorphometrical Analysis of Bone Ingrowth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, P.T.H.; Clayton, R.A.; Safir, O.A.; Backstein, D.J.; Gross, A.E. Structural Allograft as an Option for Treating Infected Hip Arthroplasty with Massive Bone Loss. Clin. Orthop. Relat. Res. 2011, 469, 1016–1023. [Google Scholar] [CrossRef] [Green Version]
- Blackley, H.R.; Davis, A.M.; Hutchison, C.R.; Gross, A.E. Proximal Femoral Allografts for Reconstruction of Bone Stock in Revision Arthroplasty of the Hip: A Nine to Fifteen-Year Follow-up. J. Bone Jt. Surg. 2001, 83, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Head, W.C.; Emerson, R.H., Jr.; Malinin, T.I. Structural Bone Grafting for Femoral Reconstruction. Clin. Orthop. Relat. Res. 1999, 369, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.J.; Garbuz, D.S.; Pugh, L.; Masri, B.A. Revision Total Knee Arthroplasty: Clinical Outcome Comparison with and Without the Use of Femoral Head Structural Allograft. J. Arthroplast. 2011, 26, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Klára, T.; Csönge, L.; Janositz, G.; Csernátony, Z.; Lacza, Z. Albumin-coated structural lyophilized bone allografts: A clinical report of 10 cases. Cell Tissue Bank. 2014, 15, 89–97. [Google Scholar] [CrossRef]
- Enneking, W.F.; Mindell, E.R. Observations on massive retrieved human allografts. J. Bone Jt. Surg. 1991, 73, 1123–1142. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, A.; Konrad, L.; Hessmann, M.H.; Küuchle, R.; Korner, J.; Rompe, J.D.; Rommens, P.M. The influence of bone allograft processing on osteoblast attachment and function. J. Orthop. Res. 2005, 23, 846–854. [Google Scholar] [CrossRef]
- Brewster, N.T.; Gillespie, W.J.; Howie, C.R.; Madabhushi, S.P.; Usmani, A.S.; Fairbairn, D.R. Mechanical considerations in impaction bone grafting. J. Bone Jt. Surg. Br. 1999, 81, 118–124. [Google Scholar] [CrossRef]
- Parvizi, J.; Saleh, K.J.; Ragland, P.S.; Pour, A.E.; Mont, M.A. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop. 2008, 79, 335–341. [Google Scholar] [CrossRef]
- Parvizi, J.; Pour, A.E.; Keshavarzi, N.R.; D’apuzzo, M.; Sharkey, P.F.; Hozack, W.J. Revision Total Hip Arthroplasty in Octogenarians: A case-control study. J. Bone Jt. Surg. 2007, 89, 2612–2618. [Google Scholar] [CrossRef]
- Blom, A.W.; Taylor, A.H.; Pattison, G.; Whitehouse, S.; Bannister, G.C. Infection after total hip arthroplasty. The Avon experience. J. Bone Jt. Surg. Br. 2003, 85, 956–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dale, H.; Hallan, G.; Espehaug, B.; I Havelin, L.; Engesæter, L.B. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop. 2009, 80, 639–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kordbacheh Changi, K.; Finkelstein, J.; Papapanou, P.N. Peri-implantitis prevalence, incidence rate, and risk factors: A study of electronic health records at a U.S. dental school. Clin. Oral Implants Res. 2019, 30, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Hull, P.D.; Johnson, S.C.; Stephen, D.J.G.; Kreder, H.J.; Jenkinson, R.J. Delayed debridement of severe open fractures is associated with a higher rate of deep infection. Bone Jt. J. 2014, 96, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Camps-Font, O.; Figueiredo, R.; Valmaseda-Castellón, E.; Gay-Escoda, C. Postoperative Infections after Dental Implant Placement: Prevalence, Clinical Features, and Treatment. Implant Dent. 2015, 24, 713–719. [Google Scholar] [CrossRef] [Green Version]
- Tägil, M.; Aspenberg, P. Impaction of cancellous bone grafts impairs osteoconduction in titanium chambers. Clin. Orthop. Relat. Res. 1998, 352, 231–238. [Google Scholar]
- Buttaro, M.A.; de la Rosa, D.M.; Comba, F.; Piccaluga, F. High Failure Rate with the GAP II Ring and Impacted Allograft Bone in Severe Acetabular Defects. Clin. Orthop. Relat. Res. 2012, 470, 3148–3155. [Google Scholar] [CrossRef] [Green Version]
- Duffy, G.P.; O’Connor, M.I.; Brodersen, M.P. Fatigue Failure of the GAP Ring. J. Arthroplast. 2007, 22, 711–714. [Google Scholar] [CrossRef]
- Ding, H.; Mao, Y.; Yu, B.; Zhu, Z.; Li, H.; Yu, B.; Huang, J. The use of morselized allografts without impaction and cemented cage support in acetabular revision surgery: A 4- to 9-year follow-up. J. Orthop. Surg. Res. 2015, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Isefuku, S.; Joyner, C.J.; Simpson, A.H.R.W. Gentamicin May Have an Adverse Effect on Osteogenesis. J. Orthop. Trauma 2003, 17, 212–216. [Google Scholar] [CrossRef]
- Coraça-Huber, D.C.; Kreidl, L.; Steixner, S.; Hinz, M.; Dammerer, D.; Fille, M. Identification and Morphological Characterization of Biofilms Formed by Strains Causing Infection in Orthopedic Implants. Pathogens 2020, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Penesyan, A.; Gillings, M.; Paulsen, I.T. Antibiotic Discovery: Combatting Bacterial Resistance in Cells and in Biofilm Communities. Molecules 2015, 20, 5286–5298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto-Lambertz, C.; Yagdiran, A.; Wallscheid, F.; Eysel, P.; Jung, N. Periprosthetic Infection in Joint Replacement. Dtsch. Arztebl. Int. 2017, 114, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials 2016, 81, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Liu, Y.; Guo, J.; Wu, H.; Wang, J.; Wu, G. Biomaterials with Antibacterial and Osteoinductive Properties to Repair Infected Bone Defects. Int. J. Mol. Sci. 2016, 17, 334. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, T.F.; Kuehl, R.; Coenye, T.; Metsemakers, W.-J.; Morgenstern, M.; Schwarz, E.M.; Riool, M.; Zaat, S.A.; Khana, N.; Kates, S.L.; et al. Orthopaedic device-related infection: Current and future interventions for improved prevention and treatment. EFORT Open Rev. 2016, 1, 89–99. [Google Scholar] [CrossRef]
- Anagnostakos, K.; Schröder, K. Antibiotic-Impregnated Bone Grafts in Orthopaedic and Trauma Surgery: A Systematic Review of the Literature. Int. J. Biomater. 2012, 2012, 538061. [Google Scholar] [CrossRef]
- Coraça-Huber, D.C.; Ammann, C.G.; Nogler, M.; Fille, M.; Frommelt, L.; Kühn, K.-D.; Fölsch, C. Lyophilized allogeneic bone tissue as an antibiotic carrier. Cell Tissue Bank. 2016, 17, 629–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraf, S.K.; Yadav, A.; Nagwani, S.; Sen, M.R. Decal bone matrix as a local antibiotic delivery vehicle in a MRSA-infected bone model: An experimental study. Indian J. Orthop. 2010, 44, 246–251. [Google Scholar] [CrossRef]
- Wichelhaus, T.A.; Dingeldein, E.; Rauschmann, M.; Kluge, S.; Dieterich, R.; Schäfer, V.; Brade, V. Elution characteristics of vancomycin, teicoplanin, gentamicin and clindamycin from calcium sulphate beads. J. Antimicrob. Chemother. 2001, 48, 117–119. [Google Scholar] [CrossRef] [Green Version]
- Evaniew, N.; Tan, V.; Parasu, N.; Jurriaans, E.; Finlay, K.; Deheshi, B.; Ghert, M. Use of a Calcium Sulfate–Calcium Phosphate Synthetic Bone Graft Composite in the Surgical Management of Primary Bone Tumors. Orthopedics 2013, 36, e216–e222. [Google Scholar] [CrossRef] [Green Version]
- Roberts, R.; McConoughey, S.J.; Calhoun, J.H. Size and composition of synthetic calcium sulfate beads influence dissolution and elution rates in vitro. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 667–673. [Google Scholar] [CrossRef]
- Coraça-Huber, D.C.; Wurm, A.; Fille, M.; Hausdorfer, J.; Nogler, M.; Vogt, S.; Kühn, K.-D. Antibiotic-loaded calcium carbonate/calcium sulfate granules as co-adjuvant for bone grafting. J. Mater. Sci. Mater. Med. 2015, 26, 19. [Google Scholar] [CrossRef]
- Lindner, C.; Pröhl, A.; Abels, M.; Löffler, T.; Batinic, M.; Jung, O.; Barbeck, M. Specialized Histological and Histomorphometrical Analytical Methods for Biocompatibility Testing of Biomaterials for Maxillofacial Surgery in (Pre-) Clinical Studies. Vivo 2020, 34, 3137–3152. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Jung, O.; Xiong, X.; Krastev, R.; Korzinskas, T.; Najman, S.; Radenković, M.; Wegner, N.; Knyazeva, M.; Walther, F. Balancing Purification and Ultrastructure of Naturally Derived Bone Blocks for Bone Regeneration: Report of the Purification Effort of Two Bone Blocks. Materials 2019, 12, 3234. [Google Scholar] [CrossRef] [Green Version]
- Korzinskas, T.; Jung, O.; Smeets, R.; Stojanovic, S.; Najman, S.; Glenske, K.; Hahn, M.; Wenisch, S.; Schnettler, R.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Macrophage Response of a Non-Resorbable PTFE Membrane for Guided Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 2952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawil, G.; Barbeck, M.; Unger, R.; Tawil, P.; Witte, F. Sinus Floor Elevation Using the Lateral Approach and Window Repositioning and a Xenogeneic Bone Substitute as a Grafting Material: A Histologic, Histomorphometric, and Radiographic Analysis. Int. J. Oral Maxillofac. Implants 2018, 33, 1089–1096. [Google Scholar] [CrossRef]
- Barbeck, M.; Najman, S.; Stojanovic, S.; Mitić, Z.; Živković, J.M.; Choukroun, J.; Kovačević, P.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Addition of blood to a phycogenic bone substitute leads to increased in vivo vascularization. Biomed. Mater. 2015, 10, 055007. [Google Scholar] [CrossRef]
- Barbeck, M.; Udeabor, S.; Lorenz, J.; Schlee, M.; Holthaus, M.G.; Raetscho, N.; Choukroun, J.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. High-Temperature Sintering of Xenogeneic Bone Substitutes Leads to Increased Multinucleated Giant Cell Formation: In Vivo and Preliminary Clinical Results. J. Oral Implantol. 2015, 41, e212–e222. [Google Scholar] [CrossRef]
- Sorger, J.I.; Hornicek, F.J.; Zavatta, M.; Menzner, J.P.; Gebhardt, M.C.; Tomford, W.W.; Mankin, H.J. Allograft Fractures Revisited. Clin. Orthop. Relat. Res. 2001, 382, 66–74. [Google Scholar] [CrossRef]
- Parrish, F.F. Allograft Replacement of All or Part of the End of a Long Bone Following Excision of a Tumor. J. Bone Jt. Surg. 1973, 55, 1–22. [Google Scholar] [CrossRef]
- Buchholz, H.W.; Engelbrecht, H. Depot effects of various antibiotics mixed with palacos resins. Chirurg 1970, 41, 511–515. [Google Scholar]
- Winkler, H.; Kaudela, K.; Stoiber, A.; Menschik, F. Bone grafts impregnated with antibiotics as a tool for treating infected implants in orthopedic surgery—One stage revision results. Cell Tissue Bank. 2006, 7, 319–323. [Google Scholar] [CrossRef]
- Barckman, J.; Baas, J.; Sorensen, M.; Lange, J.; Bechtold, J.E.; Soballe, K. Does tobramycin impregnation of allograft bone affect implant fixation?—An experimental study in 12 dogs. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 173–180. [Google Scholar] [CrossRef]
- Buttaro, M.A.; I Gimenez, M.; Greco, G.; Barcan, L.; Piccaluga, F. High active local levels of vancomycin without nephrotoxicity released from impacted bone allografts in 20 revision hip arthroplasties. Acta Orthop. 2005, 76, 336–340. [Google Scholar] [CrossRef]
- Toms, A.D.; Barker, R.L.; Jones, R.S.; Kuiper, J.H. Impaction Bone-Grafting in Revision Joint Replacement Surgery. J. Bone Jt. Surg. 2004, 86, 2050–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oakes, D.A.; Cabanela, M.E. Impaction Bone Grafting for Revision Hip Arthroplasty: Biology and Clinical Applications. J. Am. Acad. Orthop. Surg. 2006, 14, 620–628. [Google Scholar] [CrossRef]
- Lindsey, R.W.; Probe, R.; Miclau, T.; Alexander, J.W.; Perren, S.M. The effects of antibiotic-impregnated autogeneic cancellous bone graft on bone healing. Clin. Orthop. Relat. Res. 1993, 291, 303–312. [Google Scholar] [CrossRef]
- Goldberg, V.M. Selection of Bone Grafts for Revision Total Hip Arthroplasty. Clin. Orthop. Relat. Res. 2000, 381, 68–76. [Google Scholar] [CrossRef]
- Coraça-Huber, D.C.; Putzer, D.; Fille, M.; Hausdorfer, J.; Nogler, M.; Kühn, K.-D. Gentamicin palmitate as a new antibiotic formulation for mixing with bone tissue and local release. Cell Tissue Bank. 2014, 15, 139–144. [Google Scholar] [CrossRef]
- Coraça-Huber, D.C.; Hausdorfer, J.; Fille, M.; Nogler, M. Effect of storage temperature on gentamicin release from antibiotic-coated bone chips. Cell Tissue Bank. 2013, 14, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Witsø, E.; Persen, L.; Benum, P.; Bergh, K. Cortical allograft as a vehicle for antibiotic delivery. Acta Orthop. 2005, 76, 481–486. [Google Scholar] [CrossRef]
- Lew, W.; Moore, W. Antibiotic-Impregnated Grafts for Aortic Reconstruction. Semin. Vasc. Surg. 2011, 24, 211–219. [Google Scholar] [CrossRef]
- Chervu, A.; Moore, W.S.; Chvapil, M.; Henderson, T. Efficacy and duration of antistaphylococcal activity comparing three antibiotics bonded to Dacron vascular grafts with a collagen release system. J. Vasc. Surg. 1991, 13, 897–901. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.P.; Stefko, J.; Lumpkin, J.A.; Rosenblatt, J. The Effect of Electrostatic Charge Interactions on Release Rates of Gentamicin from Collagen Matrices. Pharm. Res. 1995, 12, 1205–1210. [Google Scholar] [CrossRef]
- Gordon, R.; Regamey, C.; Kirby, W. Serum Protein Binding of Erythromycin, Lincomycin, and Clindamycin. J. Pharm. Sci. 1973, 62, 1074–1077. [Google Scholar] [CrossRef]
- Albrecht, L.M.; Rybak, M.J.; Warbasse, L.H.; Edwards, D.J. Vancomycin Protein Binding in Patients with Infections Caused by Staphylococcus Aureus. DICP 1991, 25, 713–715. [Google Scholar] [CrossRef]
- Bailey, D.N.; Briggs, J.R. Gentamicin and Tobramycin Binding to Human Serum In Vitro. J. Anal. Toxicol. 2004, 28, 187–189. [Google Scholar] [CrossRef]
- Boman, G.; Ringberger, V.A. Binding of rifampicin by human plasma proteins. Eur. J. Clin. Pharmacol. 1974, 7, 369–373. [Google Scholar] [CrossRef]
- Opatowski, L.; Mandel, J.; Varon, E.; Boëlle, P.Y.; Temime, L.; Guillemot, D. Antibiotic dose impact on resistance selection in the community: A mathematical model of beta-lactams and Streptococcus pneumoniae dynamics. Antimicrob. Agents Chemother. 2010, 54, 2330–2337. [Google Scholar] [CrossRef] [Green Version]
- Raymond, B. Five rules for resistance management in the antibiotic apocalypse, a road map for integrated microbial management. Evol. Appl. 2019, 12, 1079–1091. [Google Scholar] [CrossRef]
- Holmes, R.L.; Jorgensen, J.H. Inhibitory Activities of 11 Antimicrobial Agents and Bactericidal Activities of Vancomycin and Daptomycin against Invasive Methicillin-Resistant Staphylococcus aureus Isolates Obtained from 1999 through 2006. Antimicrob. Agents Chemother. 2008, 52, 757–760. [Google Scholar] [CrossRef] [Green Version]
- Klemm, K. Gentamicin-PMMA-beads in treating bone and soft tissue infections (author’s transl). Zent. Chir. 1979, 104, 934–942. [Google Scholar]
- Klemm, K. The use of antibiotic-containing bead chains in the treatment of chronic bone infections. Clin. Microbiol. Infect. 2001, 7, 28–31. [Google Scholar] [CrossRef] [Green Version]
- McLaren, A.C. Alternative Materials to Acrylic Bone Cement for Delivery of Depot Antibiotics in Orthopaedic Infections. Clin. Orthop. Relat. Res. 2004, 427, 101–106. [Google Scholar] [CrossRef]
- Jiranek, W.A.; Hanssen, A.D.; Greenwald, A.S. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J. Bone Jt. Surg. Am. 2006, 88, 2487–2500. [Google Scholar] [CrossRef]
- Gallo, J.; Holinka, M.; Moucha, C.S. Antibacterial Surface Treatment for Orthopaedic Implants. Int. J. Mol. Sci. 2014, 15, 13849–13880. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Meng, G.; Han, F.; Li, X.; Chen, B.; Xu, Q.; Zhu, X.; Chu, Z.; Kong, M.; Huang, Q. Nanocontainers made of Various Materials with Tunable Shape and Size. Sci. Rep. 2013, 3, 2238. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Cho, J.; Shivapooja, P.; Ista, L.K.; López, G.P. Nanopatterned Smart Polymer Surfaces for Controlled Attachment, Killing, and Release of Bacteria. ACS Appl. Mater. Interfaces 2013, 5, 9295–9304. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priyadarshini, B.M.; Dikshit, V.; Zhang, Y. 3D-printed Bioreactors for In Vitro Modeling and Analysis. Int. J. Bioprint. 2020, 6, 267. [Google Scholar] [CrossRef]
- Perić Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Pejakić, M.; Schnettler, R.; Gosau, M.; Smeets, R.; Jung, O.; Barbeck, M. An introduction to bone tissue engineering. Int. J. Artif. Organs 2020, 43, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Dard, M.; Kokkinopoulou, M.; Markl, J.; Booms, P.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Small-sized granules of biphasic bone substitutes support fast implant bed vascularization. Biomatter 2015, 5, e1056943. [Google Scholar] [CrossRef] [Green Version]
- Barbeck, M.; Booms, P.; Unger, R.; Hoffmann, V.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Multinucleated giant cells in the implant bed of bone substitutes are foreign body giant cells-New insights into the material-mediated healing process. J. Biomed. Mater. Res. A 2017, 105, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Motta, A.; Migliaresi, C.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Heterogeneity of biomaterial-induced multinucleated giant cells: Possible importance for the regeneration process? J. Biomed. Mater. Res. A 2016, 104, 413–418. [Google Scholar] [CrossRef]



| Study Groups | p Values |
|---|---|
| Vancomycin vs. Clindamycin | 0.6871 |
| Vancomycin vs. Gentamycin | 0.6316 |
| Vancomycin vs. Vancomycin&Rifampicin | 0.9986 |
| Vancomycin vs. Control | 0.4277 |
| Clindamycin vs. Gentamycin | 0.0960 |
| Clindamycin vs. Vancomycin&Rifampicin | 0.8393 |
| Clindamycin vs. Control | 0.0607 |
| Gentamycin vs. Vancomycin&Rifampicin | 0.4777 |
| Gentamycin vs. Control | 0.9954 |
| Vancomycin&Rifampicin vs. Control | 0.3019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coraça-Huber, D.C.; Steixner, S.J.M.; Najman, S.; Stojanovic, S.; Finze, R.; Rimashevskiy, D.; Saginova, D.; Barbeck, M.; Schnettler, R. Lyophilized Human Bone Allograft as an Antibiotic Carrier: An In Vitro and In Vivo Study. Antibiotics 2022, 11, 969. https://doi.org/10.3390/antibiotics11070969
Coraça-Huber DC, Steixner SJM, Najman S, Stojanovic S, Finze R, Rimashevskiy D, Saginova D, Barbeck M, Schnettler R. Lyophilized Human Bone Allograft as an Antibiotic Carrier: An In Vitro and In Vivo Study. Antibiotics. 2022; 11(7):969. https://doi.org/10.3390/antibiotics11070969
Chicago/Turabian StyleCoraça-Huber, Débora C., Stephan J. M. Steixner, Stevo Najman, Sanja Stojanovic, Ronja Finze, Denis Rimashevskiy, Dina Saginova, Mike Barbeck, and Reinhard Schnettler. 2022. "Lyophilized Human Bone Allograft as an Antibiotic Carrier: An In Vitro and In Vivo Study" Antibiotics 11, no. 7: 969. https://doi.org/10.3390/antibiotics11070969







