The Effect of Heavy Metals on Conjugation Efficiency of an F-Plasmid in Escherichia coli
Abstract
:1. Introduction
2. Results
2.1. Effect of Heavy Metals on Growth
2.2. Conjugation of the F-Plasmid Is Inhibited by Copper Sulfate
2.3. The Effect of CuSO4 on Conjugation Is Not Due to Lower Expression from the PY Promoter
2.4. CuSO4 Is Required during the Mating to Strongly Inhibit Conjugation
3. Discussion
4. Materials and Methods
4.1. Strains, Culturing Conditions, and Chemicals
4.2. Growth Assay
4.3. Liquid Mating Assay
4.4. Construction of a PY-lacZ Fusion Strain
4.5. β-Galactosidase Assay
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- von Wintersdorff, C.J.H.; Penders, J.; van Niekerk, J.M.; Mills, N.D.; Majumder, S.; van Alphen, L.B.; Savelkoul, P.H.M.; Wolffs, P.F.G. Dissemination of Antimicrobial Resistance in Microbial Ecosystems through Horizontal Gene Transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef] [PubMed]
- Salloum, T.; Panossian, B.; Bitar, I.; Hrabak, J.; Araj, G.F.; Tokajian, S. First Report of Plasmid-Mediated Colistin Resistance Mcr-8.1 Gene from a Clinical Klebsiella Pneumoniae Isolate from Lebanon. Antimicrob. Resist. Infect. Control 2020, 9, 94. [Google Scholar] [CrossRef]
- Rozwandowicz, M.; Brouwer, M.S.M.; Fischer, J.; Wagenaar, J.A.; Gonzalez-Zorn, B.; Guerra, B.; Mevius, D.J.; Hordijk, J. Plasmids Carrying Antimicrobial Resistance Genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 1121–1137. [Google Scholar] [CrossRef]
- Francia, M.V.; Varsaki, A.; Garcillán-Barcia, M.P.; Latorre, A.; Drainas, C.; de la Cruz, F. A Classification Scheme for Mobilization Regions of Bacterial Plasmids. FEMS Microbiol. Rev. 2004, 28, 79–100. [Google Scholar] [CrossRef]
- Smillie, C.; Garcillán-Barcia, M.P.; Francia, M.V.; Rocha, E.P.C.; de la Cruz, F. Mobility of Plasmids. Microbiol. Mol. Biol. Rev. 2010, 74, 434–452. [Google Scholar] [CrossRef]
- Alalam, H.; Graf, F.E.; Palm, M.; Abadikhah, M.; Zackrisson, M.; Boström, J.; Fransson, A.; Hadjineophytou, C.; Persson, L.; Stenberg, S.; et al. A High-Throughput Method for Screening for Genes Controlling Bacterial Conjugation of Antibiotic Resistance. mSystems 2020, 5, e01226-20. [Google Scholar] [CrossRef]
- Gibert, M.; Paytubi, S.; Beltrán, S.; Juárez, A.; Balsalobre, C.; Madrid, C. Growth Phase-Dependent Control of R27 Conjugation Is Mediated by the Interplay between the Plasmid-Encoded Regulatory Circuit TrhR/TrhY-HtdA and the CAMP Regulon. Environ. Microbiol. 2016, 18, 5277–5287. [Google Scholar] [CrossRef]
- Silverman, P.M.; Wickersham, E.; Harris, R. Regulation of the F Plasmid Tra Y Promoter in Escherichia coli by Host and Plasmid Factors. J. Mol. Biol. 1991, 218, 119–128. [Google Scholar] [CrossRef]
- Strohmaier, H.; Noiges, R.; Kotschan, S.; Sawers, G.; Högenauer, G.; Zechner, E.L.; Koraimann, G. Signal Transduction and Bacterial Conjugation: Characterization of the Role of ArcA in Regulating Conjugative Transfer of the Resistance Plasmid R1. J. Mol. Biol. 1998, 277, 309–316. [Google Scholar] [CrossRef]
- Graf, F.E.; Palm, M.; Warringer, J.; Farewell, A. Inhibiting Conjugation as a Tool in the Fight against Antibiotic Resistance. Drug Dev. Res. 2019, 80, 19–23. [Google Scholar] [CrossRef]
- Fernandez-Lopez, R.; Machón, C.; Longshaw, C.M.; Martin, S.; Molin, S.; Zechner, E.L.; Espinosa, M.; Lanka, E.; de la Cruz, F. Unsaturated Fatty Acids Are Inhibitors of Bacterial Conjugation. Microbiology 2005, 151, 3517–3526. [Google Scholar] [CrossRef]
- García-Cazorla, Y.; Getino, M.; Sanabria-Ríos, D.J.; Carballeira, N.M.; de la Cruz, F.; Arechaga, I.; Cabezón, E. Conjugation Inhibitors Compete with Palmitic Acid for Binding to the Conjugative Traffic ATPase TrwD, Providing a Mechanism to Inhibit Bacterial Conjugation. J. Biol. Chem. 2018, 293, 16923–16930. [Google Scholar] [CrossRef]
- Getino, M.; Sanabria-Ríos, D.J.; Fernández-López, R.; Campos-Gómez, J.; Sánchez-López, J.M.; Fernández, A.; Carballeira, N.M.; de la Cruz, F. Synthetic Fatty Acids Prevent Plasmid-Mediated Horizontal Gene Transfer. mBio 2015, 6, e01032-15. [Google Scholar] [CrossRef]
- Getino, M.; Fernández-López, R.; Palencia-Gándara, C.; Campos-Gómez, J.; Sánchez-López, J.M.; Martínez, M.; Fernández, A.; de la Cruz, F. Tanzawaic Acids, a Chemically Novel Set of Bacterial Conjugation Inhibitors. PLoS ONE 2016, 11, e0148098. [Google Scholar] [CrossRef]
- Jutkina, J.; Rutgersson, C.; Flach, C.-F.; Joakim Larsson, D.G. An Assay for Determining Minimal Concentrations of Antibiotics That Drive Horizontal Transfer of Resistance. Sci. Total Environ. 2016, 548–549, 131–138. [Google Scholar] [CrossRef]
- Shun-Mei, E.; Zeng, J.-M.; Yuan, H.; Lu, Y.; Cai, R.-X.; Chen, C. Sub-Inhibitory Concentrations of Fluoroquinolones Increase Conjugation Frequency. Microb. Pathog. 2018, 114, 57–62. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2000; ISBN 978-0-429-19112-1. [Google Scholar]
- Nicholson, F.A.; Smith, S.R.; Alloway, B.J.; Carlton-Smith, C.; Chambers, B.J. An Inventory of Heavy Metals Inputs to Agricultural Soils in England and Wales. Sci. Total Environ. 2003, 311, 205–219. [Google Scholar] [CrossRef]
- Hobman, J.L.; Crossman, L.C.Y. 2015 Bacterial Antimicrobial Metal Ion Resistance. J. Med. Microbiol. 2015, 64, 471–497. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Macomber, L.; Imlay, J.A. The Iron-Sulfur Clusters of Dehydratases Are Primary Intracellular Targets of Copper Toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef]
- Rouch, D.A.; Lee, B.T.O.; Morby, A.P. Understanding Cellular Responses to Toxic Agents: A Model for Mechanism-Choice in Bacterial Metal Resistance. J. Ind. Microbiol. 1995, 14, 132–141. [Google Scholar] [CrossRef]
- Merchant, S.S.; Helmann, J.D. Elemental Economy: Microbial Strategies for Optimizing Growth in the Face of Nutrient Limitation. In Advances in Microbial Physiology; Poole, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2012; Chapter 2; Volume 60, pp. 91–210. [Google Scholar]
- Chandrangsu, P.; Rensing, C.; Helmann, J.D. Metal Homeostasis and Resistance in Bacteria. Nat. Rev. Microbiol. 2017, 15, 338–350. [Google Scholar] [CrossRef]
- Argudín, M.A.; Hoefer, A.; Butaye, P. Heavy Metal Resistance in Bacteria from Animals. Res. Vet. Sci. 2019, 122, 132–147. [Google Scholar] [CrossRef]
- Pal, C.; Asiani, K.; Arya, S.; Rensing, C.; Stekel, D.J.; Larsson, D.G.J.; Hobman, J.L. Metal Resistance and Its Association With Antibiotic Resistance. Adv. Microb. Physiol. 2017, 70, 261–313. [Google Scholar] [CrossRef]
- Gullberg, E.; Albrecht, L.M.; Karlsson, C.; Sandegren, L.; Andersson, D.I. Selection of a Multidrug Resistance Plasmid by Sublethal Levels of Antibiotics and Heavy Metals. mBio 2014, 5, e01918-14. [Google Scholar] [CrossRef]
- Buberg, M.L.; Witsø, I.L.; L’Abée-Lund, T.M.; Wasteson, Y. Zinc and Copper Reduce Conjugative Transfer of Resistance Plasmids from Extended-Spectrum Beta-Lactamase-Producing Escherichia coli. Microb. Drug Resist. 2020, 26, 842–849. [Google Scholar] [CrossRef]
- Klümper, U.; Dechesne, A.; Riber, L.; Brandt, K.K.; Gülay, A.; Sørensen, S.J.; Smets, B.F. Metal Stressors Consistently Modulate Bacterial Conjugal Plasmid Uptake Potential in a Phylogenetically Conserved Manner. ISME J. 2017, 11, 152–165. [Google Scholar] [CrossRef]
- Pu, Q.; Fan, X.-T.; Li, H.; An, X.-L.; Lassen, S.B.; Su, J.-Q. Cadmium Enhances Conjugative Plasmid Transfer to a Fresh Water Microbial Community. Environ. Pollut. 2021, 268, 115903. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Song, H.; Lu, J.; Yuan, Z.; Guo, J. Copper Nanoparticles and Copper Ions Promote Horizontal Transfer of Plasmid-Mediated Multi-Antibiotic Resistance Genes across Bacterial Genera. Environ. Int. 2019, 129, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, A.Z.; Cen, T.; Li, X.; He, M.; Li, D.; Chen, J. Sub-Inhibitory Concentrations of Heavy Metals Facilitate the Horizontal Transfer of Plasmid-Mediated Antibiotic Resistance Genes in Water Environment. Environ. Pollut. 2018, 237, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.T. Effect of Zn2+ on Bacterial Conjugation: Increase in Ability of F—Cells to Form Mating Pairs. J. Bacteriol. 1973, 115, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Parra, B.; Tortella, G.R.; Cuozzo, S.; Martínez, M. Negative Effect of Copper Nanoparticles on the Conjugation Frequency of Conjugative Catabolic Plasmids. Ecotoxicol. Environ. Saf. 2019, 169, 662–668. [Google Scholar] [CrossRef]
- Li, J.; Ren, X.; Fan, B.; Huang, Z.; Wang, W.; Zhou, H.; Lou, Z.; Ding, H.; Lyu, J.; Tan, G. Zinc Toxicity and Iron-Sulfur Cluster Biogenesis in Escherichia coli. Appl. Environ. Microbiol. 2019, 85, e01967-18. [Google Scholar] [CrossRef]
- Franke, S.; Grass, G.; Rensing, C.; Nies, D.H. Molecular Analysis of the Copper-Transporting Efflux System CusCFBA of Escherichia coli. J. Bacteriol. 2003, 185, 3804–3812. [Google Scholar] [CrossRef]
- Gualco, L.; Roveta, S.; Marchese, A.; Debbia, E.A. Pleiotropic Effect of Sodium Arsenite on Escherichia coli. Res. Microbiol. 2004, 155, 275–282. [Google Scholar] [CrossRef]
- Helbig, K.; Bleuel, C.; Krauss, G.J.; Nies, D.H. Glutathione and Transition-Metal Homeostasis in Escherichia coli. J. Bacteriol. 2008, 190, 5431–5438. [Google Scholar] [CrossRef]
- Hossain, S.T.; Mallick, I.; Mukherjee, S.K. Cadmium Toxicity in Escherichia coli: Cell Morphology, Z-Ring Formation and Intracellular Oxidative Balance. Ecotoxicol. Environ. Saf. 2012, 86, 54–59. [Google Scholar] [CrossRef]
- Qin, W.; Zhao, J.; Yu, X.; Liu, X.; Chu, X.; Tian, J.; Wu, N. Improving Cadmium Resistance in Escherichia coli Through Continuous Genome Evolution. Front. Microbiol. 2019, 10, 278. [Google Scholar] [CrossRef]
- Sengupta, S.; Mondal, A.; Dutta, D.; Parrack, P. HflX Protein Protects Escherichia coli from Manganese Stress. J. Biosci. 2018, 43, 1001–1013. [Google Scholar] [CrossRef]
- Silver, S.; Johnseine, P.; Whitney, E.; Clark, D. Manganese-Resistant Mutants of Escherichia coli: Physiological and Genetic Studies. J. Bacteriol. 1972, 110, 186–195. [Google Scholar] [CrossRef]
- Sütterlin, S.; Téllez-Castillo, C.J.; Anselem, L.; Yin, H.; Bray, J.E.; Maiden, M.C.J. Heavy Metal Susceptibility of Escherichia coli Isolated from Urine Samples from Sweden, Germany, and Spain. Antimicrob. Agents Chemother. 2018, 62, e00209-18. [Google Scholar] [CrossRef]
- Tetaz, T.J.; Luke, R.K. Plasmid-Controlled Resistance to Copper in Escherichia coli. J. Bacteriol. 1983, 154, 1263–1268. [Google Scholar] [CrossRef]
- Buxton, R.S.; Drury, L.S.; Curtis, C.A. Dye Sensitivity Correlated with Envelope Protein Changes in Dye (SfrA) Mutants of Escherichia coli K12 Defective in the Expression of the Sex Factor F. J. Gen. Microbiol. 1983, 129, 3363–3370. [Google Scholar] [CrossRef]
- McEwen, J.; Silverman, P. Chromosomal Mutations of Escherichia Coli That Alter Expression of Conjugative Plasmid Functions. Proc. Natl. Acad. Sci. USA 1980, 77, 513–517. [Google Scholar] [CrossRef]
- Raivio, T.L.; Leblanc, S.K.D.; Price, N.L. The Escherichia coli Cpx Envelope Stress Response Regulates Genes of Diverse Function That Impact Antibiotic Resistance and Membrane Integrity. J. Bacteriol. 2013, 195, 2755–2767. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic Copper as an Antimicrobial Surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef]
- Frenoy, A.; Bonhoeffer, S. Death and Population Dynamics Affect Mutation Rate Estimates and Evolvability under Stress in Bacteria. PLoS Biol. 2018, 16, e2005056. [Google Scholar] [CrossRef]
- Salah, I.P.; Parkin, I.; Allan, E. Copper as an Antimicrobial Agent: Recent Advances. RSC Adv. 2021, 11, 18179–18186. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, J.; Zhou, B.; Li, P.; Hu, X.; Zhu, Z.; Tan, Y.; Chang, C.; Lü, J.; Song, B. Anomalous Behavior of Membrane Fluidity Caused by Copper-Copper Bond Coupled Phospholipids. Sci. Rep. 2018, 8, 14093. [Google Scholar] [CrossRef]
- Costa, T.R.D.; Ilangovan, A.; Ukleja, M.; Redzej, A.; Santini, J.M.; Smith, T.K.; Egelman, E.H.; Waksman, G. Structure of the Bacterial Sex F Pilus Reveals an Assembly of a Stoichiometric Protein-Phospholipid Complex. Cell 2016, 166, 1436–1444.e10. [Google Scholar] [CrossRef]
- Fried, L.; Lassak, J.; Jung, K. A Comprehensive Toolbox for the Rapid Construction of LacZ Fusion Reporters. J. Microbiol. Methods 2012, 91, 537–543. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-Step Inactivation of Chromosomal Genes in Escherichia coli K-12 Using PCR Products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Miller, J.H. A Short Course in Bacterial Genetics; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1992. [Google Scholar]
- Albertson, N.H.; Nyström, T. Effects of Starvation for Exogenous Carbon on Functional MRNA Stability and Rate of Peptide Chain Elongation in Escherichia coli. FEMS Microbiol. Lett. 1994, 117, 181–187. [Google Scholar] [CrossRef]
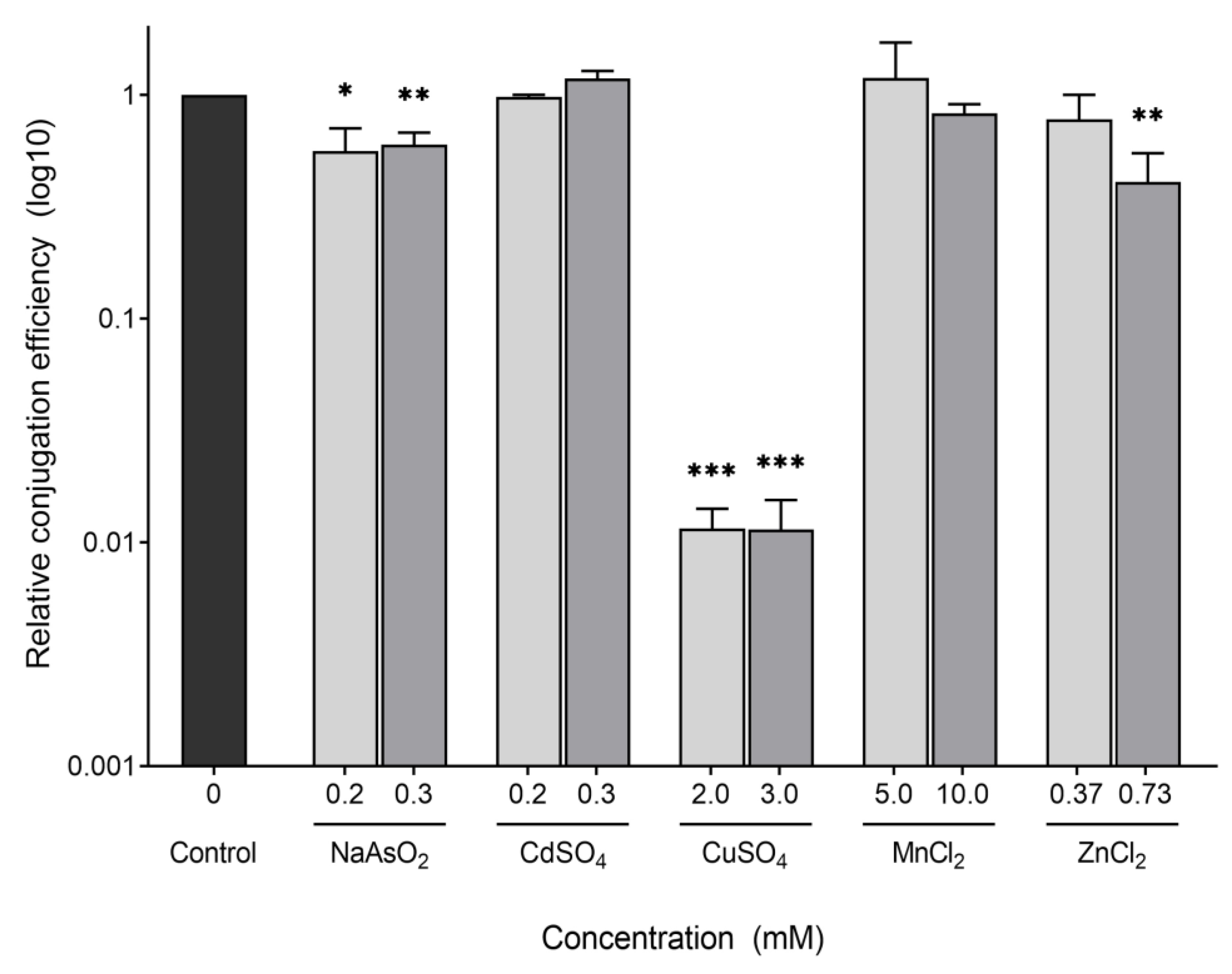
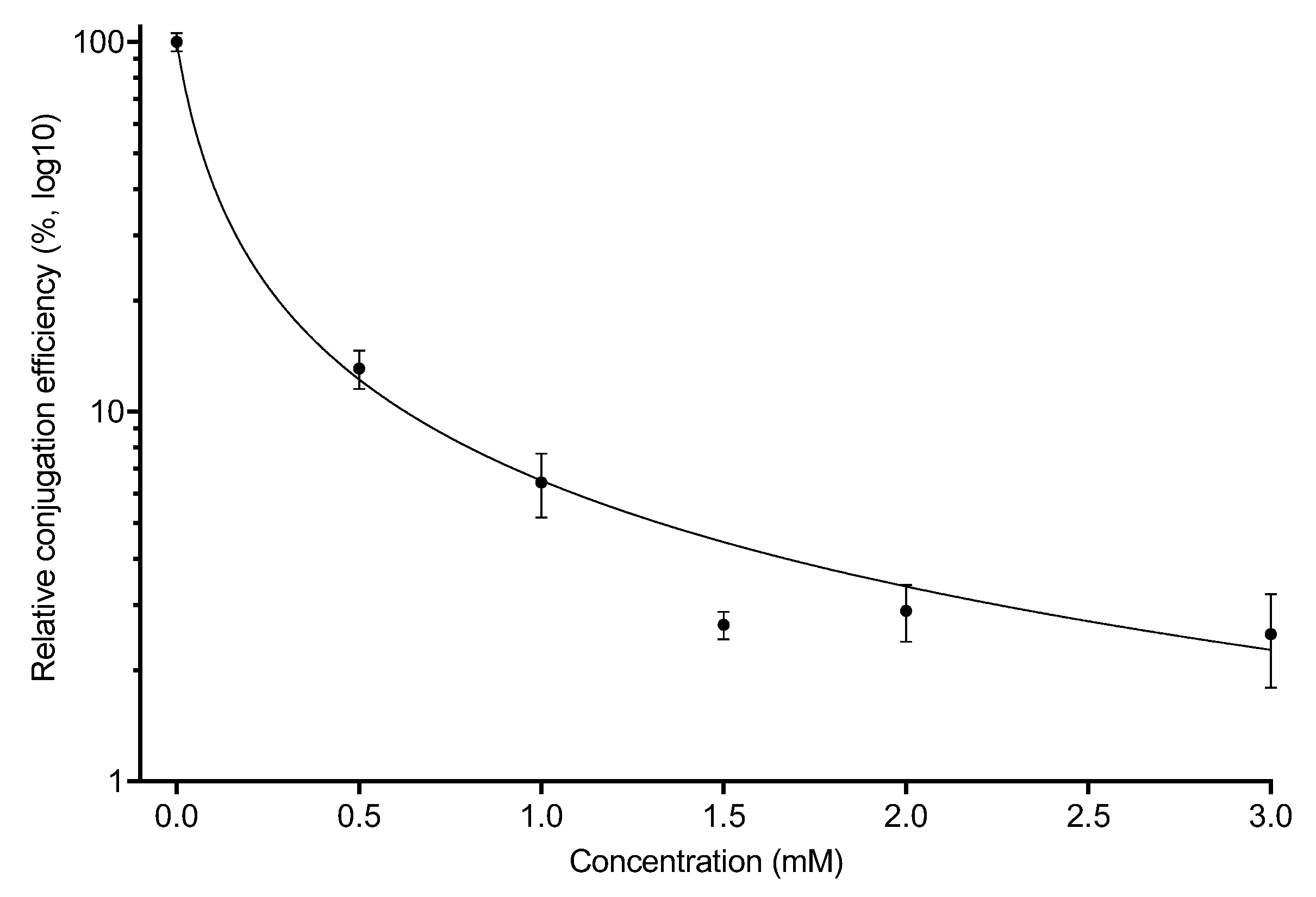
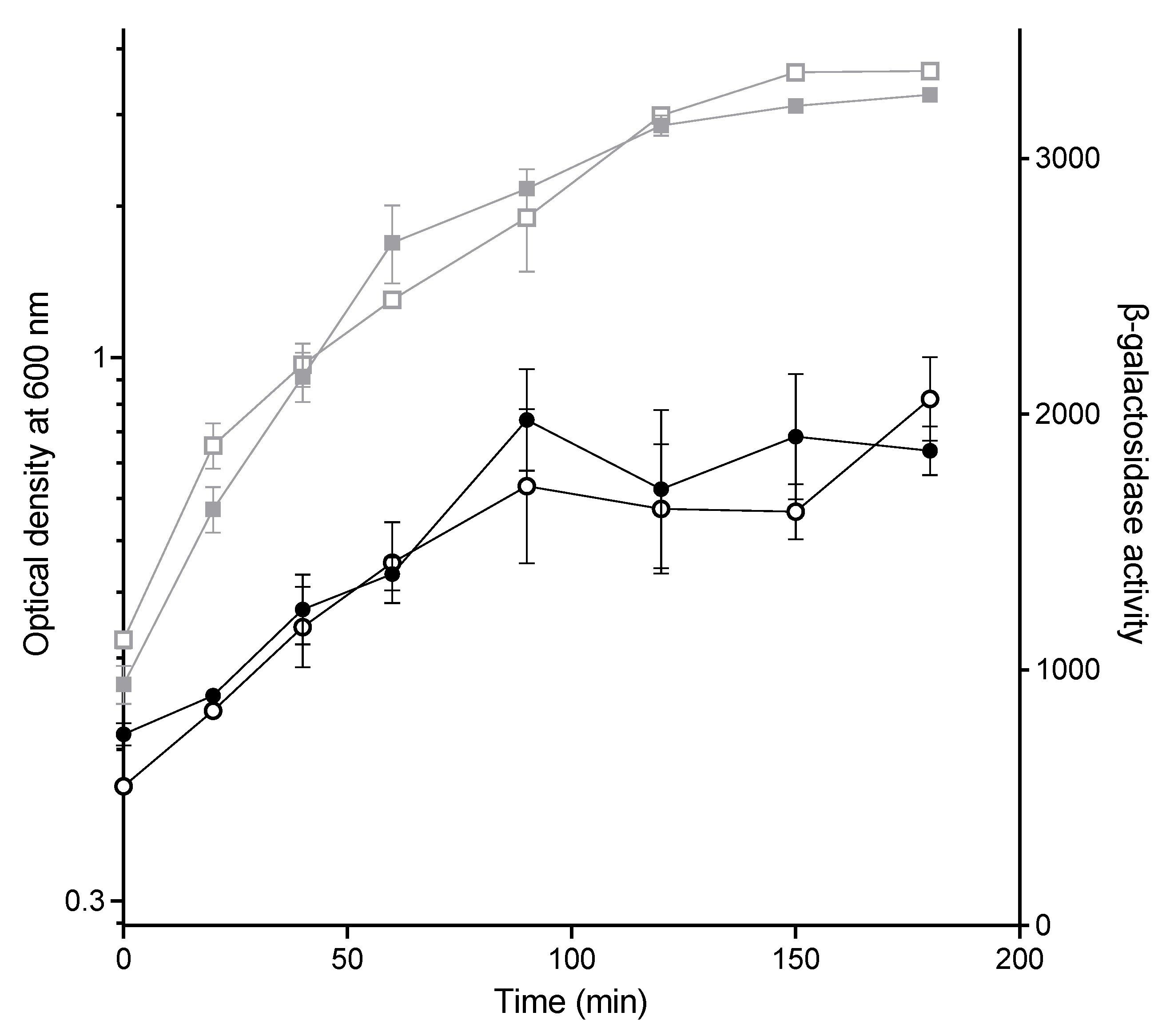

| Compound | Chemical Formula | CAS Number | Initial Concentrations Tested (mM) | Concentrations Tested for Conjugation (mM) |
|---|---|---|---|---|
| Cadmium sulfate | CdSO4 | 10124-36-4 | 0.2, 0.3, 0.4, 0.5 and 0.6 | 0.2, 0.3 |
| Copper sulfate | CuSO4 | 7758-98-7 | 0.5, 1.0, 1.5, 2.0 and 3.0 | 2.0, 3.0 |
| Manganese chloride | MnCl2 | 13446-34-9 | 5.0, 10.0, 15.0, 20.0 and 30.0 | 5.0, 10.0 |
| Sodium arsenite | NaAsO2 | 7784-46-5 | 0.2, 1.0, 2.0, 3.5 and 5.0 | 0.2, 0.3 |
| Zinc chloride | ZnCl2 | 7646-85-7 | 0.37, 0.55, 0.73, 1.1 and 1.47 | 0.55, 0.73 |
| Strain | Genotype | Resistance(s) | Source/Reference |
|---|---|---|---|
| HA4 | BW25113 araB::ChlR | Chromosomal ChlR | [8] |
| HA14 | BW25113 argC::KanR [F’ proAB lacIq Z∆M15 Tn10] | Chromosomal KanRPlasmid TetR | [8] |
| LF1 | MG1655 rpsL150 Plac::rpsL-neo-kan::lacZ∆1–100bp | Chromosomal KanR | [55] |
| MG1655 | F-λ-ilvG-rfb-50rph-1 | - | Lab stock |
| PAS3 | MG1655 rpsL150 PY-lacZ [F′ proAB lacIq Z∆M15 Tn10] | Chromosomal StrRPlasmid TetR | This study |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10] | Plasmid TetR | Stratagene, Inc. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palm, M.; Fransson, A.; Hultén, J.; Búcaro Stenman, K.; Allouche, A.; Chiang, O.E.; Constandse, M.L.; van Dijk, K.J.; Icli, S.; Klimesova, B.; et al. The Effect of Heavy Metals on Conjugation Efficiency of an F-Plasmid in Escherichia coli. Antibiotics 2022, 11, 1123. https://doi.org/10.3390/antibiotics11081123
Palm M, Fransson A, Hultén J, Búcaro Stenman K, Allouche A, Chiang OE, Constandse ML, van Dijk KJ, Icli S, Klimesova B, et al. The Effect of Heavy Metals on Conjugation Efficiency of an F-Plasmid in Escherichia coli. Antibiotics. 2022; 11(8):1123. https://doi.org/10.3390/antibiotics11081123
Chicago/Turabian StylePalm, Martin, Alfred Fransson, Julia Hultén, Karolina Búcaro Stenman, Amina Allouche, Oscar E. Chiang, Mirthe L. Constandse, Karlijn J. van Dijk, Suheda Icli, Bela Klimesova, and et al. 2022. "The Effect of Heavy Metals on Conjugation Efficiency of an F-Plasmid in Escherichia coli" Antibiotics 11, no. 8: 1123. https://doi.org/10.3390/antibiotics11081123






