Characterization of blaKPC-2 and blaNDM-1 Plasmids of a K. pneumoniae ST11 Outbreak Clone
Abstract
:1. Introduction
2. Results
2.1. A Multidrug-Resistant K. pneumoniae ST11 Clone Spread during the COVID-19 Pandemic
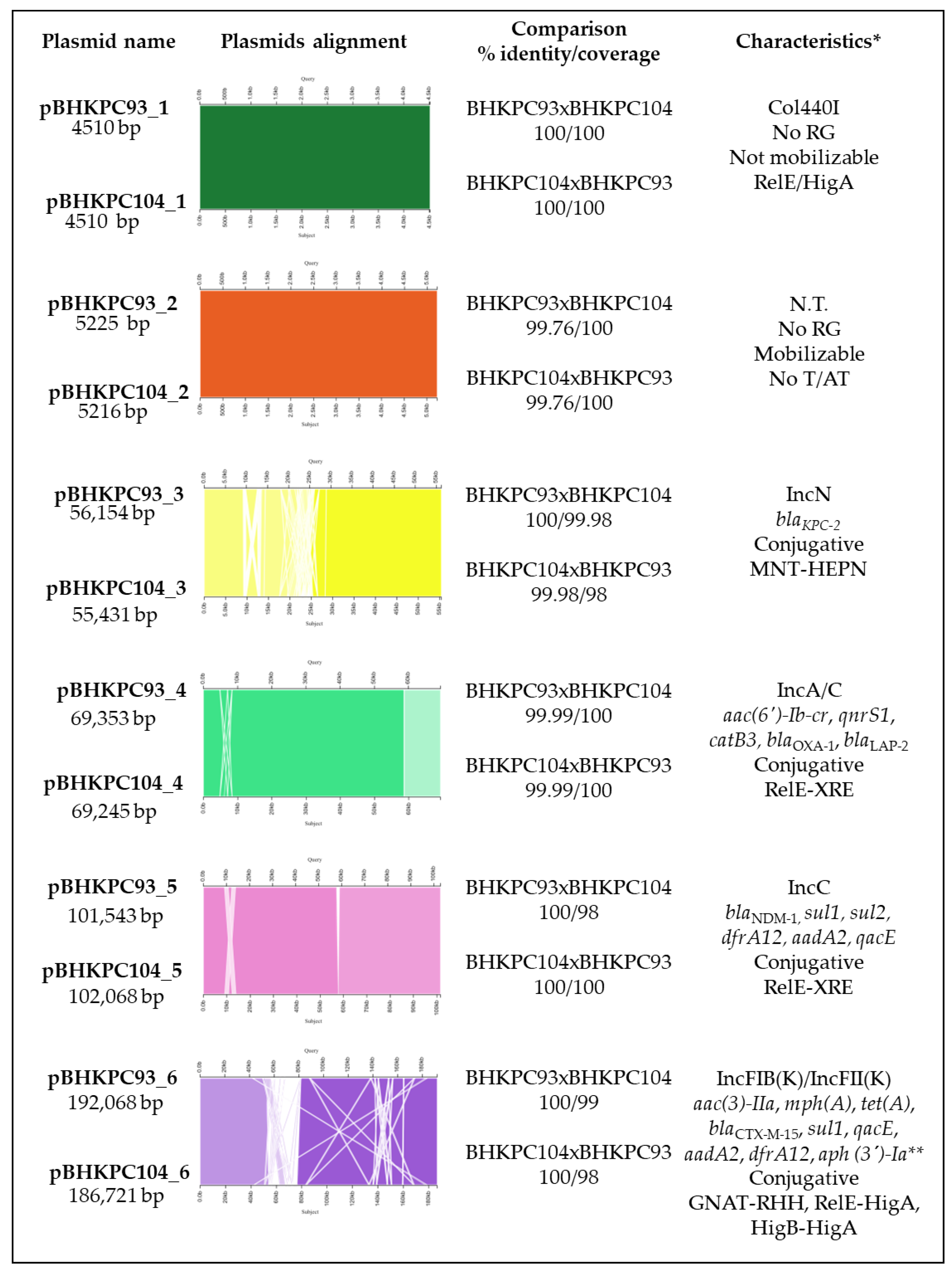
2.2. The K. pneumoniae Isolates Have 16 Resistance Genes Distributed in Four Out of Their Six Plasmids
2.3. A Retrospective Search of blaKPC Plasmids
2.4. The Impact of blaKPC Plasmid Presence in E. coli J53
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates
4.2. Antimicrobial Susceptibility Test
4.3. Genomic DNA Extractions
4.4. blaKPC and blaNDM Genes and Tn4401 Detection
4.5. Detection of the Clonality and Plasmid Sizes
4.6. Determination of the Mucoid Phenotypes
4.7. Genome Sequencing
4.8. Quality Analysis and Genome Assembly
4.9. Genomes Similarity Analysis
4.10. Code Availability
4.11. Multilocus Sequence Typing (MLST)
4.12. Plasmids Analysis
4.13. Conjugation Assay
4.14. Determination of the Growth Curve and Doubling Time
4.15. Determination of the Plasmid Copy Number
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kattan, J.N.; Villegas, M.V.; Quinn, J.P. New Developments in Carbapenems. Clin. Microbiol. Infect. 2008, 14, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Kopotsa, K.; Osei Sekyere, J.; Mbelle, N.M. Plasmid Evolution in Carbapenemase-Producing Enterobacteriaceae: A Review. Ann. N. Y. Acad. Sci. 2019, 1457, 61–91. [Google Scholar] [CrossRef] [PubMed]
- Taggar, G.; Rheman, M.A.; Boerlin, P.; Diarra, M.S. Molecular Epidemiology of Carbapenemases in Enterobacteriales from Humans, Animals, Food and the Environment. Antibiotics 2020, 9, 693. [Google Scholar] [CrossRef]
- Chen, L.; Mathema, B.; Chavda, K.D.; DeLeo, F.R.; Bonomo, R.A.; Kreiswirth, B.N. Carbapenemase-Producing Klebsiella Pneumoniae: Molecular and Genetic Decoding. Trends Microbiol. 2014, 22, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Buehrle, D.J.; Shields, R.K.; Clarke, L.G.; Potoski, B.A.; Clancy, C.J.; Hong Nguyen, M. Carbapenem-Resistant Pseudomonas Aeruginosa Bacteremia: Risk Factors for Mortality and Microbiologic Treatment Failure. Antimicrob. Agents Chemother. 2017, 61, e01243-16. [Google Scholar] [CrossRef]
- Cai, B.; Echols, R.; Magee, G.; Ferreira, J.C.A.; Morgan, G.; Ariyasu, M.; Sawada, T.; Nagata, T. Prevalence of Carbapenem-Resistant Gram-Negative Infections in the United States Predominated by Acinetobacter Baumannii and Pseudomonas Aeruginosa. Open Forum. Infect. Dis. 2017, 4, ofx176. [Google Scholar] [CrossRef]
- Swathi, C.H.; Chikala, R.; Ratnakar, K.S.; Sritharan, V. A Structural, Epidemiological & Genetic Overview of Klebsiella Pneumoniae Carbapenemases (KPCs). Indian J. Med. Res. 2016, 144, 21–31. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The Versatile β-Lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef]
- Gniadek, T.J.; Carroll, K.C.; Simner, P.J. Carbapenem-Resistant Non-Glucose-Fermenting Gram-Negative Bacilli: The Missing Piece to the Puzzle. J. Clin. Microbiol. 2016, 54, 1700. [Google Scholar] [CrossRef]
- Andrade, L.N.; Lúcia, A.; Darini, C. Bacilos Gram-Negativos Produtores de Beta-Lactamases: Que Bla Bla Bla é Esse? J. Infect. Control 2017, 6, 16–25. [Google Scholar]
- Tzouvelekis, L.S.; Markogiannakis, A.; Psichogiou, M.; Tassios, P.T.; Daikos, G.L. Carbapenemases in Klebsiella Pneumoniae and Other Enterobacteriaceae: An Evolving Crisis of Global Dimensions. Clin. Microbiol. Rev. 2012, 25, 682–707. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular Mechanisms of Antibiotic Resistance. Nature Rev. Microbiol. 2014, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Vivas, R.; Dolabella, S.S.; Barbosa, A.A.T.; Jain, S. Prevalence of Klebsiella Pneumoniae Carbapenemase—And New Delhi Metallo-Beta-Lactamase-Positive K. Pneumoniae in Sergipe, Brazil, and Combination Therapy as a Potential Treatment Option. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200064. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K.; et al. Clinical Epidemiology of the Global Expansion of Klebsiella Pneumoniae Carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Rozales, F.P.; Ribeiro, V.B.; Magagnin, C.M.; Pagano, M.; Lutz, L.; Falci, D.R.; Machado, A.; Barth, A.L.; Zavascki, A.P. Emergence of NDM-1-Producing Enterobacteriaceae in Porto Alegre, Brazil. Int. J. Infect. Dis. 2014, 25, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, J.L.M.; Gales, A.C. Antimicrobial Resistance in Enterobacteriaceae in Brazil: Focus on β-Lactams and Polymyxins. Braz. J. Microbiol. 2016, 47, 31–37. [Google Scholar] [CrossRef]
- Wink, P.L.; Martins, A.S.; Volpato, F.; Zavascki, A.P.; Barth, A.L. Increased Frequency of Bla NDM in a Tertiary Care Hospital in Southern Brazil. Braz. J. Microbiol. 2021, 52, 299–301. [Google Scholar] [CrossRef]
- Carvalho-Assef, A.P.D.; Pereira, P.S.; Albano, R.; Berião, G.C.; Chagas, T.; Timm, L.N.; Da Silva, R.C.F.; Falci, D.; Asensi, M.D. Isolation of NDM-Producing Providencia Rettgeri in Brazil. J. Antimicrob. Chemother. 2013, 68, 2956–2957. [Google Scholar] [CrossRef]
- da Silva, I.R.; Aires, C.A.M.; Conceição-Neto, O.C.; de Oliveira Santos, I.C.; Ferreira Pereira, N.; Moreno Senna, J.P.; Carvalho-Assef, A.P.D.A.; Asensi, M.D.; Rocha-De-Souza, C.M. Distribution of Clinical NDM-1-Producing Gram-Negative Bacteria in Brazil. Microb. Drug Resist. 2019, 25, 394–399. [Google Scholar] [CrossRef]
- PAHO. Epidemological Alert: Emergence and Increase of New Combinations of Carbapenemases in Enterobacterales in Latin and Caribbean. 22 October 2021. Available online: https://www.paho.org/en/documents/epidemiological-alert-emergence-and-increase-new-combinations-carbapenemases (accessed on 8 March 2023).
- BRASIL.MINISTERIO DA SAUDE. Vigilancia em saúde: Nota técnica n.74/2022-CGLAB/DAEUS/SVS/MS. Available online: https://brcast.org.br/wp-content/uploads/2022/09/SEI_MS-0028220258-Nota-Tecnica-NDM-e-coproducao-carbapenemase.pdf (accessed on 8 March 2023).
- Shakil, S.; Azhar, E.I.; Tabrez, S.; Kamal, M.A.; Jabir, N.R.; Abuzenadah, A.M.; Damanhouri, G.A.; Alam, Q. New Delhi Metallo-β-Lactamase (NDM-1): An Updates. J. Chemother. 2013, 23, 263–265. [Google Scholar] [CrossRef]
- Guimarães, T.; Nouér, S.A.; Martins, R.C.R.; Neto, L.V.P.; Martins, W.M.B.S.; Barbosa, A.C.N.; Ferreira, A.L.P.; Costa, S.F.; Gales, A.C. Ceftazidime-Avibactam as Salvage Therapy for Infections Caused by Enterobacteriales Coresistant to Carbapenems and Polymyxins. Antimicrob. Agents Chemother. 2019, 63, e00528-19. [Google Scholar] [CrossRef] [PubMed]
- Davido, B.; Fellous, L.; Lawrence, C.; Maxime, V.; Rottman, M.; Dinha, A. Ceftazidime-Avibactam and Aztreonam, an Interesting Strategy to Overcome -Lactam Resistance Conferred by Metallo--Lactamases in Enterobacteriaceae and Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2017, 61, e01008-17. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Daikos, G.L.; Tiseo, G.; Bassoulis, D.; Giordano, C.; Galfo, V.; Leonildi, A.; Tagliaferri, E.; Barnini, S.; Sani, S.; et al. Efficacy of Ceftazidime-Avibactam Plus Aztreonam in Patients with Bloodstream Infections Caused by Metallo-β-Lactamase–Producing Enterobacterales. Clin. Infect. Dis. 2021, 72, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Yamano, Y.; Yamano, Y. In Vitro Activity of Cefiderocol Against a Broad Range of Clinically Important Gram-Negative Bacteria. Clin. Infect. Dis. 2019, 69, S544–S551. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Mutakabbir, J.C.; Alosaimy, S.; Morrisette, T.; Kebriaei, R.; Rybak, M.J. Cefiderocol: A Novel Siderophore Cephalosporin against Multidrug-Resistant Gram-Negative Pathogens. Pharmacother. J. Hum. Pharmacol. Drug. Ther. 2020, 40, 1228–1247. [Google Scholar] [CrossRef]
- Sansone, P.; Giaccari, L.G.; Coppolino, F.; Aurilio, C.; Barbarisi, A.; Passavanti, M.B.; Pota, V.; Pace, M.C. Cefiderocol for Carbapenem-Resistant Bacteria: Handle with Care! A Review of the Real-World Evidence. Antibiotics 2022, 11, 904. [Google Scholar] [CrossRef]
- Bianco, G.; Boattini, M.; Comini, S.; Casale, R.; Iannaccone, M.; Cavallo, R.; Costa, C. Occurrence of Multi-Carbapenemases Producers among Carbapenemase-Producing Enterobacterales and in Vitro Activity of Combinations Including Cefiderocol, Ceftazidime-Avibactam, Meropenem-Vaborbactam, and Aztreonam in the COVID-19 Era. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 573–580. [Google Scholar] [CrossRef]
- Maraki, S.; Mavromanolaki, V.E.; Stafylaki, D.; Scoulica, E. In Vitro Activity of Newer β-Lactam/β-Lactamase Inhibitor Combinations, Cefiderocol, Plazomicin and Comparators against Carbapenemase-Producing Klebsiella Pneumoniae Isolates. J. Chemother. 2023. [Google Scholar] [CrossRef]
- Chen, F.J.; Lauderdale, T.L.; Ho, M.; Lo, H.J. The Roles of Mutations in GyrA, ParC, and OmpK35 in Fluoroquinolone Resistance in Klebsiella Pneumoniae. Microb. Drug Resist. 2004, 9, 265–271. [Google Scholar] [CrossRef]
- Akya, A.; Lorestani, R.C.; Elahi, A.; Ghadiri, K. The Impact of Mutations in Topoisomerase Genes and the Plasmid-Mediated Quinolone Resistance (PMQR) Determinants on the Resistance to Fluoroquinolones in Klebsiella Pneumoniae. Arch. Clin. Infect. Dis. 2017, 12, 57290. [Google Scholar] [CrossRef]
- de Souza, R.C.; Dabul, A.N.G.; Boralli, C.M.D.S.; Zuvanov, L.; Camargo, I.L.B.D.C. Dissemination of BlaKPC-2 in an NTEKPC by an IncX5 Plasmid. Plasmid 2019, 106, 102446. [Google Scholar] [CrossRef] [PubMed]
- al Mana, H.; Sundararaju, S.; Tsui, C.K.M.; Perez-Lopez, A.; Yassine, H.; al Thani, A.; Al-Ansari, K.; Eltai, N.O. Whole-Genome Sequencing for Molecular Characterization of Carbapenem-Resistant Enterobacteriaceae Causing Lower Urinary Tract Infection among Pediatric Patients. Antibiotics 2021, 10, 972. [Google Scholar] [CrossRef] [PubMed]
- Özad Düzgün, A. From Turkey: First Report of KPC-3- and CTX-M-27-Producing Multidrug-Resistant Klebsiella Pneumoniae ST147 Clone Carrying OmpK36 and Ompk37 Porin Mutations. Microb. Drug Resist. 2021, 27, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Sellera, F.P.; Fuga, B.; Fontana, H.; Esposito, F.; Cardoso, B.; Konno, S.; Berl, C.; Cappellanes, M.H.; Cortez, M.; Ikeda, M.; et al. Detection of IncN-pST15 One-health Plasmid Harbouring Bla in a Hypermucoviscous Klebsiella Pneumoniae CG258 Isolated from an Infected Dog, Brazil. Transbound. Emerg. Dis. 2021, 68, 3083. [Google Scholar] [CrossRef]
- Rada, A.M.; de la Cadena, E.; Agudelo, C.; Capataz, C.; Orozco, N.; Pallares, C.; Dinh, A.Q.; Panesso, D.; Ríos, R.; Diaz, L.; et al. Dynamics of BlaKPC-2 Dissemination from Non-CG258 Klebsiella Pneumoniae to Other Enterobacterales via IncN Plasmids in an Area of High Endemicity. Antimicrob. Agents Chemother. 2020, 64, e01743-20. [Google Scholar] [CrossRef]
- Quiles, M.G.; Rocchetti, T.T.; Fehlberg, L.C.; Kusano, E.J.U.; Chebabo, A.; Pereira, R.M.G.; Gales, A.C.; Pignatari, A.C.C. Unusual Association of NDM-1 with KPC-2 and ArmA among Brazilian Enterobacteriaceae Isolates. Braz. J. Med. Biol. Res. 2014, 48, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Ponce, F.; Dantas, K.; Becerra, J.; Melocco, G.; Esposito, F.; Cardoso, B.; Rodrigues, L.; Lima, K.; de Lima, A.V.; Sellera, F.P.; et al. Detecting KPC-2 and NDM-1 Coexpression in Klebsiella Pneumoniae Complex from Human and Animal Hosts in South America. Microbiol. Spectr. 2022, 10, e0115922. [Google Scholar] [CrossRef]
- Pereira, P.S.; Borghi, M.; Albano, R.M.; Lopes, J.C.O.; Silveira, M.C.; Marques, E.A.; Oliveira, J.C.R.; Asensi, M.D.; Carvalho-Assef, A.P.D.A. Coproduction of NDM-1 and KPC-2 in Enterobacter Hormaechei from Brazil. Microb. Drug Resist. 2015, 21, 234–236. [Google Scholar] [CrossRef]
- Firmo, E.F.; Beltrão, E.M.B.; da Silva, F.R.F.; Alves, L.C.; Brayner, F.A.; Veras, D.L.; Lopes, A.C.S. Association of BlaNDM-1 with BlaKPC-2 and Aminoglycoside-Modifying Enzyme Genes among Klebsiella Pneumoniae, Proteus Mirabilis and Serratia Marcescens Clinical Isolates in Brazil. J. Glob. Antimicrob. Resist. 2020, 21, 255–261. [Google Scholar] [CrossRef]
- de Oliveira, É.M.; Beltrão, E.M.B.; Scavuzzi, A.M.L.; Barros, J.F.; Lopes, A.C.S. High Plasmid Variability, and the Presence of IncFIB, IncQ, IncA/C, IncHI1B, and IncL/M in Clinical Isolates of Klebsiella Pneumoniae with Bla KPC and Bla NDM from Patients at a Public Hospital in Brazil. Rev. Soc. Bras. Med. Trop. 2020, 53, 1–8. [Google Scholar] [CrossRef]
- Gruber, T.M.; Göttig, S.; Mark, L.; Christ, S.; Kempf, V.A.J.; Wichelhaus, T.A.; Hamprecht, A. Pathogenicity of Pan-Drug-Resistant Serratia Marcescens Harbouring BlaNDM-1. J. Antimicrob. Chemother. 2015, 70, 1026–1030. [Google Scholar] [CrossRef]
- Villa, L.; Guerra, B.; Schmoger, S.; Fischer, J.; Helmuth, R.; Zong, Z.; García-Fernández, A.; Carattoli, A. IncA/C Plasmid Carrying BlaNDM-1, BlaCMY-16, and FosA3 in a Salmonella Enterica Serovar Corvallis Strain Isolated from a Migratory Wild Bird in Germany. Antimicrob. Agents Chemother. 2015, 59, 6597. [Google Scholar] [CrossRef]
- Molnár, S.; Flonta, M.M.M.; Almaş, A.; Buzea, M.; Licker, M.; Rus, M.; Földes, A.; Székely, E. Dissemination of NDM-1 Carbapenemase-Producer Providencia Stuartii Strains in Romanian Hospitals: A Multicentre Study. J. Hosp. Infect. 2019, 103, 165–169. [Google Scholar] [CrossRef]
- Solgi, H.; Giske, C.G.; Badmasti, F.; Aghamohammad, S.; Havaei, S.A.; Sabeti, S.; Mostafavizadeh, K.; Shahcheraghi, F. Emergence of Carbapenem Resistant Escherichia Coli Isolates Producing BlaNDM and BlaOXA-48-like Carried on IncA/C and IncL/M Plasmids at Two Iranian University Hospitals. Infect. Genet. Evol. 2017, 55, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Wailan, A.M.; Sartor, A.L.; Zowawi, H.M.; Perry, J.D.; Paterson, D.L.; Sidjabat, H.E. Genetic Contexts of BlaNDM-1 in Patients Carrying Multiple NDM-Producing Strains. Antimicrob. Agents Chemother. 2015, 59, 7405–7410. [Google Scholar] [CrossRef]
- Qamar, M.U.; Ejaz, H.; Walsh, T.R.; Shah, A.A.; Al Farraj, D.A.; Alkufeidy, R.M.; Alkubaisi, N.A.; Saleem, S.; Jahan, S. Clonal Relatedness and Plasmid Profiling of Extensively Drug-Resistant New Delhi Metallo-β-Lactamase-Producing Klebsiella Pneumoniae Clinical Isolates. Futur. Microbiol. 2021, 16, 229–239. [Google Scholar] [CrossRef]
- Gomez-Simmonds, A.; Annavajhala, M.K.; Tang, N.; Rozenberg, F.D.; Ahmad, M.; Park, H.; Lopatkin, A.J.; Uhlemann, A.C. Population Structure of BlaKPC-Harbouring IncN Plasmids at a New York City Medical Centre and Evidence for Multi-Species Horizontal Transmission. J. Antimicrob. Chemother. 2022, 77, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, T.; Cantamessa, R.; Melo, L.; Lincopan, N.; Fernandes, M.R.; Cerdeira, L.; Fraga, E.; Dropa, M.; Sato, M.I.Z. International High-Risk Clones of Klebsiella Pneumoniae KPC-2/CC258 and Escherichia Coli CTX-M-15/CC10 in Urban Lake Waters. Sci. Total Environ. 2017, 598, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Chaparro, P.J.; Cerdeira, L.T.; Queiroz, M.G.; De Lima, C.P.S.; Levy, C.E.; Pavez, M.; Lincopan, N.; Gonçalves, E.C.; Mamizuka, E.M.; Sampaio, J.L.M.; et al. Complete Nucleotide Sequences of Two BlaKPC-2-Bearing IncN Plasmids Isolated from Sequence Type 442 Klebsiella Pneumoniae Clinical Strains Four Years Apart. Antimicrob. Agents Chemother. 2014, 58, 2958–2960. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Aschbacher, R.; March, A.; Larcher, C.; Livermore, D.M.; Woodford, N. Complete Nucleotide Sequence of the IncN Plasmid PKOX105 Encoding VIM-1, QnrS1 and SHV-12 Proteins in Enterobacteriaceae from Bolzano, Italy Compared with IncN Plasmids Encoding KPC Enzymes in the USA. J. Antimicrob. Chemother. 2010, 65, 2070–2075. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, R.A.; Johnson, R.C.; Conlan, S.; Ramsburg, A.M.; Dekker, J.P.; Lau, A.F.; Khil, P.; Odom, R.T.; Deming, C.; Park, M.; et al. Genomic Analysis of Hospital Plumbing Reveals Diverse Reservoir of Bacterial Plasmids Conferring Carbapenem Resistance. mBio 2018, 9, e02011-17. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, C.; Bischoff, P.; Bender, J.; Kola, A.; Gastmeier, P.; Hummel, M.; Klefisch, F.R.; Schoenrath, F.; Frühauf, A.; Pfeifer, Y. Plasmid-Mediated Transmission of KPC-2 Carbapenemase in Enterobacteriaceae in Critically Ill Patients. Front. Microbiol. 2019, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Imirzalioglu, C.; Hain, T.; Kaase, M.; Gatermann, S.; Exner, M.; Mielke, M.; Hauri, A.; Dragneva, Y.; Bill, R.; et al. Complete Nucleotide Sequence of a Citrobacter Freundii Plasmid Carrying KPC-2 in a Unique Genetic Environment. Genome Announc. 2014, 2, e01157-14. [Google Scholar] [CrossRef]
- Bitar, I.; Caltagirone, M.; Villa, L.; Marchetti, V.M.; Nucleo, E.; Sarti, M.; Migliavacca, R.; Carattoli, A. Interplay among IncA and BlaKPC-Carrying Plasmids in Citrobacter Freundii. Antimicrob. Agents Chemother. 2019, 63, e02609-18. [Google Scholar] [CrossRef] [PubMed]
- Horne, T.; Orr, V.T.; Hall, J.P. How Do Interactions between Mobile Genetic Elements Affect Horizontal Gene Transfer? Curr. Opin. Microbiol. 2023, 73, 102282. [Google Scholar] [CrossRef] [PubMed]
- Gama, J.A.; Zilhão, R.; Dionisio, F. Plasmid Interactions Can Improve Plasmid Persistence in Bacterial Populations. Front. Microbiol. 2020, 11, 2033. [Google Scholar] [CrossRef] [PubMed]
- Guglielmini, J.; Van Melderen, L. Bacterial Toxin-Antitoxin Systems. Mob. Genet. Elements 2011, 1, 283–306. [Google Scholar] [CrossRef]
- Anantharaman, V.; Aravind, L. New Connections in the Prokaryotic Toxin-Antitoxin Network: Relationship with the Eukaryotic Nonsense-Mediated RNA Decay System. Genome Biol. 2003, 4, R81. [Google Scholar] [CrossRef]
- Leplae, R.; Geeraerts, D.; Hallez, R.; Guglielmini, J.; Drze, P.; Van Melderen, L. Diversity of Bacterial Type II Toxin-Antitoxin Systems: A Comprehensive Search and Functional Analysis of Novel Families. Nucleic Acids Res. 2011, 39, 5513–5525. [Google Scholar] [CrossRef]
- Palazzo, I.C.V.; Rehder, A.; Darini, A.L.C. Quantitative Disk Diffusion as a Convenient Method for Determining Minimum Inhibitory Concentrations of Oxacillin for Staphylococci Strains. J. Microbiol. Methods. 2007, 71, 186–190. [Google Scholar] [CrossRef]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for Detection of Acquired Carbapenemase Genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Naas, T.; Cuzon, G.; Villegas, M.V.; Lartigue, M.F.; Quinn, J.P.; Nordmann, P. Genetic Structures at the Origin of Acquisition of the β-Lactamase BlaKPC Gene. Antimicrob. Agents Chemother. 2008, 52, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Vuotto, C.; Longo, F.; Pascolini, C.; Donelli, G.; Balice, M.P.; Libori, M.F.; Tiracchia, V.; Salvia, A.; Varaldo, P.E. Biofilm Formation and Antibiotic Resistance in Klebsiella Pneumoniae Urinary Strains. J. Appl. Microbiol. 2017, 123, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- D’Apolito, D.; Arena, F.; Conte, V.; de Angelis, L.H.; di Mento, G.; Carreca, A.P.; Cuscino, N.; Russelli, G.; Iannolo, G.; Barbera, F.; et al. Phenotypical and Molecular Assessment of the Virulence Potential of KPC-3-Producing Klebsiella Pneumoniae ST392 Clinical Isolates. Microbiol. Res. 2020, 240, 126551. [Google Scholar] [CrossRef]
- Diancourt, L.; Passet, V.; Verhoef, J.; Grimont, P.A.D.; Brisse, S. Multilocus Sequence Typing of Klebsiella Pneumoniae Nosocomial Isolates. J. Clin. Microbiol. 2005, 43, 4178. [Google Scholar] [CrossRef]
- Woodall, C.A. DNA Transfer by Bacterial Conjugation. Methods Mol. Biol. 2003, 235, 61–65. [Google Scholar] [CrossRef]
- Škulj, M.; Okršlar, V.; Jalen, Š.; Jevševar, S.; Slanc, P.; Štrukelj, B.; Menart, V. Improved Determination of Plasmid Copy Number Using Quantitative Real-Time PCR for Monitoring Fermentation Processes. Microb. Cell Fact. 2008, 7, 6. [Google Scholar] [CrossRef]
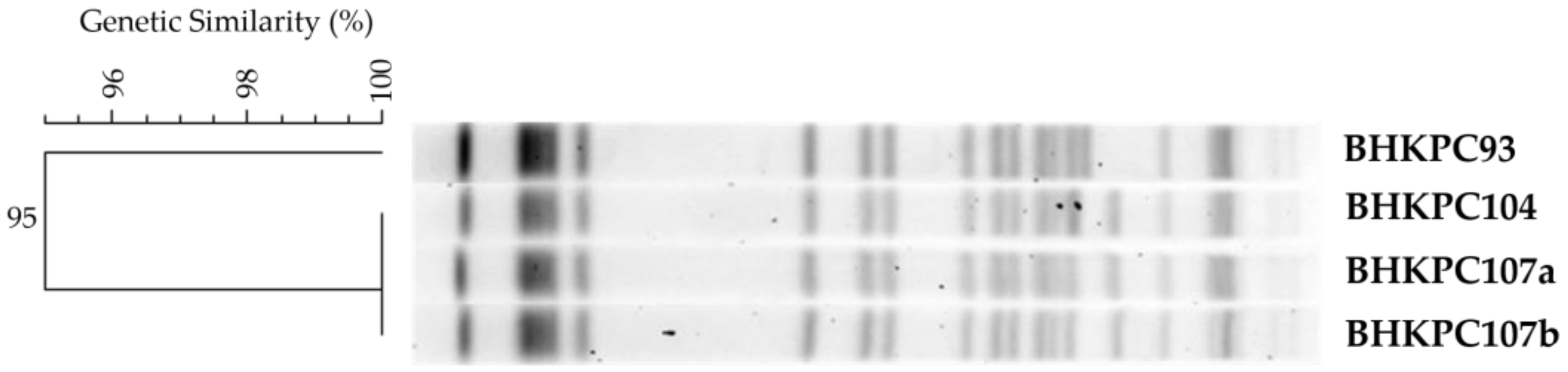
| BHKPC93 | BHKPC104 | BHKPC107a | BHKPC107b | |||||
|---|---|---|---|---|---|---|---|---|
| Isolates’ general data | ||||||||
| Species | K. pneumoniae | K. pneumoniae | K. pneumoniae | K. pneumoniae | ||||
| Isolation date (DD/MM/YYYY) | 11/12/2020 | 30/12/2020 | 01/01/2021 | 07/01/2021 | ||||
| Clinical specimen | Catheter tip | Urine | Deep tissue | Deep tissue | ||||
| Antibiotics tested (susceptibility/MIC) | ||||||||
| Nalidixic Acid | - | - | R | - | - | - | - | - |
| Amikacin | S | - | S | - | S | - | S | - |
| Amoxicillin/Clavulanic Acid | R | - | R | - | R | - | R | - |
| Ampicillin/Sulbactam | R | - | R | - | R | - | R | - |
| Cefepime | R | - | R | - | R | - | R | - |
| Ceftazidime | R | - | R | - | R | - | R | - |
| Ceftriaxone | R | - | R | - | R | - | R | - |
| Cefuroxime | R | - | R | - | R | - | R | - |
| Axetil Cefuroxime | R | - | R | - | R | - | R | - |
| Ciprofloxacin | R | - | R | - | R | - | R | - |
| Colistin | R | 16 mg/L | R | 16 mg/L | R | 16 mg/L | R | 16 mg/L |
| Ertapenem | R | - | R | - | R | - | R | - |
| Gentamicin | R | - | R | - | R | - | S | - |
| Imipenem | R | 64 mg/L | R | 128 mg/L | R | 64 mg/L | R | 64 mg/L |
| Meropenem | R | 128 mg/L | R | 256 mg/L | R | 256 mg/L | R | 256 mg/L |
| Nitrofurantoin | - | - | R | - | - | - | - | - |
| Norfloxacin | - | - | R | - | - | - | - | - |
| Piperacillin/Tazobactam | R | - | R | - | R | - | R | - |
| Polymyxin B | R | 8 mg/L | R | 8 mg/L | R | 8 mg/L | R | 8 mg/L |
| Tigecycline | - | 1 mg/L | - | 2 mg/L | - | 1 mg/L | - | 0.5 mg/L |
| Trimethoprim/Sulfamethoxazole | R | - | R | - | R | - | R | - |
| Conjugation Rate | Doubling Time (min) | Imipenem MIC (mg/L) | Meropenem MIC (mg/L) | PCN (blaKPC Plasmid/blaNDM Plasmid) | |
|---|---|---|---|---|---|
| E. coli J53 | - | 111 ± 16 | 0.125 | 0.0625 | -/- |
| K. pneumoniae BHKPC93 | - | - | 64 | 128 | 20.1 ± 0.1/1.86 ± 0.1 |
| K. pneumoniae BHKPC104 | - | - | 128 | 256 | 7.14 ± 0.03/1.85 ± 0.07 |
| E. coli J53 + pBHKPC93_3 | 2.1 × 10−5 | 111 ± 13 | 2 | 2 | 1.87 ± 0.05/- |
| E. coli J53 + pBHKPC104_3 | 1.7 × 10−5 | 112 ± 14 | 2 | 2 | 0.78 ± 0.02/- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boralli, C.M.d.S.; Paganini, J.A.; Meneses, R.S.; Mata, C.P.S.M.d.; Leite, E.M.M.; Schürch, A.C.; Paganelli, F.L.; Willems, R.J.L.; Camargo, I.L.B.C. Characterization of blaKPC-2 and blaNDM-1 Plasmids of a K. pneumoniae ST11 Outbreak Clone. Antibiotics 2023, 12, 926. https://doi.org/10.3390/antibiotics12050926
Boralli CMdS, Paganini JA, Meneses RS, Mata CPSMd, Leite EMM, Schürch AC, Paganelli FL, Willems RJL, Camargo ILBC. Characterization of blaKPC-2 and blaNDM-1 Plasmids of a K. pneumoniae ST11 Outbreak Clone. Antibiotics. 2023; 12(5):926. https://doi.org/10.3390/antibiotics12050926
Chicago/Turabian StyleBoralli, Camila Maria dos Santos, Julian Andres Paganini, Rodrigo Silva Meneses, Camila Pacheco Silveira Martins da Mata, Edna Marilea Meireles Leite, Anita C. Schürch, Fernanda L. Paganelli, Rob J. L. Willems, and Ilana Lopes Baratella Cunha Camargo. 2023. "Characterization of blaKPC-2 and blaNDM-1 Plasmids of a K. pneumoniae ST11 Outbreak Clone" Antibiotics 12, no. 5: 926. https://doi.org/10.3390/antibiotics12050926





