Combating Bacterial Biofilms: Current and Emerging Antibiofilm Strategies for Treating Persistent Infections
Abstract
:1. Introduction
2. Role of Biofilms in Persistent Infections: An Overview
3. Bacterial Biofilm Formation and Characteristics by Infection Site
3.1. Surface-Located Bacterial Biofilms
3.2. Tissue-Located Bacterial Biofilms
4. Role of Key Biofilm Components in Infections
4.1. Exopolysaccharides
4.2. Extracellular DNA
5. Key Host Innate Immune Responses against Biofilm
5.1. Nitric Oxide
5.2. Innate Antimicrobial Peptides
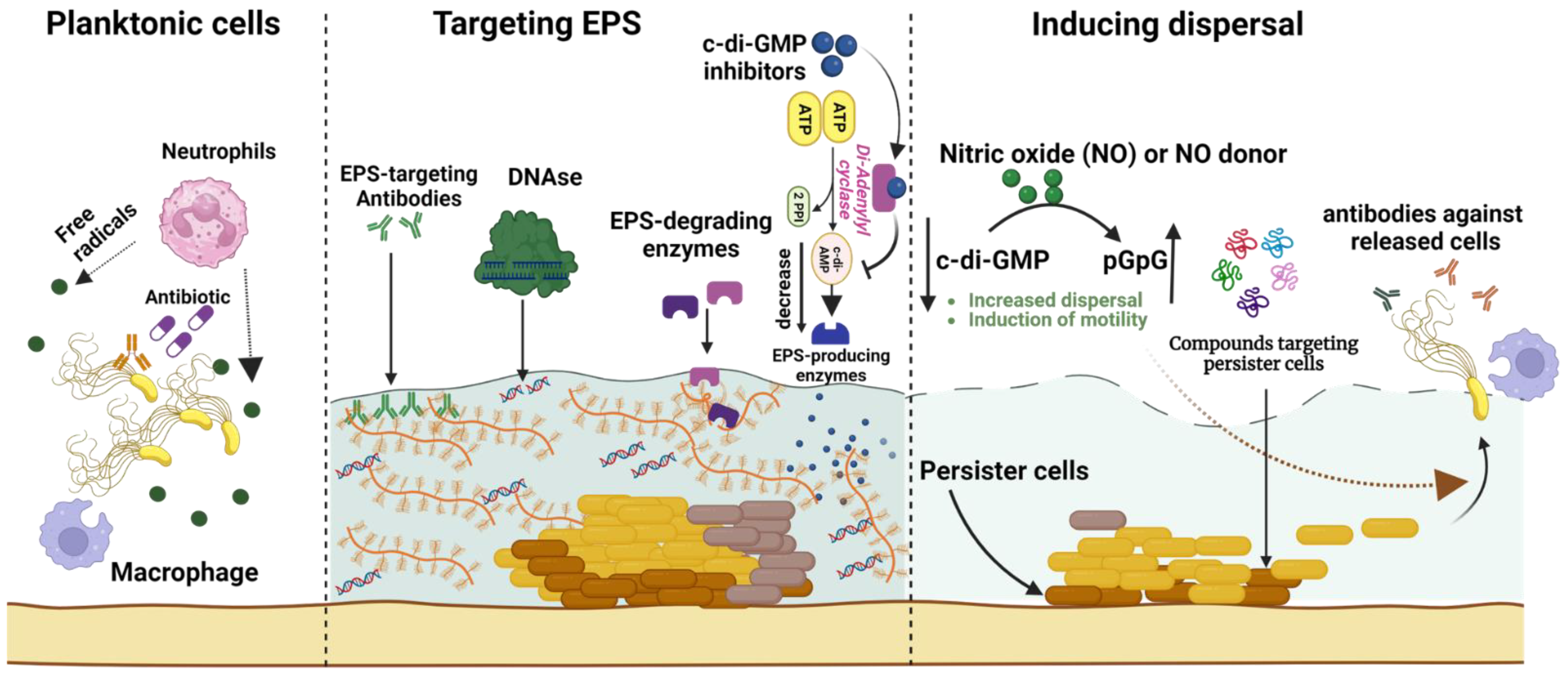
6. Biofilm Eradication Strategies
6.1. Targeting Extracellular Polymeric Substances
6.1.1. Small Molecule Inhibitors
6.1.2. Enzymes Degrading Extracellular Polymeric Substances
| Antibiofilm Agent | Target Pathogen | Antibiofilm Mode of Action | Study Model | Reference |
|---|---|---|---|---|
| Exopolysaccharide-targeting agents | ||||
| Quinoxaline derivative | Streptococcus mutans | Glucosyltransferase inhibitor | Anticaries rat | [41] |
| Oxazole derivative | S. mutans | Antagonizing glucosyltransferases | Dental caries rat | [46] |
| Dispersin B | Staphylococcus spp. | Inhibited skin colonization, detachment of Staphylococcal cells from skin | In vivo pig model | [47] |
| Endolysins | S. aureus | Peptidoglycan hydrolases | System MRSA infection in mice | [48] |
| Dornase alfa | Pseudomonas aeruginosa | Dissolving cystic fibrosis sputum and fibrillar structures | Cystic fibrosis sputum | [49] |
| DNABII antibodies | Haemophilus influenzae | Targeting epitopes of DNABII found in extracellular DNA | Chinchilla and murine | [50] |
| α-amylase | S. aureus and P. aeruginosa | Exopolysaccharide disruption | Danio rerio | [51] |
| Biofilm dispersion-targeting agents | ||||
| Nitric oxide | P. aeruginosa | Biofilm dispersion Reduced biofilm tolerance to antibiotics | Cystic fibrosis sputum | [52] |
| Cephalosporin-3′-diazeniumdiolates | P. aeruginosa | Biofilm dispersion; increases biofilm susceptibility to antibiotics | Microtiter plates | [53] |
| Nitroxides | P. aeruginosa | Promotes biofilm dispersal, inhibits biofilm formation, increases swarming motility | Flow chambers | [54] |
| Autoinducing peptide inhibitor | S. aureus | Quorum sensing inhibitor | RN9222 cell line | [55] |
| Natural peptide Capsicumicine | S. epidermidis | Disassembly of biofilm matrix | SKH1 mice | [56] |
| Biofilm persister-targeting agents | ||||
| TM5 peptide | P. aeruginosa and S. aureus | Antipersister agent | Laboratory settings | [57] |
| Rifampin + Fosfomycin | S. aureus (Methicillin-resistant) | Cure of cage-associated infections | A foreign body infection model using guinea pigs | [58] |
| Acyldepsipeptide ADEP4 | S. aureus | Activation of ClpP protease which kills growing and persister cells | Mouse model of a chronic infection | [59] |
| Glycosylated cationic peptides | S. aureus (Methicillin-resistant) | Bactericidal against persister cells and disperses biofilm mass | Ex vivo wounded human skin infection | [60] |
6.1.3. Antibodies and Nucleic-Acid-Binding Proteins
6.2. Biofilm Dispersion-Based Strategies
6.2.1. c-di-GMP Biosynthesis Inhibitors
6.2.2. Quorum Sensing Inhibiting Peptides
6.3. Targeting Biofilm Metabolism and Dormancy
6.3.1. Metabolic Inhibitors
6.3.2. Antipersister Peptides
7. Emerging Antibiofilm Technologies
7.1. Antibiofilm Nanoparticles
7.2. Antibiofilm Surface Coatings
7.3. Antimicrobial Microneedles
8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Hall-Stoodley, L.; Stoodley, P. Evolving concepts in biofilm infections. Cell. Microbiol. 2009, 11, 1034–1043. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [Green Version]
- Moser, C.; Pedersen, H.T.; Lerche, C.J.; Kolpen, M.; Line, L.; Thomsen, K.; Høiby, N.; Jensen, P.Ø. Biofilms and host response - helpful or harmful. APMIS 2017, 125, 320–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N. Dental caries. Nat. Rev. Dis. Prim. 2017, 3, 17030. [Google Scholar] [CrossRef] [Green Version]
- Folkesson, A. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: An evolutionary perspective. Nat. Rev. Microbiol. 2012, 10, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.R.; Tullis, E.; Castaños, C.; Castellani, C.; et al. The future of cystic fibrosis care: A global perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef] [Green Version]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21, S1–S25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- David, L.; Jean-Marc, G.; Christophe, B. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 2023, 21, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Kumar, C.G.; Anand, S.K. Significance of microbial biofilms in food industry: A review. Int. J. Food Microbiol. 1998, 42, 9–27. [Google Scholar] [CrossRef]
- Tribedi, P.; Sil, A.K. Cell surface hydrophobicity: A key component in the degradation of polyethylene succinate by Pseudomonas sp. AKS2. J. Appl. Microbiol. 2014, 116, 295–303. [Google Scholar] [CrossRef]
- Paula, A.J.; Hwang, G.; Koo, H. Dynamics of bacterial population growth in biofilms resemble spatial and structural aspects of urbanization. Nat. Commun. 2020, 11, 1354. [Google Scholar] [CrossRef] [Green Version]
- González-Rivas, F.; Ripolles-Avila, C.; Fontecha-Umaña, F.; Ríos-Castillo, A.G.; Rodríguez-Jerez, J.J. Biofilms in the spotlight: Detection, quantification, and removal methods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1261–1276. [Google Scholar] [CrossRef] [Green Version]
- Jensen, E. Complement activation by Pseudomonas aeruginosa biofilms. Microb. Pathog. 1993, 15, 377–388. [Google Scholar] [CrossRef]
- Alhede, M. Bacterial aggregate size determines phagocytosis efficiency of polymorphonuclear leukocytes. Med. Microbiol. Immunol. 2020, 209, 669–680. [Google Scholar] [CrossRef]
- Leid, J. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J. Immunol. 2005, 175, 7512–7518. [Google Scholar] [CrossRef] [Green Version]
- VL de, S.R.J.S. Staphylococci evade the innate immune response by disarming neutrophils and forming biofilms. FEBS Lett. 2020, 594, 2556–2569. [Google Scholar]
- Dengler Haunreiter, V.; Boumasmoud, M.; Häffner, N.; Wipfli, D.; Leimer, N.; Rachmühl, C.; Kühnert, D.; Achermann, Y.; Zbinden, R.; Benussi, S.; et al. In-host evolution of Staphylococcus epidermidis in a pacemaker-associated endocarditis resulting in increased antibiotic tolerance. Nat. Commun. 2019, 10, 1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franklin, M.J.; Nivens, D.E.; Weadge, J.T.; Lynne Howell, P. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front. Microbiol. 2011, 2, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectr. 2015, 3, 10.1128. [Google Scholar] [CrossRef] [Green Version]
- Puig, C.; Marti, S.; Hermans, P.W.; de Jonge, M.I.; Ardanuy, C.; Liñares, J.; Langereis, J.D. Incorporation of phosphorylcholine into the lipooligosaccharide of nontypeable Haemophilus influenzae does not correlate with the level of biofilm formation in vitro. Infect. Immun. 2014, 82, 1591–1599. [Google Scholar] [CrossRef] [Green Version]
- Harrell, J.E.; Hahn, M.M.; D’Souza, S.J.; Vasicek, E.M.; Sandala, J.L.; Gunn, J.S.; McLachlan, J.B. Salmonella Biofilm Formation, Chronic Infection, and Immunity Within the Intestine and Hepatobiliary Tract. Front. Cell. Infect. Microbiol. 2020, 10, 624622. [Google Scholar] [CrossRef]
- Gunn, J.S.; Bakaletz, L.O.; Wozniak, D.J. What’s on the outside matters: The role of the extracellular polymeric substance of gram-negative biofilms in evading host immunity and as a target for therapeutic intervention. J. Biol. Chem. 2016, 291, 12538–12546. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.; Horsman, S.R.; Charron-Mazenod, L.; Turnbull, A.L.; Mulcahy, H.; Surette, M.G.; Lewenza, S. Extracellular DNA-induced antimicrobial peptide resistance in Salmonella enterica serovar Typhimurium. BMC Microbiol. 2013, 13, 115. [Google Scholar] [CrossRef] [Green Version]
- Jones, E.A.; McGillivary, G.; Bakaletz, L.O. Extracellular DNA within a nontypeable Haemophilus influenzae-induced biofilm binds human β defensin-3 and reduces its antimicrobial activity. J. Innate Immun. 2013, 5, 24–38. [Google Scholar] [CrossRef]
- Worlitzsch, D.; Tarran, R.; Ulrich, M.; Schwab, U.; Cekici, A.; Meyer, K.C.; Birrer, P.; Bellon, G.; Berger, J.; Weiss, T.; et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest. 2002, 109, 317–325. [Google Scholar] [CrossRef]
- De Groote, M.A.; Fang, F.C. NO inhibitions: Antimicrobial properties of nitric oxide. Clin. Infect. Dis. 1995, 21 (Suppl. S2), S162–S165. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Pittman, J.E.; Ferkol, T.W. The evolution of cystic fibrosis care. Chest 2015, 148, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.K.; Parsek, M.R.; Greenberg, E.P.; Welsh, M.J. A component of innate immunity prevents bacterial biofilm development. Nature 2002, 417, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bartlett, J.A.; Di, M.E.; Bomberger, J.M.; Chan, Y.R.; Gakhar, L.; Mallampalli, R.K.; McCray, P.B.J.; Di, Y.P. SPLUNC1/BPIFA1 contributes to pulmonary host defense against Klebsiella pneumoniae respiratory infection. Am. J. Pathol. 2013, 182, 1519–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gakhar, L.; Bartlett, J.A.; Penterman, J.; Mizrachi, D.; Singh, P.K.; Mallampalli, R.K.; Ramaswamy, S.; McCray, P.B.J. PLUNC is a novel airway surfactant protein with anti-biofilm activity. PLoS One 2010, 5, e9098. [Google Scholar] [CrossRef]
- Liu, Y.; Di, M.E.; Chu, H.W.; Liu, X.; Wang, L.; Wenzel, S.; Di, Y.P. Increased susceptibility to pulmonary Pseudomonas infection in Splunc1 knockout mice. J. Immunol. 2013, 191, 4259–4268. [Google Scholar] [CrossRef] [Green Version]
- Tsou, Y.A.; Chen, C.M.; Lin, T.C.; Hu, F.W.; Tai, C.J.; Chen, H.C.; Yeh, T.H.; Harn, H.J.; Tsai, M.H.; Jan, C.I. Decreased SPLUNC1 expression is associated with Pseudomonas infection in surgically treated chronic rhinosinusitis patients who may require repeated sinus surgery. Laryngoscope 2013, 123, 845–851. [Google Scholar] [CrossRef]
- Fernicola, S.; Paiardini, A.; Giardina, G.; Rampioni, G.; Leoni, L.; Cutruzzolà, F.; Rinaldo, S. In silico discovery and in vitro validation of catechol-containing sulfonohydrazide compounds as potent inhibitors of the diguanylate cyclase PleD. J. Bacteriol. 2015, 198, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Sambanthamoorthy, K.; Sloup, R.E.; Parashar, V.; Smith, J.M.; Kim, E.E.; Semmelhack, M.F.; Neiditch, M.B.; Waters, C.M. Identification of small molecules that antagonize diguanylate cyclase enzymes to inhibit biofilm formation. Antimicrob. Agents Chemother. 2012, 56, 5202–5211. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Cui, T.; Zeng, J.; Chen, L.; Zhang, W.; Xu, X.; Cheng, L.; Li, M.; Li, J.; Zhou, X.; et al. Molecule targeting glucosyltransferase inhibits Streptococcus mutans biofilm formation and virulence. Antimicrob. Agents Chemother. 2015, 60, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Nett, J.E.; Cabezas-Olcoz, J.; Marchillo, K.; Mosher, D.F.; Andes, D.R. Targeting fibronectin to disrupt in vivo Candida albicans biofilms. Antimicrob. Agents Chemother. 2016, 60, 3152–3155. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, J.B. Biofilm matrix-degrading enzymes. Methods Mol. Biol. 2014, 1147, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Pleszczyńska, M.; Wiater, A.; Janczarek, M.; Szczodrak, J. (1,3)-α-d-Glucan hydrolases in dental biofilm prevention and control: A review. Int. J. Biol. Macromol. 2015, 79, 761–778. [Google Scholar] [CrossRef]
- Iwase, T.; Uehara, Y.; Shinji, H.; Tajima, A.; Seo, H.; Takada, K.; Agata, T.; Mizunoe, Y. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 2010, 465, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ren, Z.; Zhou, X.; Zeng, J.; Zou, J.; Li, Y. Inhibition of Streptococcus mutans biofilm formation, extracellular polysaccharide production, and virulence by an oxazole derivative. Appl. Microbiol. Biotechnol. 2016, 100, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Mlynek, K.D.; Hettiarachchi, H.; Alamneh, Y.A.; Biggemann, L.; Zurawski, D.V.; Black, C.C.; Bane, C.E.; Kim, R.K.; Granick, M.S. Extracellular polymeric substance (EPS)-degrading enzymes reduce staphylococcal surface attachment and biocide resistance on pig skin in vivo. PLoS ONE 2018, 13, e0205526. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Shen, Y.; Nelson, D.C.; Eugster, M.R.; Eichenseher, F.; Hanke, D.C.; Loessner, M.J.; Dong, S.; Pritchard, D.G.; Lee, J.C.; et al. Evolutionarily distinct bacteriophage endolysins featuring conserved peptidoglycan cleavage sites protect mice from MRSA infection. J. Antimicrob. Chemother. 2015, 70, 1453–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzenreiter, R.; Kienberger, F.; Marcos, V.; Schilcher, K.; Krautgartner, W.D.; Obermayer, A.; Huml, M.; Stoiber, W.; Hector, A.; Griese, M.; et al. Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J. Cyst. Fibros. 2012, 11, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Novotny, L.A.; Jurcisek, J.A.; Goodman, S.D.; Bakaletz, L.O. Monoclonal antibodies against DNA-binding tips of DNABII proteins disrupt biofilms in vitro and induce bacterial clearance in vivo. EBioMedicine 2016, 10, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Lakshmi, S.A.; Alexpandi, R.; Shafreen, R.M.B.; Tamilmuhilan, K.; Srivathsan, A.; Kasthuri, T.; Ravi, A.V.; Shiburaj, S.; Pandian, S.K. Evaluation of antibiofilm potential of four-domain α-amylase from Streptomyces griseus against exopolysaccharides (EPS) of bacterial pathogens using Danio rerio. Arch. Microbiol. 2022, 204, 243. [Google Scholar] [CrossRef] [PubMed]
- Howlin, R.P.; Cathie, K.; Hall-Stoodley, L.; Cornelius, V.; Duignan, C.; Allan, R.N.; Fernandez, B.O.; Barraud, N.; Bruce, K.D.; Jefferies, J.; et al. Low-Dose Nitric Oxide as Targeted Anti-biofilm Adjunctive Therapy to Treat Chronic Pseudomonas aeruginosa Infection in Cystic Fibrosis. Mol. Ther. 2017, 25, 2104–2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barraud, N.; Kardak, B.G.; Yepuri, N.R.; Howlin, R.P.; Webb, J.S.; Faust, S.N.; Kjelleberg, S.; Rice, S.A.; Kelso, M.J. Cephalosporin-3′-diazeniumdiolates: Targeted NO-donor prodrugs for dispersing bacterial biofilms. Angew. Chem. Int. Ed Engl. 2012, 51, 9057–9060. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente-Núñez, C.; Reffuveille, F.; Fairfull-Smith, K.E.; Hancock, R.E.W. Effect of nitroxides on swarming motility and biofilm formation, multicellular behaviors in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 4877–4881. [Google Scholar] [CrossRef] [Green Version]
- George, E.A.; Novick, R.P.; Muir, T.W. Cyclic peptide inhibitors of staphylococcal virulence prepared by Fmoc-based thiolactone peptide synthesis. J. Am. Chem. Soc. 2008, 130, 4914–4924. [Google Scholar] [CrossRef]
- Rafael, G.V.B.; Sophie, C.; Rafael, S.; Sylvie, N.-L.; Serge, B.; Emmanuel, G.; Aline, R.Z.; Cristina, B.G.S.; José, M.A.; Reynald, G. Capsicumicine, a new bioinspired peptide from red peppers prevents staphylococcal biofilm In Vitro and In Vivo via a matrix anti-assembly mechanism of action. Microbiol. Spectr. 2021, 9, e00471-21. [Google Scholar] [CrossRef]
- Lin, J.S.; Bekale, L.A.; Molchanova, N.; Nielsen, J.E.; Wright, M.; Bacacao, B.; Diamond, G.; Jenssen, H.; Santa Maria, P.L.; Barron, A.E. Anti-persister and anti-biofilm activity of self-assembled antimicrobial peptoid ellipsoidal micelles. ACS Infect. Dis. 2022, 8, 1823–1830. [Google Scholar] [CrossRef]
- Mihailescu, R.; Tafin, U.F.; Corvec, S.; Oliva, A.; Betrisey, B.; Borens, O.; Trampuza, A. High activity of fosfomycin and rifampin against methicillin-resistant Staphylococcus aureus biofilm in vitro and in an experimental foreign-body infection model. Antimicrob. Agents Chemother. 2014, 58, 2547–2553. [Google Scholar] [CrossRef] [Green Version]
- Conlon, B.P.; Nakayasu, E.S.; Fleck, L.E.; Lafleur, M.D.; Isabella, V.M.; Coleman, K.; Leonard, S.N.; Smith, R.D.; Adkins, J.N.; Lewis, K. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 2013, 503, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Du, Y.; Si, Z.; Liu, Y.; Turvey, M.E.; Raju, C.; Keogh, D.; Ruan, L.; Jothy, S.L.; Reghu, S.; et al. Enantiomeric glycosylated cationic block co-beta-peptides eradicate Staphylococcus aureus biofilms and antibiotic-tolerant persisters. Nat. Commun. 2019, 10, 4792. [Google Scholar] [CrossRef] [Green Version]
- Okshevsky, M.; Regina, V.R.; Meyer, R.L. Extracellular DNA as a target for biofilm control. Curr. Opin. Biotechnol. 2015, 33, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Ratjen, F. Effect of dornase alfa on inflammation and lung function: Potential role in the early treatment of cystic fibrosis. J. Cyst. Fibros. 2012, 11, 78–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, M.; Wozniak, D.J.; Stoodley, P.; Hall-Stoodley, L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev. Anti. Infect. Ther. 2015, 13, 1499–1516. [Google Scholar] [CrossRef] [Green Version]
- DiGiandomenico, A.; Warrener, P.; Hamilton, M.; Guillard, S.; Ravn, P.; Minter, R.; Camara, M.M.; Venkatraman, V.; MacGill, R.S.; Lin, J.; et al. Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening. J. Exp. Med. 2012, 209, 1273–1287. [Google Scholar] [CrossRef] [Green Version]
- Flores-Mireles, A.L.; Pinkner, J.S.; Caparon, M.G.; Hultgren, S.J. EbpA vaccine antibodies block binding of Enterococcus faecalis to fibrinogen to prevent catheter-associated bladder infection in mice. Sci. Transl Med. 2014, 6, 254ra127. [Google Scholar] [CrossRef] [Green Version]
- Brady, R.A.; O’May, G.A.; Leid, J.G.; Prior, M.L.; Costerton, J.W.; Shirtliff, M.E. Resolution of Staphylococcus aureus biofilm infection using vaccination and antibiotic treatment. Infect. Immun. 2011, 79, 1797–1803. [Google Scholar] [CrossRef] [Green Version]
- Estellés, A.; Woischnig, A.K.; Liu, K.; Stephenson, R.; Lomongsod, E.; Nguyen, D.; Zhang, J.; Heidecker, M.; Yang, Y.; Simon, R.J.; et al. A high-affinity native human antibody disrupts biofilm from Staphylococcus aureus bacteria and potentiates antibiotic efficacy in a mouse implant infection model. Antimicrob. Agents Chemother. 2016, 60, 2292–2301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novotny, L.A.; Jurcisek, J.A.; Ward, M.O.; Jordan, Z.B.; Goodman, S.D.; Bakaletz, L.O. Antibodies against the majority subunit of type IV pili disperse nontypeable Haemophilus influenzae biofilms in a LuxS-dependent manner and confer therapeutic resolution of experimental otitis media. Mol. Microbiol. 2015, 96, 276–292. [Google Scholar] [CrossRef] [Green Version]
- Jurcisek, J.A.; Hofer, L.K.; Goodman, S.D.; Bakaletz, L.O. Monoclonal antibodies that target extracellular DNABII proteins or the type IV pilus of nontypeable Haemophilus influenzae (NTHI) worked additively to disrupt 2-genera biofilms. Biofilm 2022, 4, 100096. [Google Scholar] [CrossRef]
- Römling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef]
- Simonetti, O.; Cirioni, O.; Ghiselli, R.; Goteri, G.; Scalise, A.; Orlando, F.; Silvestri, C.; Riva, A.; Saba, V.; Madanahally, K.D.; et al. RNAIII-inhibiting peptide enhances healing of wounds infected with methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2008, 52, 2205–2211. [Google Scholar] [CrossRef] [Green Version]
- Shang, D.; Han, X.; Du, W.; Kou, Z.; Jiang, F. Trp-Containing antibacterial peptides impair quorum sensing and biofilm development in multidrug-resistant Pseudomonas aeruginosa and exhibit synergistic effects with antibiotics. Front. Microbiol. 2021, 12, 611009. [Google Scholar] [CrossRef]
- Louis, M.; Clamens, T.; Tahrioui, A.; Desriac, F.; Rodrigues, S.; Rosay, T.; Harmer, N.; Diaz, S.; Barreau, M.; Racine, P.-J.; et al. Pseudomonas aeruginosa biofilm dispersion by the human atrial natriuretic peptide. Adv. Sci. 2022, 9, 2103262. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.M.; Browngardt, C.; Xiaohui, X.; Klepac-Ceraj, V.; Paster, B.J.; Burne, R.A. The effect of arginine on oral biofilm communities. Mol. Oral Microbiol. 2014, 29, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Gnanadhas, D.P.; Elango, M.; Datey, A.; Chakravortty, D. Chronic lung infection by Pseudomonas aeruginosa biofilm is cured by l-methionine in combination with antibiotic therapy. Sci. Rep. 2015, 5, 16043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Invest. 2007, 117, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Moreau-Marquis, S.; O’Toole, G.A.; Stanton, B.A. Tobramycin and FDA-approved iron chelators eliminate Pseudomonas aeruginosa biofilms on cystic fibrosis cells. Am. J. Respir. Cell. Mol. Biol. 2009, 41, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Pletzer, D.; Coleman, S.R.; Hancock, R.E.W. Anti-biofilm peptides as a new weapon in antimicrobial warfare. Curr. Opin. Microbiol. 2016, 33, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Scheper, H.; Wubbolts, J.M.; Verhagen, J.A.M.; de Visser, A.W.; van der Wal, R.J.P.; Visser, L.G.; de Boer, M.G.J.; Nibbering, P.H. SAAP-148 Eradicates MRSA persisters within mature biofilm models simulating prosthetic joint infection. Front. Microbiol. 2021, 12, 625952. [Google Scholar] [CrossRef]
- Horev, B.; Klein, M.I.; Hwang, G.; Li, Y.; Kim, D.; Koo, H.; Benoit, D.S.W. pH-activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano 2015, 9, 2390–2404. [Google Scholar] [CrossRef] [Green Version]
- Baelo, A.; Levato, R.; Julián, E.; Crespo, A.; Astola, J.; Gavaldà, J.; Engel, E.; Mateos-Timoneda, M.A.; Torrents, E. Disassembling bacterial extracellular matrix with DNase-coated nanoparticles to enhance antibiotic delivery in biofilm infections. J. Control Release 2015, 209, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Zhao, W.; Zhang, X.; Song, L.; Luan, S. Charge-switchable MOF nanocomplex for enhanced biofilm penetration and eradication. J. Hazard. Mater. 2022, 439, 129594. [Google Scholar] [CrossRef] [PubMed]
- Gilabert-Porres, J.; Martí, S.; Calatayud, L.; Ramos, V.; Rosell, A.; Borrós, S. Design of a nanostructured active surface against gram-positive and gram-negative bacteria through plasma activation and in situ silver reduction. ACS Appl. Mater. Interfaces 2016, 8, 64–73. [Google Scholar] [CrossRef]
- Ye, Z.; Sang, T.; Li, K.; Fischer, N.G.; Mutreja, I.; Echeverría, C.; Kumar, D.; Tang, Z.; Aparicio, C. Hybrid nanocoatings of self-assembled organic-inorganic amphiphiles for prevention of implant infections. Acta Biomater. 2022, 140, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Danehy, R.; Cai, H.; Ao, Z.; Pu, M.; Nusawardhana, A.; Rowe-Magnus, D.; Guo, F. Microneedle patch-mediated treatment of bacterial biofilms. ACS Appl. Mater. Interfaces 2019, 11, 14640–14646. [Google Scholar] [CrossRef]
- Lv, X.; Xu, Y.; Ruan, X.; Yang, D.; Shao, J.; Hu, Y.; Wang, W.; Cai, Y.; Tu, Y.; Dong, X. An injectable and biodegradable hydrogel incorporated with photoregulated NO generators to heal MRSA-infected wounds. Acta Biomater. 2022, 146, 107–118. [Google Scholar] [CrossRef]
- Forier, K.; Raemdonck, K.; De Smedt, S.C.; Demeester, J.; Coenye, T.; Braeckmans, K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J. Control. Release 2014, 190, 607–623. [Google Scholar] [CrossRef] [Green Version]
- Giovagnoli, S.; Pietrella, D.; Barberini, L.; Santi, C.; Carotti, A.; di Michele, A.; Ricci, M. Reshaping antibiotics through hydrophobic drug-bile acid ionic complexation enhances activity against Staphylococcus aureus biofilms. Int. J. Pharm. 2017, 528, 144–162. [Google Scholar] [CrossRef]
- De Jong, W.H.; Van Der Ven, L.T.M.; Sleijffers, A.; Park, M.V.D.Z.; Jansen, E.H.J.M.; Van Loveren, H.; Vandebriel, R.J. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials 2013, 34, 8333–8343. [Google Scholar] [CrossRef] [Green Version]
- Wen, L.; Tian, Y.; Jiang, L. Bioinspired super-wettability from fundamental research to practical applications. Angew. Chem. Int. Ed. Engl. 2015, 54, 3387–3399. [Google Scholar] [CrossRef]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for biomedical applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashbaugh, A.G.; Jiang, X.; Zheng, J.; Tsai, A.S.; Kim, W.S.; Thompson, J.M.; Miller, R.J.; Shahbazian, J.H.; Wang, Y.; Dillen, C.A.; et al. Polymeric nanofiber coating with tunable combinatorial antibiotic delivery prevents biofilm-associated infection in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, E6919–E6928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodhouse, I.; Nejati, S.; Selvamani, V.; Jiang, H.; Chittiboyina, S.; Grant, J.; Mutlu, Z.; Waimin, J.; Abutaleb, N.S.; Seleem, M.N.; et al. Flexible Microneedle Array Patch for Chronic Wound Oxygenation and Biofilm Eradication. ACS Appl. bio. Mater. 2021, 4, 5405–5415. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; Mir, M.; Utomo, E.; Donnelly, R.F. Bacterially sensitive nanoparticle-based dissolving microneedles of doxycycline for enhanced treatment of bacterial biofilm skin infection: A proof of concept study. Int. J. Pharm. X 2020, 2, 100047. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Mainardi, V.L.; Wang, H.; McCarthy, A.; Zhang, Y.S.; Chen, S.; John, J.V.; Wong, S.L.; Hollins, R.R.; Wang, G.; et al. Dissolvable microneedles coupled with nanofiber dressings eradicate biofilms via effectively delivering a database-designed antimicrobial peptide. ACS Nano 2020, 14, 11775–11786. [Google Scholar] [CrossRef]
- Cámara, M.; Green, W.; MacPhee, C.E.; Rakowska, P.D.; Raval, R.; Richardson, M.C.; Slater-Jefferies, J.; Steventon, K.; Webb, J.S. Economic significance of biofilms: A multidisciplinary and cross-sectoral challenge. NPJ Biofilms Microbiomes 2022, 8, 42. [Google Scholar] [CrossRef] [PubMed]



| Antibiofilm Agent | Target Pathogen | Antibiofilm Mode of Action | Study Model | Reference |
|---|---|---|---|---|
| Farnesol-loaded nanoparticles | Streptococcus mutans | Attenuated biofilm virulence | Dental caries disease model | [80] |
| Ciprofloxacin-loaded nanoparticles | Pseudomonas aeruginosa | Prevented biofilm formation and reduced established biofilm mass | Macrophages | [81] |
| Proteinase K and Rose-Bengal-loaded nanocomplex | Staphylococcus aureus | Biofilm eradication | Cutaneous wound infection in mouse model | [82] |
| Nanostructured silver antibacterial surfaces | S. aureus and P. aeruginosa | Antibacterial and antifouling activity | Polydimethylsiloxane films | [83] |
| AMP * nanostructures with silver nanoparticles | S. aureus (Methicillin- resistant) | In vivo antimicrobial activity | Subcutaneous infection model in rats | [84] |
| Microneedle patches with chloramphenicol-loaded nanoparticles | Vibrio vulnificus | Biofilm disruption and antibiotic penetration | In vitro biofilm model | [85] |
| BNN6 †-loaded polydopamine nanoparticles | S. aureus (Methicillin-resistant) | Decrease in biofilm cells and wound healing | In vivo wound in mouse model | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhamid, A.G.; Yousef, A.E. Combating Bacterial Biofilms: Current and Emerging Antibiofilm Strategies for Treating Persistent Infections. Antibiotics 2023, 12, 1005. https://doi.org/10.3390/antibiotics12061005
Abdelhamid AG, Yousef AE. Combating Bacterial Biofilms: Current and Emerging Antibiofilm Strategies for Treating Persistent Infections. Antibiotics. 2023; 12(6):1005. https://doi.org/10.3390/antibiotics12061005
Chicago/Turabian StyleAbdelhamid, Ahmed G., and Ahmed E. Yousef. 2023. "Combating Bacterial Biofilms: Current and Emerging Antibiofilm Strategies for Treating Persistent Infections" Antibiotics 12, no. 6: 1005. https://doi.org/10.3390/antibiotics12061005







