An Edible Biopolymeric Microcapsular Wrapping Integrating Lytic Bacteriophage Particles for Salmonella enterica: Potential for Integration into Poultry Feed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Chemicals
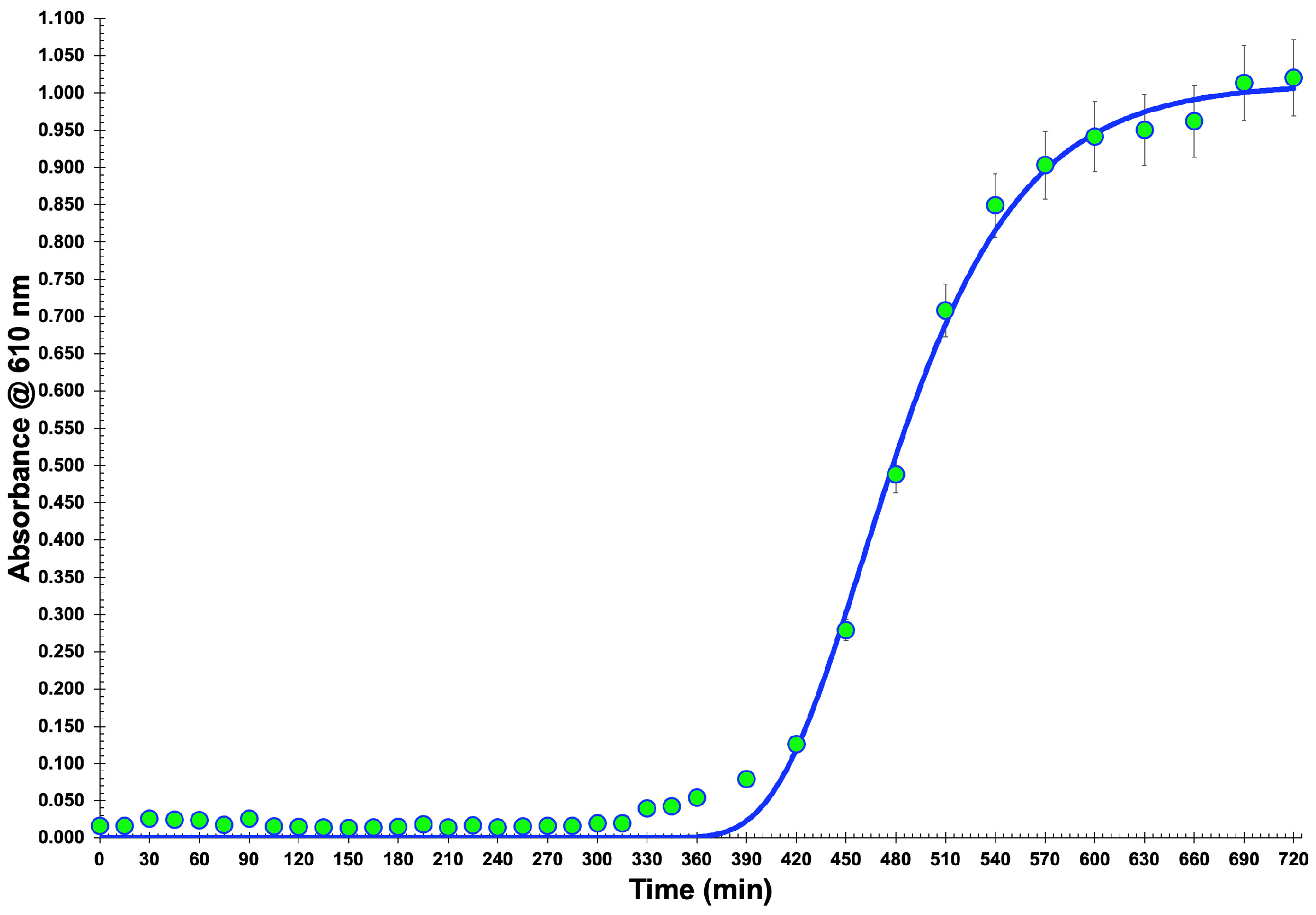
2.3. Preparation of a Salmonella enterica CCCD-S004 Growth Curve
2.4. Phage Enrichment, Isolation, Propagation, and Enumeration
2.5. Phage–PEG Precipitation
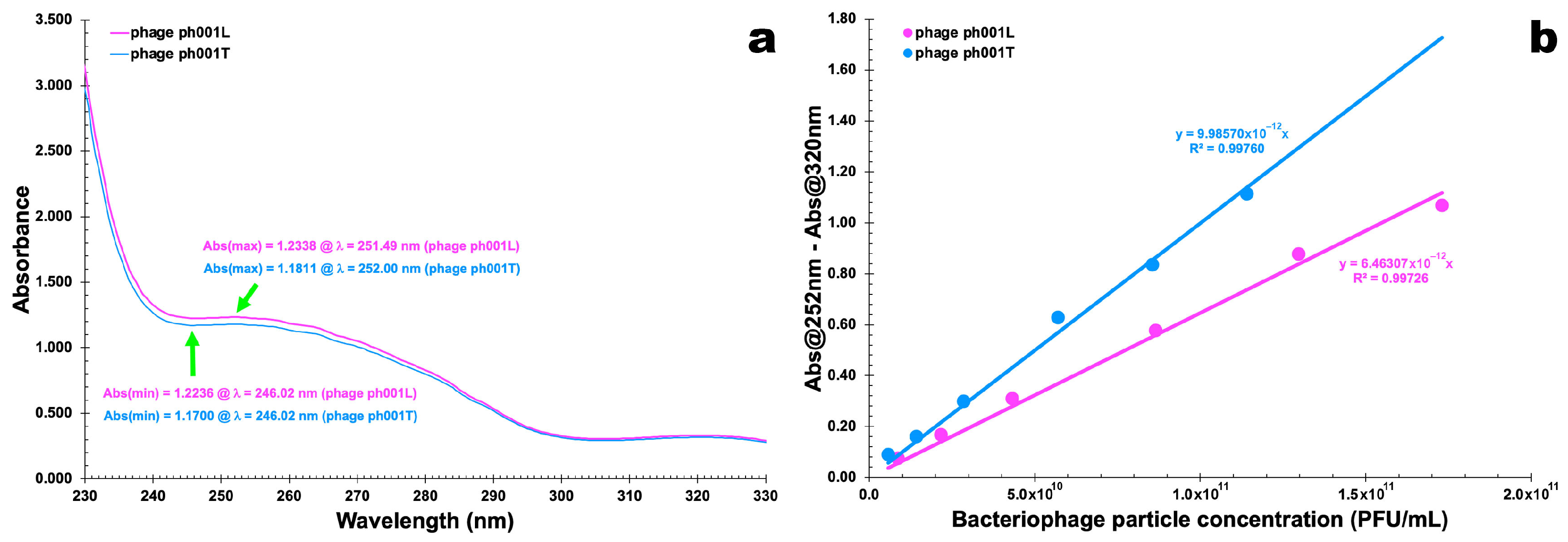
2.6. UV-Vis Spectral Scans for Determination of Phage Particle Extinction Coefficient
2.7. Transmission Electron Microscopy (TEM) Analyses
2.8. Host Range of Isolated Phage Particles: Spot Test and Efficiency of Plating (EOP)
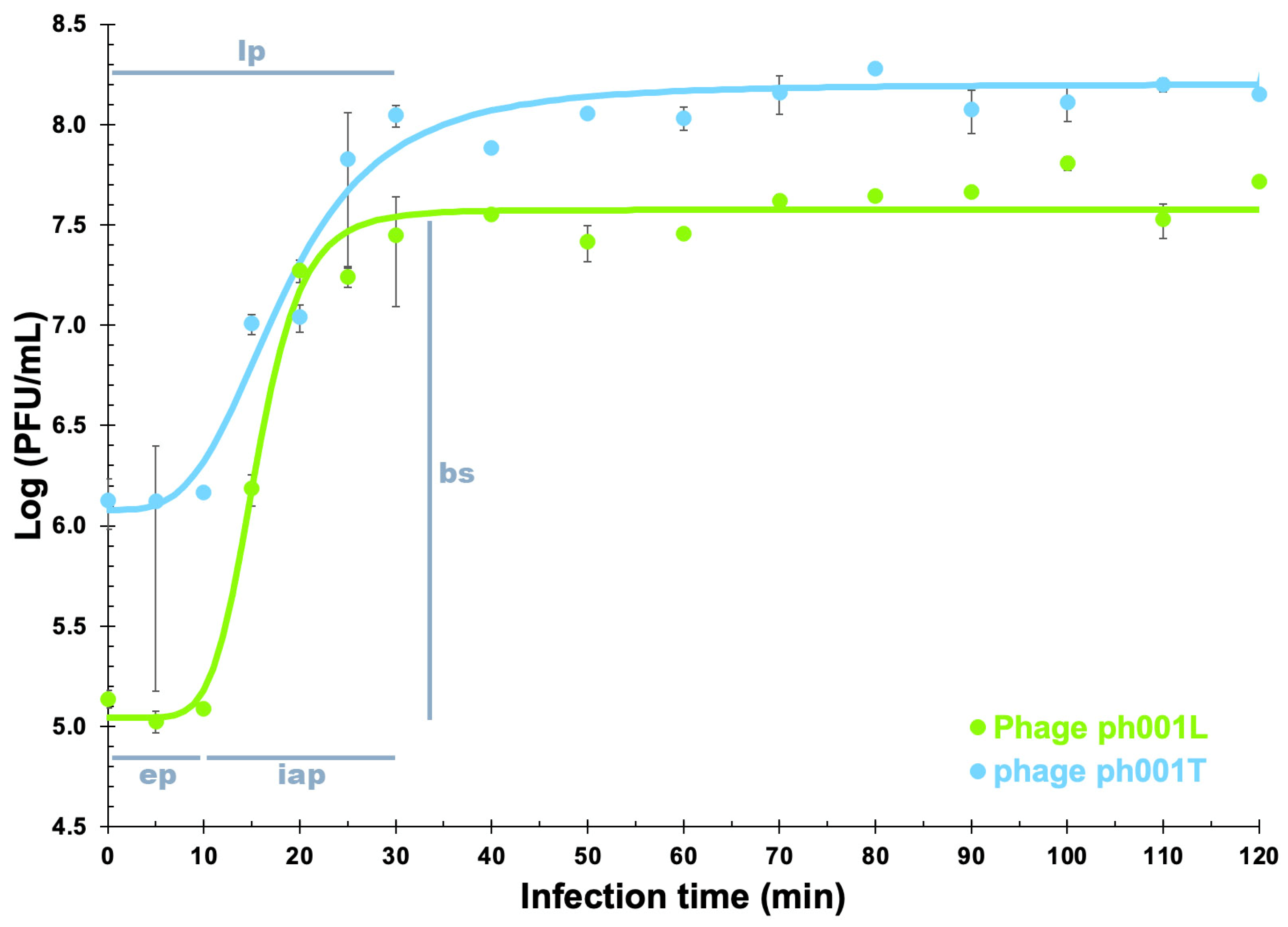
2.9. Phage One-Step Growth (OSG) Analyses
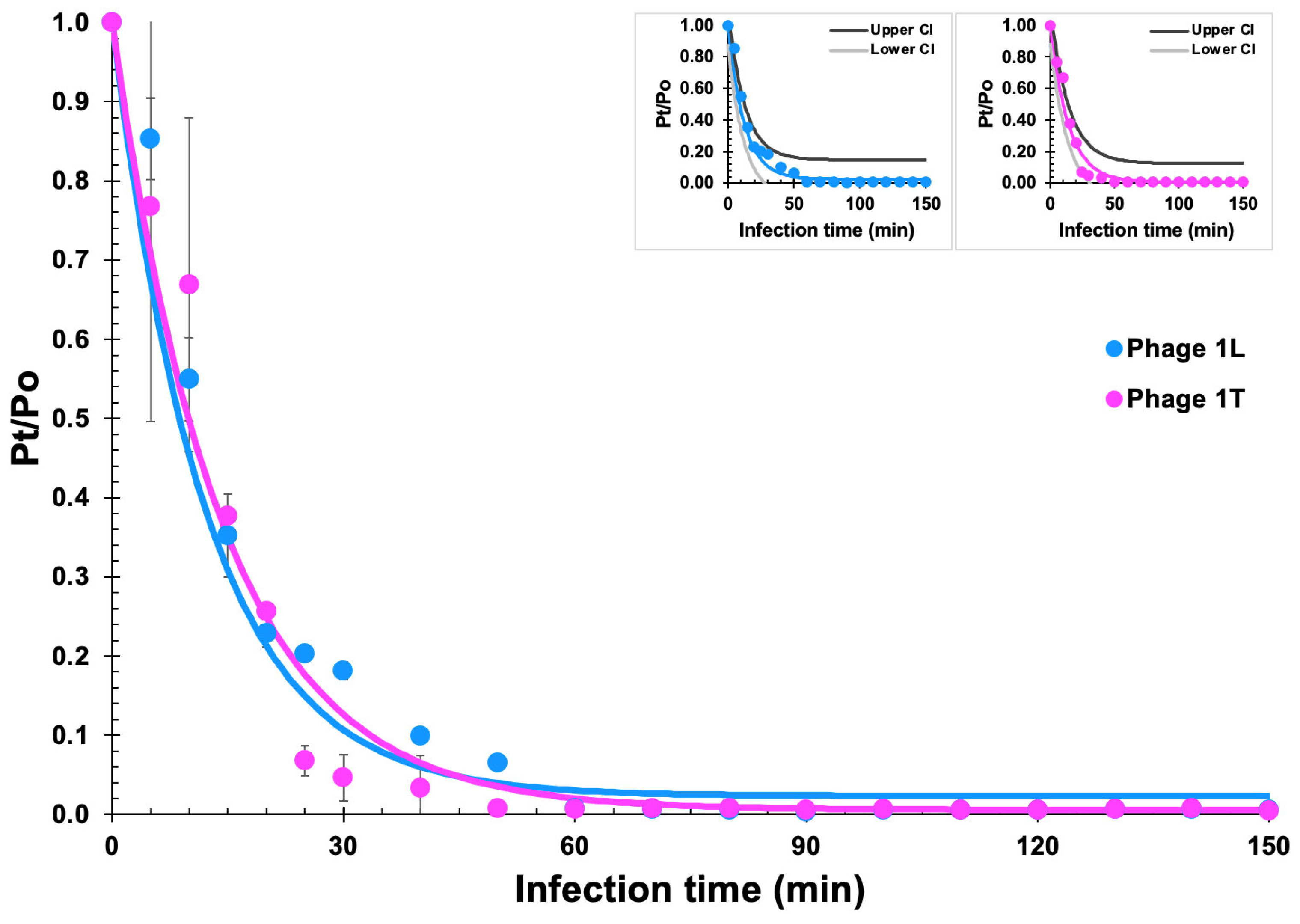
2.10. Phage Adsorption Analyses
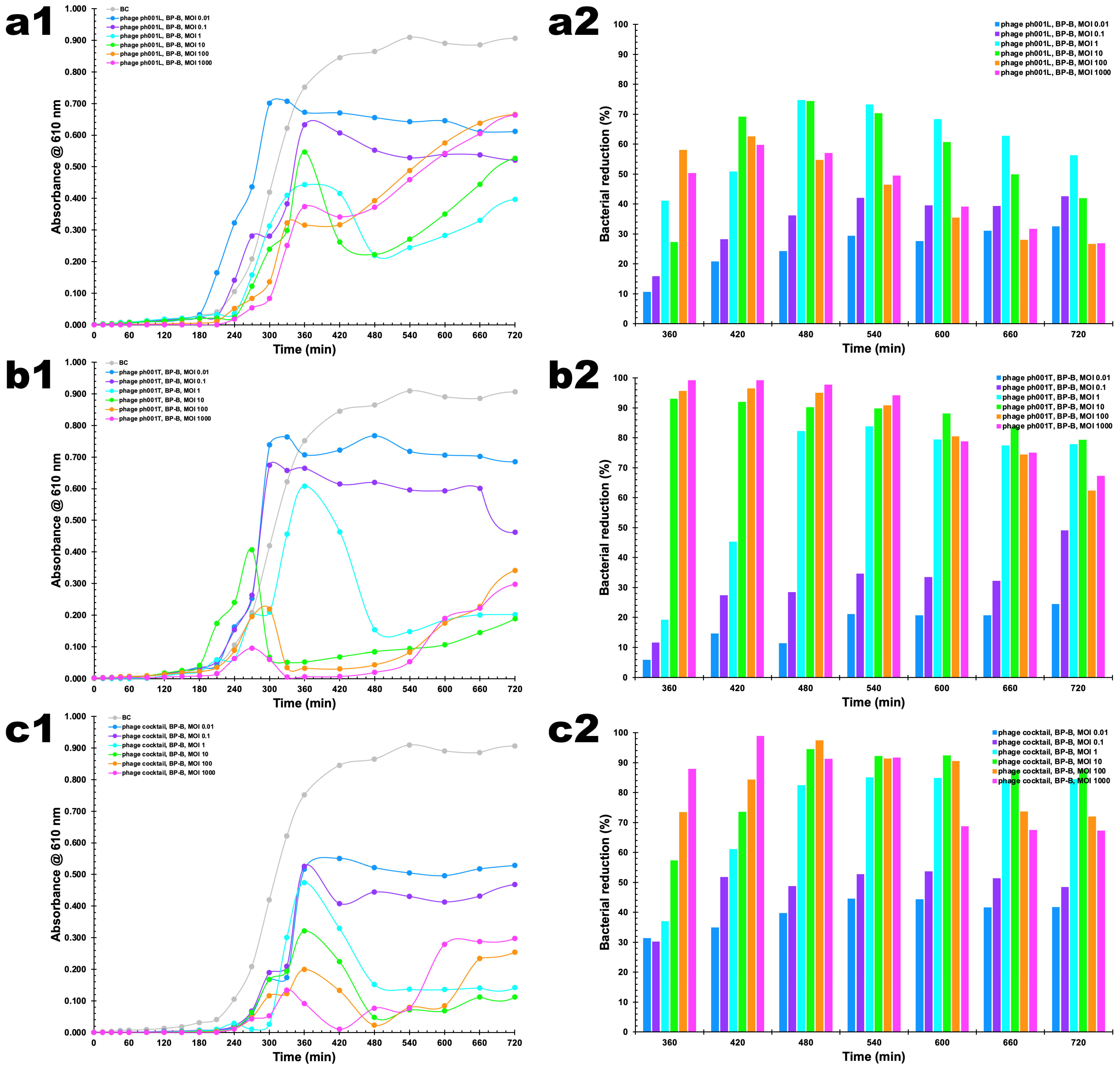
2.11. Bacteria Inactivation Experiments In Vitro by the Two Phages
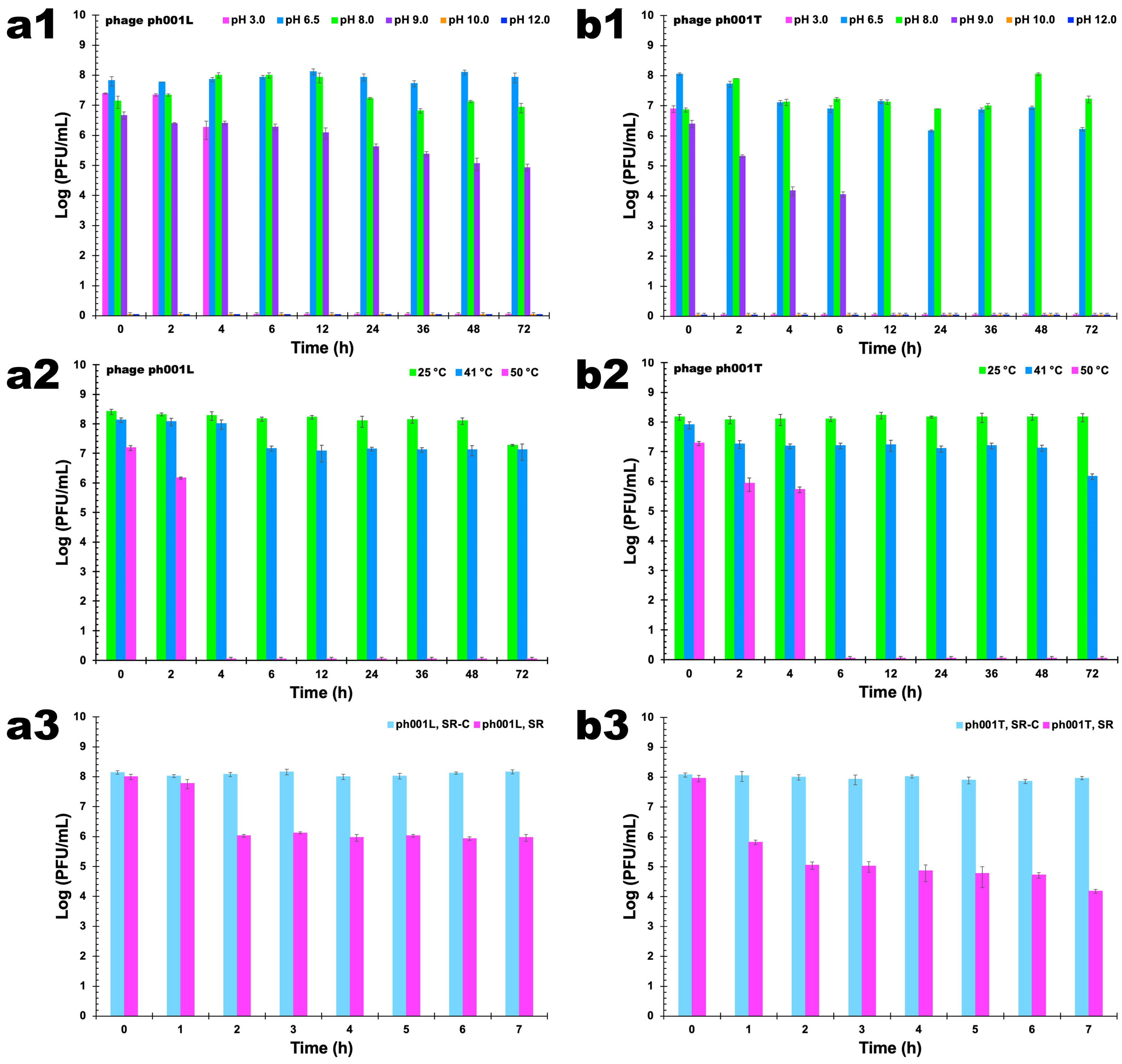
2.12. Assessment of the Outcome of Abiotic Factors upon Phage Viability
2.12.1. pH Studies
2.12.2. Temperature Studies
2.12.3. Solar Radiation Studies
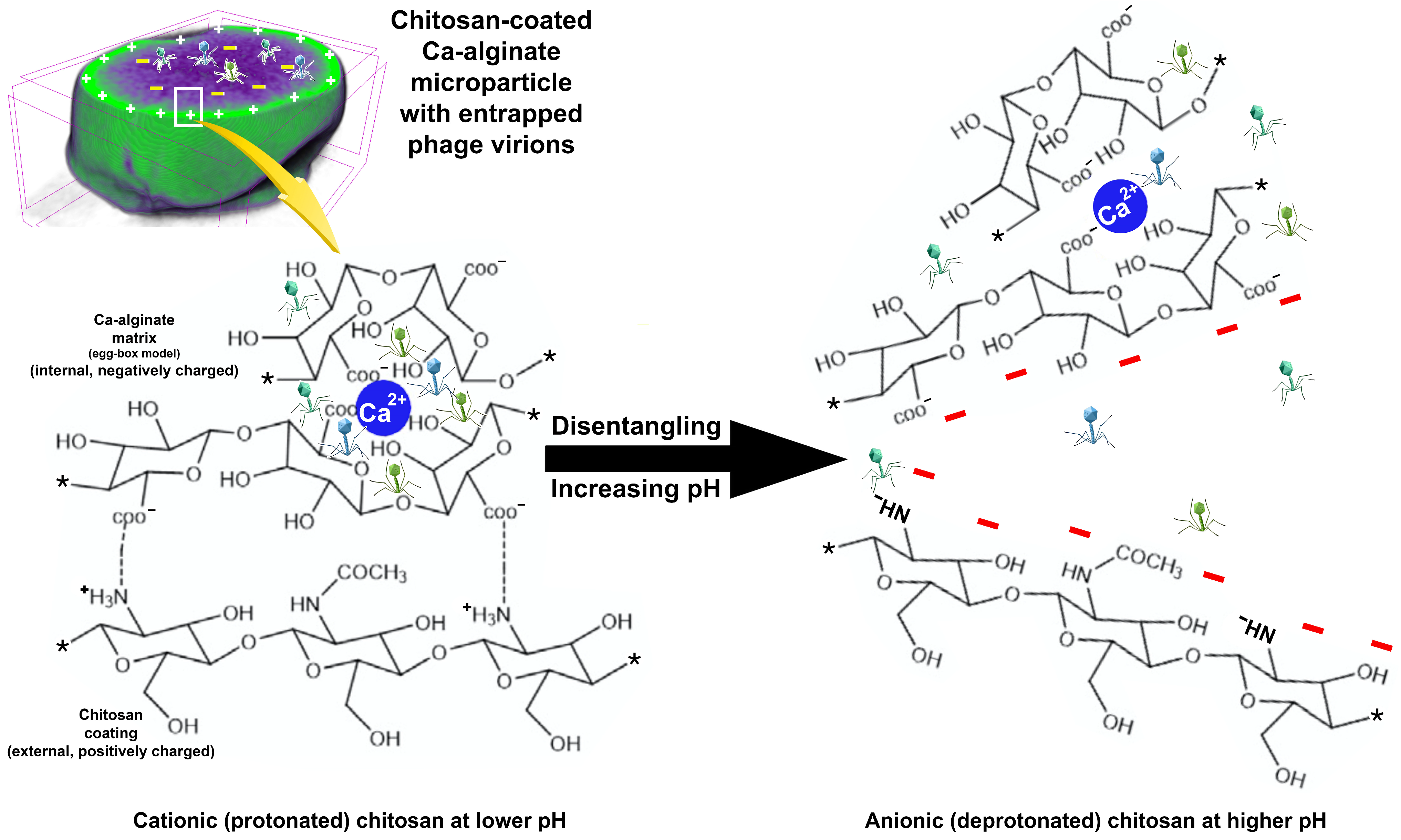
2.13. Formulation of the Edible Biopolymeric Microcapsular Wrapping (EBMW) Integrating the Bacteriophage Cocktail
2.14. Assessment of the Lytic Viability of Entrapped Bacteriophage Particles within the EBMW Formulations
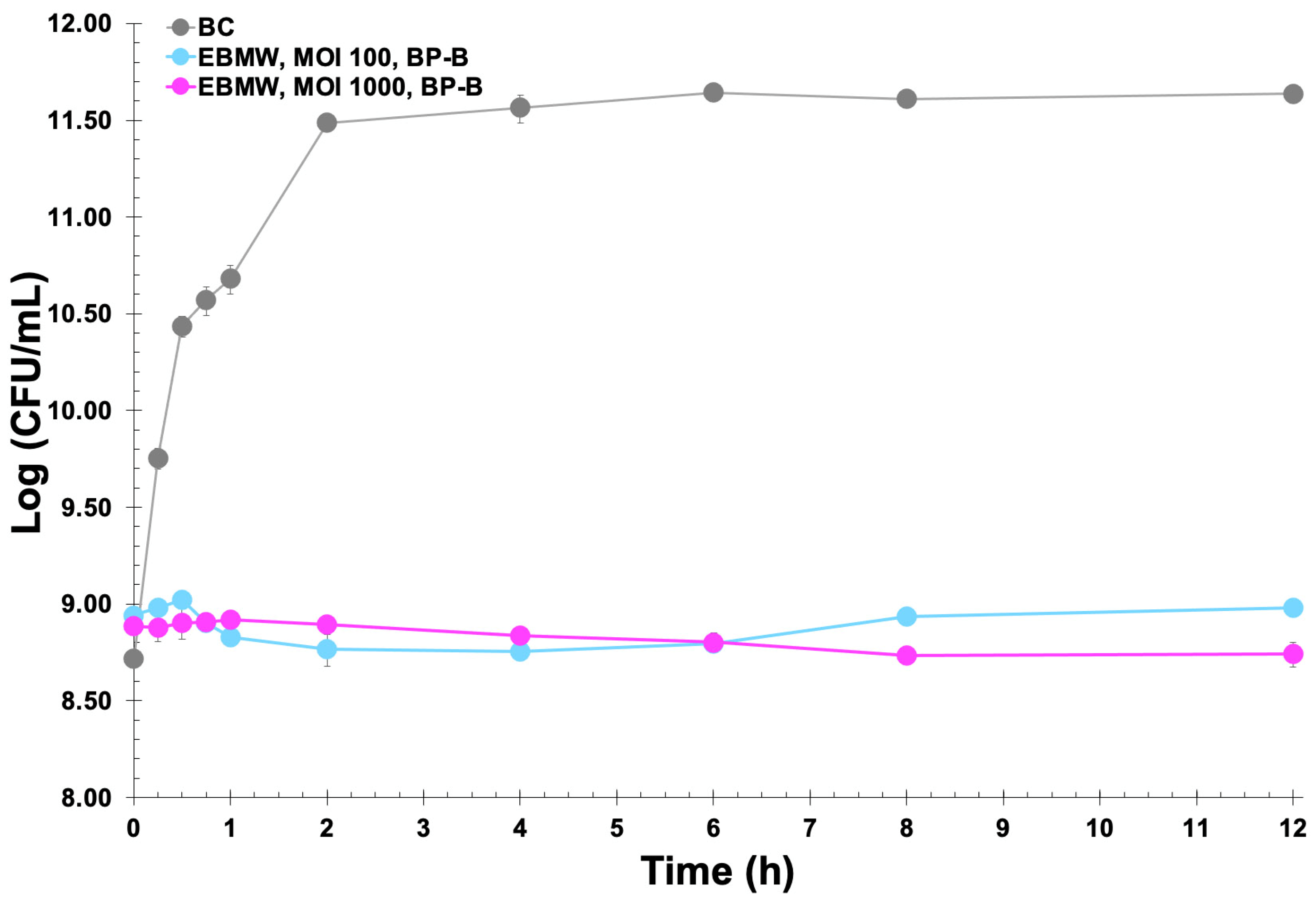
2.15. Bacteria Inactivation Experiments In Vitro by the EBMW Particles with Entrapped Phage Cocktail
2.16. Phage Release Experiments from the EBMW Particles with Entrapped Phage Cocktail
2.17. Physicochemical Characterization of EBMW Formulation
2.17.1. FTIR Tests
2.17.2. Thermal Analyses via DSC Tests
2.17.3. EDXRF Tests
2.17.4. XRT Tests
2.17.5. SEM Tests
2.18. Statistical Tests
3. Results
3.1. Bacterial Growth Curve
3.2. Phage Virion Morphology via Transmission Electron Microscopy (TEM) Analyses
3.3. Phage Particle Extinction Coefficients
3.4. Phage Host Range and Efficiency of Plating (EOP)
3.5. Phage One-Step Growth (OSG) Analyses
3.6. Phage Adsorption Analyses
3.7. In Vitro Phage–Bacteria Inactivation Assays
3.8. Assessment of the Outcome of Abiotic Factors upon Phage Viability
3.8.1. pH Studies
3.8.2. Temperature Studies
3.8.3. Solar radiation Studies
3.9. Preparation and Characterization of the Edible Biopolymeric Microcapsular Wrapping (EBMW) Integrating the Bacteriophage Cocktail
3.10. Assessment of the Lytic Viability of Entrapped Bacteriophage Particles within the EBMW Formulations
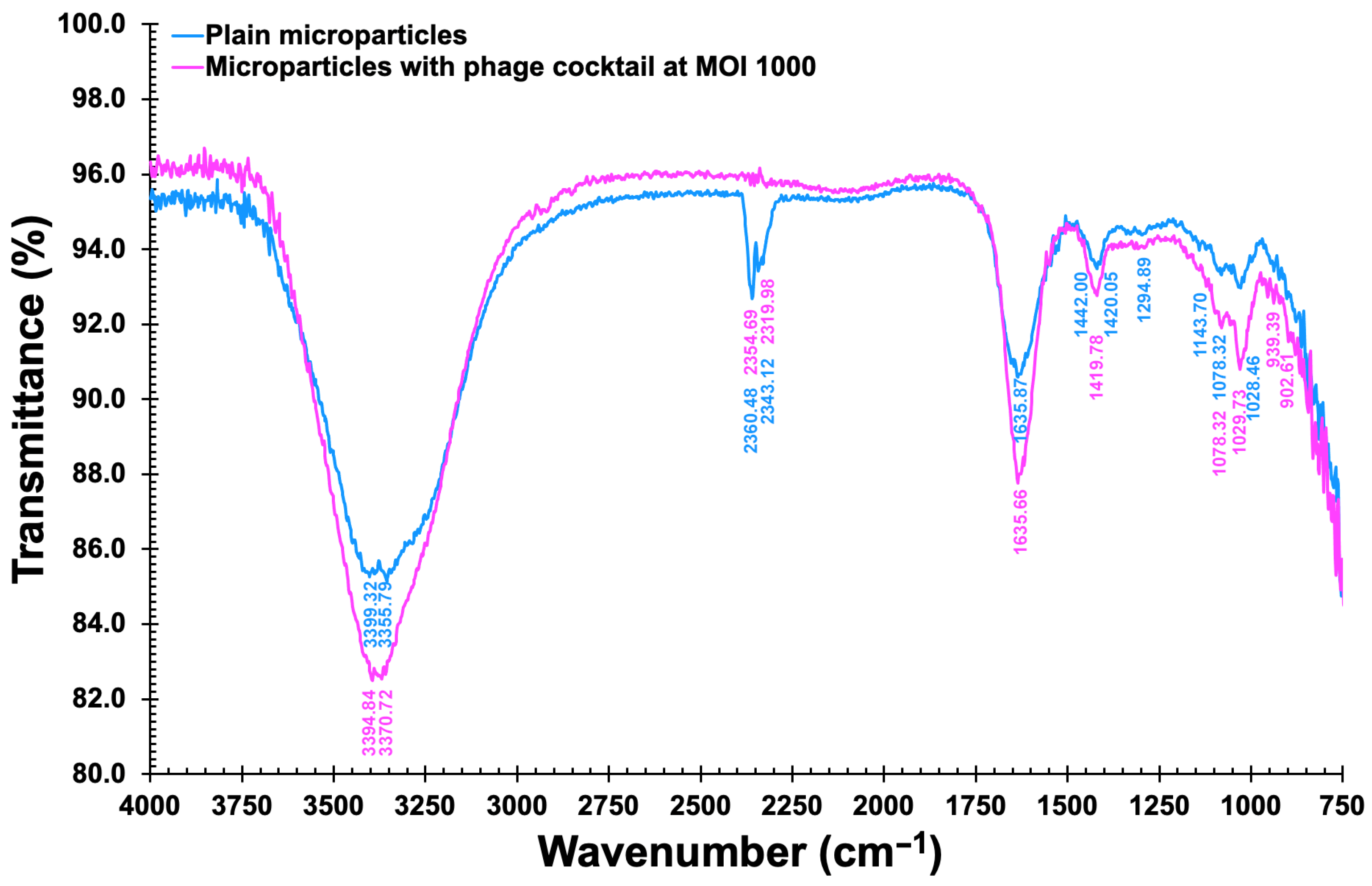
3.11. Fourier Transform InfraRed Spectrometry (FTIR) Analyses
3.12. Thermal Characterization of the EBMW Formulations via DSC
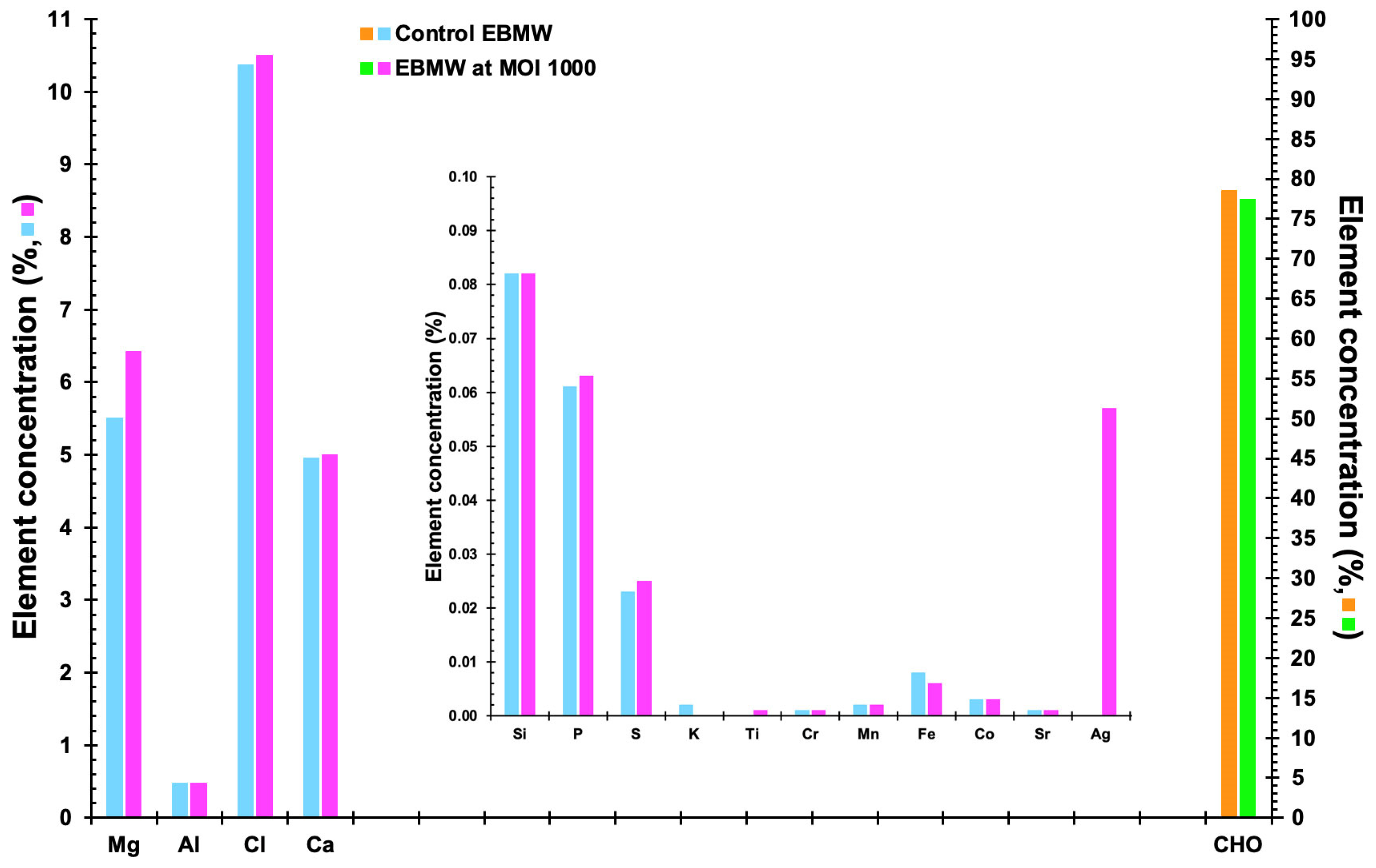
3.13. Elemental Profile of the EBMW Formulations Obtained by EDXRF
3.14. XRT Analysis of the EBMW Integrating the Cocktail of Phage Particles
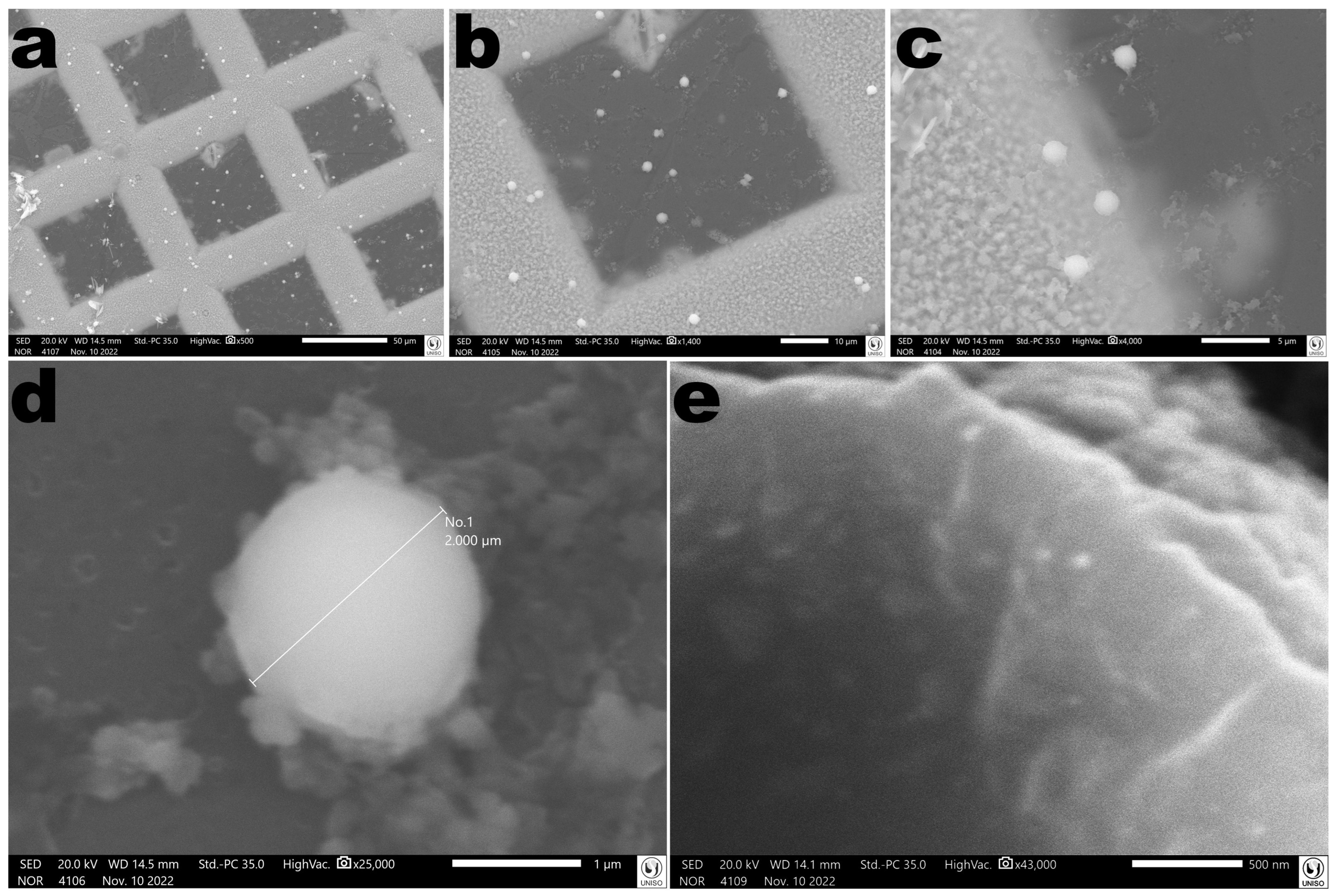
3.15. Morphological Analyses of the EBMW via SEM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ravi, Y.; Pooja, M.K.; Krushna Yadav, D.K. Review- bacteriophages in food preservation. Int. J. Pure App. Biosci. 2017, 5, 197–205. [Google Scholar] [CrossRef]
- Anany, H.; Brovko, L.Y.; El-Arabi, T.; Griffiths, M.W. 4—Bacteriophages as antimicrobials in food products: History, biology and application. In Handbook of Natural Antimicrobials for Food Safety and Quality; Taylor, T.M., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2015; pp. 69–87. [Google Scholar]
- Finger, J.A.F.F.; Baroni, W.S.G.V.; Maffei, D.F.; Bastos, D.H.M.; Pinto, U.M. Overview of Foodborne Disease Outbreaks in Brazil from 2000 to 2018. Foods 2019, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Feltes, M.M.C.; Arisseto-Bragotto, A.P.; Block, J.M. Food quality, food-borne diseases, and food safety in the Brazilian food industry. Food Qual. Saf. 2017, 1, 13–27. [Google Scholar] [CrossRef]
- Eng, S.-K.; Pusparajah, P.; Ab Mutalib, N.-S.; Ser, H.-L.; Chan, K.-G.; Lee, L.-H. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015, 8, 284–293. [Google Scholar] [CrossRef]
- Pulido-Landínez, M. Food safety—Salmonella update in broilers. Anim. Feed Sci. Technol. 2019, 250, 53–58. [Google Scholar] [CrossRef]
- Penha Filho, R.A.C.; Ferreira, J.C.; Kanashiro, A.M.I.; Darini, A.L.C.; Berchieri Junior, A. Antimicrobial susceptibility of Salmonella Gallinarum and Salmonella Pullorum isolated from ill poultry in Brazil. Cienc. Rural 2016, 46, 513–518. [Google Scholar] [CrossRef]
- Doss, J.; Culbertson, K.; Hahn, D.; Camacho, J.; Barekzi, N. A review of phage therapy against bacterial pathogens of aquatic and terrestrial organisms. Viruses 2017, 9, 50. [Google Scholar] [CrossRef]
- Żbikowska, K.; Michalczuk, M.; Dolka, B. The Use of Bacteriophages in the Poultry Industry. Animals 2020, 10, 872. [Google Scholar] [CrossRef]
- Ricke, S.C.; Lee, S.I.; Kim, S.A.; Park, S.H.; Shi, Z. Prebiotics and the poultry gastrointestinal tract microbiome. Poult. Sci. 2020, 99, 670–677. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Ahn, J.M.; Cho, J.H.; Kim, J.Y.; Kang, D.K.; Kim, S.W.; Kim, H.B.; Kim, I.H. Bacteriophage cocktail supplementation improves growth performance, gut microbiome and production traits in broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 49. [Google Scholar] [CrossRef]
- Kazi, M.; Annapure, U.S. Bacteriophage biocontrol of foodborne pathogens. J. Food Sci. Technol. 2016, 53, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Harada, L.K.; Silva, E.C.; Campos, W.F.; Del Fiol, F.S.; Vila, M.M.D.C.; Dąbrowska, K.; Krylov, V.N.; Balcão, V.M. Biotechnological applications of bacteriophages: State of the art. Microbiol. Res. 2018, 212–213, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Rios, A.C.; Moutinho, C.G.; Pinto, F.C.; Del Fiol, F.S.; Jozala, A.F.; Chaud, M.V.; Vila, M.M.D.C.; Teixeira, J.A.; Balcão, V.M. Alternatives to overcoming bacterial resistances: State-of-the-art. Microbiol. Res. 2016, 191, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Rakhuba, D.V.; Kolomiets, E.I.; Szwajcer Dey, E.; Novik, G.I. Bacteriophage receptors, mechanisms of phage adsorption and penetration into host cell. Pol. J. Microbiol. (Pol. Tow. Mikrobiol.) 2010, 59, 145–155. [Google Scholar] [CrossRef]
- García, P.; Martínez, B.; Obeso, J.M.; Rodríguez, A. Bacteriophages and their application in food safety. Lett. Appl. Microbiol. 2008, 47, 479–485. [Google Scholar] [CrossRef]
- Kimminau, E.A.; Russo, K.N.; Karnezos, T.P.; Oh, H.G.; Lee, J.J.; Tate, C.C.; Baxter, J.A.; Berghaus, R.D.; Hofacre, C.L. Bacteriophage in-feed application: A novel approach to preventing Salmonella Enteritidis colonization in chicks fed experimentally contaminated feed. J. Appl. Poult. Res. 2020, 29, 930–936. [Google Scholar] [CrossRef]
- Qadir, M.I.; Mobeen, T.; Masood, A. Phage therapy: Progress in pharmacokinetics. Braz. J. Pharm. Sci. 2018, 54, e17093. [Google Scholar] [CrossRef]
- Batalha, L.S. Encapsulation of Bacteriophages in Different Matrices and Evaluation of the Potential for Phagotherapy; Universidade Federal de Viçosa: Viçosa, MG, Brazil, 2017. [Google Scholar]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef]
- González-Menéndez, E.; Fernández, L.; Gutiérrez, D.; Pando, D.; Martínez, B.; Rodríguez, A.; García, P. Strategies to Encapsulate the Staphylococcus aureus Bacteriophage phiIPLA-RODI. Viruses 2018, 10, 495. [Google Scholar] [CrossRef]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef]
- Balcão, V.M.; Moreli, F.C.; Silva, E.C.; Belline, B.G.; Martins, L.F.; Rossi, F.P.N.; Pereira, C.; Vila, M.M.D.C.; da Silva, A.M. Isolation and Molecular Characterization of a Novel Lytic Bacteriophage That Inactivates MDR Klebsiella pneumoniae Strains. Pharmaceutics 2022, 14, 1421. [Google Scholar] [CrossRef] [PubMed]
- Balcão, V.M.; Belline, B.G.; Silva, E.C.; Almeida, P.F.F.B.; Baldo, D.Â.; Amorim, L.R.P.; Oliveira Júnior, J.M.; Vila, M.M.D.C.; Del Fiol, F.S. Isolation and Molecular Characterization of Two Novel Lytic Bacteriophages for the Biocontrol of Escherichia coli in Uter-ine Infections: In Vitro and Ex Vivo Preliminary Studies in Veterinary Medicine. Pharmaceutics 2022, 14, 2344. [Google Scholar] [CrossRef] [PubMed]
- Harada, L.K.; Silva, E.C.; Rossi, F.P.N.; Cieza, B.; Oliveira, T.J.; Pereira, C.; Tomazetto, G.; Silva, B.B.; Squina, F.M.; Vila, M.M.D.C.; et al. Characterization and in vitro testing of newly isolated lytic bacteriophages for biocontrol of Pseudomonas aeruginosa. Future Microbiol. 2022, 17, 111–141. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.C.; Oliveira, T.J.; Moreli, F.C.; Harada, L.K.; Vila, M.M.D.C.; Balcão, V.M. Newly isolated lytic bacteriophages for Staphylococcus intermedius, structurally and functionally stabilized in a hydroxyethylcellulose gel containing choline geranate: Potential for transdermal permeation in veterinary phage therapy. Res. Vet. Sci. 2021, 135, 42–58. [Google Scholar] [CrossRef]
- Rios, A.C.; Vila, M.M.D.C.; Lima, R.; Del Fiol, F.S.; Tubino, M.; Teixeira, J.A.; Balcão, V.M. Structural and functional stabilization of bacteriophage particles within the aqueous core of a W/O/W multiple emulsion: A potential biotherapeutic system for the inhalational treatment of bacterial pneumonia. Process Biochem. 2018, 64, 177–192. [Google Scholar] [CrossRef]
- Pinheiro, L.A.M.; Pereira, C.; Frazão, C.; Balcão, V.M.; Almeida, A. Efficiency of Phage φ6 for Biocontrol of Pseudomonas syringae pv. syringae: An in Vitro Preliminary Study. Microorganisms 2019, 7, 286. [Google Scholar] [CrossRef]
- Pinheiro, L.A.M.; Pereira, C.; Barreal, M.E.; Gallego, P.P.; Balcão, V.M.; Almeida, A. Use of phage ϕ6 to inactivate Pseudomonas syringae pv. actinidiae in kiwifruit plants: In vitro and ex vivo experiments. Appl. Microbiol. Biotechnol. 2019, 104, 1319–1330. [Google Scholar] [CrossRef]
- Melo, L.D.R.; Sillankorva, S.; Ackermann, H.-W.; Kropinski, A.M.; Azeredo, J.; Cerca, N. Isolation and characterization of a new Staphylococcus epidermidis broad-spectrum bacteriophage. J. Gen. Virol. 2014, 95, 506–515. [Google Scholar] [CrossRef]
- Khan Mirzaei, M.; Nilsson, A.S. Isolation of phages for phage therapy: A comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS ONE 2015, 10, e0118557. [Google Scholar] [CrossRef]
- Stuer-Lauridsen, B.; Janzen, T.; Schnabl, J.; Johansen, E. Identification of the host determinant of two prolate-headed phages infecting Lactococcus lactis. Virology 2003, 309, 10–17. [Google Scholar] [CrossRef]
- Storms, Z.J.; Sauvageau, D. Modeling tailed bacteriophage adsorption: Insight into mechanisms. Virology 2015, 485, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Takesue, S.; Ishibashi, K.; Nakahara, S. A computer simulation of the adsorption of Lactobacillus phage PL-1 to host cells: Some factors affecting the process. Agric. Biol. Chem. 1982, 46, 697–702. [Google Scholar] [CrossRef]
- Moldovan, R.; Chapman-McQuiston, E.; Wu, X.L. On kinetics of phage adsorption. Biophys. J. 2007, 93, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Balcão, V.M.; Moreira, A.R.; Moutinho, C.G.; Chaud, M.V.; Tubino, M.; Vila, M.M.D.C. Structural and functional stabilization of phage particles in carbohydrate matrices for bacterial biosensing. Enzym. Microb. Technol. 2013, 53, 55–69. [Google Scholar] [CrossRef]
- Balcão, V.M.; Barreira, S.V.P.; Nunes, T.M.; Chaud, M.V.; Tubino, M.; Vila, M.M.D.C. Carbohydrate hydrogels with stabilized phage particles for bacterial biosensing: Bacterium diffusion studies. Appl. Biochem. Biotechnol. 2014, 172, 1194–1214. [Google Scholar] [CrossRef]
- Harada, L.K.; Bonventi Júnior, W.; Silva, E.C.; Oliveira, T.J.; Moreli, F.C.; Oliveira Júnior, J.M.; Tubino, M.; Vila, M.M.D.C.; Balcão, V.M. Bacteriophage-based biosensing of Pseudomonas aeruginosa: An integrated approach for the putative real-time detection of multi-drug-resistant strains. Biosensors 2021, 11, 124. [Google Scholar] [CrossRef]
- Rocha, L.K.H.; Favaro, L.I.L.; Rios, A.C.; Silva, E.C.; Silva, W.F.; Stigliani, T.P.; Guilger, M.; Lima, R.; Oliveira, J.M.; Aranha, N.; et al. Sericin from Bombyx mori cocoons. Part I: Extraction and physicochemical-biological characterization for biopharmaceutical applications. Process Biochem. 2017, 61, 163–177. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Martins, A.C.G. Construction and test of low cost X-ray tomography scanner for physical-chemical analysis and nondestructive inspections. AIP Conf. Proc. 2009, 1139, 102–105. [Google Scholar]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical cone-beam algorithm. J. Opt. Soc. Am. A 1984, 1, 612–619. [Google Scholar] [CrossRef]
- Bates, D.M.; Watts, D.G. Nonlinear Regression Analysis and Its Applications, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 1988; ISBN 978-0471816430. [Google Scholar]
- Balcão, V.M.; Vieira, M.C.; Malcata, F.X. Adsorption of Protein from Several Commercial Lipase Preparations onto a Hollow-Fiber Membrane Module. Biotechnol. Prog. 1996, 12, 164–172. [Google Scholar] [CrossRef]
- Balcão, V.M.; Oliveira, T.A.; Xavier Malcata, F. Stability of a commercial lipase from Mucor javanicus: Kinetic modelling of pH and temperature dependencies. Biocatal. Biotransform. 1998, 16, 45–66. [Google Scholar] [CrossRef]
- Pires, D.P.; Oliveira, H.; Melo, L.D.R.; Sillankorva, S.; Azeredo, J. Bacteriophage-encoded depolymerases: Their diversity and biotechnological applications. Appl. Microbiol. Biotechnol. 2016, 100, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; São-José, C.; Azeredo, J. Phage-derived peptidoglycan degrading enzymes: Challenges and future prospects for in vivo therapy. Viruses 2018, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J. Application of UV Light Scattering to Detect Reversible Self-Association and Aggregation of Proteins in Solution. Ph.D. Thesis, University of Connecticut, Mansfield, CT, USA, 2017. [Google Scholar]
- Vekshin, N.L. Screening Hypochromism of Chromophores in Macromolecular Biostructures. Biophysics 1999, 44, 41–51. [Google Scholar]
- Manjula, B.; Varaprasad, K.; Sadiku, R.; Raju, K.M. Preparation and characterization of sodium alginate-based hydrogels and their in vitro release studies. Adv. Polym. Technol. 2013, 32, 21340. [Google Scholar] [CrossRef]
- De’Nobili, M.D.; Curto, L.M.; Delfino, J.M.; Pérez, C.D.; Bernhardt, D.; Gerschenson, L.N.; Fissore, E.N.; Rojas, A.M. Alginate utility in edible and nonedible film development and the influence of its macromolecular structure in the antioxidant activity of a pharmaceutical/food interface. In Alginic Acid: Chemical Structure, Uses and Health Benefits; Moore, A., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2014; pp. 119–169. ISBN 978-1-63463-242-3. [Google Scholar]
- Rotman, S.G.; Post, V.; Foster, A.L.; Lavigne, R.; Wagemans, J.; Trampuz, A.; Gonzalez Moreno, M.; Metsemakers, W.-J.; Grijpma, D.W.; Richards, R.G.; et al. Alginate chitosan microbeads and thermos-responsive hyaluronic acid hydrogel for phage delivery. J. Drug Deliv. Sci. Technol. 2023, 79, 103991. [Google Scholar] [CrossRef]
- Batalha, L.S.; Gontijo, M.T.P.; Teixeira, A.V.N.C.; Boggione, D.M.G.; Lopez, M.E.S.; Eller, M.R.; Mendonça, R.C.S. Encapsulation in alginate-polymers improves stability and allows controlled release of the UFV-AREG1 bacteriophage. Food Res. Int. 2021, 139, 109947. [Google Scholar] [CrossRef]
- Alves, D.; Cerqueira, M.A.; Pastrana, L.M.; Sillankorva, S. Entrapment of a phage cocktail and cinnamaldehyde on sodium alginate emulsion-based films to fight food contamination by Escherichia coli and Salmonella Enteritidis. Food Res. Int. 2020, 128, 108791. [Google Scholar] [CrossRef]
- Dicastillo, C.L.; Settier-Ramírez, L.; Gavara, R.; Hernández-Muñoz, P.; Carballo, G.L. Development of biodegradable films loaded with phages with antilisterial properties. Polymers 2021, 13, 327. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Moura, M.R.; Glenn, G.M.; Aouada, F.A. Thermal, microstructural, and spectroscopic analysis of Ca2+ alginate/clay nanocomposite hydrogel beads. J. Mol. Liq. 2018, 265, 327–336. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. β-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf. B Biointerfaces 2010, 79, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zou, L.-Q.; Niu, J.; Liu, W.; Peng, S.-F.; Liu, C.-M. The Stability, sustained release and cellular antioxidant activity of curcumin nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef]
- Darandale, S.S.; Vavia, P.R. Cyclodextrin-based nanosponges of curcumin: Formulation and physicochemical characterization. J. Incl. Phenom. Macrocycl. Chem. 2012, 75, 315–322. [Google Scholar] [CrossRef]
- Gangwar, R.K.; Dhumale, V.A.; Kumari, D.; Nakate, U.T.; Gosavi, S.W.; Sharma, R.B.; Kale, S.N.; Datar, S. Conjugation of curcumin with PVP capped gold nanoparticles for improving bioavailability. Mater. Sci. Eng. C 2012, 32, 2659–2663. [Google Scholar] [CrossRef]
- Li, J.; Lee, I.W.; Shin, G.H.; Chen, X.; Park, H.J. Curcumin-Eudragit® E PO solid dispersion: A simple and potent method to solve the problems of curcumin. Eur. J. Pharm. Biopharm. 2015, 94, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef]
- Sujima Anbu, A.; Sahi, S.V.; Venkatachalam, P. Synthesis of Bioactive Chemicals Cross-linked Sodium Tripolyphosphate (TPP)—Chitosan Nanoparticles for Enhanced Cytotoxic Activity against Human Ovarian Cancer cell Line (PA-1). J. Nanomed. Nanotechnol. 2016, 7, 1000418. [Google Scholar] [CrossRef]
- Rowbotham, J.S.; Dyer, P.W.; Greenwell, H.C.; Selby, D.; Theodorou, M.K. Copper(II)-mediated thermolysis of alginates: A model kinetic study on the influence of metal ions in the thermochemical processing of macroalgae. Interface Focus 2013, 3, 20120046. [Google Scholar] [CrossRef]
- Lozano-Vazquez, G.; Lobato-Calleros, C.; Escalona-Buendia, H.; Chavez, G.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Effect of the weight ratio of alginate-modified tapioca starch on the physicochemical properties and release kinetics of chlorogenic acid containing beads. Food Hydrocoll. 2015, 48, 301–311. [Google Scholar] [CrossRef]
- Laia, A.G.S.; Costa, E.S., Jr.; Costa, H.S. A study of sodium alginate and calcium chloride interaction through films for intervertebral disc regeneration uses. In Proceedings of the 21 CBECIMAT: Brazilian Congress of Engineering and Materials Science, Cuiabá, MT, Brazil, 9–13 November 2014. [Google Scholar]
- Lopes, S.; Bueno, L.; Aguiar Jr, F.; Finkler, C. Preparation and characterization of alginate and gelatin microcapsules containing Lactobacillus rhamnosus. An. Acad. Bras. Ciênc. 2017, 89, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.A.; An, I.B.; Lim, D.G.; Lim, J.Y.; Lee, S.Y.; Shim, W.S.; Kang, N.G.; Jeong, S.H. Effects of pH and buffer concentration on the thermal stability of etanercept using DSC and DLS. Biol. Pharm. Bull. 2014, 37, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Vinner, G.K.; Rezaie-Yazdi, Z.; Leppanen, M.; Stapley, A.G.F.; Leaper, M.C.; Malik, D.J. Microencapsulation of Salmonella-Specific Bacteriophage Felix O1 Using Spray-Drying in a pH-Responsive Formulation and Direct Compression Tableting of Powders into a Solid Oral Dosage Form. Pharmaceuticals 2019, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Petsong, K.; Benjakul, S.; Vongkamjan, K. Optimization of wall material for phage encapsulation via freeze-drying and antimicrobial efficacy of microencapsulated phage against Salmonella. J. Food Sci. Technol. 2021, 58, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.Y.K.; Kwok, P.C.L.; Khanal, D.; Morales, S.; Kutter, E.; Li, J.; Chan, H.K. Inhalable bacteriophage powders: Glass transition temperature and bioactivity stabilization. Bioeng. Transl. Med. 2020, 5, e10159. [Google Scholar] [CrossRef] [PubMed]
- Fertah, M.; Belfkira, A.; Dahmane, E.M.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Theiler, J. Estimating fractal dimension. J. Opt. Soc. Am. A 1990, 7, 1055–1073. [Google Scholar] [CrossRef]
- Hughes, K.A.; Sutherland, I.W.; Clark, J.; Jones, M.V. Bacteriophage and associated polysaccharide depolymerases—Novel tools for study of bacterial biofilms. J. Appl. Microbiol. 2002, 85, 583–590. [Google Scholar] [CrossRef]
- Hyman, P. Phages for Phage Therapy: Isolation, Characterization, and Host Range Breadth. Pharmaceuticals 2019, 12, 35. [Google Scholar] [CrossRef]
- Ross, A.; Ward, S.; Hyman, P. More Is Better: Selecting for Broad Host Range Bacteriophages. Front. Microbiol. 2016, 7, 1352. [Google Scholar] [CrossRef]
- Hendrix, R.W.; Smith, M.C.M.; Burns, R.N.; Ford, M.E.; Hatfull, G.F. Evolutionary relationships among diverse bacteriophages and prophages: All the world’s a phage. Proc. Natl. Acad. Sci. USA 1999, 96, 2192–2197. [Google Scholar] [CrossRef]
- Göller, P.C.; Elsener, T.; Lorgé, D.; Radulovic, N.; Bernardi, V.; Naumann, A.; Amri, N.; Khatchatourova, E.; Coutinho, F.H.; Loessner, M.J.; et al. Multi-species host range of staphylococcal phages isolated from wastewater. Nat. Commun. 2021, 12, 6965. [Google Scholar] [CrossRef]
- Letarov, A.V.; Letarova, M.A. The Burden of Survivors: How Can Phage Infection Impact Non-Infected Bacteria? Int. J. Mol. Sci. 2023, 24, 2733. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Moreirinha, C.; Lewicka, M.; Almeida, P.; Clemente, C.; Cunha, Â.; Delgadillo, I.; Romalde, J.L.; Nunes, M.L.; Almeida, A. Bacteriophages with potential to inactivate Salmonella Typhimurium: Use of single phage suspensions and phage cocktails. Virus Res. 2016, 220, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T.; Culler, R.R. Optimizing bacteriophage plaque fecundity. J. Theor. Biol. 2007, 249, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Mateus, L.; Costa, L.; Silva, Y.J.; Pereira, C.; Cunha, A.; Almeida, A. Efficiency of phage cocktails in the inactivation of Vibrio in aquaculture. Aquaculture 2014, 424–425, 167–173. [Google Scholar] [CrossRef]
- Abedon, S.T. Lysis from without. Bacteriophage 2011, 1, 46–49. [Google Scholar] [CrossRef]
- Letarov, A.V.; Kulikov, E.E. Adsorption of Bacteriophages on Bacterial Cells. Biochemistry 2017, 82, 1632–1658. [Google Scholar] [CrossRef]
- Ahiwale, S.S.; Bankar, A.V.; Tagunde, S.N.; Zinjarde, S.; Ackermann, H.W.; Kapadnis, B.P. Isolation and characterization of a rare waterborne lytic phage of Salmonella enterica serovar Paratyphi B. Can. J. Microbiol. 2013, 59, 318–323. [Google Scholar] [CrossRef]
- Hussein, M.N.; Jahell, W.A.; Al-Shekake, T.J.M.; Al-khozai, Z.M.F. Evaluation of Lytic Bacteriophages for Control of Multidrug-Resistant Salmonella Typhi. Ann. Rom. Soc. Cell Biol. 2021, 25, 11359–11372. [Google Scholar]
- Santos, S.B.; Carvalho, C.; Azeredo, J.; Ferreira, E.C. Population dynamics of a Salmonella lytic phage and its host: Implications of the host bacterial growth rate in modelling. PLoS ONE 2014, 9, e102507. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Yi, Z.; Zhang, Y.; Wang, Y.; Zhu, H.; Afayibo, D.J.A.; Li, T.; Tian, M.; Qi, J.; Ding, C.; et al. Characterization of a Broad-Host-Range Lytic Phage SHWT1 Against Multidrug-Resistant Salmonella and Evaluation of Its Therapeutic Efficacy in vitro and in vivo. Front. Vet. Sci. 2021, 8, 683853. [Google Scholar] [CrossRef] [PubMed]
- Capparelli, R.; Nocerino, N.; Iannaccone, M.; Ercolini, D.; Parlato, M.; Chiara, M.; Iannelli, D. Bacteriophage Therapy of Salmonella enterica: A Fresh Appraisal of Bacteriophage Therapy. J. Infect. Dis. 2010, 201, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Balcão, V.M.; Basu, A.; Cieza, B.; Rossi, F.N.; Pereira, C.; Vila, M.M.D.C.; Setubal, J.C.; Ha, T.; da Silva, A.M. Pseudomonas-tailed lytic phages: Genome mechanical analysis and putative correlation with virion morphogenesis yield. Future Microbiol. 2022, 17, 1009–1026. [Google Scholar] [CrossRef]
- Chi-Hsin, H.; ChongYi, L.; JongKang, L.; Chan-Shing, L. Control of the eel (Anguilla japonica) pathogens, Aeromonas hydrophila and Edwardsiella tarda, by bacteriophages. J. Fish. Soc. Taiwan 2000, 27, 21–31. [Google Scholar]
- Pasharawipas, T.; Manopvisetcharean, J.; Flegel, T.W. Phage treatment of Vibrio harveyi: A general concept of protection against bacterial infection. Res. J. Microbiol. 2011, 6, 560–567. [Google Scholar] [CrossRef]
- Prasad, Y.; Arpana; Kumar, D.; Sharma, A.K. Lytic bacteriophages specific to Flavobacterium columnare rescue catfish, Clarias batrachus (Linn.) from columnaris disease. J. Environ. Biol. 2011, 32, 161–168. [Google Scholar]
- Pereira, C.; Moreirinha, C.; Lewicka, M.; Almeida, P.; Clemente, C.; Romalde, J.L.; Nunes, M.L.; Almeida, A. Characterization and in vitro evaluation of new bacteriophages for the biocontrol of Escherichia coli. Virus Res. 2017, 227, 171–182. [Google Scholar] [CrossRef]
- Duarte, J.; Pereira, C.; Moreirinha, C.; Salvio, R.; Lopes, A.; Wang, D.; Almeida, A. New insights on phage efficacy to control Aeromonas salmonicida in aquaculture systems: An in vitro preliminary study. Aquaculture 2018, 495, 970–982. [Google Scholar] [CrossRef]
- Costa, P.; Pereira, C.; Gomes, A.T.P.C.; Almeida, A. Efficiency of single phage suspensions and phage cocktail in the inactivation of Escherichia coli and Salmonella Typhimurium: An in vitro preliminary study. Microorganisms 2019, 7, 94. [Google Scholar] [CrossRef]
- Forti, F.; Roach, D.R.; Cafora, M.; Pasini, M.E.; Horner, D.S.; Fiscarelli, E.V.; Rossitto, M.; Cariani, L.; Briani, F.; Debarbieux, L.; et al. Design of a Broad-Range Bacteriophage Cocktail That Reduces Pseudomonas aeruginosa Biofilms and Treats Acute Infections in Two Animal Models. Antimicrob. Agents Chemother. 2018, 62, e02573-17. [Google Scholar] [CrossRef] [PubMed]
- Campos, W.F.; Silva, E.C.; Oliveira, T.J.; Oliveira, J.M., Jr.; Tubino, M.; Pereira, C.; Vila, M.M.D.C.; Balcão, V.M. Transdermal permeation of bacteriophage particles by choline oleate: Potential for treatment of soft-tissue infections. Future Microbiol. 2020, 15, 881–896. [Google Scholar] [CrossRef] [PubMed]






| Component | EBMW Formulation | |||||
|---|---|---|---|---|---|---|
| EBMW 1: No Phages | EBMW 2: MOI 1 | EBMW 3: MOI 10 | EBMW 4: MOI 100 | EBMW 5: MOI 1000 | ||
| Phage ph001L (amount of virions) | - | 8.63 × 107 | 5.18 × 108 | 5.18 × 109 | 9.94 × 109 | |
| Phage ph001T (amount of virions) | - | 5.70 × 107 | 5.70 × 108 | 5.70 × 109 | 1.09 × 1010 | |
| Dispersion A | Phage buffer (mL) | 5 | 5 | 5 | 5 | 5 |
| Gelatin (mg) | 50 | 50 | 50 | 50 | 50 | |
| Phage cocktail (virions) | - | 1.43 × 108 | 1.09 × 109 | 1.09 × 1010 | 2.09 × 1010 | |
| Sodium alginate at 2% (w/w) (mL) | 20 | 20 | 20 | 20 | 20 | |
| Dispersion B | Calcium chloride 2.65 mol dm−3 with chitosan at 0.3% (w/w) (mL) | 10 | 10 | 10 | 10 | 10 |
| Dimension | Phage ph001L | Phage ph001T |
|---|---|---|
| Head length (nm) | 56.1 ± 1.7 | 52.6 ± 1.7 |
| Head width (nm) | 56.9 ± 2.6 | 57.1 ± 0.0 |
| Tail length (nm) | 217.9 ± 1.8 | 185.2 ± 2.7 |
| Tail thickness (nm) | 16.8 ± 1.4 | 12.8 ± 1.9 |
| Phage Suspension Volume (μL) | Dilution Volume (μL) | Virion | Number of Phage Virions Withdrawn from Suspension | Virion Particle Concentration (PFU/mL) | Absorbance at 251 (or 252) nm | Absorbance at 320 nm | Absorbance at 251 (or 252) nm—Absorbance at 320 nm |
|---|---|---|---|---|---|---|---|
| 10 | 2000 | ph001L | 1.73 × 1010 | 8.65 × 109 | 0.106 | 0.032 | 0.075 |
| ph001T | 1.14 × 1010 | 5.70 × 109 | 0.135 | 0.045 | 0.090 | ||
| 25 | 2000 | ph001L | 4.33 × 1010 | 2.16 × 1010 | 0.250 | 0.082 | 0.168 |
| ph001T | 2.85 × 1010 | 1.43 × 1010 | 0.226 | 0.065 | 0.161 | ||
| 50 | 2000 | ph001L | 8.65 × 1010 | 4.33 × 1010 | 0.451 | 0.141 | 0.310 |
| ph001T | 5.70 × 1010 | 2.85 × 1010 | 0.429 | 0.130 | 0.299 | ||
| 100 | 2000 | ph001L | 1.73 × 1011 | 8.65 × 1010 | 0.841 | 0.263 | 0.577 |
| ph001T | 1.14 × 1011 | 5.70 × 1010 | 0.905 | 0.277 | 0.628 | ||
| 150 | 2000 | ph001L | 2.60 × 1011 | 1.30 × 1011 | 1.272 | 0.394 | 0.878 |
| ph001T | 1.71 × 1011 | 8.55 × 1010 | 1.210 | 0.374 | 0.836 | ||
| 200 | 2000 | ph001L | 3.46 × 1011 | 1.73 × 1011 | 1.544 | 0.476 | 1.068 |
| ph001T | 2.28 × 1011 | 1.14 × 1011 | 1.610 | 0.495 | 1.115 |
| Bacterial Strains | Source | Phage ph001L | Phage ph001T | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Spot Test | Titre in Target Bacteria (PFU/mL) | EOP (%) | Score | Spot Test | Titre in Target Bacteria (PFU/mL) | EOP (%) | Score | ||
| Salmonella enterica CCCD-S004 | Collection, CEFAR | + | 1.42 × 1012 | 100 (host) | High | + | 1.82 × 1012 | 100 (host) | High |
| Salmonella enterica subsp. Enteritidis ATCC 13076 | Collection, ATCC | + | 6.87 × 1011 | 48.4 | Moderate | + | 6.06 × 1011 | 33.3 | Moderate |
| Pseudomonas syringae pv. Garcae IBSBF-158 | Collection, IBSBF | + | 2.10 × 1011 | 14.8 | Moderate | + | 3.26 × 1011 | 17.9 | Moderate |
| Escherichia coli ATCC 25922 | Collection, ATCC | + | 2.18 × 1011 | 15.4 | Moderate | + | 1.78 × 1011 | 9.8 | Moderate |
| Escherichia coli ATCC 8739 | Collection, ATCC | + | 1.89 × 1011 | 13.3 | Moderate | + | 2.25 × 1011 | 12.4 | Moderate |
| Klebsiella pneumoniae ATCC-13883 | Collection, ATCC | + | 1.04 × 1011 | 7.3 | Moderate | + | 4.12 × 1011 | 22.7 | Moderate |
| Klebsiella pneumoniae NCTC-13439 | Collection, NCTC | + | 9.41 × 1010 | 6.6 | Moderate | + | 3.29 × 1011 | 18.1 | Moderate |
| Aeromonas hydrophyla ATCC-7966 | Collection, ATCC | − | − | − | − | − | − | − | − |
| Enterococcus faecalis ATCC-29212 | Collection, ATCC | − | − | − | − | − | − | − | − |
| Proteus mirabilis ATCC 25933 | Collection, ATCC | − | − | − | − | − | − | − | − |
| Pseudomonas aeruginosa ATCC 27853 | Collection, ATCC | − | − | − | − | − | − | − | − |
| Pseudomonas aeruginosa ATCC 9027 | Collection, ATCC | − | − | − | − | − | − | − | − |
| Staphylococcus aureus ATCC 25923 | Collection, ATCC | − | − | − | − | − | − | − | − |
| Staphylococcus aureus ATCC 6538 | Collection, ATCC | − | − | − | − | − | − | − | − |
| Salmonella enterica subsp. Enterica serovar Typhimurium ATCC 14028 | Collection, ATCC | − | − | − | − | − | − | − | − |
| Pseudomonas aeruginosa CCCD-P004 | Collection, CEFAR | − | − | − | − | − | − | − | − |
| Bacillus cereus ATCC 14579 | Collection, CEFAR | − | − | − | − | − | − | − | − |
| Proteus mirabilis CCCD-P001 | Collection, CEFAR | − | − | − | − | − | − | − | − |
| Salmonella thyphimurium ATCC 13311 | Collection, ATCC | − | − | − | − | − | − | − | − |
| Parameter | EBMW Entrapping the Cocktail of Virion Particles at MOI 1000 | |
|---|---|---|
| Bi-Dimensional (2D) Morphological Analysis | Three-Dimensional (3D) Morphological Analysis | |
| Number of layers | - | 101.0 |
| Pixel size (µm) | - | 6.70 |
| Total VOI (volume of interest), TV (µm3) | 6.45 × 109 | 6.45 × 109 |
| Object volume, Obj.V (µm3) | 8.67 × 108 | 8.66 × 108 |
| Percent object volume, Obj.V/TV (%) | 13.44 | 13.42 |
| Total VOI surface, TS (µm2) | 2.74 × 107 | 2.74 × 107 |
| Object surface, Obj.S (µm2) | 6.13 × 106 | 5.41 × 106 |
| Total intersection surface, i.S (µm2) | 0 | 1.92 × 106 |
| Object surface/volume ratio, Obj.S/Obj.V (µm−1) | 7.07 × 10−3 | 6.25 × 10−3 |
| Mean number of objects per slice, Obj.N | 1.15 | - |
| Average object area per slice, Av.Obj.Ar (µm2) | 1.20 × 106 | - |
| Average moment of inertia (x), Av.MMI (x) (µm4) | 1.79 × 1011 | 1.51 × 1014 |
| Average moment of inertia (y), Av.MMI (y) (µm4) | 1.00 × 1011 | 9.83 × 1013 |
| Average moment of inertia (z), Av.MMI (z) (µm4) | - | 1.90 × 1014 |
| Mean eccentricity, Ecc | 0.69 | - |
| Cross-sectional thickness, Cs.Th (µm) | 573.70 | - |
| Object surface density, Obj.S/TV (µm−1) | - | 8.38 × 10−4 |
| Mean surface convexity index, SCv.I (µm−1) | 2.17 × 10−4 | 3.14 × 10−3 |
| Degree of anisotropy, DA | - | 2.75 (0.64) |
| Eigenvalue 1 | - | 2.86 × 10−2 |
| Eigenvalue 2 | - | 4.32 × 10−2 |
| Eigenvalue 3 | - | 7.88 × 10−2 |
| Number of closed pores, Po.N (cl) | - | 0 |
| Volume of closed pores, Po.V (cl) (µm3) | - | 0 |
| Surface of closed pores, Po.S (cl) (µm2) | - | 0 |
| Closed porosity (percent), Po (cl) (%) | 9.47 × 10−4 | 0 |
| Mean fractal dimension, FD | 1.02 | 2.04 |
| Volume of open pore space, Po.V (op) (µm3) | - | 5.58 × 109 |
| Open porosity (percent), Po (op) (%) | - | 86.58 |
| Total volume of pore space, Po.V (tot) (µm3) | - | 5.58 × 109 |
| Total porosity (percent), Po(tot) (%) | - | 86.58 |
| Euler number, Eu.N | - | 1 |
| Connectivity, Conn | - | 3 |
| Connectivity density, Conn.Dn (µm−3) | - | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.O.; Barros, N.M.A.; Guerrero, B.R.; Emencheta, S.C.; Baldo, D.Â.; Oliveira Jr., J.M.; Vila, M.M.D.C.; Balcão, V.M. An Edible Biopolymeric Microcapsular Wrapping Integrating Lytic Bacteriophage Particles for Salmonella enterica: Potential for Integration into Poultry Feed. Antibiotics 2023, 12, 988. https://doi.org/10.3390/antibiotics12060988
Pereira AO, Barros NMA, Guerrero BR, Emencheta SC, Baldo DÂ, Oliveira Jr. JM, Vila MMDC, Balcão VM. An Edible Biopolymeric Microcapsular Wrapping Integrating Lytic Bacteriophage Particles for Salmonella enterica: Potential for Integration into Poultry Feed. Antibiotics. 2023; 12(6):988. https://doi.org/10.3390/antibiotics12060988
Chicago/Turabian StylePereira, Arthur O., Nicole M. A. Barros, Bruna R. Guerrero, Stephen C. Emencheta, Denicezar Â. Baldo, José M. Oliveira Jr., Marta M. D. C. Vila, and Victor M. Balcão. 2023. "An Edible Biopolymeric Microcapsular Wrapping Integrating Lytic Bacteriophage Particles for Salmonella enterica: Potential for Integration into Poultry Feed" Antibiotics 12, no. 6: 988. https://doi.org/10.3390/antibiotics12060988








