Symptoms, Treatment, and Outcomes of COVID-19 Patients Coinfected with Clostridioides difficile: Single-Center Study from NE Romania during the COVID-19 Pandemic
Abstract
:1. Introduction
2. Results
2.1. Baseline Patient Characteristics and Hospitalization Data
2.2. Analysis of Clinical and Paraclinical Features of CDI in Group A Patients
3. Discussion
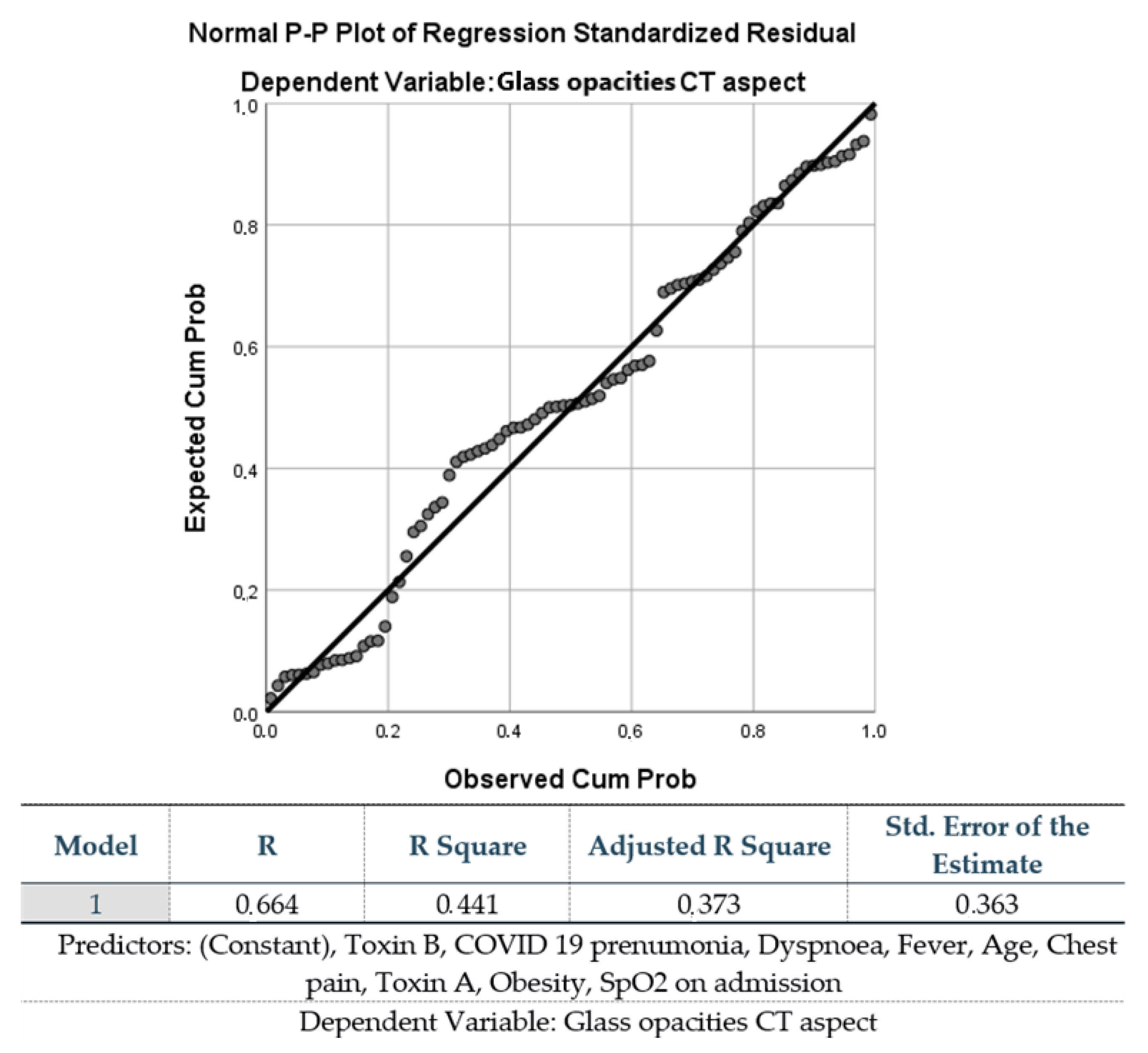
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus Disease 2019–COVID-19. Clin. Microbiol. Rev. 2020, 33, e00028-20. [Google Scholar] [CrossRef] [PubMed]
- Miftode, E.; Luca, C.; Manciuc, C.; Vâță, A.; Hunea, I.; Miftode, L.; Bădescu, A.; Dorneanu, O. COVID-19: A Course through Stormy Waters. Med. Surg. J. 2020, 124, 351–362. [Google Scholar]
- Dixon, B.E.; Wools-Kaloustian, K.; Fadel, W.F.; Duszynski, T.J.; Yiannoutsos, C.; Halverson, P.K.; Menachemi, N. Symptoms and Symptom Clusters Associated with SARS-CoV-2 Infection in Community-Based Populations: Results from a Statewide Epidemiological Study. MedRxiv Prepr. Serv. Health Sci. 2020, 16, e0241875. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cao, Q.; Qin, L.; Wang, X.; Cheng, Z.; Pan, A.; Dai, J.; Sun, Q.; Zhao, F.; Qu, J.; et al. Clinical Characteristics and Imaging Manifestations of the 2019 Novel Coronavirus Disease (COVID-19): A Multi-Center Study in Wenzhou City, Zhejiang, China. J. Infect. 2020, 80, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, P.A.; Citron, D.M.; Tyrrell, K.L.; Finegold, S.M. Reclassification of Clostridium difficile as Clostridioides Difficile (Hall and O’Toole 1935) Prévot 1938. Anaerobe 2016, 40, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Clostridium Difficile. Available online: https://www.ecdc.europa.eu/en/publications-data/directory-HAI-clostridium-difficile (accessed on 22 May 2023).
- Leffler, D.A.; Lamont, J.T. Clostridium difficile Infection. N. Engl. J. Med. 2015, 372, 1539–1548. [Google Scholar] [CrossRef] [Green Version]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef]
- Abushaheen, M.A.; Muzaheed; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; et al. Antimicrobial Resistance, Mechanisms and Its Clinical Significance. Dis. Mon. 2020, 66, 100971. [Google Scholar] [CrossRef] [PubMed]
- Lingas, E.C. Empiric Antibiotics in COVID 19: A Narrative Review. Cureus 2022, 14, e25596. [Google Scholar] [CrossRef]
- Azimirad, M.; Noori, M.; Raeisi, H.; Yadegar, A.; Shahrokh, S.; Asadzadeh Aghdaei, H.; Bentivegna, E.; Martelletti, P.; Petrosillo, N.; Zali, M.R. How Does COVID-19 Pandemic Impact on Incidence of Clostridioides Difficile Infection and Exacerbation of Its Gastrointestinal Symptoms? Front. Med. 2021, 8, 775063. [Google Scholar] [CrossRef]
- Bentivegna, E.; Alessio, G.; Spuntarelli, V.; Luciani, M.; Santino, I.; Simmaco, M.; Martelletti, P. Impact of COVID-19 Prevention Measures on Risk of Health Care-Associated Clostridium difficile Infection. Am. J. Infect. Control 2021, 49, 640–642. [Google Scholar] [CrossRef]
- Ponce-Alonso, M.; Sáez de la Fuente, J.; Rincón-Carlavilla, A.; Moreno-Nunez, P.; Martínez-García, L.; Escudero-Sánchez, R.; Pintor, R.; García-Fernández, S.; Cobo, J. Impact of the Coronavirus Disease 2019 (COVID-19) Pandemic on Nosocomial Clostridioides Difficile Infection. Infect. Control. Hosp. Epidemiol. 2021, 42, 406–410. [Google Scholar] [CrossRef]
- Ochoa-Hein, E.; Rajme-López, S.; Rodríguez-Aldama, J.C.; Huertas-Jiménez, M.A.; Chávez-Ríos, A.R.; de Paz-García, R.; Haro-Osnaya, A.; González-Colín, K.K.; González-González, R.; González-Lara, M.F.; et al. Substantial Reduction of Healthcare Facility-Onset Clostridioides Difficile Infection (HO-CDI) Rates after Conversion of a Hospital for Exclusive Treatment of COVID-19 Patients. Am. J. Infect. Control. 2021, 49, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Consumption in the EU/EEA (ESAC-Net)—Annual Epidemiological Report for 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-consumption-europe-2020 (accessed on 11 June 2023).
- Sipos, S.; Vlad, C.; Prejbeanu, R.; Haragus, H.; Vlad, D.; Cristian, H.; Dumitrascu, C.; Popescu, R.; Dumitrascu, V.; Predescu, V. Impact of COVID-19 Prevention Measures on Clostridioides Difficile Infections in a Regional Acute Care Hospital. Exp. Ther. Med. 2021, 22, 1215. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet Lond. Engl. 2020, 395, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miftode, I.-L.; Nastase, E.V.; Miftode, R.-Ș.; Miftode, E.G.; Iancu, L.S.; Luncă, C.; Anton Păduraru, D.-T.; Costache, I.-I.; Stafie, C.-S.; Dorneanu, O.-S. Insights into Multidrug-Resistant K. Pneumoniae Urinary Tract Infections: From Susceptibility to Mortality. Exp. Ther. Med. 2021, 22, 1086. [Google Scholar] [CrossRef]
- Miftode, I.-L.; Pasare, M.-A.; Miftode, R.-S.; Nastase, E.; Plesca, C.E.; Lunca, C.; Miftode, E.-G.; Timpau, A.-S.; Iancu, L.S.; Dorneanu, O.S. What Doesn’t Kill Them Makes Them Stronger: The Impact of the Resistance Patterns of Urinary Enterobacterales Isolates in Patients from a Tertiary Hospital in Eastern Europe. Antibiotics 2022, 11, 548. [Google Scholar] [CrossRef]
- Miftode, I.-L.; Leca, D.; Miftode, R.-S.; Roşu, F.; Plesca, C.; Loghin, I.; Timpau, A.S.; Mitu, I.; Mititiuc, I.; Dorneanu, O.; et al. The Clash of the Titans: COVID-19, Carbapenem-Resistant Enterobacterales, and First Mcr-1-Mediated Colistin Resistance in Humans in Romania. Antibiotics 2023, 12, 324. [Google Scholar] [CrossRef]
- Cao, W.; Li, T. COVID-19: Towards Understanding of Pathogenesis. Cell Res. 2020, 30, 367–369. [Google Scholar] [CrossRef]
- Blaser, M.J. Antibiotic Use and Its Consequences for the Normal Microbiome. Science 2016, 352, 544–545. [Google Scholar] [CrossRef] [Green Version]
- Aljeldah, M.M. Antimicrobial Resistance and Its Spread Is a Global Threat. Antibiotics 2022, 11, 1082. [Google Scholar] [CrossRef]
- Irfan, M.; Almotiri, A.; AlZeyadi, Z.A. Antimicrobial Resistance and Its Drivers—A Review. Antibiotics 2022, 11, 1362. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotic Resistance Crisis. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W. Treatment of Community-Acquired Pneumonia During the Coronavirus Disease 2019 (COVID-19) Pandemic. Ann. Intern. Med. 2020, 173, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, C.J.; Pho, M.T.; Pitrak, D.; Ridgway, J.P.; Pettit, N.N. Community-Acquired Coinfection in Coronavirus Disease 2019: A Retrospective Observational Experience. Clin. Infect. Dis. 2021, 72, 1450–1452. [Google Scholar] [CrossRef]
- Czepiel, J.; Kędzierska, J.; Biesiada, G.; Birczyńska, M.; Perucki, W.; Nowak, P.; Garlicki, A. Epidemiology of Clostridium difficile Infection: Results of a Hospital-Based Study in Krakow, Poland. Epidemiol. Infect. 2015, 143, 3235–3243. [Google Scholar] [CrossRef]
- Antibiotic Resistance Threats in the United States, 2013. Available online: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 18 May 2023).
- Marra, A.R.; Perencevich, E.N.; Nelson, R.E.; Samore, M.; Khader, K.; Chiang, H.-Y.; Chorazy, M.L.; Herwaldt, L.A.; Diekema, D.J.; Kuxhausen, M.F.; et al. Incidence and Outcomes Associated with Clostridium difficile Infections: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2020, 3, e1917597. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Huang, Y.; Li, Y.; Nie, Y. Clinical Characteristics of Clostridium difficile-Associated Diarrhea among Patients in a Tertiary Care Center in China. Pak. J. Med. Sci. 2016, 32, 736–741. [Google Scholar] [CrossRef]
- Lamberti, L.M.; Fischer Walker, C.L.; Black, R.E. Systematic Review of Diarrhea Duration and Severity in Children and Adults in Low- and Middle-Income Countries. BMC Public Health 2012, 12, 276. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Vaishnavi, C.; Kochhar, R.; Mahmood, S. Toxigenic Clostridium difficile Isolates from Clinically Significant Diarrhoea in Patients from a Tertiary Care Centre. Indian J. Med. Res. 2017, 145, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Bagdasarian, N.; Rao, K.; Malani, P.N. Diagnosis and Treatment of Clostridium difficile in Adults: A Systematic Review. JAMA 2015, 313, 398. [Google Scholar] [CrossRef] [PubMed]
- Mejía, F.; Medina, C.; Cornejo, E.; Morello, E.; Vásquez, S.; Alave, J.; Schwalb, A.; Málaga, G. Oxygen Saturation as a Predictor of Mortality in Hospitalized Adult Patients with COVID-19 in a Public Hospital in Lima, Peru. PLoS ONE 2020, 15, e0244171. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Savidge, T.; Feng, H. The Enterotoxicity of Clostridium difficile Toxins. Toxins 2010, 2, 1848–1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyras, D.; O’Connor, J.R.; Howarth, P.M.; Sambol, S.P.; Carter, G.P.; Phumoonna, T.; Poon, R.; Adams, V.; Vedantam, G.; Johnson, S.; et al. Toxin B Is Essential for Virulence of Clostridium difficile. Nature 2009, 458, 1176–1179. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Lee, K.; Rajyaguru, U.; Jones, C.H.; Janezic, S.; Rupnik, M.; Anderson, A.S.; Liberator, P. Ribotype Classification of Clostridioides Difficile Isolates Is Not Predictive of the Amino Acid Sequence Diversity of the Toxin Virulence Factors TcdA and TcdB. Front. Microbiol. 2020, 11, 1310. [Google Scholar] [CrossRef]
- Carter, G.P.; Chakravorty, A.; Pham Nguyen, T.A.; Mileto, S.; Schreiber, F.; Li, L.; Howarth, P.; Clare, S.; Cunningham, B.; Sambol, S.P.; et al. Defining the Roles of TcdA and TcdB in Localized Gastrointestinal Disease, Systemic Organ Damage, and the Host Response during Clostridium difficile Infections. mBio 2015, 6, e00551-15. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Pollock, N.R.; Banz, A.; Lantz, A.; Xu, H.; Gu, L.; Gerding, D.N.; Garey, K.W.; Gonzales-Luna, A.J.; Zhao, M.; et al. Toxin A–Predominant Pathogenic Clostridioides Difficile: A Novel Clinical Phenotype. Clin. Infect. Dis. 2020, 70, 2628–2633. [Google Scholar] [CrossRef]
- Djuikoue, I.C.; Tambo, E.; Tazemda, G.; Njajou, O.; Makoudjou, D.; Sokeng, V.; Wandji, M.; Tomi, C.; Nanfack, A.; Dayomo, A.; et al. Evaluation of Inpatients Clostridium difficile Prevalence and Risk Factors in Cameroon. Infect. Dis. Poverty 2020, 9, 122. [Google Scholar] [CrossRef]
- Zeng, F.; Huang, Y.; Guo, Y.; Yin, M.; Chen, X.; Xiao, L.; Deng, G. Association of Inflammatory Markers with the Severity of COVID-19: A Meta-Analysis. Int. J. Infect. Dis. 2020, 96, 467–474. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Timpau, A.-S.; Miftode, R.-S.; Leca, D.; Timpau, R.; Miftode, I.-L.; Petris, A.O.; Costache, I.I.; Mitu, O.; Nicolae, A.; Oancea, A.; et al. A Real Pandora’s Box in Pandemic Times: A Narrative Review on the Acute Cardiac Injury Due to COVID-19. Life 2022, 12, 1085. [Google Scholar] [CrossRef] [PubMed]
- Timpau, A.-S.; Miftode, R.-S.; Costache, I.-I.; Petris, A.O.; Miftode, I.-L.; Gheorghe, L.; Timpau, R.; Miftode, I.D.; Prepeliuc, C.S.; Coman, I.; et al. An Overview of the Impact of Bacterial Infections and the Associated Mortality Predictors in Patients with COVID-19 Admitted to a Tertiary Center from Eastern Europe. Antibiotics 2023, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Nseir, W.; Khamisy-Farah, R.; Amara, A.; Farah, R. The Prognostic Value of Inflammatory Markers in Clostridium difficile-Associated Diarrhea. Isr. Med. Assoc. J. IMAJ 2019, 21, 658–661. [Google Scholar]
- Solomon, K.; Martin, A.J.; O’Donoghue, C.; Chen, X.; Fenelon, L.; Fanning, S.; Kelly, C.P.; Kyne, L. Mortality in Patients with Clostridium difficile Infection Correlates with Host Pro-Inflammatory and Humoral Immune Responses. J. Med. Microbiol. 2013, 62, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Granata, G.; Bartoloni, A.; Codeluppi, M.; Contadini, I.; Cristini, F.; Fantoni, M.; Ferraresi, A.; Fornabaio, C.; Grasselli, S.; Lagi, F.; et al. The Burden of Clostridioides Difficile Infection during the COVID-19 Pandemic: A Retrospective Case-Control Study in Italian Hospitals (CloVid). J. Clin. Med. 2020, 9, 3855. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239. [Google Scholar] [CrossRef]
- Bertsimas, D.; Lukin, G.; Mingardi, L.; Nohadani, O.; Orfanoudaki, A.; Stellato, B.; Wiberg, H.; Gonzalez-Garcia, S.; Parra-Calderón, C.L.; Robinson, K.; et al. COVID-19 Mortality Risk Assessment: An International Multi-Center Study. PLoS ONE 2020, 15, 1–13. [Google Scholar] [CrossRef]
- Zhao, W.; Zhong, Z.; Xie, X.; Yu, Q.; Liu, J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. Am. J. Roentgenol. 2020, 214, 1072–1077. [Google Scholar] [CrossRef]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 2020, 296, E32–E40. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Zhang, H.; Xie, J.; Lin, M.; Ying, L.; Pang, P.; Ji, W. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology 2020, 296, E115–E117. [Google Scholar] [CrossRef]
- Martínez Chamorro, E.; Díez Tascón, A.; Ibáñez Sanz, L.; Ossaba Vélez, S.; Borruel Nacenta, S. Diagnóstico radiológico del paciente con COVID-19. Radiología 2021, 63, 56–73. [Google Scholar] [CrossRef]
- No Time to Wait: Securing the Future from Drug-Resistant Infections. Available online: https://www.who.int/docs/default-source/documents/no-time-to-wait-securing-the-future-from-drug-resistant-infections-en.pdf (accessed on 18 May 2023).
- Rawson, T.M.; Moore, L.S.P.; Castro-Sanchez, E.; Charani, E.; Davies, F.; Satta, G.; Ellington, M.J.; Holmes, A.H. COVID-19 and the Potential Long-Term Impact on Antimicrobial Resistance. J. Antimicrob. Chemother. 2020, 75, 1681–1684. [Google Scholar] [CrossRef]
- Spernovasilis, N.A.; Kofteridis, D.P. COVID-19 and Antimicrobial Stewardship: What Is the Interplay? Infect. Control Hosp. Epidemiol. 2021, 42, 378–379. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.J.; Loman, N.; Bogaert, D.; O’Grady, J. Co-Infections: Potentially Lethal and Unexplored in COVID-19. Lancet Microbe 2020, 1, e11. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.; Tillotson, G.; Polistico, J.; Salimnia, H.; Cranis, M.; Moshos, J.; Cullen, L.; Jabbo, L.; Diebel, L.; Chopra, T. Clostridioides Difficile in COVID-19 Patients, Detroit, Michigan, USA, March–April 2020. Emerg. Infect. Dis. 2020, 26, 2272–2274. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Grinspan, L.T.; Fu, Y.; Adams-Sommer, V.; Willey, D.K.; Patel, G.; Grinspan, A.M. Hospital-Onset Clostridioides Difficile Infections during the COVID-19 Pandemic. Infect. Control Hosp. Epidemiol. 2021, 42, 1165–1166. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antibiotic Resistance: Multi-Country Public Awareness Survey; World Health Organization: Geneva, Switzerland, 2015; ISBN 978-92-4-150981-7. [Google Scholar]
- Portero de la Cruz, S.; Cebrino, J. Prevalence and Determinants of Antibiotic Consumption in the Elderly during 2006–2017. Int. J. Environ. Res. Public. Health 2020, 17, 3243. [Google Scholar] [CrossRef]
- Johnson, S.; Louie, T.J.; Gerding, D.N.; Cornely, O.A.; Chasan-Taber, S.; Fitts, D.; Gelone, S.P.; Broom, C.; Davidson, D.M.; for the Polymer Alternative for CDI Treatment (PACT) Investigators. Vancomycin, Metronidazole, or Tolevamer for Clostridium difficile Infection: Results from Two Multinational, Randomized, Controlled Trials. Clin. Infect. Dis. 2014, 59, 345–354. [Google Scholar] [CrossRef] [Green Version]
- Di, X.; Bai, N.; Zhang, X.; Liu, B.; Ni, W.; Wang, J.; Wang, K.; Liang, B.; Liu, Y.; Wang, R. A Meta-Analysis of Metronidazole and Vancomycin for the Treatment of Clostridium difficile Infection, Stratified by Disease Severity. Braz. J. Infect. Dis. 2015, 19, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Liang, W.; Jiang, M.; Guan, W.; Zhan, C.; Wang, T.; Tang, C.; Sang, L.; Liu, J.; Ni, Z.; et al. Risk Factors of Fatal Outcome in Hospitalized Subjects with Coronavirus Disease 2019 From a Nationwide Analysis in China. Chest 2020, 158, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Covassin, N.; Fan, Z.; Singh, P.; Gao, W.; Li, G.; Kara, T.; Somers, V.K. Association Between Hypoxemia and Mortality in Patients With COVID-19. Mayo Clin. Proc. 2020, 95, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jin, J.; Luo, W.; Gan, Y.; Chen, B.; Li, W. Risk Factors for Predicting Mortality of COVID-19 Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0243124. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Chinn, J.; De Ferrante, M.; Kirby, K.A.; Hohmann, S.F.; Amin, A. Male Gender Is a Predictor of Higher Mortality in Hospitalized Adults with COVID-19. PLoS ONE 2021, 16, e0254066. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Soto, M.C.; Ortega-Cáceres, G.; Arroyo-Hernández, H. Sex Differences in COVID-19 Fatality Rate and Risk of Death: An Analysis in 73 Countries, 2020–2021. Infez. Med. 2021, 29, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Ahrenfeldt, L.J.; Otavova, M.; Christensen, K.; Lindahl-Jacobsen, R. Sex and Age Differences in COVID-19 Mortality in Europe. Wien. Klin. Wochenschr. 2021, 133, 393–398. [Google Scholar] [CrossRef]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male Sex Identified by Global COVID-19 Meta-Analysis as a Risk Factor for Death and ITU Admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef]
- Sieurin, J.; Brandén, G.; Magnusson, C.; Hergens, M.-P.; Kosidou, K. A Population-Based Cohort Study of Sex and Risk of Severe Outcomes in COVID-19. Eur. J. Epidemiol. 2022, 37, 1159–1169. [Google Scholar] [CrossRef]
- Ling, J.; Geetha, S.D.; Amundsen, T.; Zamarripa, A.; Badillo, R.; Thosani, N.; Kannadath, B.S.; Guha, S.; Fallon, M.B. S0159 Role of Gender in the Incidence and Mortality of Clostridium difficile Infections in the United States: Results from the National Inpatient Sample Database 2012–2017. Am. J. Gastroenterol. 2020, 115, S68. [Google Scholar] [CrossRef]
- Redelings, M.D.; Sorvillo, F.; Mascola, L. Increase in Clostridium difficile—Related Mortality Rates, United States, 1999–2004. Emerg. Infect. Dis. 2007, 13, 1417–1419. [Google Scholar] [CrossRef] [Green Version]
- Maslennikov, R.; Ivashkin, V.; Ufimtseva, A.; Poluektova, E.; Ulyanin, A. Clostridioides Difficile Co-Infection in Patients with COVID-19. Future Microbiol. 2022, 17, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Lett, J.; Lattimer, C.; Tillotson, G. Transitions of Care in Clostridioides Difficile Infection: A Need of the Hour. Ther. Adv. Gastroenterol. 2022, 15, 175628482210786. [Google Scholar] [CrossRef] [PubMed]
| Symptomatology | COVID-19 Severe Form of Disease (N = 36) | COVID-19 Mild/Moderate Form of Disease (N = 50) | T-Value | p-Value |
|---|---|---|---|---|
| Fever | 19 (52.7%) | 26 (52%) | 0.70 | 0.944 |
| Shivers | 13 (36.1%) | 15 (30%) | 0.591 | 0.550 |
| Productive cough | 8 (22.2%) | 11 (22%) | 0.024 | 0.981 |
| Dry cough | 20 (55.5%) | 17 (34%) | 2.016 | 0.047 |
| Asthenia | 22 (61.1%) | 27 (54%) | 0.651 | 0.517 |
| Myalgia/arthralgia | 10 (27.7%) | 9 (18%) | 1.046 | 0.299 |
| Chest pain | 8 (22.2%) | 11 (22%) | 0.024 | 0.981 |
| Odynophagia | 2 (5.5%) | 6 (12%) | 83.98 | 0.289 |
| Headache | 9 (25%) | 14 (28%) | 84 | 0.760 |
| Anosmia | 3 (8.3%) | 6 (12%) | 84 | 0.589 |
| Ageusia | 2 (5.5%) | 6 (12%) | 83.98 | 0.289 |
| Mean of Severe Form of COVID-19 (N = 36) | Mean of Non Severe Form of COVID-19 (N = 50) | t-Value | p-Value | |
|---|---|---|---|---|
| WBC (cells/µL) | 10796.67 | 8253.20 | 1.914 | 0.06 |
| Neutrophils (%) | 77.55 | 67.52 | 2.722 | 0.008 |
| Lymphocytes (%) | 13.95 | 23.06 | −3.074 | 0.03 |
| Thrombocytes (103 cells/µL) | 288.79 | 256.10 | 1.085 | 0.2 |
| LDH (UI/L) | 369.89 | 274.33 | 2.483 | 0.01 |
| D-dimer (FEU µg/mL) | 143.45 | 151.56 | −0.065 | 0.9 |
| Interleukine-6 (pg/mL) | 25.69 | 43.22 | −0.76 | 0.5 |
| Ferritin (ng/mL) | 939.76 | 804.42 | 0.37 | 0.7 |
| CRP on admission (mg/dL) | 98.87 | 71.11 | 1.979 | 0.05 |
| CRP at discharge (mg/dL) | 46.58 | 55.14 | −0.477 | 0.6 |
| ESR (mm/h) | 80.00 | 60.73 | 2.437 | 0.01 |
| Fibrinogen (mg/dL) | 9.01 | 7.36 | 0.319 | 0.7 |
| INR | 1.16 | 1.24 | −0.58 | 0.5 |
| Glucose (mg/dL) | 140.92 | 118.09 | 1.992 | 0.05 |
| Creatinine (mg/dL) | 1.21 | 1.39 | −0.6 | 0.5 |
| ALT (U/L) | 52.72 | 53.16 | −0.042 | 0.9 |
| COVID-19 Treatment | Mild/Moderate COVID-19 N = 50 | Severe COVID-19 N = 36 | p-Value | OR | CI 95% |
|---|---|---|---|---|---|
| Cephalosporins (3rd generation) | 37 (74%) | 20 (55.6%) | 0.009 | 5.5914 | 1.4926–20.9459 |
| Aminopenicillins | 3 (6%) | 3 (8.3%) | - | - | - |
| Macrolides | 6 (12%) | 2 (5.6%) | 0.277 | 3.15 | 0.6176–16.0669 |
| Carbapenems | 14 (28%) | 21 (58.3%) | 0.04 | 2.45 | 1.0848–5.5335 |
| Linezolid | 3 (6%) | 4 (11.1%) | - | - | - |
| Favipiravir | 2 (4%) | 0 | - | - | - |
| Lopinavirum + ritonavirum | 27 (54%) | 12 (33.3%) | 0.079 | 0.4259 | 0.1752–1.0357 |
| Antibiotic Treatment | GROUP A N = 86 | GROUP B N = 86 | Total N = 172 | p-Value | OR | CI 95% |
|---|---|---|---|---|---|---|
| Cephalosporins (3rd gen.) | 57 (66.2%) | 64 (74.4%) | 121 (70.3%) | 0.05 | 0.5887 | 0.3287–0.9621 |
| Aminopenicillins | 6 (6.9%) | 10 (11.6%) | 16 (9.3%) | 0.299 | 0.5182 | 0.1816–1.4789 |
| Macrolides | 8 (9.3%) | 7 (8.1%) | 15 (8.7%) | 0.790 | 1.2842 | 0.4491–3.6723 |
| Carbapenems | 35 (40.6%) | 15 (17.4%) | 50 (35.3%) | 0.005 | 2.5926 | 1.3197–5.0933 |
| Linezolid | 7 (8.1%) | 2 (2.3%) | 9 (5.2%) | 0.088 | 0.2456 | 0.0499–1.2099 |
| Fluoroquinolones | 1 (1.1%) | 6 (6.9%) | 7 (4%) | 0.055 | 0.1435 | 0.017–1.2122 |
| Association of antibiotics | 26 (30%) | 19 (22%) | 45 (52.3%) | 0.504 | 1.3076 | 0.675–2.5332 |
| Parameters | GROUP A (N = 86) | GROUP B (N = 86) | t-Value | p-Value |
|---|---|---|---|---|
| Length of hospital stay (days) (mean) | 17.31 | 11.56 | −2.9467 | 0.0036 |
| Transfer to ICU | 3 (3.4%) | 10 (11.6%) | −1.581 | 0.117 |
| Fatalities | 3 (3.4%) | 6 (6.9%) | −3.484 | 0.496 |
| Oxygen therapy | 54 (62.7%) | 28 (32.5%) | 4.246 | 0.001 |
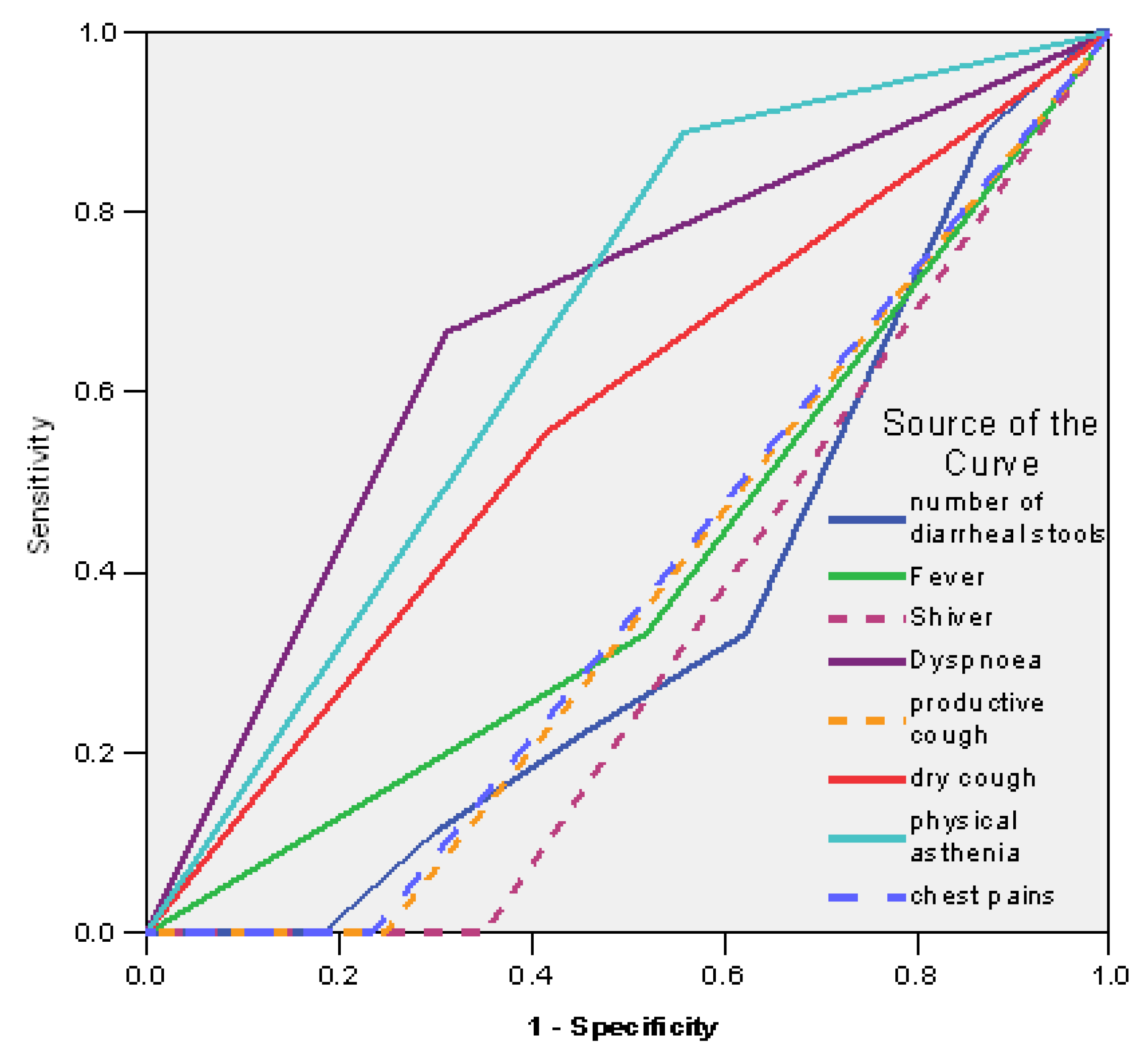
| Test Result Variables | AUC | Std. Error (a) | Asymptotic Sig. (b) | Asymptotic 95% Confidence Interval |
|---|---|---|---|---|
| No diarrhea | 0.354 | 0.081 | 0.152 | 0.194–0.513 |
| Fever | 0.407 | 0.098 | 0.363 | 0.214–0.599 |
| Shivers | 0.325 | 0.074 | 0.087 | 0.180–0.469 |
| Dyspnea | 0.677 | 0.096 | 0.083 | 0.489–0.866 |
| Productive cough | 0.377 | 0.082 | 0.228 | 0.215–0.538 |
| Dry cough | 0.570 | 0.102 | 0.494 | 0.371–0.769 |
| Asthenia | 0.665 | 0.083 | 0.106 | 0.502–0.828 |
| Chest pains | 0.383 | 0.083 | 0.253 | 0.220–0.547 |
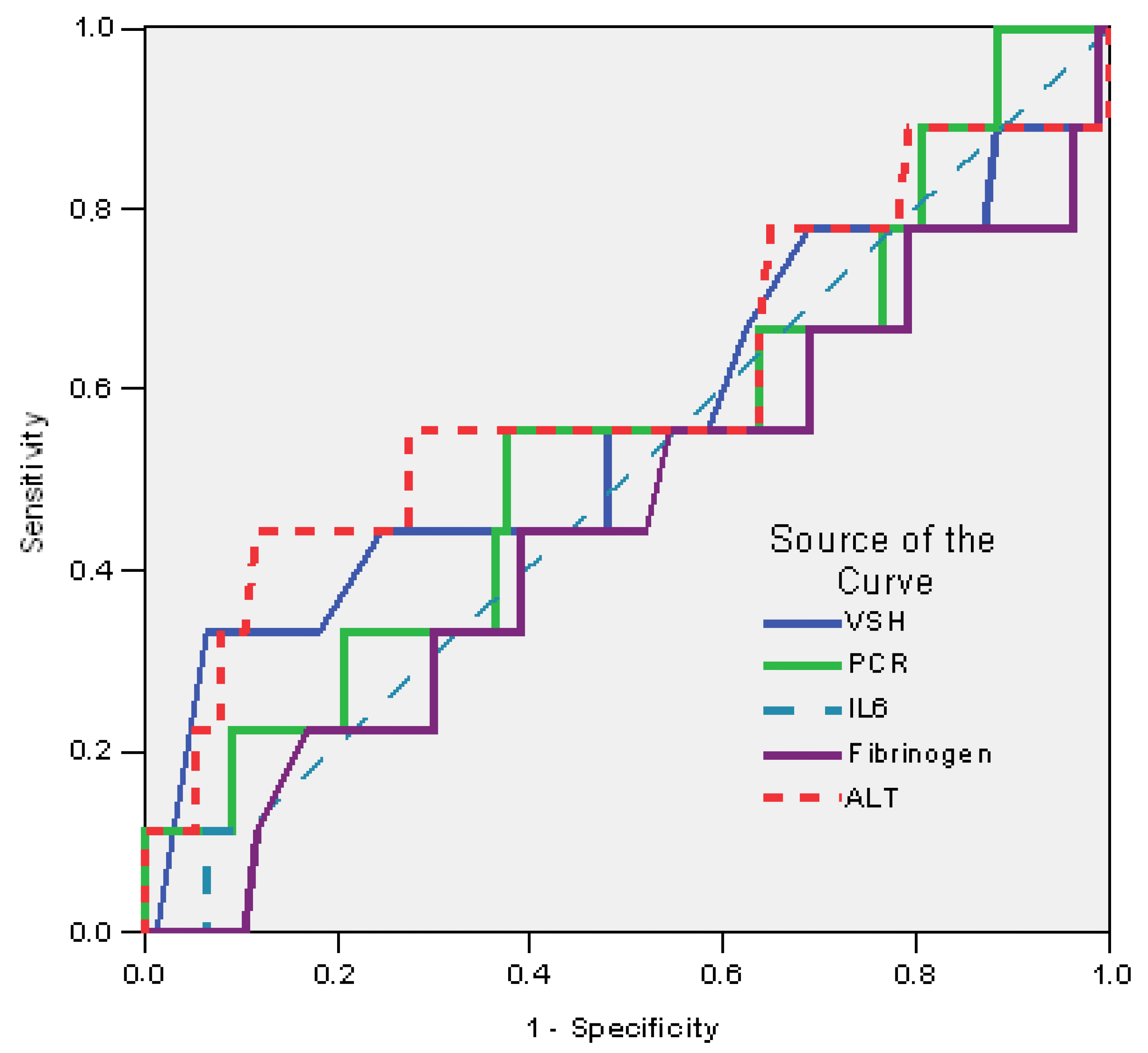
| Test Result Variable(s) | AUC | Std. Error (a) | Asymptotic Sig. (b) | Asymptotic 95% Confidence Interval |
|---|---|---|---|---|
| ESR | 0.563 | 0.120 | 0.539 | 0.328–0.797 |
| CRP | 0.541 | 0.108 | 0.688 | 0.330–0.752 |
| IL-6 | 0.502 | 0.102 | 0.983 | 0.302–0.702 |
| Fibrinogen | 0.455 | 0.109 | 0.662 | 0.241–0.670 |
| ALT | 0.602 | 0.120 | 0.317 | 0.367–0.837 |
| Variable | Favourable Outcome Patients N = 83 | Deceased Patients N = 3 | Fisher’s Test p-Value |
|---|---|---|---|
| Male gender | 42 (50.6%) | 3 (100%) | 0.017 |
| Urban area of residence | 54 (65%) | 1 (33.3%) | 0.093 |
| Mean age | 60.8 | 77.33 | 0.001 |
| Associated pathologies: | |||
| DM | 20 (24%) | 0 (0%) | 0.093 |
| CV | 48 (57.8%) | 0 (0%) | 0.078 |
| Neurological | 14 (16.8%) | 1 (33.3%) | 0.125 |
| Respiratory | 3 (3.6%) | 0 (0%) | 0.109 |
| Oncological | 9 (10.8%) | 2 (66.6%) | 0.303 |
| Previous antibiotic treatment | 25 (30.1%) | 1 (33.3%) | 0.269 |
| Hospitalization length (mean) | 17.36 | 16 | 0.275 |
| ICU admission | 3 (3.6%) | 0 (0%) | 0.227 |
| Severe COVID-19 form | 35 (42.1%) | 2 (66.6%) | 0.230 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stămăteanu, L.O.; Miftode, I.L.; Pleșca, C.E.; Dorneanu, O.S.; Roșu, M.F.; Miftode, I.D.; Obreja, M.; Miftode, E.G. Symptoms, Treatment, and Outcomes of COVID-19 Patients Coinfected with Clostridioides difficile: Single-Center Study from NE Romania during the COVID-19 Pandemic. Antibiotics 2023, 12, 1091. https://doi.org/10.3390/antibiotics12071091
Stămăteanu LO, Miftode IL, Pleșca CE, Dorneanu OS, Roșu MF, Miftode ID, Obreja M, Miftode EG. Symptoms, Treatment, and Outcomes of COVID-19 Patients Coinfected with Clostridioides difficile: Single-Center Study from NE Romania during the COVID-19 Pandemic. Antibiotics. 2023; 12(7):1091. https://doi.org/10.3390/antibiotics12071091
Chicago/Turabian StyleStămăteanu, Lidia Oana, Ionela Larisa Miftode, Claudia Elena Pleșca, Olivia Simona Dorneanu, Manuel Florin Roșu, Ioana Diandra Miftode, Maria Obreja, and Egidia Gabriela Miftode. 2023. "Symptoms, Treatment, and Outcomes of COVID-19 Patients Coinfected with Clostridioides difficile: Single-Center Study from NE Romania during the COVID-19 Pandemic" Antibiotics 12, no. 7: 1091. https://doi.org/10.3390/antibiotics12071091






