Management of Polypharmacy and Potential Drug–Drug Interactions in Patients with Mycobacterial Infection: A 1-Year Experience of a Multidisciplinary Outpatient Clinic
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics and TB Treatment
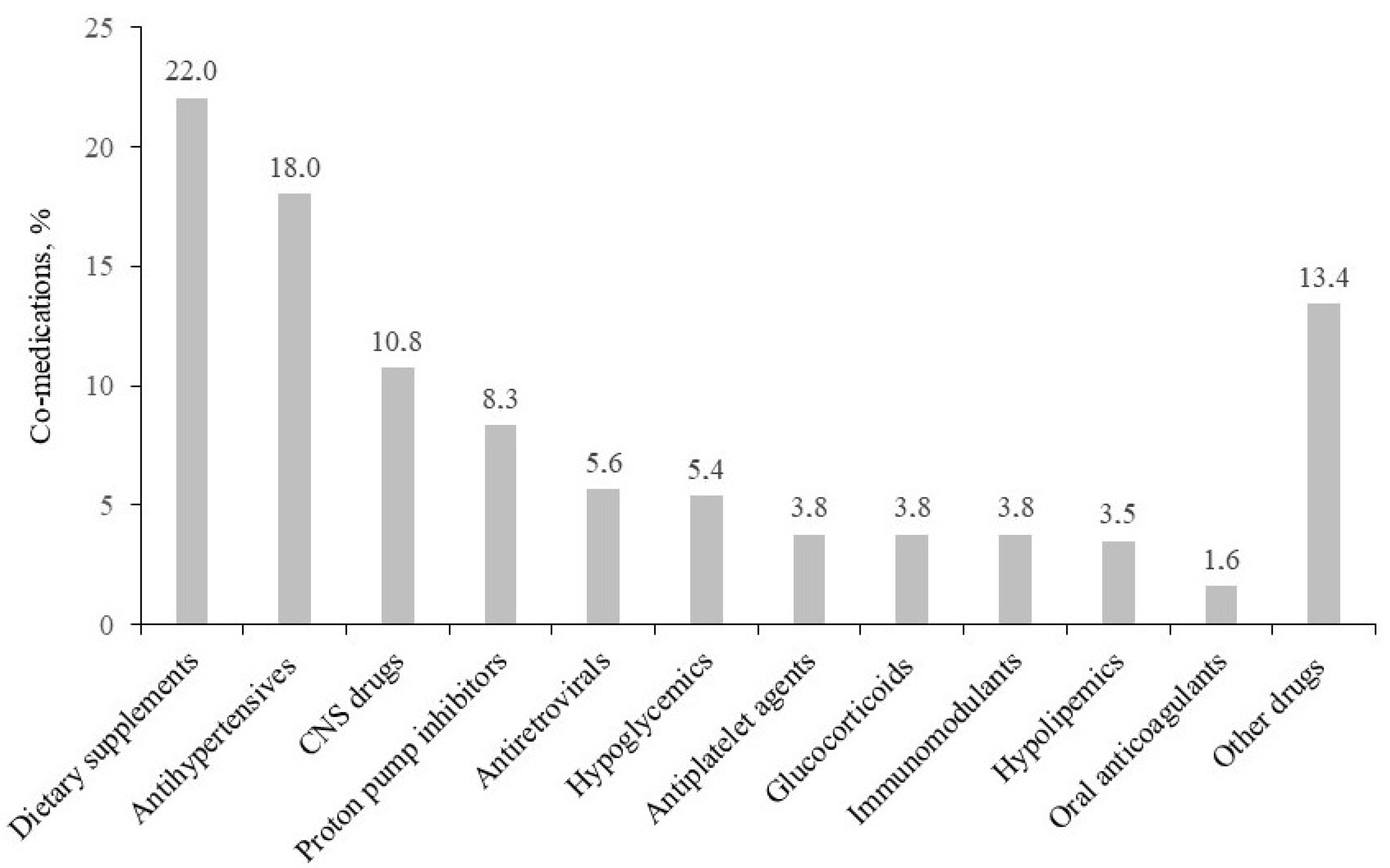
2.2. Co-Medications, ACB Scale, and pDDIs
2.3. Proposed Actions Identified during the GAP-MyTB Visits
2.3.1. TDM of Rifampicin
2.3.2. NAT2 Phenotyping
3. Discussion
4. Materials and Methods
4.1. Patient Selection and Study Design
4.2. Assessment of Rifampicin Plasma Concentrations and TDM
4.3. Pharmacogenetic Tests
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imperial, M.Z.; Nedelman, J.R.; Conradie, F.; Savic, R.M. Proposed Linezolid Dosing Strategies to Minimize Adverse Events for Treatment of Extensively Drug-Resistant Tuberculosis. Clin. Infect. Dis. 2022, 74, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Conradie, F.; Diacon, A.H.; Ngubane, N.; Howell, P.; Everitt, D.; Crook, A.M.; Mendel, C.M.; Egizi, E.; Moreira, J.; Timm, J.; et al. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N. Engl. J. Med. 2020, 382, 893–902. [Google Scholar] [CrossRef]
- Tweed, C.D.; Dawson, R.; A Burger, D.; Conradie, A.; Crook, A.M.; Mendel, C.M.; Conradie, F.; Diacon, A.H.; E Ntinginya, N.; E Everitt, D.; et al. Bedaquiline, moxifloxacin, pretomanid, and pyrazinamide during the first 8 weeks of treatment of patients with drug-susceptible or drug-resistant pulmonary tuberculosis: A multicentre, open-label, partially randomised, phase 2b trial. Lancet Respir. Med. 2019, 7, 1048–1058. [Google Scholar] [CrossRef] [Green Version]
- Ong, C.W.M.; Migliori, G.B.; Raviglione, M.; MacGregor-Skinner, G.; Sotgiu, G.; Alffenaar, J.W.; Tiberi, S.; Adlhoch, C.; Alonzi, T.; Archuleta, S.; et al. Epidemic and pandemic viral infections: Impact on tuberculosis and the lung: A consensus by the World Association for Infectious Diseases and Immunological Disorders (WAidid), Global Tuberculosis Network (GTN), and members of the European Society of Clinical Microbiology and Infectious Diseases Study Group for Mycobacterial Infections (ESGMYC). Eur. Respir. J. 2020, 56, 2001727. [Google Scholar] [PubMed]
- Gopalaswamy, R.; Shanmugam, S.; Mondal, R.; Subbian, S. Of tuberculosis and non- tuberculous mycobacterial infections—A comparative analysis of epidemiology, diagnosis and treatment. J. Biomed. Sci. 2020, 27, 74. [Google Scholar] [CrossRef]
- Sahasrabudhe, V.; Zhu, T.; Vaz, A.; Tse, S. Drug metabolism and drug interactions: Potential application to antituberculosis drugs. J. Infect. Dis. 2015, 211 (Suppl. S3), S107–S114. [Google Scholar] [CrossRef] [Green Version]
- Riccardi, N.; Canetti, D.; Rodari, P.; Besozzi, G.; Saderi, L.; Dettori, M.; Codecasa, L.R.; Sotgiu, G. Tuberculosis and pharmacological interactions: A narrative review. Curr. Res. Pharmacol. Drug Discov. 2020, 2, 100007. [Google Scholar] [CrossRef]
- Hu, M.; Zheng, C.; Gao, F. Use of bedaquiline and delamanid in diabetes patients: Clinical and pharmacological considerations. Drug Des. Devel. Ther. 2016, 10, 3983–3994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dooley, K.E.; Rosenkranz, S.L.; Conradie, F.; Moran, L.; Hafner, R.; von Groote-Bidlingmaier, F.; Lama, J.R.; Shenje, J.; De Los Rios, J.; Comins, K.; et al. QT effects of bedaquiline, delamanid, or both in patients with rifampicin-resistant tuberculosis: A phase 2, open-label, randomised, controlled trial. Lancet Infect. Dis. 2021, 21, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, S. Metabolism-based Drug-drug Interactions in Patients with Chronic Respiratory Diseases: A Review Focusing on Drugs Affecting the Respiratory System. Curr. Drug Metab. 2020, 21, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Noor, S.; Ismail, M.; Ali, Z. Potential drug-drug interactions among pneumonia patients: Do these matter in clinical perspectives? BMC Pharmacol. Toxicol. 2019, 20, 45. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.; Kurian, S.J.; Mukherjee, N.; Thomas, R.B.; Keerthanaa, B.; Chaithra; Sekhar, S.M.; Subeesh, V.; Banerjee, M.; Varma, M.; et al. Potential drug-drug interactions among hospitalised TB patients. Int. J. Tuberc. Lung Dis. 2022, 26, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Resende, N.H.D.; Miranda, S.S.D.; Ceccato, M.D.G.B.; Reis, A.M.M.; Haddad, J.P.A.; Silva, D.I.D.; Carvalho, W.D.S. Assessment of factors associated with potential drug-drug interactions in patients with tuberculosis and HIV/AIDS. Rev. Soc. Bras. Med. Trop. 2021, 54, e01032021. [Google Scholar] [CrossRef]
- Noor, S.; Ismail, M.; Khan, F. Drug safety in hospitalized patients with tuberculosis: Drug interactions and adverse drug effects. Clin. Respir. J. 2021, 15, 97–108. [Google Scholar] [CrossRef]
- Salahudeen, M.S.; Duffull, S.B.; Nishtala, P.S. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: A systematic review. BMC Geriatr. 2015, 15, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gervasoni, C.; Formenti, T.; Cattaneo, D. Management of Polypharmacy and Drug-Drug Interactions in HIV Patients: A 2-year Experience of a Multidisciplinary Outpatient Clinic. AIDS Rev. 2019, 21, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, D.; Oreni, L.; Meraviglia, P.; Minisci, D.; Astuti, N.; Antinori, S.; Gori, A.; Gervasoni, C. Polypharmacy and aging in people living with HIV: 6 years of experience in a multidisciplinary outpatient clinic. Drugs Aging 2023, 40, 665–674. [Google Scholar] [CrossRef]
- Metzger, N.L.; Momary, K.M. A patient with HIV and tuberculosis with diminished clopidogrel response. Int. J. STD AIDS 2014, 25, 532–534. [Google Scholar] [CrossRef]
- Li, T.-Y.; Liu, W.; Chen, K.; Liang, S.-Y.; Liu, F. The influence of combination use of CYP450 inducers on the pharmacokinetics of voriconazole: A systematic review. J. Clin. Pharm. Ther. 2017, 42, 135–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwiesow, J.N.; Iseman, M.D.; Peloquin, C.A. Concomitant use of voriconazole and rifabutin in a patient with multiple infections. Pharmacotherapy 2008, 28, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Schnoll-Sussman, F.; Niec, R.; Katz, P.O. Proton pump inhibitors: The good, bad, and ugly. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, D.; Pasina, L.; Maggioni, A.P.; Oreni, L.; Conti, F.; Pezzati, L.; Casalini, G.; Bonazzetti, C.; Morena, V.; Ridolfo, A.; et al. Drug-Drug Interactions and Prescription Appropriateness at Hospital Discharge: Experience with COVID-19 Patients. Drugs Aging 2021, 38, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Perisetti, A.; Gajendran, M.; Jean-Louis, F.; Bansal, P.; Dwivedi, A.K.; Goyal, H. Pre-hospitalization proton pump inhibitor use and clinical outcomes in COVID-19. Eur. J. Gastroenterol. Hepatol. 2022, 34, 137–141. [Google Scholar] [CrossRef]
- Lee, S.W.; Ha, E.K.; Yeniova, A.; Moon, S.Y.; Kim, S.Y.; Koh, H.Y.; Yang, J.M.; Jeong, S.J.; Moon, S.J.; Cho, J.Y.; et al. Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: A nationwide cohort study with propensity score matching. Gut 2021, 70, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Haastrup, P.F.; Thompson, W.; Søndergaard, J.; Jarbøl, D. Side Effects of Long-Term Proton Pump Inhibitor Use: A Review. Basic Clin. Pharmacol. Toxicol. 2018, 123, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Shawaqfeh, B.; Hughes, C.M.; McGuinness, B.; Barry, H.E. A systematic review of interventions to reduce anticholinergic burden in older people with dementia in primary care. Int. J. Geriatr. Psychiatry 2022, 37. [Google Scholar] [CrossRef]
- Stewart, C.; Gallacher, K.; Nakham, A.; Cruickshank, M.; Newlands, R.; Bond, C.; Myint, P.K.; Bhattacharya, D.; Mair, F.S. Barriers and facilitators to reducing anticholinergic burden: A qualitative systematic review. Int. J. Clin. Pharm. 2021, 43, 1451–1460. [Google Scholar] [CrossRef]
- Cresswell, F.V.; Meya, D.B.; Kagimu, E.; Grint, D.; Brake, L.T.; Kasibante, J.; Martyn, E.; Rutakingirwa, M.; Quinn, C.M.; Okirwoth, M.; et al. High-Dose Oral and Intravenous Rifampicin for the Treatment of Tuberculous Meningitis in Predominantly Human Immunodeficiency Virus (HIV)-Positive Ugandan Adults: A Phase II Open-Label Randomized Controlled Trial. Clin. Infect. Dis. 2021, 73, 876–884. [Google Scholar] [CrossRef]
- Ramachandran, G.; Chandrasekaran, P.; Gaikwad, S.; Agibothu Kupparam, H.K.; Thiruvengadam, K.; Gupte, N.; Paradkar, M.; Dhanasekaran, K.; Sivaramakrishnan, G.N.; Kagal, A.; et al. Subtherapeutic Rifampicin Concentration Is Associated with Unfavorable Tuberculosis Treatment Outcomes. Clin. Infect. Dis. 2020, 70, 1463–1470. [Google Scholar] [CrossRef]
- Thomas, L.; Raju, A.P.; Chaithra Varma, M.; Saravu, K.; Banerjee, M.; Sv, C.S.; Mallayasamy, S.; Rao, M. Influence of N-acetyltransferase 2 (NAT2) genotype/single nucleotide polymorphisms on clearance of isoniazid in tuberculosis patients: A systematic review of population pharmacokinetic models. Eur. J. Clin. Pharmacol. 2022, 78, 1535–1553. [Google Scholar] [CrossRef]
- Gausi, K.; Ignatius, E.H.; Sun, X.; Kim, S.; Moran, L.; Wiesner, L.; von Groote-Bidlingmaier, F.; Hafner, R.; Donahue, K.; Vanker, N.; et al. Semimechanistic Model of the Bactericidal Activity of High-Dose Isoniazid against Multidrug-Resistant Tuberculosis: Results from a Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 1327–1335. [Google Scholar] [CrossRef]
- Cattaneo, D.; Formenti, T.; Astuti, N.; Meraviglia, P.; Ridolfo, A.; Gervasoni, C. How relevant are the drug-drug interactions between antiretroviral boosted-based regimens and calcium channel blockers in real life? J. Antimicrob. Chemother. 2018, 73, 2271–2273. [Google Scholar] [CrossRef] [Green Version]
- Gervasoni, C.; Resnati, C.; Formenti, T.; Fossati, A.; Minisci, D.; Meraviglia, P.; Cattaneo, D. The relevance of drug-drug interactions in clinical practice: The case of concomitant boosted protease inhibitors plus alpha-1 blocker administration. Antivir. Ther. 2018, 23, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Waterhouse, D.; Ardrey, A.; Ward, S.A. Quantification of rifampicin in human plasma and cerebrospinal fluid by a highly sensitive and rapid liquid chromatographic-tandem mass spectrometric method. J. Pharm. Biomed. Anal. 2012, 70, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Lemaitre, F. Has the Time Come for Systematic Therapeutic Drug Monitoring of First-Line and WHO Group A Antituberculosis Drugs? Ther. Drug Monit. 2022, 44, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Magis-Escurra, C.; Later-Nijland, H.; Alffenaar, J.; Broeders, J.; Burger, D.; van Crevel, R.; Boeree, M.; Donders, A.; van Altena, R.; van der Werf, T.; et al. Population pharmacokinetics and limited sampling strategy for first-line tuberculosis drugs and moxifloxacin. Int. J. Antimicrob. Agents 2014, 44, 229–234. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Clinical Features |
|---|---|
| Patients, n | 52 |
| Females, n (%) | 22 (42.3%) |
| Mean age, years | 61 ± 16 |
| HIV co-infection, n (%) | 8 (15.4%) |
| Ethnicity (%) | Caucasian (69%), Asian (10%), Hispanic (8%), Black (8%), Arab (6%) |
| TB disease, n (%) | 21 (40.4%) |
| Localization (n) | Pulmonary (n = 11), ocular (n = 4), abdominal (n = 2), cerebral (n = 1), renal (n = 1), pulmonary/abdominal (n = 1), pulmonary/cerebral (n = 1) |
| TB infection, n (%) | 13 (25.0%) |
| NTM, n (%) | 18 (34.6%) |
| Anti-tubercular treatments (n) | Rifampicin (n = 30), ethambutol (n = 29), isoniazid (n = 29), rifabutin (n = 17), azithromycin (n = 16), pyrazinamide (n = 12), linezolid (n = 2), amikacin (n = 2), levofloxacin (n = 2),clarithromycin (n = 1), bedaquiline (n = 1), moxifloxacin (n = 1), clofazimine (n = 1) |
| Type of pDDI | Overall | TB Drugs | Co-Medications |
|---|---|---|---|
| Drugs, n | 10.4 ± 3.7 | 2.8 ± 1.0 | 7.8 ± 3.9 |
| pDDIs | 262 | 178 | 84 |
| Red-flag pDDIs | 5 | 3 Azithromycin/lithium/omeprazole Isoniazid/clopidogrel/rabeprazole Voriconazole//rifabutine | 2 Methotrexate/omeprazole Clopidogrel/rabeprazole |
| Orange-flag pDDIs | 189 | 130 | 59 |
| Yellow-flag pDDIs | 68 | 45 | 23 |
| ACB ≥ 3 | 9 (17.3%) | 5 patients ≤ 65 years and 4 patients > 65 years | |
| Diagnostic Intervention | Frequency, n (%) |
|---|---|
| Perform electrocardiogram | 27 (51.9%) |
| Perform therapeutic drug monitoring | 21 (40.4%) |
| Monitor serum electrolytes | 17 (32.7%) |
| Perform pharmacogenetic test | 11 (21.2%) |
| Monitor metabolic assessment | 8 (15.4%) |
| Monitor blood pressure | 8 (15.4%) |
| Monitor liver function | 7 (13.5%) |
| Monitor renal function | 2 (3.8%) |
| Monitor thyroid hormones | 2 (3.8%) |
| Monitor respiratory functionality | 2 (3.8%) |
| Changes in pharmacologic therapies | Frequency, n (%) |
| Reduce/stop proton pump inhibitor | 19 (36.5%) |
| Reduce/change statin | 7 (13.5%) |
| Reduce anticholinergic burden | 4 (7.7%) |
| Change timing of daily drug intake | 3 (5.8%) |
| Reduce/stop benzodiazepine | 2 (3.8%) |
| Change antiplatelet | 2 (3.8%) |
| Change bisphosphonate | 1 (1.9%) |
| Change oral anticoagulant | 1 (1.9%) |
| Stop diuretic | 1 (1.9%) |
| Patients with no suggestions | 2 (3.8%) |
| Diagnostic Intervention | Data |
|---|---|
| TDM of rifampicin, n (% of treated patients) | 26 (86.7%) |
| Mean dose of rifampicin before TDM, mg/day | 645 ± 101 |
| [Rifampicin] C2, mg/L | 9.9 ± 5.5 |
| [Rifampicin] C4, mg/L | 8.1 ± 2.8 |
| [Rifampicin] C6, mg/L | 5.6 ± 3.1 |
| [Rifampicin] Cmax, mg/L | 10.4 ± 4.9 |
| Rifampicin AUC0–24, mg×h/L | 65.6 ± 31.0 |
| [Rifampicin] Cmax < 8 mg/L, % | 38.5% |
| [Rifampicin] Cmax > 24 mg/L, % | 0% |
| Mean dose of rifampicin after TDM, mg/day | 793 ± 189 (+38%) * |
| Time to rifampicin dose change, days | 11.0 ± 11.3 |
| Geno-phenotyping of NAT2, n (%) -Rapid NAT2 acetylators -Intermediate NAT2 acetylators -Slow NAT2 acetylators | 27 (93.1%) 2 (7.4%) 12 (44.4%) 13 (48.2%) |
| Isoniazid dose modifications, n (%) -Dose reduction -Dose increase | 5 (18.5%) 3 (toxicity) 2 (rapid acetylators) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cattaneo, D.; Torre, A.; Schiuma, M.; Civati, A.; Lazzarin, S.; Rizzardini, G.; Gori, A.; Antinori, S.; Gervasoni, C. Management of Polypharmacy and Potential Drug–Drug Interactions in Patients with Mycobacterial Infection: A 1-Year Experience of a Multidisciplinary Outpatient Clinic. Antibiotics 2023, 12, 1171. https://doi.org/10.3390/antibiotics12071171
Cattaneo D, Torre A, Schiuma M, Civati A, Lazzarin S, Rizzardini G, Gori A, Antinori S, Gervasoni C. Management of Polypharmacy and Potential Drug–Drug Interactions in Patients with Mycobacterial Infection: A 1-Year Experience of a Multidisciplinary Outpatient Clinic. Antibiotics. 2023; 12(7):1171. https://doi.org/10.3390/antibiotics12071171
Chicago/Turabian StyleCattaneo, Dario, Alessandro Torre, Marco Schiuma, Aurora Civati, Samuel Lazzarin, Giuliano Rizzardini, Andrea Gori, Spinello Antinori, and Cristina Gervasoni. 2023. "Management of Polypharmacy and Potential Drug–Drug Interactions in Patients with Mycobacterial Infection: A 1-Year Experience of a Multidisciplinary Outpatient Clinic" Antibiotics 12, no. 7: 1171. https://doi.org/10.3390/antibiotics12071171






