Returning to Nature for the Next Generation of Antimicrobial Therapeutics
Abstract
:1. Introduction
2. Natural Product Antibiotic Discovery
2.1. Reviving Natural Product Antibiotic Discovery in Traditional Antibiotic Producers
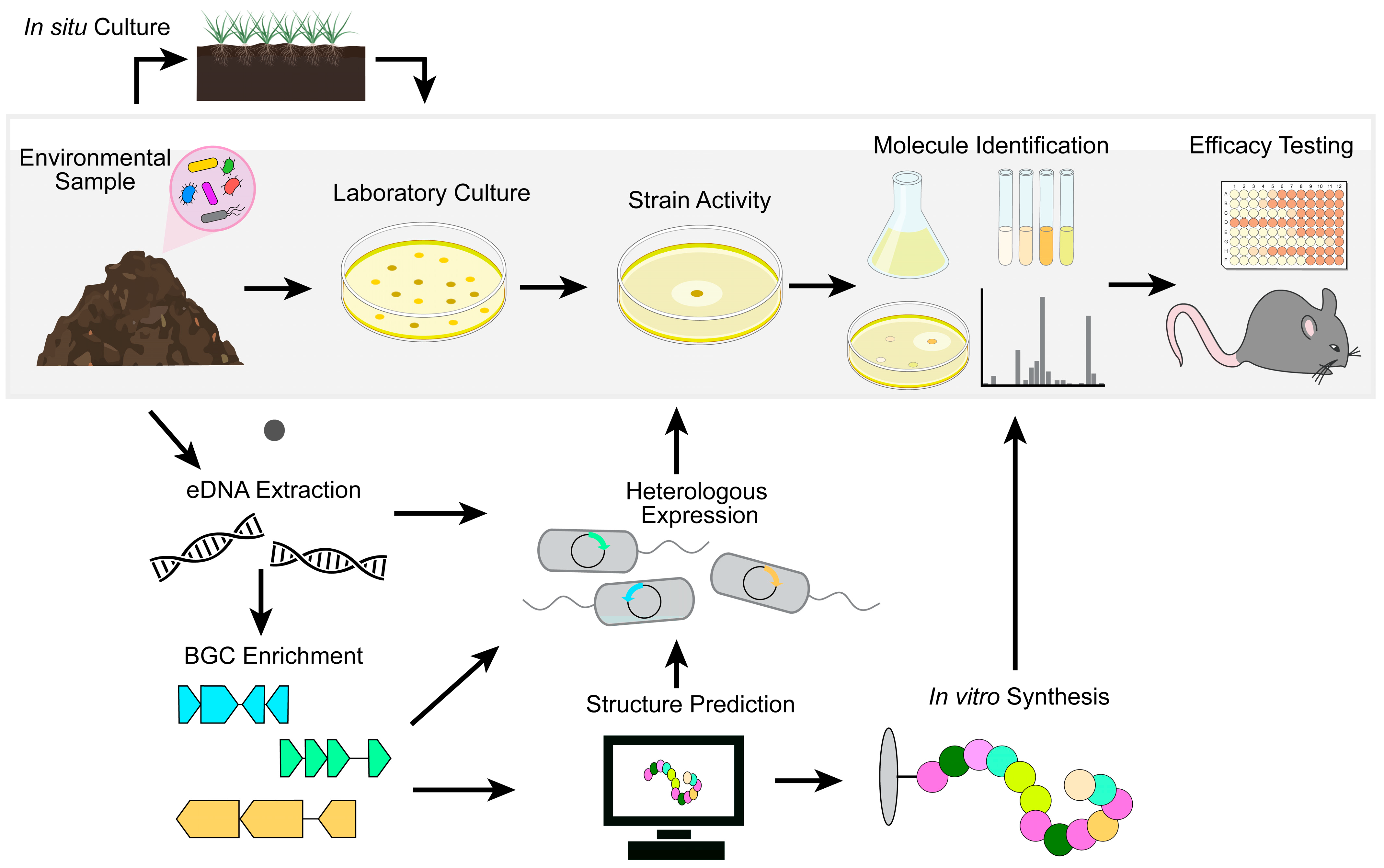
2.2. In Situ Cultivation of Previously Unculturable Microbes
2.3. Culture-Independent Mining for Natural Products
| Compound | Structure | Target | Discovery Approach | Comment | Ref. |
|---|---|---|---|---|---|
| Pestalone | 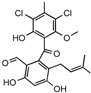 | Unknown | Produced by a marine fungus when co-cultured with a marine bacterium. | Potent activity against MRSA and VRE. | [36,40] |
| Corbomycin | 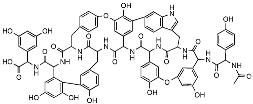 | Autolysin inhibition | Phylogenetic analysis of BGCs and resistance determinants predicted production of this novel glycopeptide. | Activity against Gram-positive bacteria. Low levels of resistance development. | [50,51] |
| Teixobactin | 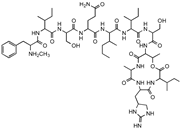 | Lipid II Lipid III | In situ cultivation of a previously unculturable microbe. | Activity against Gram-positive bacteria. Low frequency of resistance. | [96] |
| Darobactin |  | BamA | In situ cultivation of a previously unculturable microbe. | Isolated from a nematode symbiont. Activity against Gram-negative bacteria. | [52,53] |
| Lassomycin | 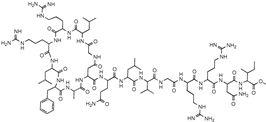 | ClpC 1P1P2 | In situ cultivation of a previously unculturable microbe. | Narrow spectrum M. tuberculosis activity. | [67] |
| Malacidin A | 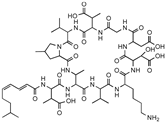 | Lipid II | Sequence-guided and culture-independent mining of BGCs. | Structurally distinct calcium-dependent antibiotic. Activity against Gram-positive bacteria. | [82] |
| MBA6 | 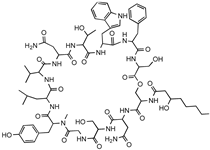 | Menaquinone | Sequence-guided and culture-independent mining of BGCs. | Menaquinone-targeting antimicrobials contain a conserved binding motif. Activity against Gram-positive bacteria. | [86] |
3. Microbiota-Based Therapeutics
3.1. Colonization Resistance
3.2. Fecal Microbiota Transplantation and Bacterial Consortia
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial Competition: Surviving and Thriving in the Microbial Jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Aminov, R.I. The Role of Antibiotics and Antibiotic Resistance in Nature. Environ. Microbiol. 2009, 11, 2970–2988. [Google Scholar] [CrossRef]
- Abdelghani, Z.; Hourani, N.; Zaidan, Z.; Dbaibo, G.; Mrad, M.; Hage-Sleiman, R. Therapeutic Applications and Biological Activities of Bacterial Bioactive Extracts. Arch. Microbiol. 2021, 203, 4755–4776. [Google Scholar] [CrossRef]
- Ramírez-Rendon, D.; Passari, A.K.; Ruiz-Villafán, B.; Rodríguez-Sanoja, R.; Sánchez, S.; Demain, A.L. Impact of Novel Microbial Secondary Metabolites on the Pharma Industry. Appl. Microbiol. Biotechnol. 2022, 106, 1855–1878. [Google Scholar] [CrossRef]
- Lewis, K. Recover the Lost Art of Drug Discovery. Nature 2012, 485, 439–440. [Google Scholar] [CrossRef]
- Brown, E.D.; Wright, G.D. Antibacterial Drug Discovery in the Resistance Era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef]
- Lewis, K. Platforms for Antibiotic Discovery. Nat. Rev. Drug Discov. 2013, 12, 371–387. [Google Scholar]
- Baltz, R.H. Antimicrobials from Actinomycetes:Back to the Future. Microbe 2007, 2, 125–131. [Google Scholar]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for Bad Bugs: Confronting the Challenges of Antibacterial Discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef]
- Silver, L.L. Challenges of Antibacterial Discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef] [Green Version]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for Emerging Pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef]
- Smith, P.A.; Koehler, M.F.T.; Girgis, H.S.; Yan, D.; Chen, Y.; Chen, Y.; Crawford, J.J.; Durk, M.R.; Higuchi, R.I.; Kang, J.; et al. Optimized Arylomycins Are a New Class of Gram-Negative Antibiotics. Nature 2018, 561, 189–194. [Google Scholar] [CrossRef]
- Roberts, K.D.; Zhu, Y.; Azad, M.A.K.; Han, M.-L.; Wang, J.; Wang, L.; Yu, H.H.; Horne, A.S.; Pinson, J.-A.; Rudd, D.; et al. A Synthetic Lipopeptide Targeting Top-Priority Multidrug-Resistant Gram-Negative Pathogens. Nat. Commun. 2022, 13, 1625. [Google Scholar] [CrossRef]
- Silver, L.L. Multi-Targeting by Monotherapeutic Antibacterials. Nat. Rev. Drug Discov. 2007, 6, 41–55. [Google Scholar] [CrossRef]
- Lewis, K. The Science of Antibiotic Discovery. Cell 2020, 181, 29–45. [Google Scholar] [CrossRef]
- Silver, L.L. A Gestalt Approach to Gram-Negative Entry. Bioorganic Med. Chem. 2016, 24, 6379–6389. [Google Scholar] [CrossRef]
- Hughes, D.; Karlén, A. Discovery and Preclinical Development of New Antibiotics. Upsala J. Med. Sci. 2014, 119, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Buffie, C.G.; Pamer, E.G. Microbiota-Mediated Colonization Resistance against Intestinal Pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sassone-Corsi, M.; Raffatellu, M. No Vacancy: How Beneficial Microbes Cooperate with Immunity To Provide Colonization Resistance to Pathogens. J. Immunol. 2015, 194, 4081–4087. [Google Scholar] [CrossRef] [Green Version]
- Ducarmon, Q.R.; Zwittink, R.D.; Hornung, B.V.H.; van Schaik, W.; Young, V.B.; Kuijper, E.J. Gut Microbiota and Colonization Resistance against Bacterial Enteric Infection. Microbiol. Mol. Biol. Rev. 2019, 83, e00007-19. [Google Scholar] [CrossRef] [PubMed]
- Simeis, D.D.; Serra, S. Actinomycetes: A Never-Ending Source of Bioactive Compounds—An Overview on Antibiotics Production. Antibiotics 2021, 10, 483. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. AntiSMASH 5.0: Updates to the Secondary Metabolite Genome Mining Pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.; Hwang, S.; Kim, J.; Cho, S.; Palsson, B.; Cho, B.-K. Mini Review: Genome Mining Approaches for the Identification of Secondary Metabolite Biosynthetic Gene Clusters in Streptomyces. Comput. Struct. Biotechnol. J. 2020, 18, 1548–1556. [Google Scholar] [CrossRef]
- Watve, M.G.; Tickoo, R.; Jog, M.M.; Bhole, B.D. How Many Antibiotics Are Produced by the Genus Streptomyces? Arch. Microbiol. 2001, 176, 386–390. [Google Scholar] [CrossRef]
- Cibichakravarthy, B.; Jose, P.A. Biosynthetic Potential of Streptomyces Rationalizes Genome-Based Bioprospecting. Antibiotics 2021, 10, 873. [Google Scholar] [CrossRef]
- Pauli, G.F.; Chen, S.-N.; Friesen, J.B.; McAlpine, J.B.; Jaki, B.U. Analysis and Purification of Bioactive Natural Products: The AnaPurNa Study. J. Nat. Prod. 2012, 75, 1243–1255. [Google Scholar] [CrossRef]
- Culp, E.J.; Yim, G.; Waglechner, N.; Wang, W.; Pawlowski, A.C.; Wright, G.D. Hidden Antibiotics in Actinomycetes Can Be Identified by Inactivation of Gene Clusters for Common Antibiotics. Nat. Biotechnol. 2019, 37, 1149–1154. [Google Scholar] [CrossRef]
- Sharma, R.; Jamwal, V.; Singh, V.P.; Wazir, P.; Awasthi, P.; Singh, D.; Vishwakarma, R.A.; Gandhi, S.G.; Chaubey, A. Revelation and Cloning of Valinomycin Synthetase Genes in Streptomyces Lavendulae ACR-DA1 and Their Expression Analysis under Different Fermentation and Elicitation Conditions. J. Biotechnol. 2017, 253, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Kim, J.; Oh, C.-H. Different Effects of Acidic PH Shock on the Prodiginine Production in Streptomyces Coelicolor M511 and SJM1 Mutants. J. Microbiol. Biotech. 2013, 23, 1454–1459. [Google Scholar] [CrossRef] [Green Version]
- Ripa, F.A.; Nikkon, F.; Zaman, S.; Khondkar, P. Optimal Conditions for Antimicrobial Metabolites Production from a New Streptomyces sp. RUPA-08PR Isolated from Bangladeshi Soil. Mycobiology 2009, 37, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Tomm, H.A.; Ucciferri, L.; Ross, A.C. Advances in Microbial Culturing Conditions to Activate Silent Biosynthetic Gene Clusters for Novel Metabolite Production. J. Ind. Microbiol. Biot. 2019, 46, 1381–1400. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Edwards, C. Effects of Metals on a Range of Streptomyces Species. Appl. Environ. Microb. 1989, 55, 2030–2035. [Google Scholar] [CrossRef]
- Akhter, N.; Liu, Y.; Auckloo, B.N.; Shi, Y.; Wang, K.; Chen, J.; Wu, X.; Wu, B. Stress-Driven Discovery of New Angucycline-Type Antibiotics from a Marine Streptomyces Pratensis NA-ZhouS1. Mar. Drugs 2018, 16, 331. [Google Scholar] [CrossRef] [Green Version]
- Auckloo, B.N.; Pan, C.; Akhter, N.; Wu, B.; Wu, X.; He, S. Stress-Driven Discovery of Novel Cryptic Antibiotics from a Marine Fungus Penicillium sp. BB1122. Front. Microbiol. 2017, 8, 1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, B.K.; Seyedsayamdost, M.R. Antibiotic Dialogues: Induction of Silent Biosynthetic Gene Clusters by Exogenous Small Molecules. FEMS Microbiol. Rev. 2017, 41, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Craney, A.; Ozimok, C.; Pimentel-Elardo, S.M.; Capretta, A.; Nodwell, J.R. Chemical Perturbation of Secondary Metabolism Demonstrates Important Links to Primary Metabolism. Chem. Biol. 2012, 19, 1020–1027. [Google Scholar] [CrossRef] [Green Version]
- Pimentel-Elardo, S.M.; Sørensen, D.; Ho, L.; Ziko, M.; Bueler, S.A.; Lu, S.; Tao, J.; Moser, A.; Lee, R.; Agard, D.; et al. Activity-Independent Discovery of Secondary Metabolites Using Chemical Elicitation and Cheminformatic Inference. ACS Chem. Biol. 2015, 10, 2616–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.A.; Wang, X. Use of Bacterial Co-Cultures for the Efficient Production of Chemicals. Curr. Opin. Biotech. 2018, 53, 33–38. [Google Scholar] [CrossRef]
- Ueda, K.; Beppu, T. Antibiotics in Microbial Coculture. J. Antibiot. 2017, 70, 361–365. [Google Scholar] [CrossRef]
- Park, H.B.; Kwon, H.C.; Lee, C.-H.; Yang, H.O. Glionitrin A, an Antibiotic−Antitumor Metabolite Derived from Competitive Interaction between Abandoned Mine Microbes. J. Nat. Prod. 2009, 72, 248–252. [Google Scholar] [CrossRef]
- Sugiyama, R.; Nishimura, S.; Ozaki, T.; Asamizu, S.; Onaka, H.; Kakeya, H. Discovery and Total Synthesis of Streptoaminals: Antimicrobial (5,5)-Spirohemiaminals from the Combined-Culture of Streptomyces nigrescens and Tsukamurella pulmonis. Angew. Chem. Int. Ed. 2016, 55, 10278–10282. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, S.; Onaka, H.; Abe, I. Activation of Silent Biosynthetic Pathways and Discovery of Novel Secondary Metabolites in Actinomycetes by Co-Culture with Mycolic Acid-Containing Bacteria. J. Ind. Microbiol. Biot. 2019, 46, 363–374. [Google Scholar] [CrossRef]
- Augner, D.; Krut, O.; Slavov, N.; Gerbino, D.C.; Sahl, H.-G.; Benting, J.; Nising, C.F.; Hillebrand, S.; Kronke, M.; Schmalz, H.-G. On the Antibiotic and Antifungal Activity of Pestalone, Pestalachloride A, and Structurally Related Compounds. J. Nat. Prod. 2013, 76, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Oikawa, H.; Ogawa, H.; Hosono, K.; Shinmachi, F.; Takano, H.; Sakuda, S.; Beppu, T.; Ueda, K. Desferrioxamine E Produced by Streptomyces Griseus Stimulates Growth and Development of Streptomyces tanashiensis. Microbiology 2005, 151, 2899–2905. [Google Scholar] [CrossRef]
- Cueto, M.; Jensen, P.R.; Kauffman, C.; Fenical, W.; Lobkovsky, E.; Clardy, J. Pestalone, a New Antibiotic Produced by a Marine Fungus in Response to Bacterial Challenge. J. Nat. Prod. 2001, 64, 1444–1446. [Google Scholar] [CrossRef]
- Eto, D.; Watanabe, K.; Saeki, H.; Oinuma, K.; Otani, K.; Nobukuni, M.; Shiratori-Takano, H.; Takano, H.; Beppu, T.; Ueda, K. Divergent Effects of Desferrioxamine on Bacterial Growth and Characteristics. J. Antibiot. 2013, 66, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.; Kim, W.; Chung, J.; Lee, Y.; Cho, S.; Jang, K.-S.; Kim, S.C.; Palsson, B.; Cho, B.-K. Iron Competition Triggers Antibiotic Biosynthesis in Streptomyces Coelicolor during Coculture with Myxococcus Xanthus. ISME J. 2020, 14, 1111–1124. [Google Scholar] [CrossRef] [Green Version]
- Amano, S.; Morota, T.; Kano, Y.; Narita, H.; Hashidzume, T.; Yamamoto, S.; Mizutani, K.; Sakuda, S.; Furihata, K.; Takano-Shiratori, H.; et al. Promomycin, a Polyether Promoting Antibiotic Production in Streptomyces spp. J. Antibiot. 2010, 63, 486–491. [Google Scholar] [CrossRef] [Green Version]
- Amano, S.; Sakurai, T.; Endo, K.; Takano, H.; Beppu, T.; Furihata, K.; Sakuda, S.; Ueda, K. A Cryptic Antibiotic Triggered by Monensin. J. Antibiot. 2011, 64, 703. [Google Scholar] [CrossRef]
- Baltz, R.H. Marcel Faber Roundtable: Is Our Antibiotic Pipeline Unproductive Because of Starvation, Constipation or Lack of Inspiration? J. Ind. Microbiol. Biotechnol. 2006, 33, 507–513. [Google Scholar] [CrossRef]
- Caesar, L.K.; Montaser, R.; Keller, N.P.; Kelleher, N.L. Metabolomics and Genomics in Natural Products Research: Complementary Tools for Targeting New Chemical Entities. Nat. Prod. Rep. 2021, 38, 2041–2065. [Google Scholar] [CrossRef]
- Grienke, U.; Foster, P.A.; Zwirchmayr, J.; Tahir, A.; Rollinger, J.M.; Mikros, E. 1H NMR-MS-Based Heterocovariance as a Drug Discovery Tool for Fishing Bioactive Compounds out of a Complex Mixture of Structural Analogues. Sci. Rep. 2019, 9, 11113. [Google Scholar] [CrossRef] [Green Version]
- Volynkina, I.A.; Zakalyukina, Y.V.; Alferova, V.A.; Belik, A.R.; Yagoda, D.K.; Nikandrova, A.A.; Buyuklyan, Y.A.; Udalov, A.V.; Golovin, E.V.; Kryakvin, M.A.; et al. Mechanism-Based Approach to New Antibiotic Producers Screening among Actinomycetes in the Course of the Citizen Science Project. Antibiotics 2022, 11, 1198. [Google Scholar] [CrossRef]
- Martinez-Fructuoso, L.; Arends, S.J.R.; Freire, V.F.; Evans, J.R.; DeVries, S.; Peyser, B.D.; Akee, R.K.; Thornburg, C.C.; Kumar, R.; Ensel, S.; et al. Screen for New Antimicrobial Natural Products from the NCI Program for Natural Product Discovery Prefractionated Extract Library. ACS Infect. Dis. 2023, 9, 1245–1256. [Google Scholar] [CrossRef]
- Medema, M.H.; Fischbach, M.A. Computational Approaches to Natural Product Discovery. Nat. Chem. Biol. 2015, 11, 639–648. [Google Scholar] [CrossRef]
- Culp, E.J.; Waglechner, N.; Wang, W.; Fiebig-Comyn, A.A.; Hsu, Y.-P.; Koteva, K.; Sychantha, D.; Coombes, B.K.; Nieuwenhze, M.S.V.; Brun, Y.V.; et al. Evolution-Guided Discovery of Antibiotics That Inhibit Peptidoglycan Remodelling. Nature 2020, 578, 582–587. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, J.; Qiao, Y.; Lin, B.; Deng, Z.; Kong, L.; You, D. Genome Mining and Metabolic Profiling Reveal Cytotoxic Cyclodipeptides in Streptomyces Hygrospinosus Var. Beijingensis. Antibiotics 2022, 11, 1463. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Vizcaíno, A.; González, V.; Braña, A.F.; Palacios, J.J.; Otero, L.; Fernández, J.; Molina, A.; Kulik, A.; Vázquez, F.; Acuña, J.L.; et al. Pharmacological Potential of Phylogenetically Diverse Actinobacteria Isolated from Deep-Sea Coral Ecosystems of the Submarine Avilés Canyon in the Cantabrian Sea. Microb. Ecol. 2017, 73, 338–352. [Google Scholar] [CrossRef]
- Hui, M.L.-Y.; Tan, L.T.-H.; Letchumanan, V.; He, Y.-W.; Fang, C.-M.; Chan, K.-G.; Law, J.W.-F.; Lee, L.-H. The Extremophilic Actinobacteria: From Microbes to Medicine. Antibiotics 2021, 10, 682. [Google Scholar] [CrossRef]
- Imai, Y.; Meyer, K.J.; Iinishi, A.; Favre-Godal, Q.; Green, R.; Manuse, S.; Caboni, M.; Mori, M.; Niles, S.; Ghiglieri, M.; et al. A New Antibiotic Selectively Kills Gram-Negative Pathogens. Nature 2019, 459–464. [Google Scholar] [CrossRef]
- Kaur, H.; Jakob, R.P.; Marzinek, J.K.; Green, R.; Imai, Y.; Bolla, J.R.; Agustoni, E.; Robinson, C.V.; Bond, P.J.; Lewis, K.; et al. The Antibiotic Darobactin Mimics a β-Strand to Inhibit Outer Membrane Insertase. Nature 2021, 593, 125–129. [Google Scholar] [CrossRef]
- Ghequire, M.G.K.; Swings, T.; Michiels, J.; Buchanan, S.K.; Mot, R. de Hitting with a BAM: Selective Killing by Lectin-like Bacteriocins. mBio 2018, 9, e02138-17. [Google Scholar] [CrossRef] [Green Version]
- Storek, K.M.; Auerbach, M.R.; Shi, H.; Garcia, N.K.; Sun, D.; Nickerson, N.N.; Vij, R.; Lin, Z.; Chiang, N.; Schneider, K.; et al. Monoclonal Antibody Targeting the β-Barrel Assembly Machine of Escherichia Coli Is Bactericidal. Proc. Natl. Acad. Sci. USA 2018, 115, 3692–3697. [Google Scholar] [CrossRef] [Green Version]
- Hart, E.M.; Mitchell, A.M.; Konovalova, A.; Grabowicz, M.; Sheng, J.; Han, X.; Rodriguez-Rivera, F.P.; Schwaid, A.G.; Malinverni, J.C.; Balibar, C.J.; et al. A Small-Molecule Inhibitor of BamA Impervious to Efflux and the Outer Membrane Permeability Barrier. Proc. Natl. Acad. Sci. USA 2019, 116, 201912345. [Google Scholar] [CrossRef] [Green Version]
- Keseler, I.M.; Gama-Castro, S.; Mackie, A.; Billington, R.; Bonavides-Martínez, C.; Caspi, R.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Muñiz-Rascado, L.; et al. The EcoCyc Database in 2021. Front. Microbiol. 2021, 12, 711077. [Google Scholar] [CrossRef]
- Stewart, E.J. Growing Unculturable Bacteria. J. Bacteriol. 2012, 194, 4151–4160. [Google Scholar] [CrossRef] [Green Version]
- Lagier, J.-C.; Dubourg, G.; Million, M.; Cadoret, F.; Bilen, M.; Fenollar, F.; Levasseur, A.; Rolain, J.-M.; Fournier, P.-E.; Raoult, D. Culturing the Human Microbiota and Culturomics. Nat. Rev. Microbiol. 2018, 16, 540–550. [Google Scholar] [CrossRef] [Green Version]
- Browne, H.P.; Forster, S.C.; Anonye, B.O.; Kumar, N.; Neville, B.A.; Stares, M.D.; Goulding, D.; Lawley, T.D. Culturing of ‘Unculturable’ Human Microbiota Reveals Novel Taxa and Extensive Sporulation. Nature 2016, 533, 543–546. [Google Scholar] [CrossRef] [Green Version]
- Ueda, K.; Yamashita, A.; Ishikawa, J.; Shimada, M.; Watsuji, T.; Morimura, K.; Ikeda, H.; Hattori, M.; Beppu, T. Genome Sequence of Symbiobacterium Thermophilum, an Uncultivable Bacterium That Depends on Microbial Commensalism. Nucleic Acids Res. 2004, 32, 4937–4944. [Google Scholar] [CrossRef]
- Bae, J.-W.; Rhee, S.-K.; Park, J.R.; Kim, B.-C.; Park, Y.-H. Isolation of Uncultivated Anaerobic Thermophiles from Compost by Supplementing Cell Extract of Geobacillus Toebii in Enrichment Culture Medium. Extremophiles 2005, 9, 477–485. [Google Scholar] [CrossRef]
- Nichols, D.; Lewis, K.; Orjala, J.; Mo, S.; Ortenberg, R.; O’Connor, P.; Zhao, C.; Vouros, P.; Kaeberlein, T.; Epstein, S.S. Short Peptide Induces an “Uncultivable” Microorganism to Grow In Vitro. Appl. Environ. Microb. 2008, 74, 4889–4897. [Google Scholar] [CrossRef] [Green Version]
- Kaeberlein, T.; Lewis, K.; Epstein, S.S. Isolating “Uncultivable” Microorganisms in Pure Culture in a Simulated Natural Environment. Science 2002, 296, 1127–1129. [Google Scholar] [CrossRef] [Green Version]
- Nichols, D.; Cahoon, N.; Trakhtenberg, E.M.; Pham, L.; Mehta, A.; Belanger, A.; Kanigan, T.; Lewis, K.; Epstein, S.S. Use of Ichip for High-Throughput In Situ Cultivation of “Uncultivable” Microbial Species. Appl. Environ. Microb. 2010, 76, 2445–2450. [Google Scholar] [CrossRef] [Green Version]
- Zengler, K.; Toledo, G.; Rappé, M.; Elkins, J.; Mathur, E.J.; Short, J.M.; Keller, M. Cultivating the Uncultured. Proc. Natl. Acad. Sci. USA 2002, 99, 15681–15686. [Google Scholar] [CrossRef]
- Buerger, S.; Spoering, A.; Gavrish, E.; Leslin, C.; Ling, L.; Epstein, S.S. Microbial Scout Hypothesis and Microbial Discovery. Appl. Environ. Microb. 2012, 78, 3229–3233. [Google Scholar] [CrossRef] [Green Version]
- Buerger, S.; Spoering, A.; Gavrish, E.; Leslin, C.; Ling, L.; Epstein, S.S. Microbial Scout Hypothesis, Stochastic Exit from Dormancy, and the Nature of Slow Growers. Appl. Environ. Microb. 2012, 78, 3221–3228. [Google Scholar] [CrossRef] [Green Version]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A New Antibiotic Kills Pathogens without Detectable Resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef]
- Shukla, R.; Lavore, F.; Maity, S.; Derks, M.G.N.; Jones, C.R.; Vermeulen, B.J.A.; Melcrová, A.; Morris, M.A.; Becker, L.M.; Wang, X.; et al. Teixobactin Kills Bacteria by a Two-Pronged Attack on the Cell Envelope. Nature 2022, 608, 390–396. [Google Scholar] [CrossRef]
- Gavrish, E.; Sit, C.S.; Cao, S.; Kandror, O.; Spoering, A.; Peoples, A.; Ling, L.; Fetterman, A.; Hughes, D.; Bissell, A.; et al. Lassomycin, a Ribosomally Synthesized Cyclic Peptide, Kills Mycobacterium Tuberculosis by Targeting the ATP-Dependent Protease ClpC1P1P2. Chem. Biol. 2014, 21, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Steen, A.D.; Crits-Christoph, A.; Carini, P.; DeAngelis, K.M.; Fierer, N.; Lloyd, K.G.; Thrash, J.C. High Proportions of Bacteria and Archaea across Most Biomes Remain Uncultured. ISME J. 2019, 13, 3126–3130. [Google Scholar] [CrossRef] [Green Version]
- Bodor, A.; Bounedjoum, N.; Vincze, G.E.; Kis, Á.E.; Laczi, K.; Bende, G.; Szilágyi, Á.; Kovács, T.; Perei, K.; Rákhely, G. Challenges of Unculturable Bacteria: Environmental Perspectives. Rev. Environ. Sci. Bio Technol. 2020, 19, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Brady, S.F.; Chao, C.J.; Handelsman, J.; Clardy, J. Cloning and Heterologous Expression of a Natural Product Biosynthetic Gene Cluster from EDNA. Org. Lett. 2001, 3, 1981–1984. [Google Scholar] [CrossRef]
- Brady, S.F.; Clardy, J. Palmitoylputrescine, an Antibiotic Isolated from the Heterologous Expression of DNA Extracted from Bromeliad Tank Water. J. Nat. Prod. 2004, 67, 1283–1286. [Google Scholar] [CrossRef]
- Brady, S.F.; Chao, C.J.; Clardy, J. New Natural Product Families from an Environmental DNA (EDNA) Gene Cluster. J. Am. Chem. Soc. 2002, 124, 9968–9969. [Google Scholar] [CrossRef]
- Handelsman, J. Metagenomics: Application of Genomics to Uncultured Microorganisms. Microbiol. Mol. Biol. Rev. 2004, 68, 669–685. [Google Scholar] [CrossRef] [Green Version]
- Garcia, J.A.L.; Fernández-Guerra, A.; Casamayor, E.O. A Close Relationship between Primary Nucleotides Sequence Structure and the Composition of Functional Genes in the Genome of Prokaryotes. Mol. Phylogenet Evol. 2011, 61, 650–658. [Google Scholar] [CrossRef]
- Owen, J.G.; Reddy, B.V.B.; Ternei, M.A.; Charlop-Powers, Z.; Calle, P.Y.; Kim, J.H.; Brady, S.F. Mapping Gene Clusters within Arrayed Metagenomic Libraries to Expand the Structural Diversity of Biomedically Relevant Natural Products. Proc. Natl. Acad. Sci. USA 2013, 110, 11797–11802. [Google Scholar] [CrossRef]
- Katz, M.; Hover, B.M.; Brady, S.F. Culture-Independent Discovery of Natural Products from Soil Metagenomes. J. Ind. Microbiol. Biot. 2016, 43, 129–141. [Google Scholar] [CrossRef]
- Li, L.; Maclntyre, L.W.; Brady, S.F. Refactoring Biosynthetic Gene Clusters for Heterologous Production of Microbial Natural Products. Curr. Opin. Biotech. 2021, 69, 145–152. [Google Scholar] [CrossRef]
- Tan, G.-Y.; Liu, T. Rational Synthetic Pathway Refactoring of Natural Products Biosynthesis in Actinobacteria. Metab. Eng. 2017, 39, 228–236. [Google Scholar] [CrossRef]
- Hover, B.M.; Kim, S.-H.; Katz, M.; Charlop-Powers, Z.; Owen, J.G.; Ternei, M.A.; Maniko, J.; Estrela, A.B.; Molina, H.; Park, S.; et al. Culture-Independent Discovery of the Malacidins as Calcium-Dependent Antibiotics with Activity against Multidrug-Resistant Gram-Positive Pathogens. Nat. Microbiol. 2018, 3, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Owen, J.G.; Charlop-Powers, Z.; Smith, A.G.; Ternei, M.A.; Calle, P.Y.; Reddy, B.V.B.; Montiel, D.; Brady, S.F. Multiplexed Metagenome Mining Using Short DNA Sequence Tags Facilitates Targeted Discovery of Epoxyketone Proteasome Inhibitors. Proc. Natl. Acad. Sci. USA 2015, 112, 4221–4226. [Google Scholar] [CrossRef]
- Sun, Z.; Shang, Z.; Forelli, N.; Po, K.H.L.; Chen, S.; Brady, S.F.; Li, X. Total Synthesis of Malacidin A by Β-Hydroxyaspartic Acid Ligation-Mediated Cyclization and Absolute Structure Establishment. Angew. Chem. Int. Ed. 2020, 59, 19868–19872. [Google Scholar] [CrossRef]
- Kovalenko, N.; Howard, G.K.; Swain, J.A.; Hermant, Y.; Cameron, A.J.; Cook, G.M.; Ferguson, S.A.; Stubbing, L.A.; Harris, P.W.R.; Brimble, M.A. A Concise Synthetic Strategy Towards the Novel Calcium-Dependent Lipopeptide Antibiotic, Malacidin A and Analogues. Front. Chem. 2021, 9, 687875. [Google Scholar] [CrossRef]
- Li, L.; Koirala, B.; Hernandez, Y.; MacIntyre, L.W.; Ternei, M.A.; Russo, R.; Brady, S.F. Identification of Structurally Diverse Menaquinone-Binding Antibiotics with in Vivo Activity against Multidrug-Resistant Pathogens. Nat. Microbiol. 2022, 7, 120–131. [Google Scholar] [CrossRef]
- Chu, J.; Vila-Farres, X.; Inoyama, D.; Ternei, M.; Cohen, L.J.; Gordon, E.A.; Reddy, B.V.B.; Charlop-Powers, Z.; Zebroski, H.A.; Gallardo-Macias, R.; et al. Discovery of MRSA Active Antibiotics Using Primary Sequence from the Human Microbiome. Nat. Chem. Biol. 2016, 12, 1004–1006. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Koirala, B.; Hernandez, Y.; Zimmerman, M.; Park, S.; Perlin, D.S.; Brady, S.F. A Naturally Inspired Antibiotic to Target Multidrug-Resistant Pathogens. Nature 2022, 601, 606–611. [Google Scholar] [CrossRef]
- Miller, R.D.; Iinishi, A.; Modaresi, S.M.; Yoo, B.-K.; Curtis, T.D.; Lariviere, P.J.; Liang, L.; Son, S.; Nicolau, S.; Bargabos, R.; et al. Computational Identification of a Systemic Antibiotic for Gram-Negative Bacteria. Nat. Microbiol. 2022, 7, 1661–1672. [Google Scholar] [CrossRef]
- Kaur, N.; Chen, C.-C.; Luther, J.; Kao, J.Y. Intestinal Dysbiosis in Inflammatory Bowel Disease. Gut Microbes 2011, 2, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of Antibiotics on Gut Microbiota. Digest Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Pamer, E.G. Interbacterial Mechanisms of Colonization Resistance and the Strategies Pathogens Use to Overcome Them. Mucosal Immunol. 2019, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bohnhoff, M.; Drake, B.L.; Miller, C.P. Effect of Streptomycin on Susceptibility of Intestinal Tract to Experimental Salmonella Infection. Proc. Soc. Exp. Biol. Med. 1954, 86, 132–137. [Google Scholar] [CrossRef]
- RABIU, B.A.; GIBSON, G.R. Carbohydrates: A Limit on Bacterial Diversity within the Colon. Biol. Rev. 2002, 77, 443–453. [Google Scholar] [CrossRef]
- Bauer, M.A.; Kainz, K.; Carmona-Gutierrez, D.; Madeo, F. Microbial Wars: Competition in Ecological Niches and within the Microbiome. Microb. Cell 2018, 5, 215–219. [Google Scholar] [CrossRef]
- Celis, A.I.; Relman, D.A. Competitors versus Collaborators: Micronutrient Processing by Pathogenic and Commensal Human-Associated Gut Bacteria. Mol. Cell 2020, 78, 570–576. [Google Scholar] [CrossRef]
- Kamada, N.; Kim, Y.-G.; Sham, H.P.; Vallance, B.A.; Puente, J.L.; Martens, E.C.; Núñez, G. Regulated Virulence Controls the Ability of a Pathogen to Compete with the Gut Microbiota. Science 2012, 336, 1325–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maltby, R.; Leatham-Jensen, M.P.; Gibson, T.; Cohen, P.S.; Conway, T. Nutritional Basis for Colonization Resistance by Human Commensal Escherichia Coli Strains HS and Nissle 1917 against E. coli O157:H7 in the Mouse Intestine. PLoS ONE 2013, 8, e53957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickard, J.M.; Maurice, C.F.; Kinnebrew, M.A.; Abt, M.C.; Schenten, D.; Golovkina, T.V.; Bogatyrev, S.R.; Ismagilov, R.F.; Pamer, E.G.; Turnbaugh, P.J.; et al. Rapid Fucosylation of Intestinal Epithelium Sustains Host–Commensal Symbiosis in Sickness. Nature 2014, 514, 638–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowley, C.A.; Anderson, C.J.; Kendall, M.M. Ethanolamine Influences Human Commensal Escherichia Coli Growth, Gene Expression, and Competition with Enterohemorrhagic E. coli O157:H7. mBio 2018, 9, e01429-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swann, J.R.; Want, E.J.; Geier, F.M.; Spagou, K.; Wilson, I.D.; Sidaway, J.E.; Nicholson, J.K.; Holmes, E. Systemic Gut Microbial Modulation of Bile Acid Metabolism in Host Tissue Compartments. Proc. Natl. Acad. Sci. USA 2011, 108, 4523–4530. [Google Scholar] [CrossRef] [PubMed]
- Funabashi, M.; Grove, T.L.; Wang, M.; Varma, Y.; McFadden, M.E.; Brown, L.C.; Guo, C.; Higginbottom, S.; Almo, S.C.; Fischbach, M.A. A Metabolic Pathway for Bile Acid Dehydroxylation by the Gut Microbiome. Nature 2020, 582, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Sorg, J.A.; Sonenshein, A.L. Bile Salts and Glycine as Cogerminants for Clostridium Difficile Spores. J. Bacteriol. 2008, 190, 2505–2512. [Google Scholar] [CrossRef] [Green Version]
- Buffie, C.G.; Bucci, V.; Stein, R.R.; McKenney, P.T.; Ling, L.; Gobourne, A.; No, D.; Liu, H.; Kinnebrew, M.; Viale, A.; et al. Precision Microbiome Restoration of Bile Acid-Mediated Resistance to Clostridium Difficile. Nature 2015, 517, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Sorg, J.A.; Sonenshein, A.L. Inhibiting the Initiation of Clostridium Difficile Spore Germination Using Analogs of Chenodeoxycholic Acid, a Bile Acid. J. Bacteriol. 2010, 192, 4983–4990. [Google Scholar] [CrossRef] [Green Version]
- Weingarden, A.R.; Chen, C.; Zhang, N.; Graiziger, C.T.; Dosa, P.I.; Steer, C.J.; Shaughnessy, M.K.; Johnson, J.R.; Sadowsky, M.J.; Khoruts, A. Ursodeoxycholic Acid Inhibits Clostridium Difficile Spore Germination and Vegetative Growth, and Prevents the Recurrence of Ileal Pouchitis Associated With the Infection. J. Clin. Gastroenterol. 2016, 50, 624–630. [Google Scholar] [CrossRef] [Green Version]
- Coyne, M.J.; Comstock, L.E. Type VI Secretion Systems and the Gut Microbiota. Microbiol. Spectr. 2019, 7, 1–7. [Google Scholar] [CrossRef]
- Serapio-Palacios, A.; Woodward, S.E.; Vogt, S.L.; Deng, W.; Creus-Cuadros, A.; Huus, K.E.; Cirstea, M.; Gerrie, M.; Barcik, W.; Yu, H.; et al. Type VI Secretion Systems of Pathogenic and Commensal Bacteria Mediate Niche Occupancy in the Gut. Cell Rep. 2022, 39, 110731. [Google Scholar] [CrossRef] [PubMed]
- Chatzidaki-Livanis, M.; Geva-Zatorsky, N.; Comstock, L.E. Bacteroides Fragilis Type VI Secretion Systems Use Novel Effector and Immunity Proteins to Antagonize Human Gut Bacteroidales Species. Proc. Natl. Acad. Sci. USA 2016, 113, 3627–3632. [Google Scholar] [CrossRef]
- Jurėnas, D.; Journet, L. Activity, Delivery, and Diversity of Type VI Secretion Effectors. Mol. Microbiol. 2021, 115, 383–394. [Google Scholar] [CrossRef]
- Heilbronner, S.; Krismer, B.; Brötz-Oesterhelt, H.; Peschel, A. The Microbiome-Shaping Roles of Bacteriocins. Nat. Rev. Microbiol. 2021, 19, 726–739. [Google Scholar] [CrossRef]
- Lay, C.L.; Dridi, L.; Bergeron, M.G.; Ouellette, M.; Fliss, I. Nisin Is an Effective Inhibitor of Clostridium Difficile Vegetative Cells and Spore Germination. J. Med. Microbiol. 2016, 65, 169–175. [Google Scholar] [CrossRef]
- Rea, M.C.; Sit, C.S.; Clayton, E.; O’Connor, P.M.; Whittal, R.M.; Zheng, J.; Vederas, J.C.; Ross, R.P.; Hill, C. Thuricin CD, a Posttranslationally Modified Bacteriocin with a Narrow Spectrum of Activity against Clostridium Difficile. Proc. Natl. Acad. Sci. USA 2010, 107, 9352–9357. [Google Scholar] [CrossRef]
- Petersson, J.; Schreiber, O.; Hansson, G.C.; Gendler, S.J.; Velcich, A.; Lundberg, J.O.; Roos, S.; Holm, L.; Phillipson, M. Importance and Regulation of the Colonic Mucus Barrier in a Mouse Model of Colitis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 300, G327–G333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, M.; Kamada, N.; Jiao, Y.; Liu, M.Z.; Núñez, G.; Inohara, N. Protective Role of Commensals against Clostridium Difficile Infection via an IL-1β–Mediated Positive-Feedback Loop. J. Immunol. 2012, 189, 3085–3091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, X.; Fu, J.; Xu, B.; Wang, Y.; Jin, M. Interplay between Gut Microbiota and Antimicrobial Peptides. Anim. Nutr. 2020, 6, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of Colonic Regulatory T Cells by Indigenous Clostridium Species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Lécuyer, E.; Rakotobe, S.; Lengliné-Garnier, H.; Lebreton, C.; Picard, M.; Juste, C.; Fritzen, R.; Eberl, G.; McCoy, K.D.; Macpherson, A.J.; et al. Segmented Filamentous Bacterium Uses Secondary and Tertiary Lymphoid Tissues to Induce Gut IgA and Specific T Helper 17 Cell Responses. Immunity 2014, 40, 608–620. [Google Scholar] [CrossRef] [Green Version]
- Longo, D.L.; Leffler, D.A.; Lamont, J.T. Clostridium Difficile Infection. N. Engl. J. Med. 2015, 372, 1539–1548. [Google Scholar] [CrossRef] [Green Version]
- Bien, J.; Palagani, V.; Bozko, P. The Intestinal Microbiota Dysbiosis and Clostridium Difficile Infection: Is There a Relationship with Inflammatory Bowel Disease? Ther. Adv. Gastroenter 2013, 6, 53–68. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, R.J.; Wilson, R.B. Treatment of Recurrent Clostridium Difficile Colitis: A Narrative Review. Gastroenterol. Rep. 2018, 6, 21–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, B.G.; Gardner, A. Mortality and Clostridium Difficile Infection: A Review. Antimicrob. Resist. Infect. Control 2012, 1, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hocquart, M.; Lagier, J.-C.; Cassir, N.; Saidani, N.; Eldin, C.; Kerbaj, J.; Delord, M.; Valles, C.; Brouqui, P.; Raoult, D.; et al. Early Fecal Microbiota Transplantation Improves Survival in Severe Clostridium Difficile Infections. Clin. Infect. Dis. 2017, 66, 645–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eiseman, B.; Silen, W.; Bascom, G.; Kauvar, A. Fecal Enema as an Adjunct in the Treatment of Pseudomembranous Enterocolitis. Surgery 1958, 5, 854–859. [Google Scholar]
- Schwan, A.; Sjolin, S.; Trottestam, U.; Aronsson, B. Relapsing Clostridium Difficile Enterocolitis Cured by Rectal Infusion of Normal Faeces. Scand. J. Infect. Dis. 1984, 16, 211–215. [Google Scholar] [CrossRef]
- Persky, S.E.; Brandt, L.J. Treatment of Recurrent Clostridium Difficile-Associated Diarrhea by Administration of Donated Stool Directly through a Colonoscope. Am. J. Gastroenterol. 2000, 95, 3283–3285. [Google Scholar] [CrossRef]
- Aas, J.; Gessert, C.E.; Bakken, J.S. Recurrent Clostridium Difficile Colitis: Case Series Involving 18 Patients Treated with Donor Stool Administered via a Nasogastric Tube. Clin. Infect. Dis. 2003, 36, 580–585. [Google Scholar] [CrossRef] [Green Version]
- Bakken, J.S.; Borody, T.; Brandt, L.J.; Brill, J.V.; Demarco, D.C.; Franzos, M.A.; Kelly, C.; Khoruts, A.; Louie, T.; Martinelli, L.P.; et al. Treating Clostridium Difficile Infection With Fecal Microbiota Transplantation. Clin. Gastroenterol. Hepatol. 2011, 9, 1044–1049. [Google Scholar] [CrossRef] [Green Version]
- Gough, E.; Shaikh, H.; Manges, A.R. Systematic Review of Intestinal Microbiota Transplantation (Fecal Bacteriotherapy) for Recurrent Clostridium Difficile Infection. Clin. Infect. Dis. 2011, 53, 994–1002. [Google Scholar] [CrossRef] [Green Version]
- Kassam, Z.; Lee, C.H.; Yuan, Y.; Hunt, R.H. Fecal Microbiota Transplantation for Clostridium Difficile Infection: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2013, 108, 500–508. [Google Scholar] [CrossRef]
- Khoruts, A.; Sadowsky, M.J. Understanding the Mechanisms of Faecal Microbiota Transplantation. Nat. Rev. Gastroentero 2016, 13, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Seekatz, A.M.; Young, V.B. Clostridium Difficile and the Microbiota. J. Clin. Investig. 2014, 124, 4182–4189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weingarden, A.; González, A.; Vázquez-Baeza, Y.; Weiss, S.; Humphry, G.; Berg-Lyons, D.; Knights, D.; Unno, T.; Bobr, A.; Kang, J.; et al. Dynamic Changes in Short- and Long-Term Bacterial Composition Following Fecal Microbiota Transplantation for Recurrent Clostridium Difficile Infection. Microbiome 2015, 3, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broecker, F.; Klumpp, J.; Schuppler, M.; Russo, G.; Biedermann, L.; Hombach, M.; Rogler, G.; Moelling, K. Long-Term Changes of Bacterial and Viral Compositions in the Intestine of a Recovered Clostridium Difficile Patient after Fecal Microbiota Transplantation. Cold Spring Harb. Mol. Case Stud. 2016, 2, a000448. [Google Scholar] [CrossRef] [Green Version]
- Osman, M.; Stoltzner, Z.; O’Brien, K.; Ling, K.; Koelsch, E.; Dubois, N.; Amaratunga, K.; Smith, M.; Kassam, Z. Donor Efficacy in Fecal Microbiota Transplantation for Recurrent Clostridium Difficile: Evidence From a 1,999-Patient Cohort. Open Forum Infect. Dis. 2016, 3, 841. [Google Scholar] [CrossRef]
- Tariq, R.; Saha, S.; Solanky, D.; Pardi, D.S.; Khanna, S. Predictors and Management of Failed Fecal Microbiota Transplantation for Recurrent Clostridioides Difficile Infection. J. Clin. Gastroenterol. 2021, 55, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.E.; Lebwohl, B.; Cuaresma, E.; Cadwell, K.; Green, P.H.R.; Freedberg, D.E. Gut Colonization with Vancomycin-Resistant Enterococcus and Risk for Subsequent Enteric Infection. Gut Pathog. 2018, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Kates, A.E.; Thapaliya, D.; Smith, T.C.; Chorazy, M.L. Prevalence and Molecular Characterization of Staphylococcus Aureus from Human Stool Samples. Antimicrob. Resist. Infect. Control 2018, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Niki, M.; Hirai, I.; Yoshinaga, A.; Ulzii-Orshikh, L.; Nakata, A.; Yamamoto, A.; Yamamoto, M.; Yamamoto, Y. Extended-Spectrum β-Lactamase-Producing Escherichia Coli Strains in the Feces of Carriers Contribute Substantially to Urinary Tract Infections in These Patients. Infection 2011, 39, 467. [Google Scholar] [CrossRef]
- Sakka, V.; Tsiodras, S.; Galani, L.; Antoniadou, A.; Souli, M.; Galani, I.; Pantelaki, M.; Siafakas, N.; Zerva, L.; Giamarellou, H. Risk-factors and Predictors of Mortality in Patients Colonised with Vancomycin-resistant Enterococci. Clin. Microbiol. Infect. 2008, 14, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Davido, B.; Batista, R.; Fessi, H.; Michelon, H.; Escaut, L.; Lawrence, C.; Denis, M.; Perronne, C.; Salomon, J.; Dinh, A. Fecal Microbiota Transplantation to Eradicate Vancomycin-Resistant Enterococci Colonization in Case of an Outbreak. Méd. Mal. Infect. 2019, 49, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Eysenbach, L.; Allegretti, J.R.; Aroniadis, O.; Brandt, L.; Donovan, D.; Fischer, M.; Grinspan, A.; Kassam, Z.; Kelly, C.R.; Kim, C.; et al. Clearance of Vancomycin-Resistant Enterococcus Colonization With Fecal Microbiota Transplantation Among Patients With Recurrent Clostridium Difficile Infection. Open Forum Infect. Dis. 2016, 3, 2119. [Google Scholar] [CrossRef] [Green Version]
- Stripling, J.; Kumar, R.; Baddley, J.W.; Nellore, A.; Dixon, P.; Howard, D.; Ptacek, T.; Lefkowitz, E.J.; Tallaj, J.A.; Benjamin, W.H.; et al. Loss of Vancomycin-Resistant Enterococcus Fecal Dominance in an Organ Transplant Patient With Clostridium Difficile Colitis After Fecal Microbiota Transplant. Open Forum Infect. Dis. 2015, 2, ofv078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seong, H.; Lee, S.K.; Cheon, J.H.; Yong, D.E.; Koh, H.; Kang, Y.K.; Jeong, W.Y.; Lee, W.J.; Sohn, Y.; Cho, Y.; et al. Fecal Microbiota Transplantation for Multidrug-Resistant Organism: Efficacy and Response Prediction. J. Infect. 2020, 81, 719–725. [Google Scholar] [CrossRef]
- Tavoukjian, V. Faecal Microbiota Transplantation for the Decolonisation of Antibiotic-Resistant Bacteria in the Gut: A Systematic Review and Meta-Analysis. J. Hosp. Infect. 2019, 102, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Manges, A.R.; Steiner, T.S.; Wright, A.J. Fecal Microbiota Transplantation for the Intestinal Decolonization of Extensively Antimicrobial-Resistant Opportunistic Pathogens: A Review. Infect. Dis. 2016, 48, 587–592. [Google Scholar] [CrossRef]
- Singh, R.; de Groot, P.F.; Geerlings, S.E.; Hodiamont, C.J.; Belzer, C.; ten Berge, I.J.M.; de Vos, W.M.; Bemelman, F.J.; Nieuwdorp, M. Fecal Microbiota Transplantation against Intestinal Colonization by Extended Spectrum Beta-Lactamase Producing Enterobacteriaceae: A Proof of Principle Study. BMC Res. Notes 2018, 11, 190. [Google Scholar] [CrossRef] [Green Version]
- Merrick, B.; Allen, L.; Zain, N.M.M.; Forbes, B.; Shawcross, D.L.; Goldenberg, S.D. Regulation, Risk and Safety of Faecal Microbiota Transplant. Infect. Prev. Pract. 2020, 2, 100069. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Bloom, P.P.; Soto, M.T.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.-B.; Hohmann, E.L. Drug-Resistant E. Coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef]
- Tvede, M.; Rask-Madsen, J. Bacteriotherapy for Clostridium Difficile Diarrhoea. Lancet 1990, 335, 110. [Google Scholar] [CrossRef]
- Lawley, T.D.; Clare, S.; Walker, A.W.; Stares, M.D.; Connor, T.R.; Raisen, C.; Goulding, D.; Rad, R.; Schreiber, F.; Brandt, C.; et al. Targeted Restoration of the Intestinal Microbiota with a Simple, Defined Bacteriotherapy Resolves Relapsing Clostridium Difficile Disease in Mice. PLoS Pathog. 2012, 8, e1002995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dsouza, M.; Menon, R.; Crossette, E.; Bhattarai, S.K.; Schneider, J.; Kim, Y.-G.; Reddy, S.; Caballero, S.; Felix, C.; Cornacchione, L.; et al. Colonization of the Live Biotherapeutic Product VE303 and Modulation of the Microbiota and Metabolites in Healthy Volunteers. Cell Host Microbe 2022, 30, 583–598.e8. [Google Scholar] [CrossRef] [PubMed]
- Biosciences, V. Phase 2 Study of VE303 for Prevention of Recurrent Clostridium Difficile Infection—Full Text View—ClinicalTrials.Gov. Available online: https://www.clinicaltrials.gov/ct2/show/study/NCT03788434 (accessed on 19 May 2022).
- Singh, S.B.; Young, K.; Silver, L.L. What Is an “Ideal” Antibiotic? Discovery Challenges and Path Forward. Biochem. Pharmacol. 2017, 133, 63–73. [Google Scholar] [CrossRef]
- Outterson, K.; Rex, J.H.; Jinks, T.; Jackson, P.; Hallinan, J.; Karp, S.; Hung, D.T.; Franceschi, F.; Merkeley, T.; Houchens, C.; et al. Accelerating Global Innovation to Address Antibacterial Resistance: Introducing CARB-X. Nat. Rev. Drug Discov. 2016, 15, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Kostyanev, T.; Bonten, M.J.M.; O’Brien, S.; Steel, H.; Ross, S.; François, B.; Tacconelli, E.; Winterhalter, M.; Stavenger, R.A.; Karlén, A.; et al. The Innovative Medicines Initiative’s New Drugs for Bad Bugs Programme: European Public–Private Partnerships for the Development of New Strategies to Tackle Antibiotic Resistance. J. Antimicrob. Chemother. 2016, 71, 290–295. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the Sustainable Discovery and Development of New Antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.J.; Kohanski, M.A.; Collins, J.J. Role of Reactive Oxygen Species in Antibiotic Action and Resistance. Curr. Opin. Microbiol. 2009, 12, 482–489. [Google Scholar] [CrossRef] [Green Version]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef] [Green Version]
- Zeng, D.; Debabov, D.; Hartsell, T.L.; Cano, R.J.; Adams, S.; Schuyler, J.A.; McMillan, R.; Pace, J.L. Approved Glycopeptide Antibacterial Drugs: Mechanism of Action and Resistance. CSH Perspect. Med. 2016, 6, a026989. [Google Scholar] [CrossRef] [Green Version]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A.; Ikryannikova, L.N. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozyigit, I.I.; Dogan, I.; Hocaoglu-Ozyigit, A.; Yalcin, B.; Erdogan, A.; Yalcin, I.E.; Cabi, E.; Kaya, Y. Production of Secondary Metabolites Using Tissue Culture-Based Biotechnological Applications. Front. Plant Sci. 2023, 14, 1132555. [Google Scholar] [CrossRef] [PubMed]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacNair, C.R.; Tsai, C.N.; Rutherford, S.T.; Tan, M.-W. Returning to Nature for the Next Generation of Antimicrobial Therapeutics. Antibiotics 2023, 12, 1267. https://doi.org/10.3390/antibiotics12081267
MacNair CR, Tsai CN, Rutherford ST, Tan M-W. Returning to Nature for the Next Generation of Antimicrobial Therapeutics. Antibiotics. 2023; 12(8):1267. https://doi.org/10.3390/antibiotics12081267
Chicago/Turabian StyleMacNair, Craig R., Caressa N. Tsai, Steven T. Rutherford, and Man-Wah Tan. 2023. "Returning to Nature for the Next Generation of Antimicrobial Therapeutics" Antibiotics 12, no. 8: 1267. https://doi.org/10.3390/antibiotics12081267





