Retrospective Study on Staphylococcus aureus Resistance Profile and Antibiotic Use in a Pediatric Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Inclusion Criteria
2.3. Collected Variables and Microbiological Studies
2.4. Primary and Secondary Aims
2.5. Statistical Analyses
- -
- A descriptive statistical analysis: calculated median and interquartile range for numeric variables and absolute and percentage frequencies for categorical variables;
- -
- An inferential statistical analysis;
- -
- Univariate analysis for comparison of the frequency of resistance to the investigated antibiotics between categories of categorical variables using the χ2 test (or Fisher’s exact test in the case of expected absolute frequencies in at least one of the cells of the contingency table less than 5);
- -
- Non-parametric Mann–Whitney test for the comparison of numerical variables with non-normal distribution between the investigated antibiotic-resistant and non-resistant groups;
- -
- Multivariate analysis using logistic regression for the calculation of the odds ratio and relative 95% confidence interval.
3. Results
3.1. Study Population
- -
- Newborns (0–28 days): 484 patients (40%);
- -
- Infants (29–90 days): 170 patients (14%);
- -
- Children (older than 90 days): 556 patients (46%).
- -
- Pharyngeal swab in 46% of cases;
- -
- Bronchoalveolar lavage (BAL) in 35% of cases;
- -
- Central venous catheter (CVC) in 13% of cases;
- -
- Urine in 4% of cases;
- -
- Other sites in 2% of cases.
3.2. Pattern of Antibiotic Resistance in S. aureus Isolates
- -
- Pharyngeal swab in 24.6% of cases (298 patients);
- -
- Broncho-alveolar lavage (BAL) in 8.6% of cases (104 patients);
- -
- Central venous catheter (CVC) in 0.5% of cases (6 patients);
- -
- Urine in 1.32% of cases (16 patients);
- -
- Other sites in 0.6% of cases (8 patients).
- -
- Pharyngeal swab in 15.2% of cases (184 patients);
- -
- Broncho-alveolar lavage (BAL) in 16.4% of cases (199 patients);
- -
- Central venous catheter (CVC) in 0.4% of cases (5 patients);
- -
- Urine in 0.6% of cases (8 patients);
- -
- Other sites in 0.5% of cases (6 patients).
- -
- Pharyngeal swab in 3.2% of cases (39 patients);
- -
- Broncho-alveolar lavage (BAL) in 5.6% of cases (68 patients);
- -
- Central venous catheter (CVC) in 7.2% of cases (87 patients);
- -
- Urine in 1.2% of cases (13 patients);
- -
- Other sites in 0.57% of cases (7 patients).
- -
- Pharyngeal swab in 2.3% of cases (36 patients);
- -
- Broncho-alveolar lavage (BAL) in 4.4% of cases (53 patients);
- -
- Central venous catheter (CVC) in 4.9% of cases (59 patients);
- -
- Urine in 0.91% of cases (11 patients);
- -
- Other sites in 0.25% of cases (3 patients).
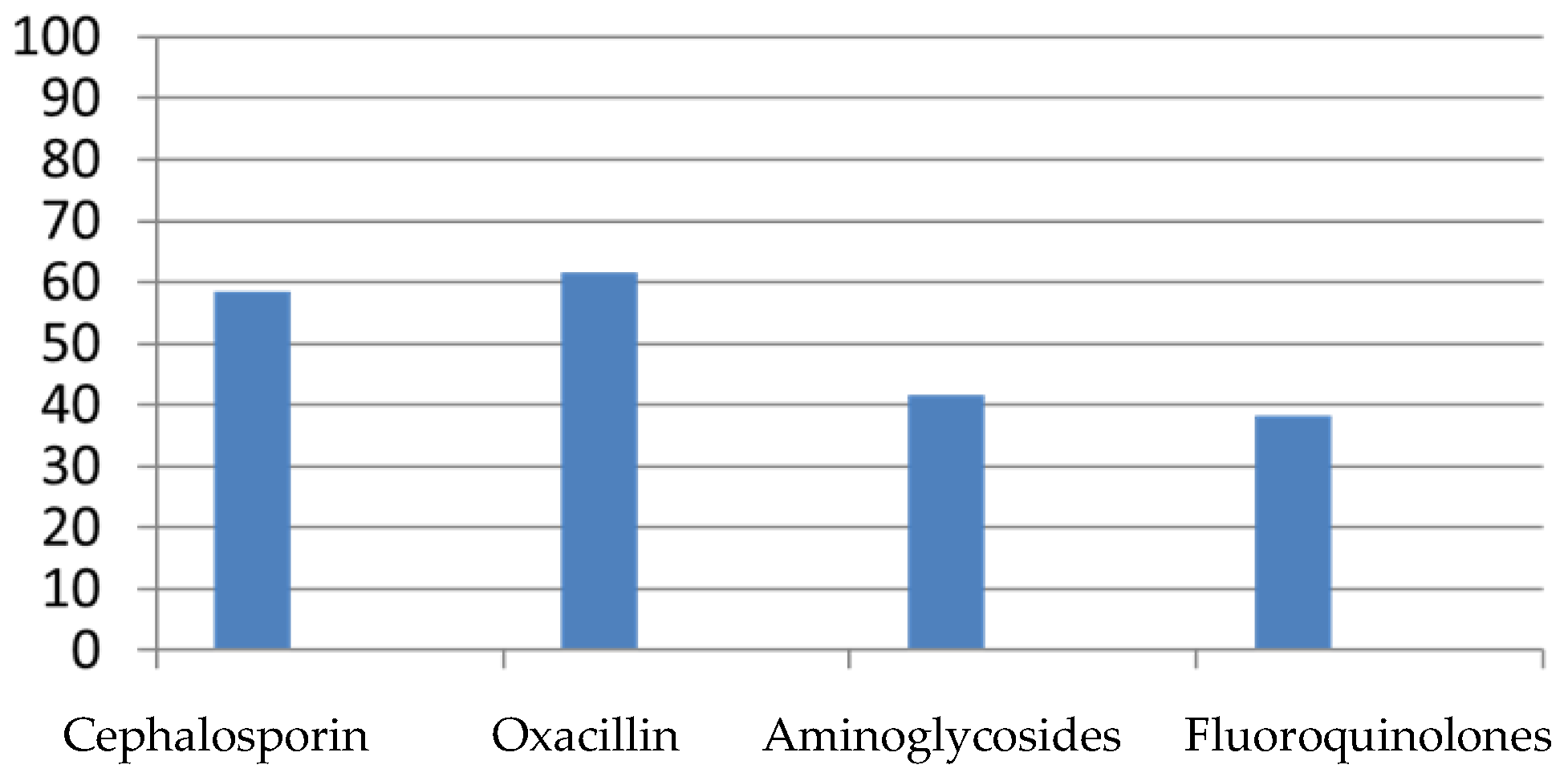
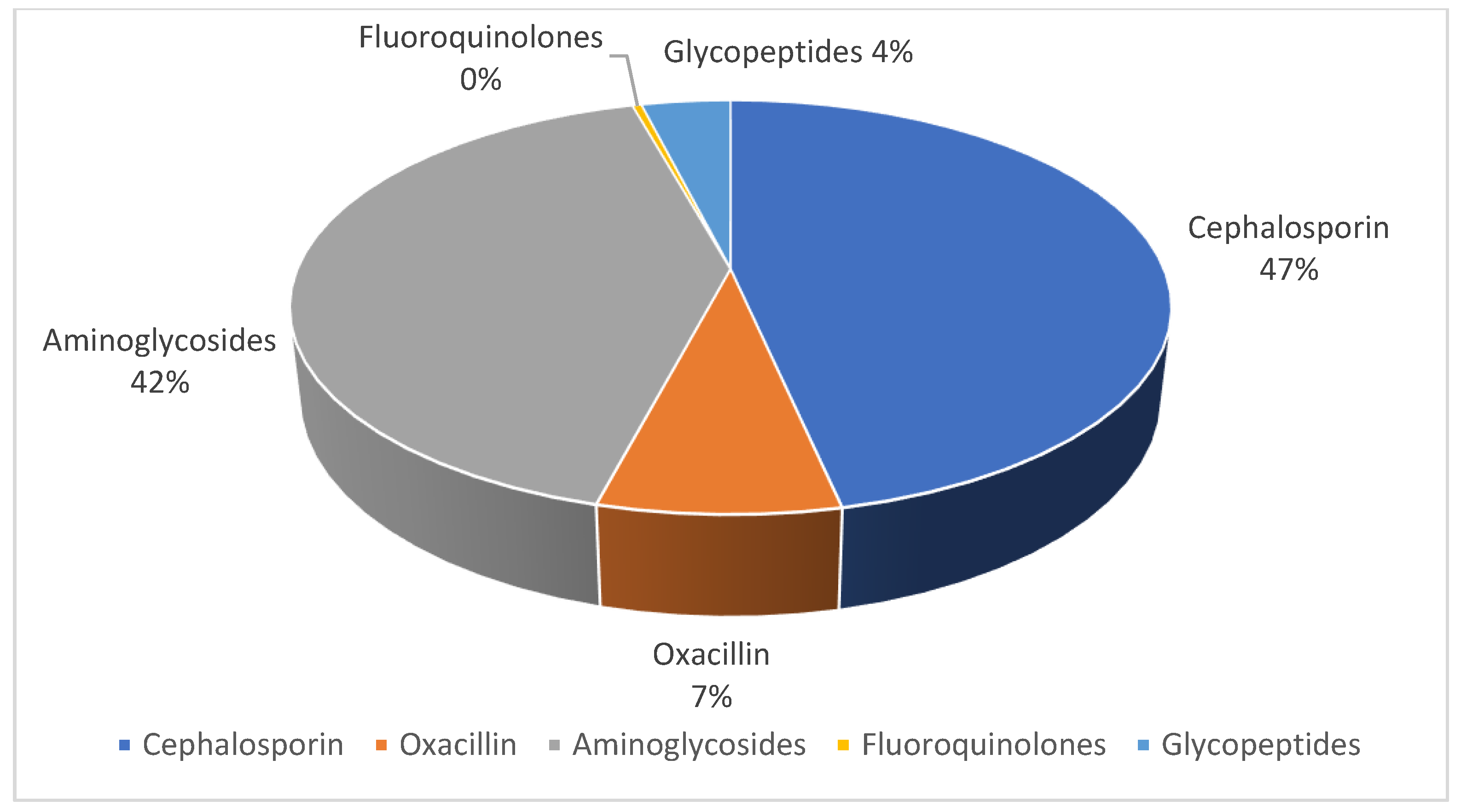
3.3. Empiric and Targeted Antibiotics Used
- -
- Oxacillin or β-lactam therapy was initiated in 48% of cases;
- -
- 42% began therapy with aminoglycosides;
- -
- Cephalosporin therapy was initiated in 9% of cases;
- -
- Fluoroquinolones were initiated in 1% of cases;
- -
- No patients began therapy with glycopeptides.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed. Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Laux, C.; Peschel, A.; Krismer, B. Staphylococcus aureus Colonization of the Human Nose and Interaction with Other Microbiome Members. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Kalu, I.C.; Kao, C.M.; Fritz, S.A. Management and Prevention of Staphylococcus aureus Infections in Children. Infect. Dis. Clin. N. Am. 2022, 36, 73–100. [Google Scholar] [CrossRef]
- Ondusko, D.S.; Nolt, D. Staphylococcus aureus . Pediatr. Rev. 2018, 39, 287–298. [Google Scholar] [CrossRef] [PubMed]
- See, P.; Bonacorsi, S.; Toumazi, A.; Doit, C.; Naudin, J.; Chomton, M.; Le Bourgeois, F.; Caseris, M.; Mariani-Kurkdjian, P.; Poncelet, G.; et al. Factors linked to Staphylococcus aureus healthcare-associated infections among pediatric intensive care unit colonized patients. Arch. Pediatr. 2023, 30, 153–157. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Barankin, B.; Leong, K.F. Staphylococcal-scalded skin syndrome: Evaluation, diagnosis, and management. World J. Pediatr. 2018, 14, 116–120. [Google Scholar] [CrossRef]
- Cassat, J.E.; Thomsen, I. Staphylococcus aureus infections in children. Curr. Opin. Infect. Dis. 2021, 34, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Gómez, N.E.; Merida-Vieyra, J.; Isunza-Alonso, O.D.; Morales-Pirela, M.G.; Colín-Martínez, O.; Juárez-Benítez, E.J.; García de la Puente, S.; Aquino-Andrade, A. Surveillance of osteoarticular infections caused by Staphylococcus aureus in a paediatric hospital in Mexico City. Front. Cell Infect. Microbiol. 2022, 12, 999268. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Campbell, A.J.; Al Yazidi, L.S.; Phuong, L.K.; Leung, C.; Best, E.J.; Webb, R.H.; Voss, L.; Athan, E.; Britton, P.N.; Bryant, P.A.; et al. Pediatric Staphylococcus aureus Bacteremia: Clinical Spectrum and Predictors of Poor Outcome. Clin. Infect. Dis. 2022, 74, 604–613. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! an Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Tsai, C.M.; Hajam, I.A.; Caldera, J.R.; Liu, G.Y. Integrating complex host-pathogen immune environments into S. aureus vaccine studies. Cell Chem. Biol. 2022, 29, 730–740. [Google Scholar] [CrossRef]
- Kumar, S.; He, G.; Kakarla, P.; Shrestha, U.; Ranjana, K.C.; Ranaweera, I.; Willmon, T.M.; Barr, S.R.; Hernandez, A.J.; Varela, M.F. Bacterial Multidrug Efflux Pumps of the Major Facilitator Superfamily as Targets for Modulation. Infect. Disord. Drug Targets 2016, 16, 28–43. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Stewardship Programmes in Health-Care Facilities in Low- and Middle-Income Countries: A WHO Practical Toolkit; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Machowska, A.; Stålsby Lundborg, C. Drivers of Irrational Use of Antibiotics in Europe. Int. J. Environ. Res. Public Health 2018, 16, 27. [Google Scholar] [CrossRef]
- Culyba, M.J.; Mo, C.J.; Kohli, R.M. Targets for Combating the Evolution of Acquired Antibiotic Resistance. Biochemistry 2015, 54, 3573–3582. [Google Scholar] [CrossRef]
- Lambert, P.A. Bacterial resistance to antibiotics: Modified target sites. Adv. Drug Deliv. Rev. 2005, 57, 1471–1485. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Vancomycin Resistance in Staphylococcus aureus. Yale J. Biol. Med. 2017, 90, 269–281. [Google Scholar] [PubMed]
- Mulligan, M.E.; Murray-Leisure, K.A.; Ribner, B.S.; Standiford, H.C.; John, J.F.; Korvick, J.A.; Kauffman, C.A.; Yu, V.L. Methicillin-resistant Staphylococcus aureus: A consensus review of the microbiology, pathogenesis, and epidemiology with implications for prevention and management. Am. J. Med. 1993, 94, 313–328. [Google Scholar] [CrossRef]
- Moran, G.J.; Krishnadasan, A.; Gorwitz, R.J.; Fosheim, G.E.; McDougal, L.K.; Carey, R.B.; Talan, D.A. EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 2006, 355, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, R.A.; Fraser, T.J. The emergence of methicillin-resistant Staphylococcus aureus in the community. Can. J. Infect. Dis. 2003, 14, 249–251. [Google Scholar] [CrossRef]
- Arikan, K.; Karadag-Oncel, E.; Aycan, A.E.; Yuksekkaya, S.; Sancak, B.; Ceyhan, M. Epidemiologic and Molecular Characteristics of Staphylococcus aureus Strains Isolated from Hospitalized Pediatric Patients. Pediatr. Infect. Dis. J. 2020, 39, 1002–1006. [Google Scholar] [CrossRef]
- Bádue Pereira, M.F.; Bando, S.Y.; Sasagawa, S.M.; da Silva, C.B.; Mimica, M.J.; Berezin, E.N. Panton-Valentine Positive Staphylococcus aureus in Community-Acquired and Hospital-Acquired Pediatric Infections. Pediatr. Infect. Dis. J. 2019, 38, 1068–1070. [Google Scholar] [CrossRef]
- Gijón, M.; Bellusci, M.; Petraitiene, B.; Noguera-Julian, A.; Glikman, D.; Saavedra-Lozano, J.; Neth, O.; Daskalaki, M.; Zilinskaite, V.; Kaiser-Labusch, P.; et al. Pediatric Community-Acquired Bone and Joint Staphylococcus Aureus Infections in Europe: Severe Infections are Associated to Panton-Valentine Leucocidin Presence. Pediatr. Infect. Dis. J. 2020, 39, e73–e76. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, L.; Hsu, L.Y.; Kurup, A. Community-associated methicillin-resistant Staphylococcus aureus: Overview and local situation. Ann. Acad. Med. Singap. 2006, 35, 479–486. [Google Scholar] [CrossRef] [PubMed]
- David, M.Z.; Daum, R.S. Community-associated methicillin-resistant Staphylococcus aureus: Epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 2010, 23, 616–687. [Google Scholar] [CrossRef] [PubMed]
- Rojo, P.; Barrios, M.; Palacios, A.; Gomez, C.; Chaves, F. Community-associated Staphylococcus aureus infections in children. Expert. Rev. Anti. Infect. Ther. 2010, 8, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Yueh, C.M.; Chi, H.; Chiu, N.C.; Huang, F.Y.; Huang, D.T.-N.; Chang, L.; Kung, Y.H.; Huang, C.Y. Etiology, clinical features, management, and outcomes of skin and soft tissue infections in hospitalized children: A 10-year review. J. Microbiol. Immunol. Infect. 2022, 55, 728–739. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 13.0. 2023. Available online: http.//www.eucast.org (accessed on 15 June 2023).
- Canty, E.; Carnahan, B.; Curley, T.; Anususinha, E.; Hamdy, R.F.; Ericson, J.E. Reduced Vancomycin Susceptibility, MRSA and Treatment Failure in Pediatric Staphylococcus aureus Bloodstream Infections. Pediatr. Infect. Dis. J. 2021, 40, 429–433. [Google Scholar] [CrossRef]
- Huang, S.H.; Chen, Y.C.; Chuang, Y.C.; Chiu, S.K.; Fung, C.P.; Lu, P.L.; Wang, L.S.; Wu, T.L.; Wang, J.T. Prevalence of vancomycin-intermediate Staphylococcus aureus (VISA) and heterogeneous VISA among methicillin-resistant S. aureus with high vancomycin minimal inhibitory concentrations in Taiwan: A multicenter surveillance study, 2012–2013. J. Microbiol. Immunol. Infect. 2016, 49, 701–707. [Google Scholar] [CrossRef]
- Dong, Q.; Liu, Y.; Li, W.; Chen, M.; Li, W.; Wang, X.; Fu, J.; Ye, X. Phenotypic and Molecular Characteristics of Community-Associated Staphylococcus aureus Infection in Neonates. Infect. Drug Resist. 2020, 13, 4589–4600. [Google Scholar] [CrossRef]
- Sheldon, L.; Jaime, K.; Deville, G.; Yogev, R.; Morfin, M.R.; Wu, E.; Adler, S.; Edge-Padbury, B.; Naberhuis-Stehouwer, S.; Bruss, J.B.; et al. Linezolid versus vancomycin for treatment of resistant Gram-positive infections in children. Pediatr. Infect. Dis. J. 2003, 22, 677–686. [Google Scholar] [CrossRef]
- Walkey, A.J.; O’Donnell, M.R.; Wiener, R.S. Linezolid vs glycopeptide antibiotics for the treatment of suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia: A meta-analysis of randomized controlled trials. Chest 2011, 139, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Keohavong, B.; Vonglokham, M.; Phoummalaysith, B.; Louangpradith, V.; Inthaphatha, S.; Kariya, T.; Saw, Y.M.; Yamamoto, E.; Hamajima, N. Antibiotic prescription for under-fives with common cold or upper respiratory tract infection in Savannakhet Province, Lao PDR. Trop. Med. Health 2019, 47, 16. [Google Scholar] [CrossRef]
- Tzialla, C.; Borghesi, A.; Serra, G.; Stronati, M.; Corsello, G. Antimicrobial therapy in neonatal intensive care unit. Ital. J. Pediatr. 2015, 41, 27. [Google Scholar] [CrossRef] [PubMed]
- Sutter, D.E.; Milburn, E.; Chukwuma, U.; Dzialowy, N.; Maranich, A.M.; Hospenthal, D.R. Changing Susceptibility of Staphylococcus aureus in a US Pediatric Population. Pediatrics 2016, 137, e20153099. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2020; European Centre for Disease Prevention and Control: Solna, Sweden, 2022.
- Llor, C.; Cots, J.M. The sale of antibiotics without prescription in pharmacies in Catalonia, Spain. Clin. Infect. Dis. 2009, 48, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net) Annual Epidemiological Report for 2021; European Centre for Disease Prevention and Control: Solna, Sweden, 2022.
- Ferrer-Bergua, L.G.; Senra, A.M.B. Rate of methicillin-resistant Staphylococcus aureus in pediatric emergency departments in Spain. An. Pediatr. (Engl. Ed.) 2021, 97, 95–102. [Google Scholar] [CrossRef]
- Khamash, D.F.; Voskertchian, A.; Tamma, P.D.; Akinboyo, I.C.; Carroll, K.C.; Milstone, A.M. Increasing Clindamycin and Trimethoprim-Sulfamethoxazole Resistance in Pediatric Staphylococcus aureus Infections. J. Pediatric. Infect. Dis. Soc. 2019, 8, 351–353. [Google Scholar] [CrossRef]
- Pacifici, G.M. Clinical Pharmacokinetics of Penicillins, Cephalosporins and Aminoglycosides in the Neonate: A Review. Pharmaceuticals 2010, 3, 2568–2591. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Zea-Vera, A.; Ochoa, T.J. Challenges in the diagnosis and management of neonatal sepsis. J. Trop. Pediatr. 2015, 61, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Procianoy, R.S.; Silveira, R.C. The challenges of neonatal sepsis management. J. Pediatr. (Rio. J.) 2020, 96 (Suppl. S1), 80–86. [Google Scholar] [CrossRef] [PubMed]
- Pérez, G.; Martiren, S.; Reijtman, V.; Romero, R.; Mastroianni, A.; Casimir, L.; Bologna, R. Community- acquired Staphylococcus aureus bacteremia in children: A cohort study for 2010–2014. Arch. Argent. Pediatr. 2016, 114, 508–513. [Google Scholar] [CrossRef] [PubMed]

| 1210 Participants | N° of Patients |
|---|---|
| Male | 702 (58%) |
| Female | 508 (42%) |
| 0–28 days | 484 (40%) |
| 29–90 days | 170 (14%) |
| >90 days | 556 (46%) |
| Neonatal ICU | 560 (46.2%) |
| Pediatric ICU | 219 (18.1%) |
| Comorbidities | 830 (68.6%) |
| Devices | 814 (67.3%) |
| Isolation Site: | Oxacillin | Cephalosporin | Aminoglycosides | Fluoroquinolones |
|---|---|---|---|---|
| PS (46%) | 24.6% | 15.2% | 3.2% | 2.3% |
| BAL (35%) | 8.6% | 16.4% | 5.6% | 4.4% |
| CVC (13%) | 0.5% | 0.4% | 7.2% | 4.9% |
| Urine (4%) | 1.32% | 0.6% | 1.2% | 0.91% |
| Other sites (2%) | 0.6% | 0.5% | 0.57% | 0.25% |
| Oxacillin Resistance (MRSA) | |||||
|---|---|---|---|---|---|
| YES | NO | ||||
| n | % | n | % | p-Value | |
| Age (days) | |||||
| 0–28 | 235 | 72.53 | 89 | 27.47 | <0.001 |
| 29–90 | 327 | 76.22 | 102 | 23.77 | |
| >90 | 280 | 61.26 | 177 | 38.73 | |
| Sex | |||||
| Male | 425 | 60.54 | 277 | 39.45 | 0.811 |
| Female | 311 | 61.22 | 197 | 38.77 | |
| ICU admission | |||||
| No | 450 | 67.56 | 216 | 32.43 | <0.001 |
| Yes | 219 | 40.25 | 325 | 59.74 | |
| ICU type | |||||
| Neonatal ICU | 144 | 59.01 | 100 | 40.98 | 0.997 |
| Paediatric ICU | 177 | 59.00 | 123 | 41.00 | |
| Previous admissions | |||||
| No | 582 | 69.70 | 253 | 30.29 | <0.001 |
| Yes | 221 | 58.93 | 154 | 41.06 | |
| Comorbidity | |||||
| No | 416 | 57.37 | 309 | 42.62 | <0.001 |
| Yes | 214 | 44.12 | 271 | 55.87 | |
| Type of comorbidity | |||||
| Haematological | 80 | 56.33 | 62 | 43.66 | 0.707 |
| Gastrointestinal | 60 | 42.25 | 55 | 38.73 | |
| Infectious | 63 | 57.27 | 47 | 42.72 | |
| Kidney | 39 | 48.14 | 42 | 51.85 | |
| Other | 19 | 51.35 | 18 | 48.64 | |
| Device | |||||
| No | 285 | 47.49 | 304 | 52.50 | 0.001 |
| Yes | 359 | 56.89 | 272 | 43.10 | |
| Average days hospitalization | 3.7 | 3.7 | |||
| Average emergency room access | 1.09 | 1.09 | |||
| Cephalosporin Resistance | |||||
|---|---|---|---|---|---|
| YES | NO | ||||
| n | % | n | % | p-Value | |
| Age (days) | |||||
| 0–28 | 322 | 61.90 | 198 | 38.07 | <0.001 |
| 29–90 | 210 | 48.38 | 224 | 51.61 | |
| >90 | 147 | 57.42 | 109 | 42.57 | |
| Sex | |||||
| Male | 446 | 63.53 | 256 | 36.46 | 0.304 |
| Female | 308 | 60.62 | 200 | 39.37 | |
| ICU admission | |||||
| No | 384 | 56.38 | 297 | 43.61 | <0.001 |
| Yes | 348 | 65.78 | 181 | 32.21 | |
| ICU type | |||||
| Neonatal ICU | 206 | 63.19 | 120 | 36.80 | 0.203 |
| Paediatric ICU | 117 | 57.63 | 86 | 42.36 | |
| Previous admissions | |||||
| No | 402 | 56.30 | 312 | 43.69 | 0.206 |
| Yes | 261 | 52.62 | 235 | 47.37 | |
| Comorbidity | |||||
| No | 467 | 60.72 | 302 | 39.27 | 0.027 |
| Yes | 239 | 54.19 | 202 | 45.80 | |
| Type of comorbidity | |||||
| Haematological | 53 | 54.08 | 45 | 45.91 | 0.620 |
| Gastrointestinal | 60 | 55.00 | 50 | 45.00 | |
| Infectious | 67 | 62.03 | 41 | 37.96 | |
| Kidney | 57 | 56.43 | 44 | 43.56 | |
| Other | 16 | 66.60 | 8 | 33.30 | |
| Device | |||||
| No | 300 | 50.08 | 299 | 49.91 | <0.001 |
| Yes | 395 | 64.64 | 216 | 35.35 | |
| Average days hospitalization | 3.7 | 3.7 | |||
| Average emergency room access | 1.09 | 1.09 | |||
| Aminoglycosides Resistance | |||||
|---|---|---|---|---|---|
| YES | NO | ||||
| n | % | n | % | p-Value | |
| Age (days) | |||||
| 0–28 | 100 | 34.60 | 189 | 65.39 | 0.039 |
| 29–90 | 172 | 41.05 | 247 | 58.94 | |
| >90 | 220 | 43.82 | 282 | 56.17 | |
| Sex | |||||
| Male | 294 | 41.88 | 408 | 58.11 | 0.249 |
| Female | 196 | 38.58 | 312 | 61.41 | |
| ICU admission | |||||
| No | 289 | 42.12 | 397 | 57.87 | 0.235 |
| Yes | 203 | 38.74 | 321 | 61.25 | |
| ICU type | |||||
| Neonatal ICU | 204 | 72.59 | 77 | 27.40 | 0.014 |
| Paediatric ICU | 152 | 62.55 | 91 | 37.44 | |
| Previous admissions | |||||
| No | 294 | 43.62 | 380 | 56.37 | 0.907 |
| Yes | 232 | 43.28 | 304 | 56.71 | |
| Comorbidity | |||||
| No | 266 | 44.93 | 326 | 55.06 | 0.002 |
| Yes | 223 | 36.08 | 395 | 63.91 | |
| Type of comorbidity | |||||
| Haematological | 34 | 29.56 | 81 | 70.43 | 0.087 |
| Gastrointestinal | 49 | 35.25 | 90 | 64.74 | |
| Infectious | 31 | 20.66 | 119 | 79.33 | |
| Kidney | 36 | 26.08 | 102 | 73.91 | |
| Other | 20 | 26.31 | 56 | 73.68 | |
| Device | |||||
| No | 357 | 56.04 | 280 | 43.95 | 0.578 |
| Yes | 312 | 54.45 | 261 | 45.54 | |
| Average days hospitalization | 3.7 | 3.7 | |||
| Average emergency room access | 1.09 | 1.09 | |||
| Fluoroquinolones Resistance | |||||
|---|---|---|---|---|---|
| YES | NO | ||||
| n | % | n | % | p-Value | |
| Age (days) | |||||
| 0–28 | 220 | 36.91 | 376 | 63.08 | 0.870 |
| 29–90 | 132 | 37.07 | 224 | 62.92 | |
| >90 | 100 | 38.75 | 158 | 61.24 | |
| Sex | |||||
| Male | 289 | 41.16 | 413 | 58.23 | 0.623 |
| Female | 202 | 39.76 | 306 | 60.23 | |
| ICU admission | |||||
| No | 273 | 39.85 | 412 | 60.14 | 0.491 |
| Yes | 199 | 37.90 | 326 | 62.02 | |
| ICU type | |||||
| Neonatal ICU | 130 | 59.09 | 90 | 40.90 | 0.662 |
| Paediatric ICU | 186 | 60.98 | 119 | 39.01 | |
| Previous admissions | |||||
| No | 217 | 30.13 | 503 | 69.86 | 0.006 |
| Yes | 185 | 37.75 | 305 | 62.24 | |
| Comorbidity | |||||
| No | 222 | 32.31 | 465 | 67.68 | 0.364 |
| Yes | 182 | 34.79 | 341 | 65.20 | |
| Type of comorbidity | |||||
| Haematological | 30 | 25.42 | 88 | 74.57 | 0.017 |
| Gastrointestinal | 39 | 33.62 | 77 | 66.37 | |
| Infectious | 49 | 44.95 | 60 | 55.04 | |
| Kidney | 32 | 29.09 | 78 | 70.90 | |
| Other | 19 | 27.14 | 51 | 72.85 | |
| Device | |||||
| No | 271 | 34.08 | 524 | 65.91 | <0.001 |
| Yes | 265 | 63.85 | 150 | 36.14 | |
| Average days hospitalization | 3.7 | 3.7 | |||
| Average emergency room access | 1.09 | 1.09 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonsenso, D.; Giaimo, M.; Pata, D.; Rizzi, A.; Fiori, B.; Spanu, T.; Ruggiero, A.; Attinà, G.; Piastra, M.; Genovese, O.; et al. Retrospective Study on Staphylococcus aureus Resistance Profile and Antibiotic Use in a Pediatric Population. Antibiotics 2023, 12, 1378. https://doi.org/10.3390/antibiotics12091378
Buonsenso D, Giaimo M, Pata D, Rizzi A, Fiori B, Spanu T, Ruggiero A, Attinà G, Piastra M, Genovese O, et al. Retrospective Study on Staphylococcus aureus Resistance Profile and Antibiotic Use in a Pediatric Population. Antibiotics. 2023; 12(9):1378. https://doi.org/10.3390/antibiotics12091378
Chicago/Turabian StyleBuonsenso, Danilo, Martina Giaimo, Davide Pata, Alessia Rizzi, Barbara Fiori, Teresa Spanu, Antonio Ruggiero, Giorgio Attinà, Marco Piastra, Orazio Genovese, and et al. 2023. "Retrospective Study on Staphylococcus aureus Resistance Profile and Antibiotic Use in a Pediatric Population" Antibiotics 12, no. 9: 1378. https://doi.org/10.3390/antibiotics12091378







