Mechanism of Action of Streptococcus downii, a New Bacterial Species with Probiotic Potential
Abstract
:1. Introduction
2. Results
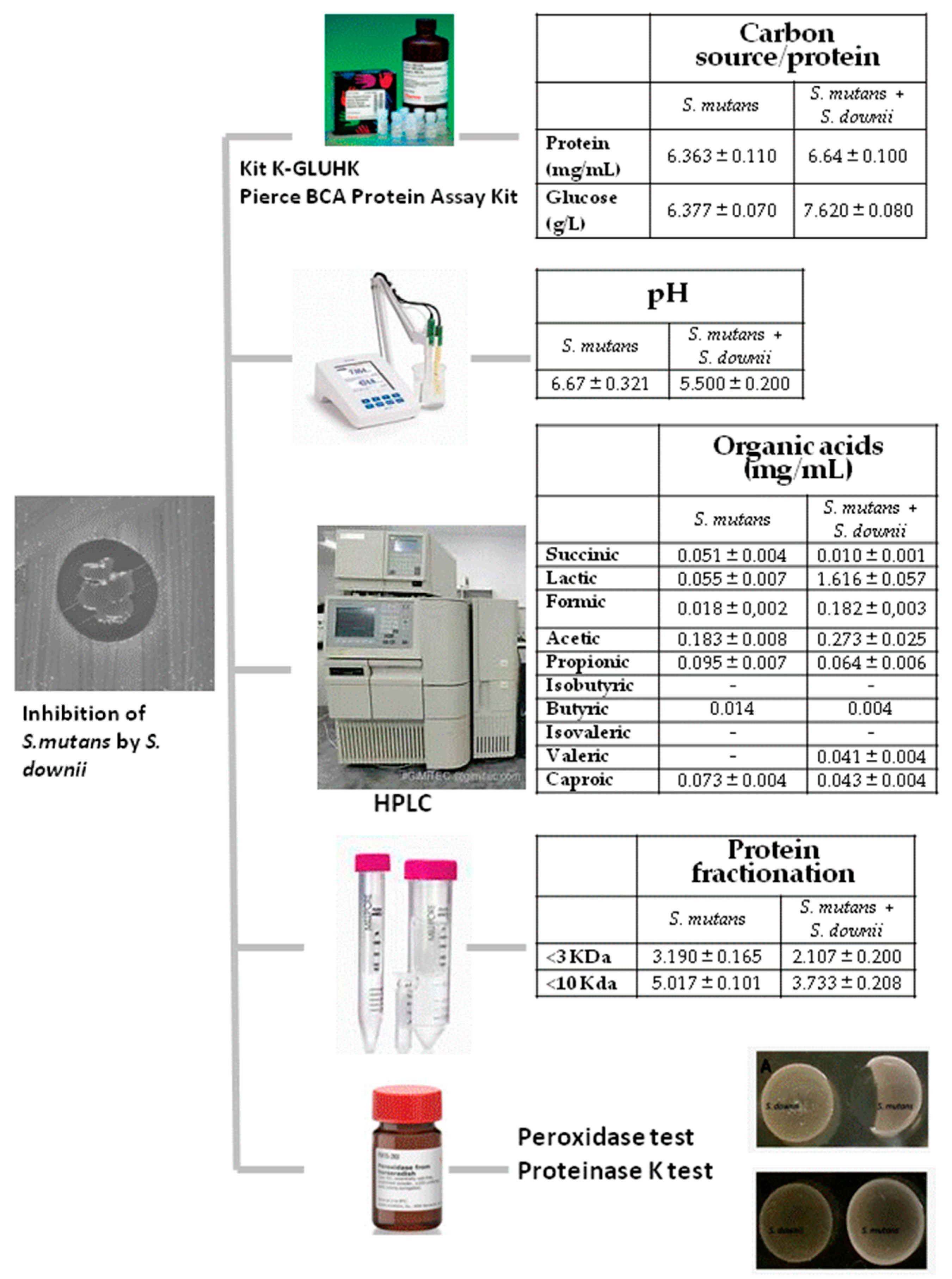
2.1. Availability of Carbon Source and Protein Content
2.2. Modification of Environmental Potential of Hydrogen (pH)
2.3. Organic Acid Production
2.4. Analysis of Protein Fractionation
2.5. Hydrogen Peroxide Production and Proteinase K Test
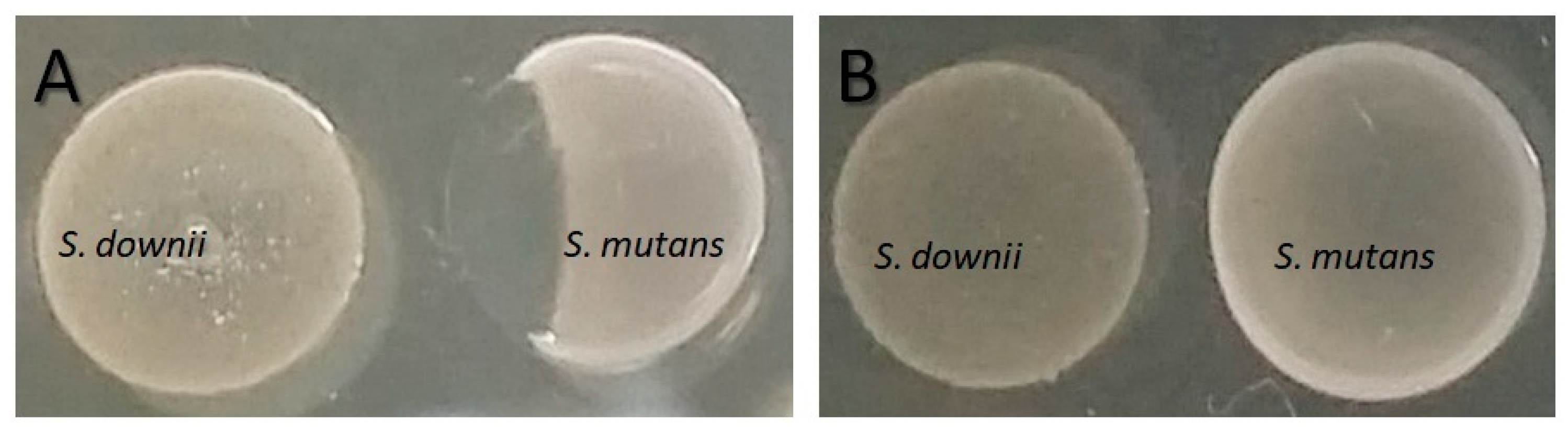
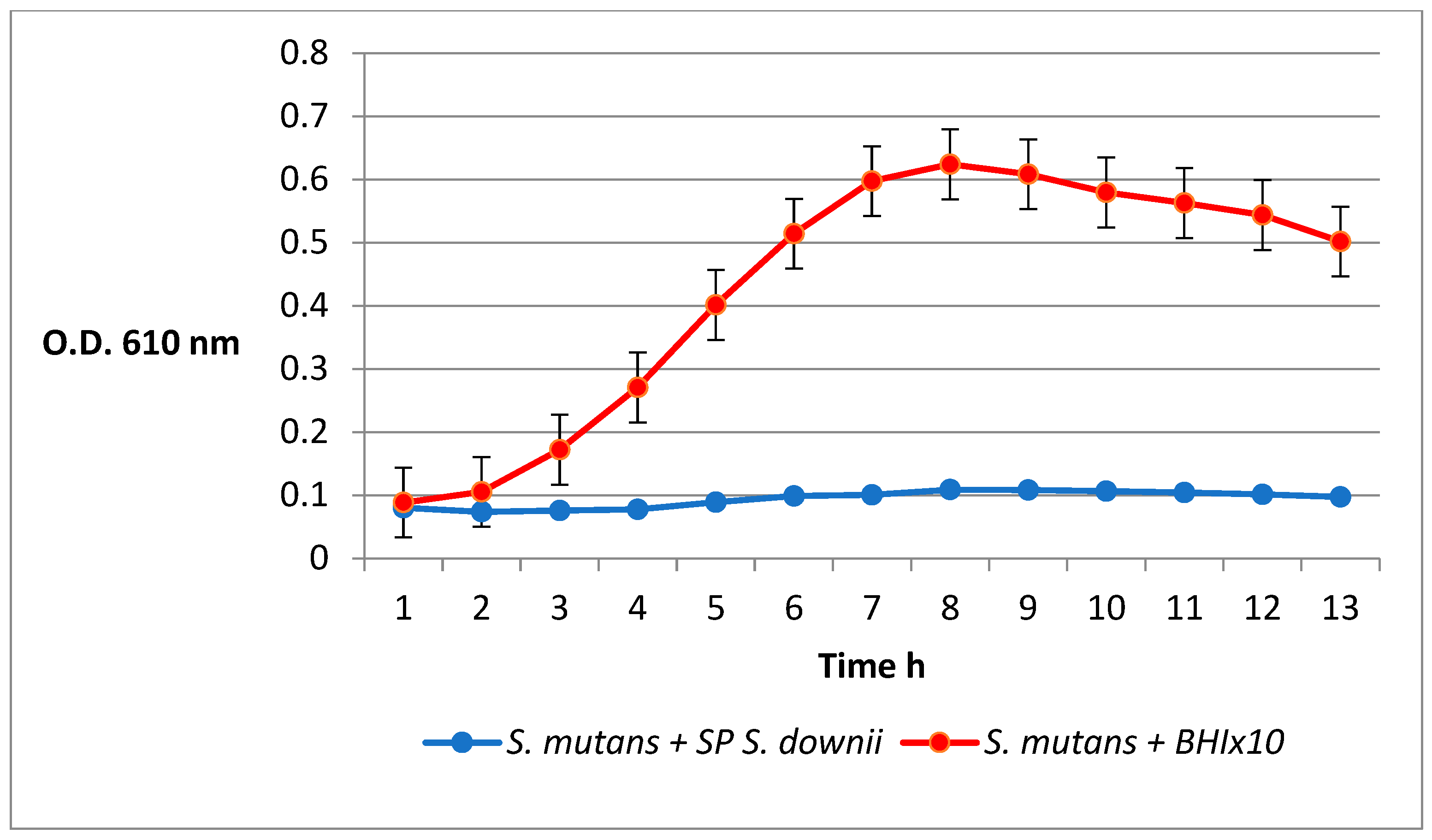
2.6. Inhibition of S. mutans by the S. downii Supernatant
3. Discussion
4. Materials and Methods
4.1. Inhibition of S. mutans by S. downii
4.2. Inhibition of S. mutans by the S. downii Supernatant
4.3. Availability of Carbon Source and Protein Content
4.4. Modification of Environmental pH
4.5. Organic Acid Production
4.6. Analysis of Protein Fractionation
4.7. Hydrogen Peroxide Production and Proteinase K Test
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belda-Ferre, P.; Alcaraz, L.D.; Cabrera-Rubio, R.; Romero, H.; Simon-Soro, A.; Pignatelli, M.; Mira, A. The oral metagenome in health and disease. ISME J. 2012, 6, 46–56. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Solbiati, J.; Frias-Lopez, J. Metatranscriptome of the oral microbiome in health and Disease. J. Dent. Res. 2018, 97, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, Z.; Hwang, G.; Koo, H. Therapeutic strategies targeting cariogenic biofilm microenvironment. Adv. Dent. Res. 2018, 29, 86–92. [Google Scholar] [CrossRef]
- Food and Agricultural Organization of the United Nations; World Health Organization. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; Food and Agricultural Organization of the United Nations: Rome, Italy, 2002. [Google Scholar]
- Kreth, J.; Merritt, J.; Shi, W.; Qi, F. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J. Bacteriol. 2005, 187, 7193–7203. [Google Scholar] [CrossRef] [PubMed]
- Kreth, J.; Zhang, Y.; Herzberg, M.C. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J. Bacteriol. 2008, 190, 4632–4640. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Palmer, R.J., Jr.; Rickard, A.H.; Jakubovics, N.S.; Chalmers, N.I.; Diaz, P.I. Bacterial interactions and successions during plaque development. Periodontology 2000 2006, 42, 47–79. [Google Scholar] [CrossRef]
- Myneni, S.R.; Brocavich, K.H.; Wang, H. Biological strategies for the prevention of periodontal disease: Probiotics and vaccines. Periodontology 2000 2020, 84, 161–175. [Google Scholar] [CrossRef]
- Burton, J.P.; Chilcott, C.N.; Moore, C.J.; Speiser, G.; Tagg, J.R. A preliminary study of the effect of probiotic Streptococcus salivarius K12 on oral malodour parameters. J. Appl. Microbiol. 2006, 100, 754–764. [Google Scholar] [CrossRef]
- Seminario-Amez, M.; López-López, J.; Estrugo-Devesa, A.; Ayuso-Montero, R.; Jané-Salas, E. Probiotics and oral health: A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e282–e288. [Google Scholar] [CrossRef]
- Lodi, C.S.; Oliveira, L.V.; Brighenti, F.L.; Delbem, A.C.B.; Martinhon, C.C.R. Effects of probiotic fermented milk on biofilms, oral microbiota, and enamel. Braz. Oral Res. 2015, 29, S1806–S83242015000100229. [Google Scholar] [CrossRef] [PubMed]
- Logares, R.; Haverkamp, T.H.; Kumar, S.; Lanzén, A.; Nederbragt, A.J.; Quince, C.; Kauserud, H. Environmental microbiology through the lens of high-throughput DNA sequencing: Synopsis of current platforms and bioinformatics approaches. J. Microbiol. Methods 2012, 91, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lamas, L.; Limeres-Posse, J.; Diz-Dios, P.; Álvarez-Fernández, M. Streptococcus downii sp. nov., isolated from the oral cavity of a teenager with Down syndrome. Int. J. Syst. Evol. Microbiol. 2020, 70, 4098–4104. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, M.; Sánchez, M.C.; Diz, P.; Martínez-Lamas, L.; Álvarez, M.; Limeres, J.; Sanz, M.; Herrera, D. In vitro anti-biofilm and antibacterial properties of Streptococcus downii sp. nov. Microorganisms 2021, 9, 450. [Google Scholar] [CrossRef]
- Bhaumik, D.; Manikandan, D.; Foxman, B. Cariogenic and oral health taxa in the oral cavity among children and adults: A scoping review. Arch. Oral Biol. 2021, 129, 105204. [Google Scholar] [CrossRef]
- Alok, A.; Singh, I.D.; Singh, S.; Kishore, M.; Jha, P.C.; Iqubal, M.A. Probiotics: A new era of biotherapy. Adv. Biomed. Res. 2017, 6, 31. [Google Scholar] [CrossRef]
- Saha, S.; Tomaro-Duchesneau, C.; Rodes, L.; Malhotra, M.; Tabrizian, M.; Prakash, S. Investigation of probiotic bacteria as dental caries and periodontal disease biotherapeutics. Benef. Microbes 2014, 5, 447–460. [Google Scholar] [CrossRef]
- Teughels, W.; Kinder Haake, S.; Sliepen, I.; Pauwels, M.; van Eldere, J.; Cassiman, J.J.; Quirynen, M. Bacteria interfere with A. actinomycetemcomitans colonization. J. Dent. Res. 2007, 86, 611–617. [Google Scholar] [CrossRef]
- Van Hoogmoed, C.G.; Geertsema-Doornbusch, G.I.; Teughels, W.; Quirynen, M.; Busscher, H.J.; van der Mei, H.C. Reduction of periodontal pathogens adhesion by antagonistic strains. Oral Microbiol. Immunol. 2008, 23, 43–48. [Google Scholar] [CrossRef]
- Ferrer, M.D.; López-López, A.; Nicolescu, T.; Perez-Vilaplana, S.; Boix-Amorós, A.; Dzidic, M.; Garcia, S.; Artacho, A.; Llena, C.; Mira, A. Topic application of the probiotic Streptococcus dentisani improves clinical and microbiological parameters associated with oral health. Front. Cell. Infect. Microbiol. 2020, 10, 465. [Google Scholar] [CrossRef]
- Chen, L.J.; Tsai, H.T.; Chen, W.J.; Hsieh, C.Y.; Wang, P.C.; Chen, C.S.; Wang, L.; Yang, C.C. In vitro antagonistic growth effects of Lactobacillus fermentum and Lactobacillus salivarius and their fermentative broth on periodontal pathogens. Braz. J. Microbiol. 2012, 43, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Poorni, S.; Srinivasan, M.R.; Nivedhitha, M.S. Probiotic Streptococcus strains in caries prevention: A systematic review. J. Conserv. Dent. 2019, 22, 123–128. [Google Scholar] [CrossRef]
- Huang, X.; Browngardt, C.M.; Jiang, M.; Ahn, S.J.; Burne, R.A.; Nascimento, M.M. Diversity in antagonistic interactions between commensaloral Streptococci and Streptococcus mutans. Caries Res. 2018, 52, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Walker, A.R.; Chakraborty, B.; Kaspar, J.R.; Nascimento, M.M.; Burne, R.A. Exploring novel probiotic mechanisms of Streptococcus A12 with functional genomics. bioRxiv 2019. [Google Scholar] [CrossRef]
- Allaker, R.P.; Ian Douglas, C. Non-conventional therapeutics for oral infections. Virulence 2015, 6, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Polonskaia, M.S. Antibiotic substances in acidophil bacteria. Mikrobiologiia 1952, 21, 303–310. [Google Scholar]
- Silva, M.; Jacobus, N.V.; Deneke, C.; Gorbach, S.L. Antimicrobial substance from a human Lactobacillus strain. Antimicrob. Agents Chemother. 1987, 31, 1231–1233. [Google Scholar] [CrossRef]
- Hillman, J.D.; Shivers, M. Interaction between wild-type, mutant and revertant forms of the bacterium Streptococcus sanguis and the bacterium Actinobacillus actinomycetemcomitans in vitro and in the gnotobiotic rat. Arch. Oral Biol. 1988, 33, 395–401. [Google Scholar] [CrossRef]
- Tong, H.; Dong, Y.; Wang, X.; Hu, Q.; Yang, F.; Yi, M.; Deng, H.; Dong, X. Redox-regulated adaptation of Streptococcus oligofermentans to hydrogen peroxide stress. mSystems 2020, 5, e00006-20. [Google Scholar] [CrossRef]
- Bao, X.; de Soet, J.J.; Tong, H.; Gao, X.; He, L.; van Loveren, C.; Deng, D.M. Streptococcus oligofermentans inhibits Streptococcus mutans in biofilms at both neutral pH and cariogenic conditions. PLoS ONE 2015, 10, e0130962. [Google Scholar] [CrossRef]
- Huang, X.; Palmer, S.R.; Ahn, S.J.; Richards, V.P.; Williams, M.L.; Nascimento, M.M.; Burne, R.A. A highly arginolytic Streptococcus species that potently antagonizes Streptococcus mutans. Appl. Environ. Microbiol. 2016, 82, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Díaz-Ropero, M.P.; Martín, R.; Rodríguez, J.M.; Xaus, J. Antimicrobial potential of four Lactobacillus strains isolated from breast milk. J. Appl. Microbiol. 2006, 101, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Terai, T.; Okumura, T.; Imai, S.; Nakao, M.; Yamaji, K.; Ito, M.; Nagata, T.; Kaneko, K.; Miyazaki, K.; Okada, A.; et al. Screening of probiotic candidates in human oral bacteria for the prevention of dental disease. PLoS ONE 2015, 10, e0128657. [Google Scholar] [CrossRef] [PubMed]
- Arihara, K.; Ogihara, S.; Mukai, T.; Itoh, M.; Kondo, Y. Salivacin 140, a novel bacteriocin from Lactobacillus salivarius subsp. salicinius T140 active against pathogenic bacteria. Lett. Appl. Microbiol. 1996, 22, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Ganzle, M.G.; Höltzel, A.; Walter, J.; Jung, G.; Hammes, W.P. Characterization of reutericyclin produced by Lactobacillus reuteri LTH2584. Appl. Environ. Microbiol. 2000, 66, 4325–4333. [Google Scholar] [CrossRef]
- Van Reenen, C.A.; Dicks, L.M.; Chikindas, M.L. Isolation, purification and partial characterization of plantaricin 423, a bacteriocin produced by Lactobacillus plantarum. J. Appl. Microbiol. 1998, 84, 1131–1137. [Google Scholar] [CrossRef]
- Baca-Castanón, M.L.; De la Garza-Ramos, M.A.; Alcázar-Pizana, A.G.; Grondin, Y.; Coronado-Mendoza, A.; Sánchez-Najera, R.I.; Cárdenas-Estrada, E.; Medina-De la Garza, C.E.; Escamilla-García, E. Antimicrobial effect of Lactobacillus reuteri on cariogenic bacteria Streptococcus gordonii, Streptococcus mutans, and periodontal diseases Actinomyces naeslundii and Tannerella forsythia. Probiotics Antimicrob. Proteins 2015, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hillman, J.D.; McDonell, E.; Cramm, T.; Hillman, C.H.; Zahradnik, R.T. A spontaneous lactate dehydrogenase deficient mutant of Streptococcus rattus for use as a probiotic in the prevention of dental caries. J. Appl. Microbiol. 2009, 107, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Hoare, A.; Marsh, P.D.; Diaz, P.I. Ecological therapeutic opportunities for oral diseases. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Hillman, J.D.; Johnson, K.P.; Yaphe, B.I. Isolation of a Streptococcus mutans strain producing a novel bacteriocin. Infect. Immun. 1984, 44, 141–144. [Google Scholar] [CrossRef]
- Walker, G.V.; Heng, N.C.; Carne, A.; Tagg, J.R.; Wescombe, P.A. Salivaricin E and abundant dextranase activity may contribute to the anti-cariogenic potential of the probiotic candidate Streptococcus salivarius JH. Microbiology 2016, 162, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Masdea, L.; Kulik, E.M.; Hauser-Gerspach, I.; Ramseier, A.M.; Filippi, A.; Waltimo, T. Antimicrobial activity of Streptococcus salivarius K12 on bacteria involved in oral malodour. Arch. Oral Biol. 2012, 57, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Zanvit, A.; Nobili, P.; Risso, P.; Fornaini, C. Cariogram outcome after 90 days of oral treatment with Streptococcus salivarius M18 in children at high risk for dental caries: Results of a randomized, controlled study. Clin. Cosmet. Investig. Dent. 2015, 7, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Camelo-Castillo, A.; Benítez-Páez, A.; Belda-Ferre, P.; Cabrera-Rubio, R.; Mira, A. Streptococcus dentisani sp. nov., a novel member of the mitis group. Int. J. Syst. Evol. Microbiol. 2014, 64, 60–65. [Google Scholar] [CrossRef] [PubMed]
- López-López, A.; Camelo-Castillo, A.; Ferrer, M.D.; Simon-Soro, Á.; Mira, A. Health-Associated niche inhabitants as oral probiotics: The case of Streptococcus dentisani. Front. Microbiol. 2017, 8, 379. [Google Scholar] [CrossRef]
- Gönczi, N.N.; Strang, O.; Bagi, Z.; Rákhely, G.; Kovács, K.L. Interactions between probiotic and oral pathogenic strains. Biol. Futur. 2021, 72, 461–471. [Google Scholar] [CrossRef]
- Nes, I.F.; Diep, D.B.; Holo, H. Bacteriocin diversity in Streptococcus and Enterococcus. J. Bacteriol. 2007, 189, 1189–1198. [Google Scholar] [CrossRef]
- Armstrong, B.D.; Herfst, C.A.; Tonial, N.C.; Wakabayashi, A.T.; Zeppa, J.J.; McCormick, J.K. Identification of a two-component Class IIb bacteriocin in Streptococcus pyogenes by recombinase-based in vivo expression technology. Sci. Rep. 2016, 6, 36233. [Google Scholar] [CrossRef]
- Heng, N.C.; Haji-Ishak, N.S.; Kalyan, A.; Wong, A.Y.; Lovrić, M.; Bridson, J.M.; Artamonova, J.; Stanton, J.A.L.; Wescombe, P.A.; Burton, J.P.; et al. Genome sequence of the bacteriocin-producing oral probiotic Streptococcus salivarius strain M18. J. Bacteriol. 2011, 193, 6402–6403. [Google Scholar] [CrossRef]
- Conrads, G.; Westenberger, J.; Lürkens, M.; Abdelbary, M.M. Isolation and Bacteriocin-Related Typing of Streptococcus dentisani. Front. Cell. Infect. Microbiol. 2019, 9, 110. [Google Scholar] [CrossRef]
- Martinez Lamas, L. Identification of Streptococcus downii sp. nov., a New Bacterial Species with Probiotic Potential. Doctoral Thesis, University of Vigo, Vigo, Spain, 2022. [Google Scholar]
- Robson, G.D.; Bell, S.D.; Kuhn, P.J.; Trinci, A.P. Glucose and penicillin concentrations in agar medium below fungal colonies. J. Gen. Microbiol. 1987, 133, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Silva, Á.; Gonzalez, N.; Terrén, A.; García, A.; Martinez-Blanch, J.F.; Illescas, V.; Morales, J.; Maroto, M.; Genovés, S.; Ramón, D.; et al. An infant milk formula supplemented with heat-treated probiotic Bifidobacterium animalis subsp. lactis CECT 8145, reduces fat deposition in C. elegans and augments acetate and lactate in a fermented infant slurry. Foods 2020, 9, E652. [Google Scholar]
- Zhu, L.; Kreth, J. The role of hydrogen peroxide in environmental adaptation of oral microbial communities. Oxidative Med. Cell. Longev. 2012, 2012, e717843. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, K.; Hallander, H.O. Production of bactericidal concentrations of hydrogen peroxide by Streptococcus sanguis. Arch. Oral Biol. 1973, 18, 423–434. [Google Scholar] [CrossRef] [PubMed]
| Organic Acids (mg/mL) | |||
|---|---|---|---|
| S. mutans | S. mutans + S. downii | p-Value | |
| Succinic | 0.051 ± 0.004 | 0.010 ± 0.001 | 0.1 |
| Lactic | 0.055 ± 0.007 | 1.616 ± 0.057 | 0.1 |
| Formic | 0.018 ± 0.002 | 0.182 ± 0.003 | 0.1 |
| Acetic | 0.183 ± 0.008 | 0.273 ± 0.025 | 0.1 |
| Propionic | 0.095 ± 0.007 | 0.064 ± 0.006 | 0.1 |
| Isobutyric | - | - | - |
| Butyric | 0.014 | 0.004 | - |
| Isovaleric | - | - | - |
| Valeric | - | 0.041 ± 0.004 | - |
| Caproic | 0.073 ± 0.004 | 0.043 ± 0.004 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Lamas, L.; García-Mato, E.; Rincón-Quintero, A.; Rivas-Mundiña, B.; Diz-Dios, P.; Álvarez-Fernández, M. Mechanism of Action of Streptococcus downii, a New Bacterial Species with Probiotic Potential. Antibiotics 2023, 12, 1472. https://doi.org/10.3390/antibiotics12091472
Martínez-Lamas L, García-Mato E, Rincón-Quintero A, Rivas-Mundiña B, Diz-Dios P, Álvarez-Fernández M. Mechanism of Action of Streptococcus downii, a New Bacterial Species with Probiotic Potential. Antibiotics. 2023; 12(9):1472. https://doi.org/10.3390/antibiotics12091472
Chicago/Turabian StyleMartínez-Lamas, Lucía, Eliane García-Mato, Anniris Rincón-Quintero, Berta Rivas-Mundiña, Pedro Diz-Dios, and Maximiliano Álvarez-Fernández. 2023. "Mechanism of Action of Streptococcus downii, a New Bacterial Species with Probiotic Potential" Antibiotics 12, no. 9: 1472. https://doi.org/10.3390/antibiotics12091472





