No Sequestration of Commonly Used Anti-Infectives in the Extracorporeal Membrane Oxygenation (ECMO) Circuit—An Ex Vivo Study
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Drug Selection
4.2. Experimental Setup
4.3. Experiment
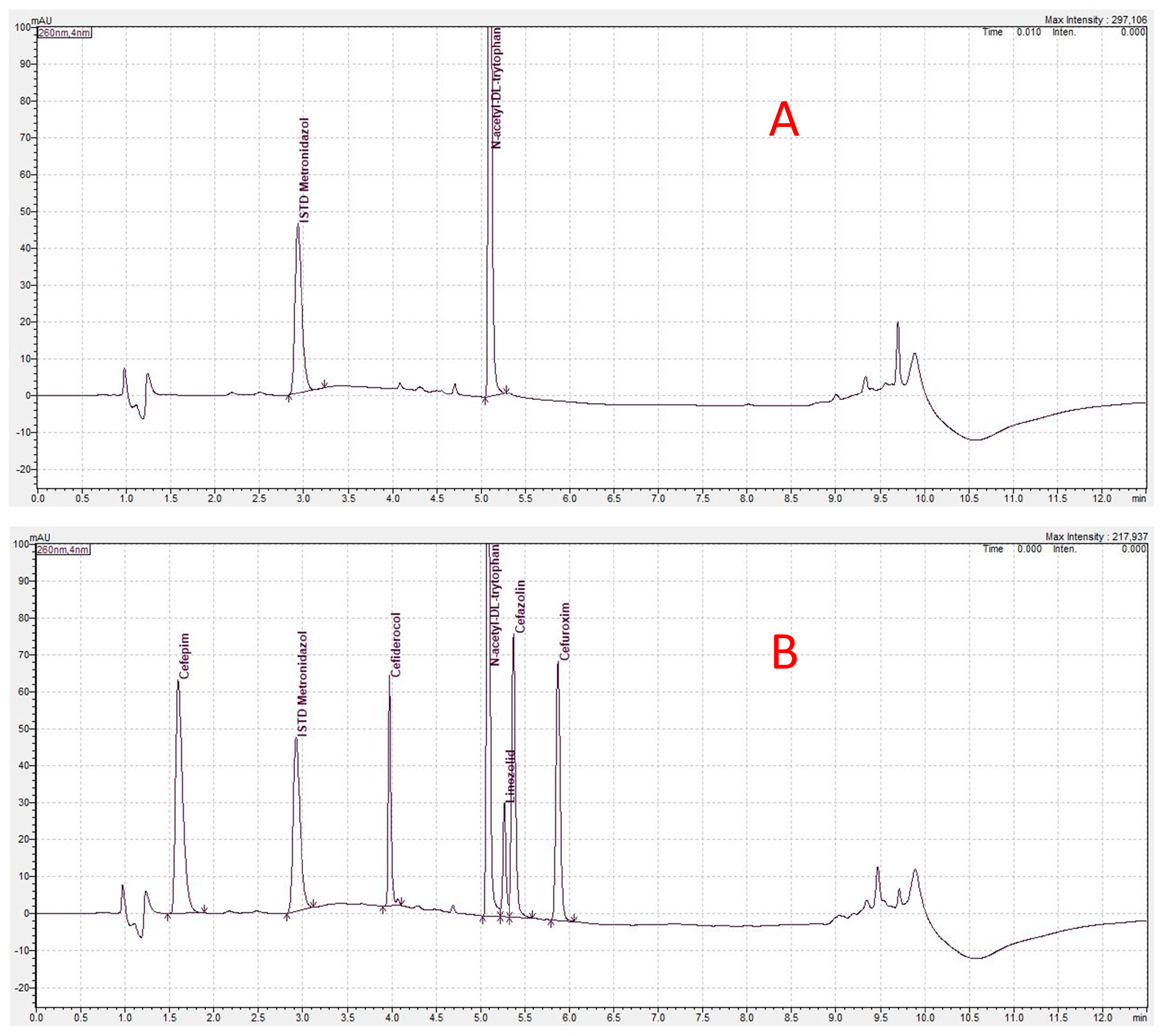
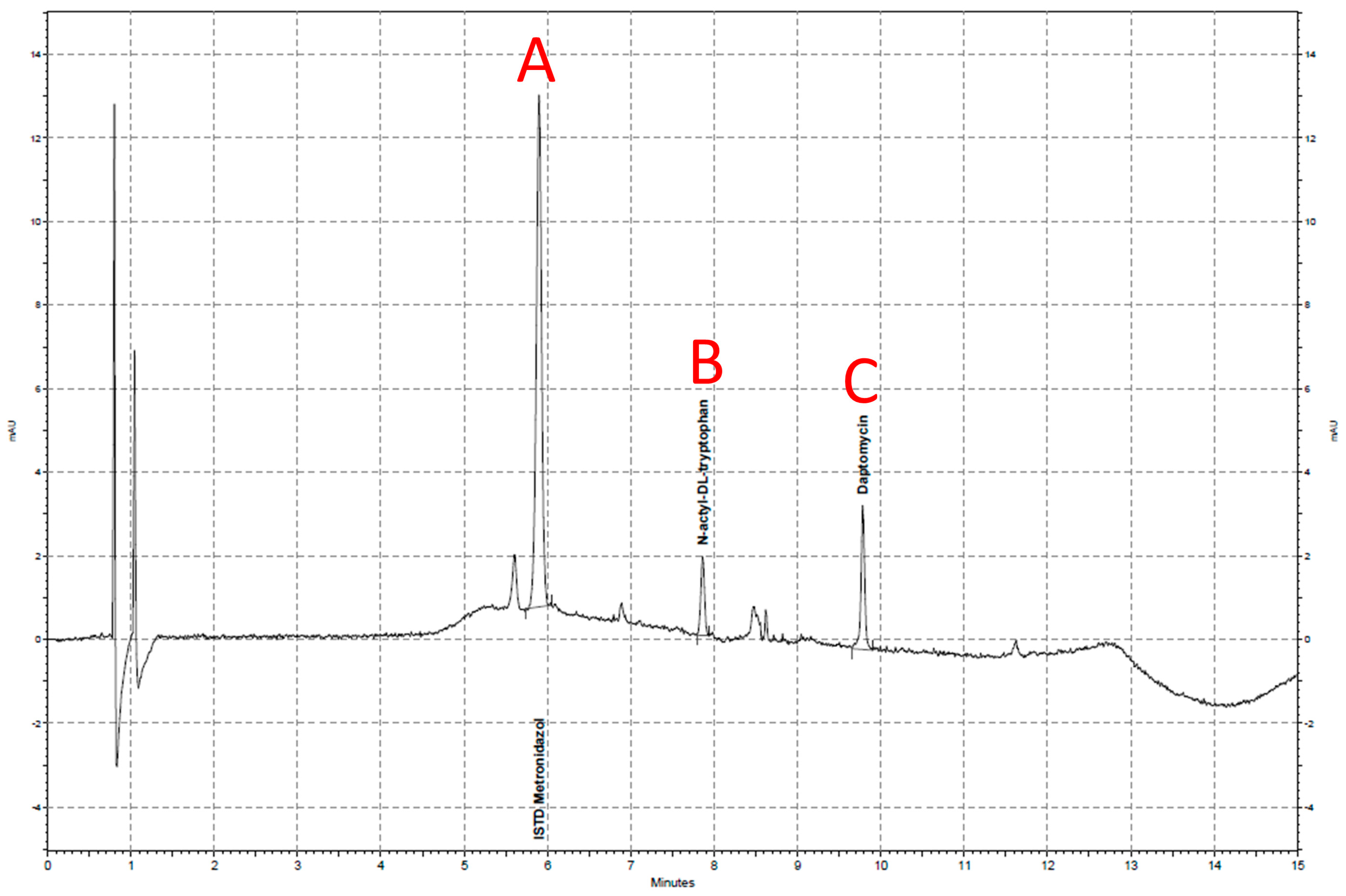
4.4. Bioanalytical Methodology
4.5. Concentration Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised Antibiotic Dosing for Patients Who Are Critically Ill: Challenges and Potential Solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Lipman, J. Pharmacokinetic Issues for Antibiotics in the Critically Ill Patient. Crit. Care Med. 2009, 37, 840–851. [Google Scholar] [CrossRef]
- Ha, M.A.; Sieg, A.C. Evaluation of Altered Drug Pharmacokinetics in Critically Ill Adults Receiving Extracorporeal Membrane Oxygenation. Pharmacotherapy 2017, 37, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Shekar, K.; Roberts, J.A.; Smith, M.T.; Fung, Y.L.; Fraser, J.F. The ECMO PK Project: An Incremental Research Approach to Advance Understanding of the Pharmacokinetic Alterations and Improve Patient Outcomes during Extracorporeal Membrane Oxygenation. BMC Anesthesiol. 2013, 13, 7. [Google Scholar] [CrossRef]
- Duceppe, M.A.; Kanji, S.; Do, A.T.; Ruo, N.; Cavayas, Y.A.; Albert, M.; Robert-Halabi, M.; Zavalkoff, S.; Dupont, P.; Samoukovic, G.; et al. Pharmacokinetics of Commonly Used Antimicrobials in Critically Ill Adults During Extracorporeal Membrane Oxygenation: A Systematic Review. Drugs 2021, 81, 1307–1329. [Google Scholar] [CrossRef]
- Mayr, V.D.; Dünser, M.W.; Greil, V.; Jochberger, S.; Luckner, G.; Ulmer, H.; Friesenecker, B.E.; Takala, J.; Hasibeder, W.R. Causes of Death and Determinants of Outcome in Critically Ill Patients. Crit. Care 2006, 10, R154. [Google Scholar] [CrossRef]
- Cies, J.J.; Moore, W.S.; Giliam, N.; Low, T.; Enache, A.; Chopra, A. Impact of Ex-Vivo Extracorporeal Membrane Oxygenation Circuitry on Daptomycin. Perfusion 2018, 33, 624–629. [Google Scholar] [CrossRef]
- Aguilar, G.; Ferriols, R.; Carbonell, J.A.; Ezquer, C.; Alonso, J.M.; Villena, A.; Puig, J.; Navarro, D.; Alós, M.; Belda, F.J. Pharmacokinetics of Anidulafungin during Venovenous Extracorporeal Membrane Oxygenation. Crit. Care 2016, 20, 325. [Google Scholar] [CrossRef]
- Kühn, D.; Metz, C.; Seiler, F.; Wehrfritz, H.; Roth, S.; Alqudrah, M.; Becker, A.; Bracht, H.; Wagenpfeil, S.; Hoffmann, M.; et al. Antibiotic Therapeutic Drug Monitoring in Intensive Care Patients Treated with Different Modalities of Extracorporeal Membrane Oxygenation (ECMO) and Renal Replacement Therapy: A Prospective, Observational Single-Center Study. Crit. Care 2020, 24, 664. [Google Scholar] [CrossRef]
- Nikolos, P.; Osorio, J.; Mohrien, K.; Rose, C. Pharmacokinetics of Linezolid for Methicillin-Resistant Staphylococcus Aureus Pneumonia in an Adult Receiving Extracorporeal Membrane Oxygenation. Am. J. Health. Syst. Pharm. 2020, 77, 877–881. [Google Scholar] [CrossRef]
- De Rosa, F.G.; Corcione, S.; Baietto, L.; Ariaudo, A.; Di Perri, G.; Ranieri, V.M.; D’Avolio, A. Pharmacokinetics of Linezolid during Extracorporeal Membrane Oxygenation. Int. J. Antimicrob. Agents 2013, 41, 590–591. [Google Scholar] [CrossRef] [PubMed]
- Shekar, K.; Roberts, J.A.; Mcdonald, C.I.; Ghassabian, S.; Anstey, C.; Wallis, S.C.; Mullany, D.V.; Fung, Y.L.; Fraser, J.F. Protein-Bound Drugs Are Prone to Sequestration in the Extracorporeal Membrane Oxygenation Circuit: Results from an Ex Vivo Study. Crit. Care 2015, 19, 164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, H.; Zhang, Q.; Ou, Q.; Zhou, H.; Sha, T.; Zeng, Z.; Wu, J.; Lu, J.; Chen, Z. Effects of Ex Vivo Extracorporeal Membrane Oxygenation Circuits on Sequestration of Antimicrobial Agents. Front. Med. 2021, 8, 748769. [Google Scholar] [CrossRef] [PubMed]
- Wildschut, E.D.; Ahsman, M.J.; Allegaert, K.; Mathot, R.A.A.; Tibboel, D. Determinants of Drug Absorption in Different ECMO Circuits. Intensive Care Med. 2010, 36, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.M.; Halwick, D.R.; Dodson, B.L.; Thompson, J.E.; Arnold, J.H. Potential Drug Sequestration during Extracorporeal Membrane Oxygenation: Results from an Ex Vivo Experiment. Intensive Care Med. 2007, 33, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Cies, J.J.; Moore, W.S.; Giliam, N.; Low, T.; Enache, A.; Chopra, A. Oxygenator Impact on Ceftaroline in Extracorporeal Membrane Oxygenation Circuits. Pediatr. Crit. Care Med. 2018, 19, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Cies, J.J.; Moore, W.S.; Giliam, N.; Low, T.; Enache, A.; Chopra, A. Oxygenator Impact on Ceftolozane and Tazobactam in Extracorporeal Membrane Oxygenation Circuits. Pediatr. Crit. Care Med. 2020, 21, 276–282. [Google Scholar] [CrossRef]
- Dagan, O.; Klein, J.; Gruenwald, C.; Bohn, D.; Barker, G.; Koren, G. Preliminary Studies of the Effects of Extracorporeal Membrane Oxygenator on the Disposition of Common Pediatric Drugs. Ther. Drug Monit. 1993, 15, 263–266. [Google Scholar] [CrossRef]
- Hinderling, P.H.; Hartmann, D. The pH Dependency of the Binding of Drugs to Plasma Proteins in Man. Ther. Drug Monit. 2005, 27, 71–85. [Google Scholar] [CrossRef]
- Doyle, A.J.; Hunt, B.J. Current Understanding of How Extracorporeal Membrane Oxygenators Activate Haemostasis and Other Blood Components. Front. Med. 2018, 5, 352. [Google Scholar] [CrossRef]
- Shekar, K.; Roberts, J.A.; Mcdonald, C.I.; Fisquet, S.; Barnett, A.G.; Mullany, D.V.; Ghassabian, S.; Wallis, S.C.; Fung, Y.L.; Smith, M.T.; et al. Sequestration of Drugs in the Circuit May Lead to Therapeutic Failure during Extracorporeal Membrane Oxygenation. Crit. Care 2012, 16, R194. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. DrugBank: A Comprehensive Resource for in Silico Drug Discovery and Exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef] [PubMed]
- Peters, F.; Schmidt, G.; Herbold, M. Methodenvalidierung im Forensisch-Toxikologischen Labor: Auswertung von Validierungsdaten Nach den Richtlinien der GTFCh Mit VALISTAT; Arvecon GmbH: Walldorf, Germany, 2003. [Google Scholar]
- Zimmer, J.; Röhr, A.C.; Kluge, S.; Faller, J.; Frey, O.R.; Wichmann, D.; König, C. Validation and Application of an Hplc-Uv Method for Routine Therapeutic Drug Monitoring of Cefiderocol. Antibiotics 2021, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Calov, S.; Munzel, F.; Roehr, A.C.; Frey, O.; Higuita, L.M.S.; Wied, P.; Rosenberger, P.; Haeberle, H.A.; Ngamsri, K.-C. Daptomycin Pharmacokinetics in Blood and Wound Fluid in Critical Ill Patients with Left Ventricle Assist Devices. Antibiot. Basel Switz. 2023, 12, 904. [Google Scholar] [CrossRef] [PubMed]
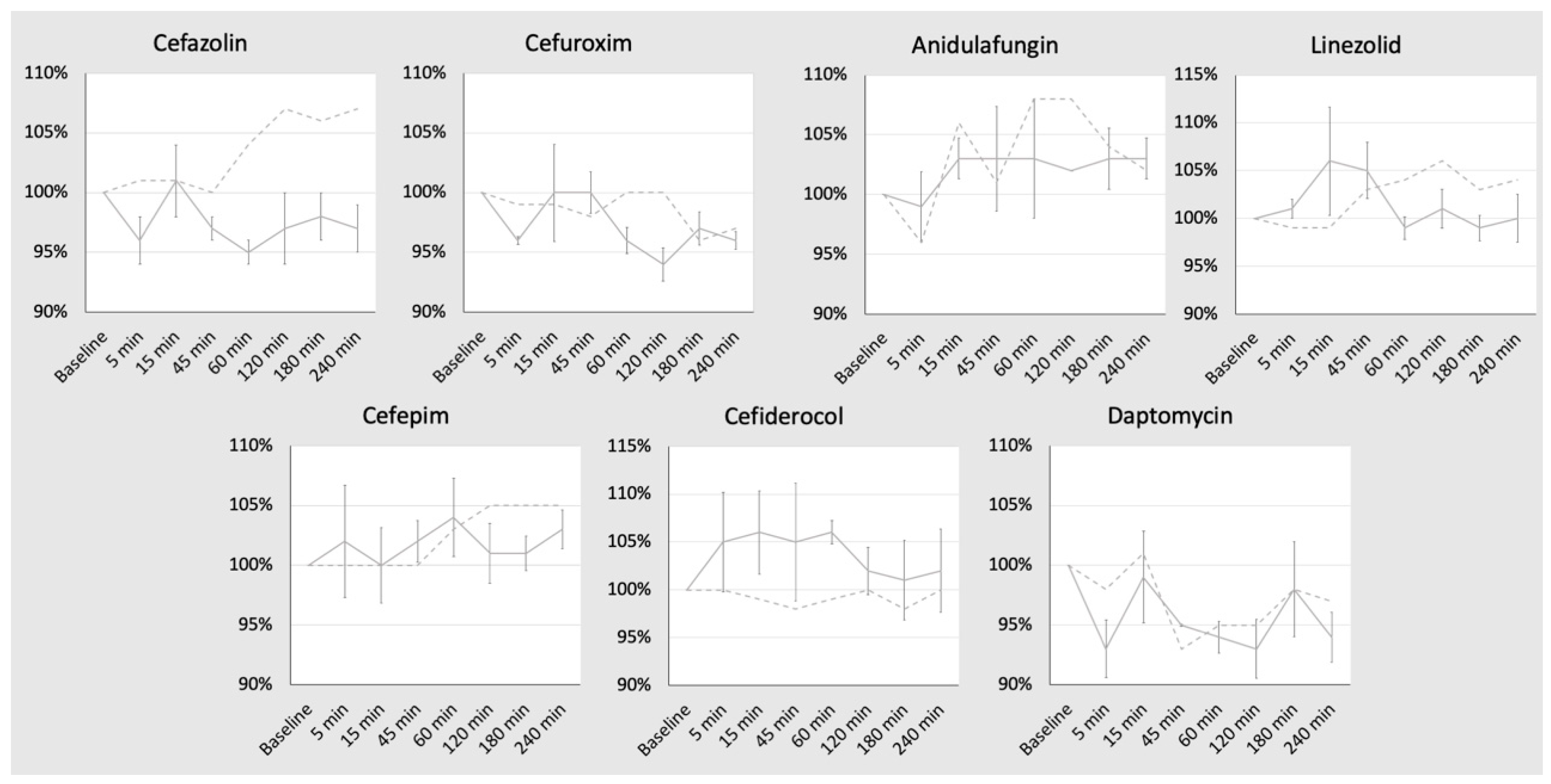

| Substance | Target Concentration (mg/L) | Baseline (0 min) (mg/L) | 5 min (mg/L) | 15 min (mg/L) | 45 min (mg/L) | 60 min (mg/L) | 120 min (mg/L) | 180 min (mg/L) | 240 min (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| Cefazolin | 100 | 89.6 ± 1.2 | 85.6 ± 2.3 | 90.6 ± 2.6 | 86.9 ± 1.4 | 84.9 ± 1.6 | 86.5 ± 2.2 | 88.1 ± 1.8 | 86.6 ± 1 |
| Cefuroxim | 75 | 72.3 ± 1 | 69.5 ± 0.8 | 72.5 ± 2 | 72.2 ± 2.1 | 69.3 ± 1.3 | 67.8 ± 0.5 | 69.8 ± 1.6 | 69.4 ± 0.5 |
| Cefepime | 100 | 100.3 ± 1.7 | 102.3 ± 3 | 100.5 ± 1.5 | 102.4 ± 1.6 | 104.4 ± 1.6 | 101 ± 2.4 | 101.1 ± 2.9 | 103.7 ± 1.3 |
| Cefiderocol | 50 | 51.9 ± 1.4 | 54.8 ± 2 | 55.2 ± 1.6 | 54.4 ± 2.8 | 55 ± 1.7 | 53 ± 1.5 | 52.6 ± 1.2 | 53.1 ± 0.7 |
| Linezolid | 15 | 14.3 ± 0.2 | 14.5 ± 0.2 | 15.2 ± 0.7 | 15 ± 0.4 | 14.2 ± 0.1 | 14.5 ± 0.3 | 14.1 ± 0.3 | 14.3 ± 0.2 |
| Daptomycin | 25 | 25.3 ± 0.5 | 23.6 ± 0.5 | 25.2 ± 0.8 | 24.1 ± 0.5 | 23.8 ± 0.2 | 23.6 ± 0.6 | 24.8 ± 0.5 | 23.8 ± 0.1 |
| Anidulafungin | 5 | 4.80 ± 0.1 | 4.75 ± 0.1 | 4.95 ± 0.1 | 4.95 ± 0.1 | 4.95 ± 0.2 | 4.90 ± 0.1 | 4.95 ± 0.2 | 4.95 ± 0.1 |
| Substance | Molecular Weight [g/mol] | logP | Protein Binding [%] | Vd [L/kg] | Vd Patient 70 kg Bodyweight [L] | Usual Bolus Dosage [mg] | Calculated cmax [mg/L] | Calculated c4h [mg/L] | Aimed Concentration [mg/L] | Amount Added [mg] | T1/2 Normal Patient [h] | Clearance Normal Patient [L/h] | Qo (Non Renal Clearance) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cefazolin | 454.51 | −0.58 | 74–86 | 0.17 | 11.9 | 2000 | 168.1 | 42.0 | 100 | 2000 | 2 | 4.1 | 0.1 |
| Cefuroxim | 424.39 | −0.16 | 50 | 0.2 | 14 | 1500 | 107.1 | 18.9 | 75 | 1500 | 1.6 | 6.1 | 0.1 |
| Cefepime | 480.56 | −0.37 | 20 | 0.27 | 18.9 | 2000 | 105.8 | 26.5 | 100 | 2000 | 2 | 6.5 | 0.15 |
| Cefiderocol | 752.21 | −2.27 | 40–60 | 0.26 | 18 | 2000 | 111.1 | 36.8 | 50 | 1000 | 2.5 | 5.2 | 0.03 |
| Linezolid | 337.35 | 0.9 | 31 | 0.65 | 45.5 | 600 | 13.1 | 7.7 | 15 | 300 | 5.1 | 6.2 | 0.7 |
| Daptomycin | 1619.71 | −0.47 | 90–94 | 0.1 | 7 | 350 | 50 | 36.1 | 25 | 500 | 8.5 | 0.2 | 0.5 |
| Anidulafungin | 1140.2 | 2.9 | 84 | 0.5 | 35 | 200 | 5.7 | 5.1 | 5 | 100 | 24 | 1.0 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Booke, H.; Friedrichson, B.; Draheim, L.; von Groote, T.C.; Frey, O.; Röhr, A.; Zacharowski, K.; Adam, E.H. No Sequestration of Commonly Used Anti-Infectives in the Extracorporeal Membrane Oxygenation (ECMO) Circuit—An Ex Vivo Study. Antibiotics 2024, 13, 373. https://doi.org/10.3390/antibiotics13040373
Booke H, Friedrichson B, Draheim L, von Groote TC, Frey O, Röhr A, Zacharowski K, Adam EH. No Sequestration of Commonly Used Anti-Infectives in the Extracorporeal Membrane Oxygenation (ECMO) Circuit—An Ex Vivo Study. Antibiotics. 2024; 13(4):373. https://doi.org/10.3390/antibiotics13040373
Chicago/Turabian StyleBooke, Hendrik, Benjamin Friedrichson, Lena Draheim, Thilo Caspar von Groote, Otto Frey, Anka Röhr, Kai Zacharowski, and Elisabeth Hannah Adam. 2024. "No Sequestration of Commonly Used Anti-Infectives in the Extracorporeal Membrane Oxygenation (ECMO) Circuit—An Ex Vivo Study" Antibiotics 13, no. 4: 373. https://doi.org/10.3390/antibiotics13040373






