Comparative Study on Antistaphylococcal Activity of Lipopeptides in Various Culture Media
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Synthesis of Lipopeptides
4.2. Antimicrobial Assays
4.2.1. MIC Assay
4.2.2. MBEC Assay
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AcOH | Acetic acid |
| AMPs | antimicrobial peptides |
| BSA | Bovine serum albumin |
| CFU | Colony forming unit |
| CLSI | Clinical and Laboratory Standards Institute |
| DCM | Dichloromethane |
| DIC | N,N’-diisopropylcarbodiimide |
| DMF | N,N-dimethylformamide |
| EDT | 1,2-ethanedithiol |
| Fmoc | 9-fluorenylmethoxycarbonyl |
| MBEC | Minimum biofilm eradication concentration |
| MIC | Minimum inhibitory concentration |
| Pal | Palmitic acid |
| PBS | Phosphate buffer saline |
| TIS | Triisopropylsilane |
| TFA | Trifluoroacetic acid |
References
- Kahl, B.C.; Becker, K. Clinical significance and pathogenesis of staphylococcal small colony variants in persistent infections. Clin. Microbiol. Rev. 2016, 29, 401–427. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lin, D.; Xu, P.; Zhang, T.; Ou, Q.; Bai, C.; Yao, Z. Non-hospital environment contamination with Staphylococcus aureus and methicillin-resistant Staphylococcus aureus: Proportion meta-analysis and features of antibiotic resistance and molecular genetics. Environ. Res. 2016, 150, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Kluytmans, J.A.J.W.; Wertheim, H.F.L. Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection 2005, 33, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Archer, N.K.; Mazaitis, M.J.; Costerton, J.W.; Leid, J.G.; Powers, M.E.; Shirtliff, M.E. Staphylococcus aureus biofilms. Virulence 2011, 2, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus colonization of the skin and antimicrobial peptides. Expert Rev. Dermatol. 2010, 5, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Staphylococcus aureus Infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Novick, R.P.; Geisinger, E. Quorum sensing in staphylococci. Annu. Rev. Genet. 2008, 42, 541–564. [Google Scholar] [CrossRef] [PubMed]
- Nusbaum, A.G.; Kirsner, R.S.; Charles, C.A. Biofilms in Dermatology. Skin Therapy Lett. 2012, 17, 1–5. [Google Scholar] [PubMed]
- Lynch, A.S.; Abbanat, D. New antibiotic agents and approaches to treat biofilm-associated infections. Expert Opin. Ther. Pat. 2010, 20, 1373–1387. [Google Scholar] [CrossRef] [PubMed]
- Dawgul, M.; Maciejewska, M.; Jaśkiewicz, M.; Karafova, A.; Kamysz, W. Antimicrobial peptides as potential tool to fight bacterial biofilm. Acta Pol. Pharm. Drug Res. 2014, 71, 39–47. [Google Scholar]
- Mansour, S.C.; Pena, O.M.; Hancock, R.E. Host defense peptides: Front-line immunomodulators. Trends Immunol. 2014, 35, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Phoenix, D.A.; Dennison, S.R.; Harris, F. Antimicrobial peptides: Their history, evolution, and functional promiscuity. Antimicrob. Pept. 2013, 1–37. [Google Scholar] [CrossRef]
- Hancock, R.E.; Lehrer, R. Cationic peptides: A new source of antibiotics. Trends Biotechnol. 1998, 16, 82–88. [Google Scholar] [CrossRef]
- Laverty, G.; McLaughlin, M.; Shaw, C.; Gorman, S.P.; Gilmore, B.F. Antimicrobial activity of short, synthetic cationic lipopeptides. Chem. Biol. Drug Des. 2010, 75, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Dawgul, M.; Baranska-Rybak, W.; Kamysz, E.; Karafova, A.; Nowicki, R.; Kamysz, W. Activity of short lipopeptides and conventional antimicrobials against planktonic cells and biofilms formed by clinical strains of Staphylococcus aureus. Future Med. Chem. 2012, 4, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests f or Bacteria That Grow Aerobically; Approved St andard—Ninth Edition; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2012; Volume 32, ISBN 1562387839. [Google Scholar]
- Giacometti, A.; Cirioni, O.; Barchiesi, F.; Del Prete, M.S.; Fortuna, M.; Caselli, F.; Scalise, G. In Vitro Susceptibility Tests for Cationic Peptides: Comparison of Broth Microdilution Methods for Bacteria That Grow Aerobically. Antimicrob. Agents Chemother. 2000, 44, 1694–1696. [Google Scholar] [CrossRef] [PubMed]
- Greber, K.E.; Dawgul, M.; Kamysz, W.; Sawicki, W. Cationic net charge and counter ion type as antimicrobial activity determinant factors of short lipopeptides. Front. Microbiol. 2017, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Makovitzki, A.; Avrahami, D.; Shai, Y.; Sela, M. Ultrashort antibacterial and antifungal lipopeptides. Proc. Natl. Acad. Sci. USA 2006, 103, 15997–16002. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Sirobhushanam, S.; Johnson, S.R.; Song, Y.; Tefft, R.; Gatto, C.; Wilkinson, B.J. Growth-environment dependent modulation of Staphylococcus aureus branched-chain to straight-chain fatty acid ratio and incorporation of unsaturated fatty acids. PLoS ONE 2016, 11, e0165300. [Google Scholar] [CrossRef] [PubMed]
- Leejae, S.; Hasap, L.; Piyawan, S.; Correspondence, V.; Voravuthikunchai, S.P. Inhibition of staphyloxanthin biosynthesis in Staphylococcus aureus by rhodomyrtone, a novel antibiotic candidate. J. Med. Microbiol. 2013, 62, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Kahlon, A.K.; Roy, S.; Sharma, A. Molecular docking studies to map the binding site of squalene synthase inhibitors on dehydrosqualene synthase of Staphylococcus aureus. J. Biomol. Struct. Dyn. 2010, 28, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Clauditz, A.; Resch, A.; Wieland, K.-P.; Peschel, A.; Götz, F. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect. Immun. 2006, 74, 4950–4953. [Google Scholar] [CrossRef] [PubMed]
- Pelz, A.; Wieland, K.-P.; Putzbach, K.; Hentschel, P.; Albert, K.; Gö Tz, F. Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus *. J. Biol. Chem. 2005, 280, 32493–32498. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.N.; Liu, G.Y.; Yeaman, M.R.; Nast, C.C.; Proctor, R.A.; McKinnell, J.; Bayer, A.S. Carotenoid-related alteration of cell membrane fluidity impacts Staphylococcus aureus susceptibility to host defense peptides. Antimicrob. Agents Chemother. 2011, 55, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Jana, M.; Luong, T.T.; Lee, C.Y. Control of glucose—and NaCl-induced biofilm formation by rbf in Staphylococcus aureus. J. Bacteriol. 2004, 186, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Fluckiger, U.; Ulrich, M.; Steinhuber, A.; Döring, G.; Mack, D.; Landmann, R.; Goerke, C.; Wolz, C. Biofilm formation, icaADBC transcription, and polysaccharide intercellular adhesin synthesis by staphylococci in a device-related infection model. Infect. Immun. 2005, 73, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Waldrop, R.; McLaren, A.; Calara, F.; McLemore, R. Biofilm growth has a threshold response to glucose in vitro. Clin. Orthop. Relat. Res. 2014, 472, 3305–3310. [Google Scholar] [CrossRef] [PubMed]
- Seidl, K.; Goerke, C.; Wolz, C.; Mack, D.; Berger-Bächi, B.; Bischoff, M. Staphylococcus aureus CcpA affects biofilm formation. Infect. Immun. 2008, 76, 2044–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, J.M.; Gill, C.J.; Hare, R.S.; Miller, G.H. Effect of NaCl supplementation of Mueller-Hinton broth on susceptibility of staphylococci to aminoglycosides. Antimicrob. Agents Chemother. 1986, 29, 152–154. [Google Scholar] [CrossRef]
- Huang, M.B.; Elaine Gay, T.; Baker, C.N.; Banerjee, S.N.; Tenoverl, F.C. Two percent sodium chloride is required for susceptibility testing of staphylococci with oxacillin when using agar-based dilution methods. J. Clin. Microbiol. 1993, 31, 2683–2688. [Google Scholar] [PubMed]
- Migoń, D.; Neubauer, D.; Sikora, K.; Jaśkiewicz, M.; Kamysz, W. Badanie aktywności przeciwdrobnoustrojowej lipopeptydów w środowisku wybranych soli. In Nauka i Przemysł. Metody Spektroskopowe w praktyce. Nowe wyzwania i możliwości. Tom I; UMCS: Lublin, Poland, 2016; pp. 336–338. ISBN 978-83-945225-0-6. (In Polish) [Google Scholar]
- Citterio, L.; Franzyk, H.; Palarasah, Y.; Andersen, T.E.; Mateiu, R.V.; Gram, L. Improved in vitro evaluation of novel antimicrobials: Potential synergy between human plasma and antibacterial peptidomimetics, AMPs and antibiotics against human pathogenic bacteria. Res. Microbiol. 2016, 167, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.A.; Hurst, M.A.; Fujii, C.A.; Kung, A.H.C.; Ho, J.F.; Cheng, F.-C.; Loury, D.J.; Fiddes, J.C. Protegrin-1: A broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 1997, 41, 1738–1742. [Google Scholar] [PubMed]
- Findlay, B.; Szelemej, P.; Zhanel, G.G.; Schweizer, F.; Beyermann, M. Guanidylation and tail effects in cationic antimicrobial lipopeptoids. PLoS ONE 2012, 7, e41141. [Google Scholar] [CrossRef] [PubMed]
- Svenson, J.; Brandsdal, B.-O.; Stensen, W.; Svendsen, J.S. Albumin binding of short cationic antimicrobial micropeptides and its influence on the in vitro bactericidal effect. J. Med. Chem. 2007, 50, 3334–3339. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K. Control of cell selectivity of antimicrobial peptides. Biochim. Biophys. Acta—Biomembr. 2009, 1788, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Oren, Z.; Shai, Y. The Effect of cyclization of magainin 2 and melittin analogues on structure, function, and model membrane interactions: Implication to their mode of action. Biochemistry 2001. [Google Scholar] [CrossRef]
- Góngora-Benítez, M.; Tulla-Puche, J.; Albericio, F. Multifaceted roles of disulfide bonds. Peptides as therapeutics. Chem. Rev. 2014, 114, 901–926. [Google Scholar] [CrossRef] [PubMed]
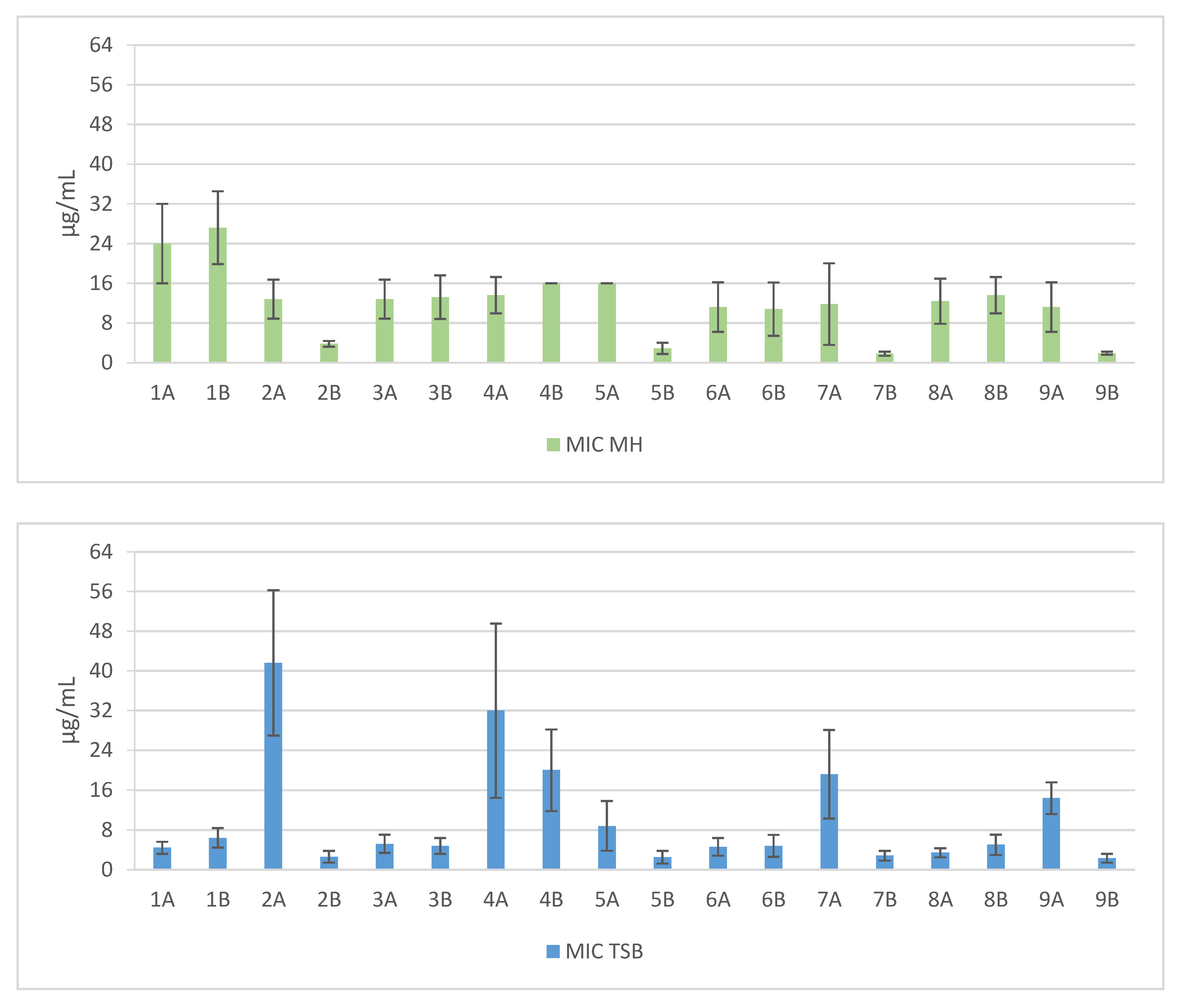
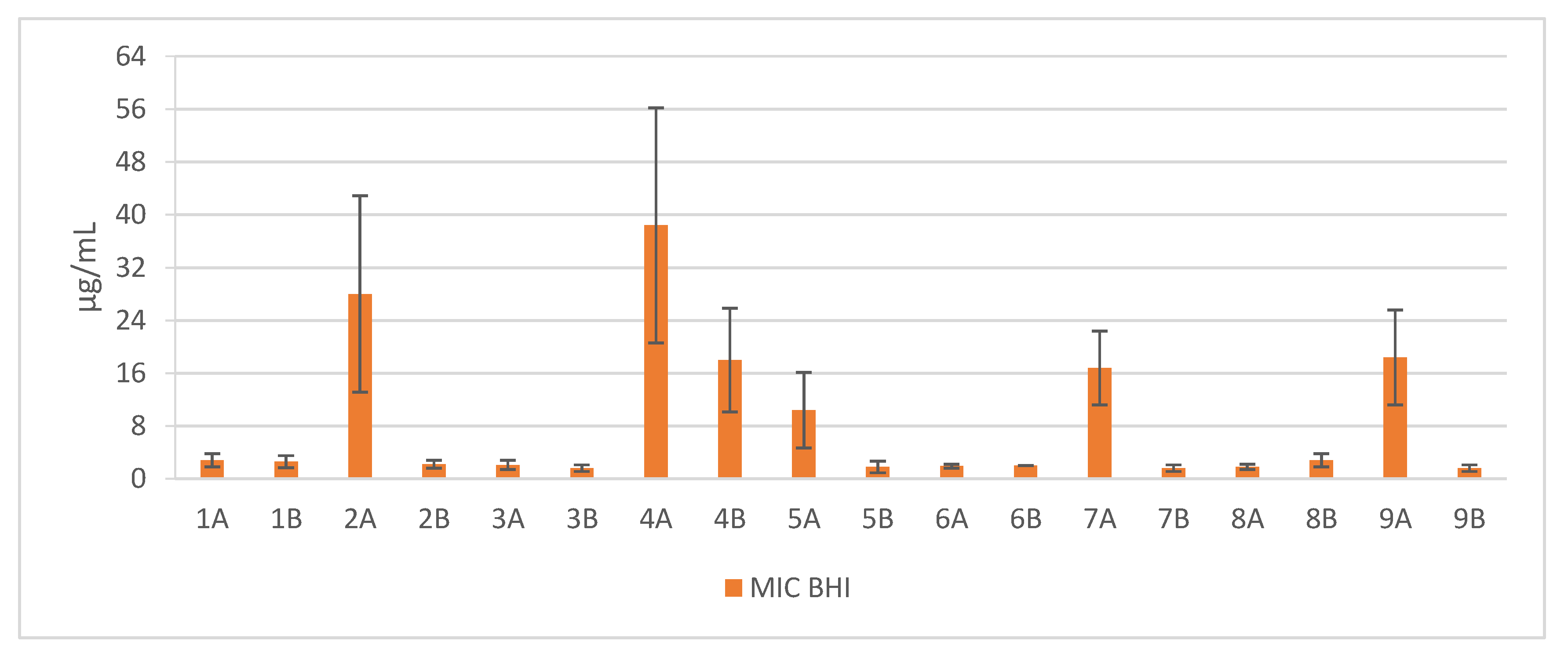
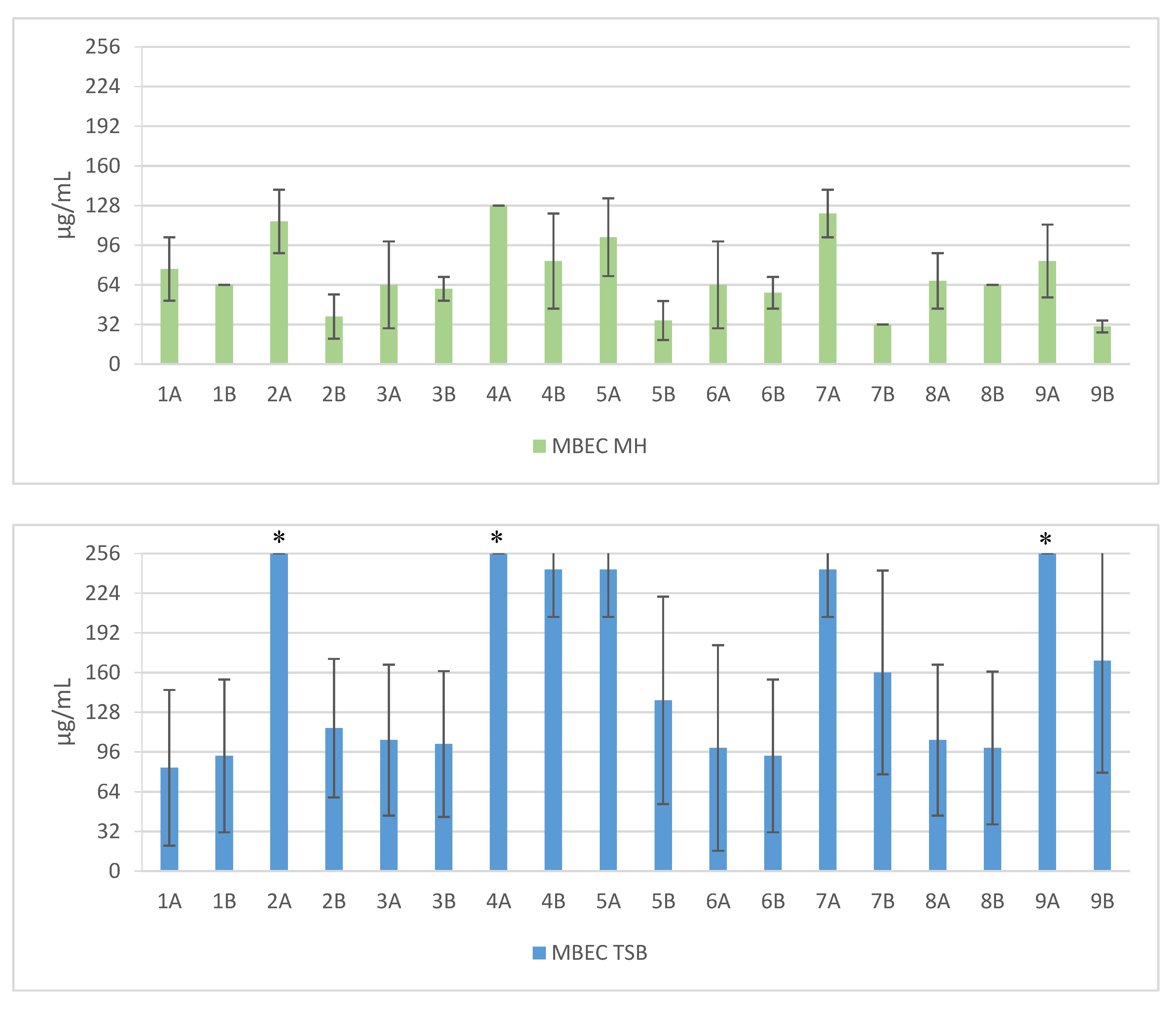


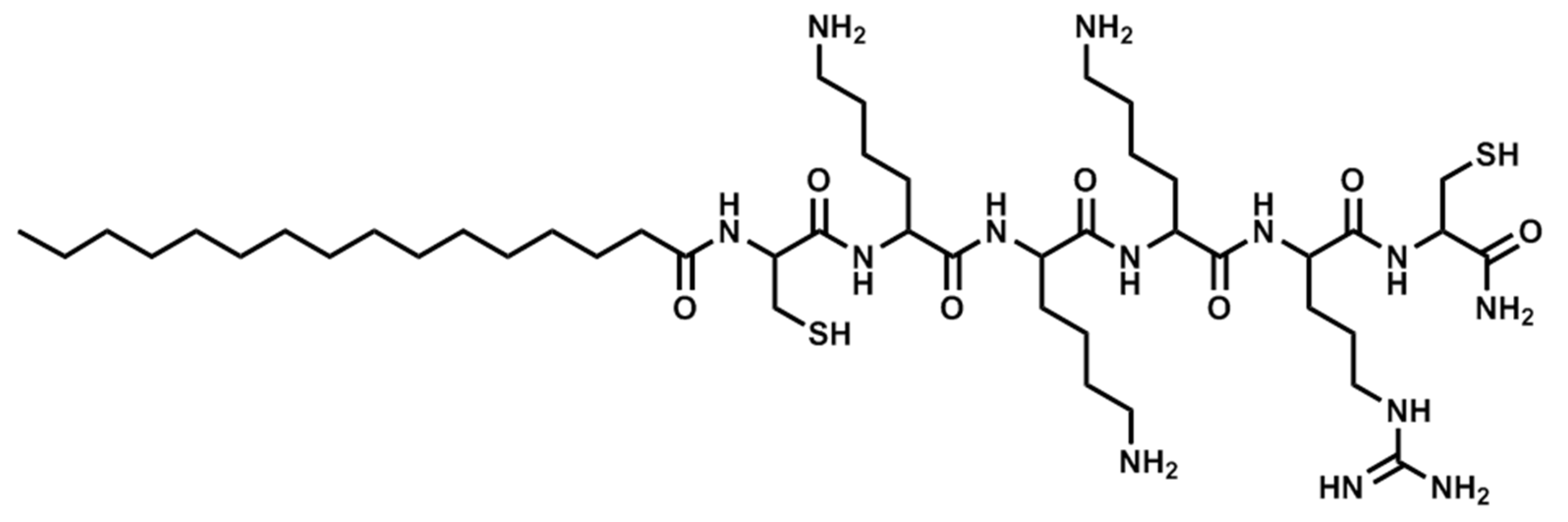
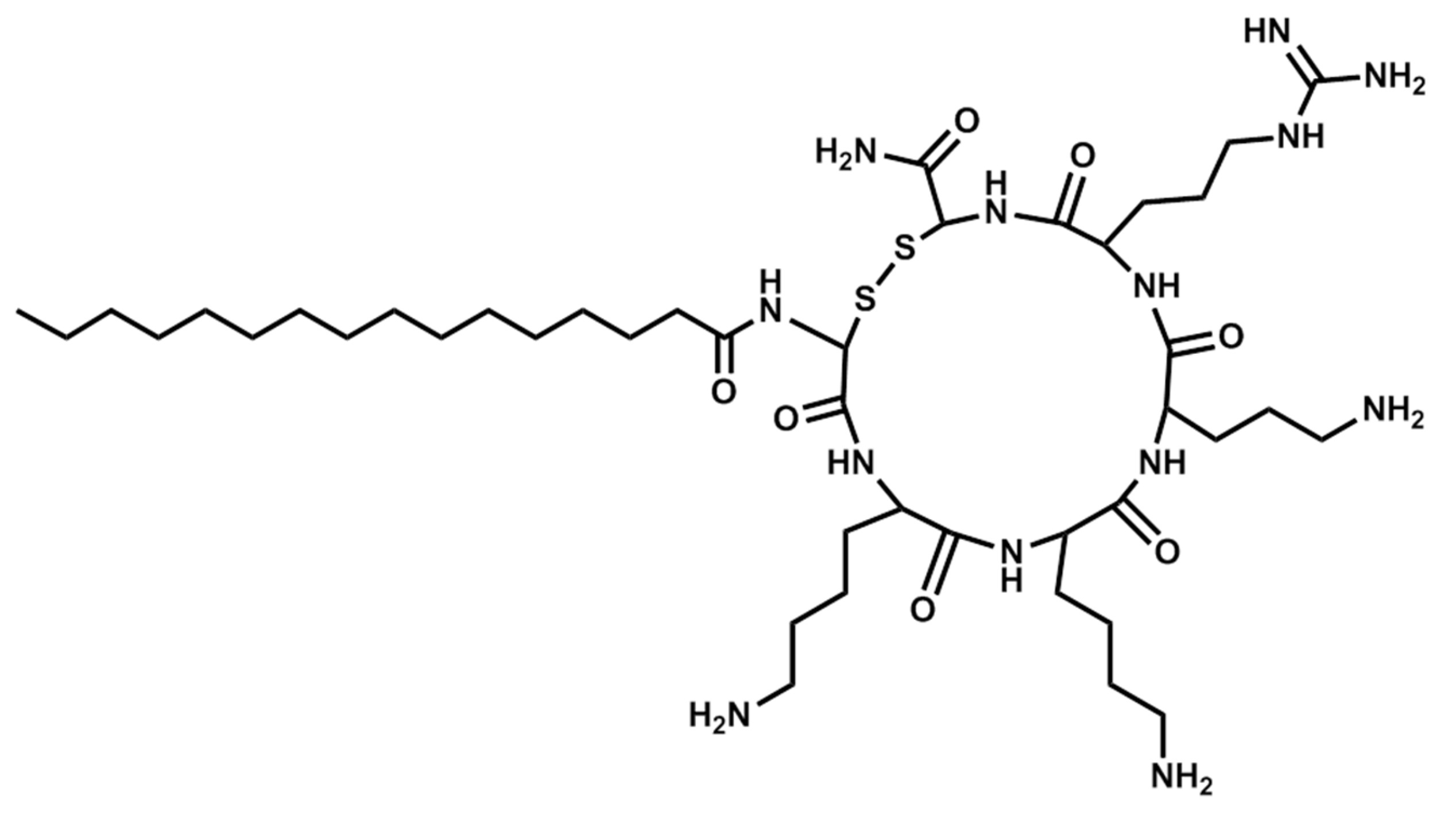
| Sequence | Structure | Solvent | Signature |
|---|---|---|---|
| Pal-KKK-NH2 | Linear | PBS | 1A |
| Pal-KKK-NH2 | Linear | AcOH/BSA | 1B |
| Pal-CKKKC-NH2 | Cyclic | PBS | 2A |
| Pal-CKKKC-NH2 | Cyclic | AcOH/BSA | 2B |
| Pal-KKKR-NH2 | Linear | PBS | 3A |
| Pal-KKKR-NH2 | Linear | AcOH/BSA | 3B |
| Pal-CKKKRC-NH2 | Linear | PBS | 4A |
| Pal-CKKKRC-NH2 | Linear | AcOH/BSA | 4B |
| Pal-CKKKRC-NH2 | Cyclic | PBS | 5A |
| Pal-CKKKRC-NH2 | Cyclic | AcOH/BSA | 5B |
| Pal-KKRK-NH2 | Linear | PBS | 6A |
| Pal-KKRK-NH2 | Linear | AcOH/BSA | 6B |
| Pal-CKKRKC-NH2 | Cyclic | PBS | 7A |
| Pal-CKKRKC-NH2 | Cyclic | AcOH/BSA | 7B |
| Pal-RKKK-NH2 | Linear | PBS | 8A |
| Pal-RKKK-NH2 | Linear | AcOH/BSA | 8B |
| Pal-RKKK-NH2 | Cyclic | PBS | 9A |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaśkiewicz, M.; Neubauer, D.; Kamysz, W. Comparative Study on Antistaphylococcal Activity of Lipopeptides in Various Culture Media. Antibiotics 2017, 6, 15. https://doi.org/10.3390/antibiotics6030015
Jaśkiewicz M, Neubauer D, Kamysz W. Comparative Study on Antistaphylococcal Activity of Lipopeptides in Various Culture Media. Antibiotics. 2017; 6(3):15. https://doi.org/10.3390/antibiotics6030015
Chicago/Turabian StyleJaśkiewicz, Maciej, Damian Neubauer, and Wojciech Kamysz. 2017. "Comparative Study on Antistaphylococcal Activity of Lipopeptides in Various Culture Media" Antibiotics 6, no. 3: 15. https://doi.org/10.3390/antibiotics6030015






