Antibiotic Prescribing for Oro-Facial Infections in the Paediatric Outpatient: A Review
Abstract
:1. Introduction
- Highlighting clinical indications of therapeutic antibiotic prescribing for orofacial infections in the paediatric outpatients;
- Presenting recommended antibiotic regimens for each clinical indication.
2. Materials and Methods
2.1. Eligibility Criteria
- Papers published in English;
- Papers published in the past 20 years (from January 1998 to December 2017);
- Clinical trials;
- Case reports and series;
- Reviews;
- Expert opinions;
- Clinical guidelines;
- Patients: paediatric outpatients having orofacial infections (odontogenic infections, periodontal infections, pericoronitis);
- Intervention: prescribing regimen of antibiotics including: name, dose and duration.
- In vitro and animal studies;
- Neonatal orofacial infections that need hospitalization;
- Paediatric dental in-patients;
- Prophylactic antibiotic prescribing.
2.2. Search Methodology
2.3. Selection Strategy
2.4. Assessment of Risk of Bias
2.5. Data Synthesis
3. Results
3.1. Search and Selection
- Prophylactic antibiotic prescribing;
- Children with chronic diseases like HIV and cardiovascular disease;
- Older guidelines when new guidelines are present;
- Non-odontogenic conditions;
- Topical use of antibiotics;
- Antimicrobials other than antibiotics;
- A recommended antibiotic regime was not mentioned.
- Adult studies;
- Non-bacterial periodontal infections;
- Topical antibiotic therapy;
- Aggressive periodontitis associated with systemic diseases;
- Antimicrobials other than antibiotics.
3.2. Clinical Indications and Recommendations for Paediatric Dental Antibiotic Prescribing
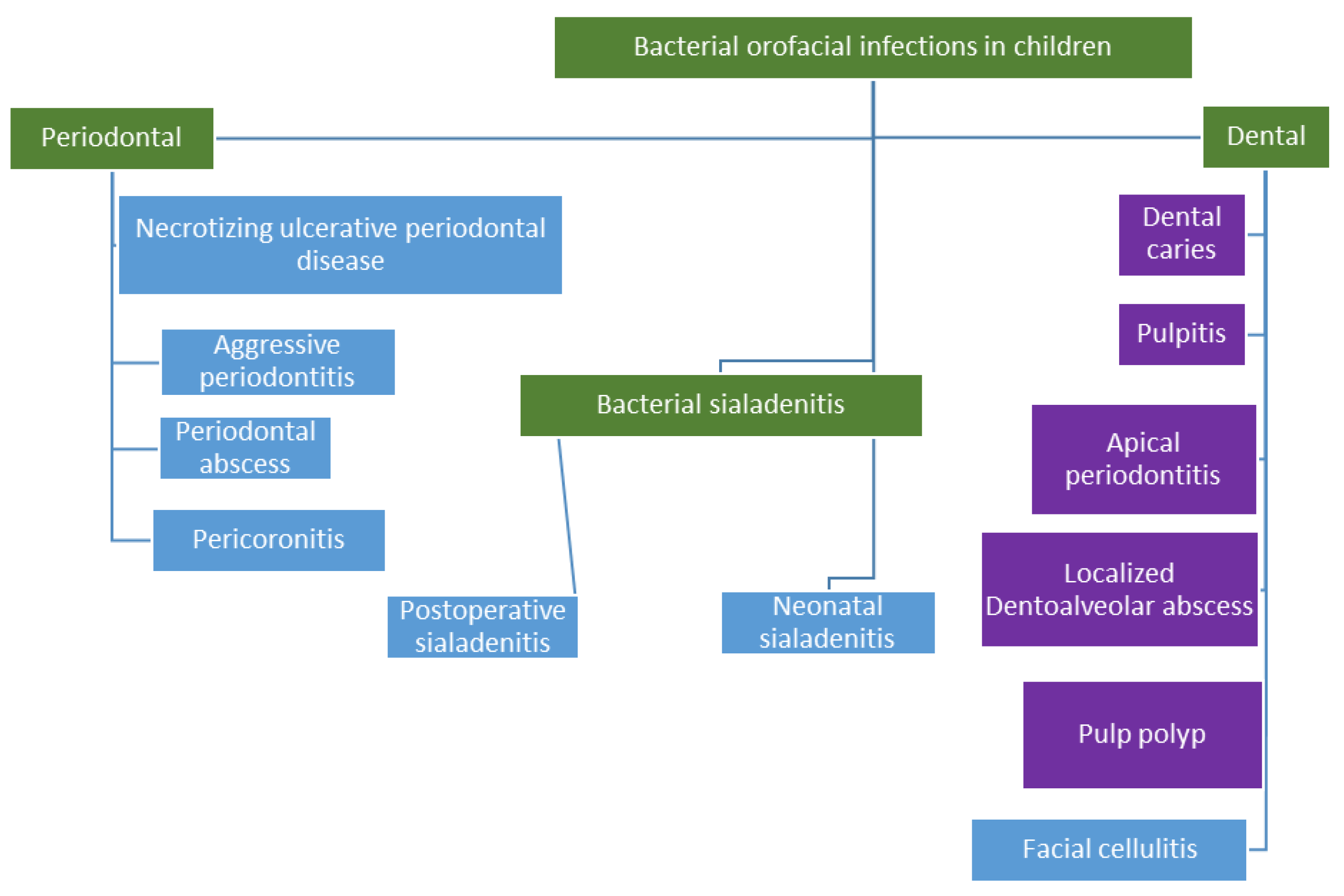
3.2.1. Dentoalveolar Infections
3.2.2. Periodontal Diseases
- Plaque-induced periodontal diseases;
- Aggressive periodontal disease;
- Periodontal disease as a manifestation of systemic diseases;
- Necrotizing periodontal diseases;
- Abscesses of the periodontium;
- Periodontal disease associated with endodontic lesions;
- Developmental or acquired periodontal deformities and conditions.
- Chronic periodontitis;
- Aggressive periodontitis;
- Necrotizing ulcerative periodontitis;
- Periodontitis associated with systemic diseases
Aggressive Periodontitis
Necrotizing Periodontal Lesions
3.3. Recommended Antibiotic Regimens
3.4. Assessment of Risk of Bias
4. Discussion
Recommendations
- Proper diagnosis is mandatory to design an appropriate treatment plan. To achieve the accurate diagnosis, history collection and clinical examination should be appropriately performed. Adjunctive diagnostic tools, like radiographs, can be of benefit, and should be used when indicated;
- It may seem more suitable to prescribe analgesics to supplement operative treatment for patients in pain;
- In case antibiotics were prescribed, children should be followed up for a few days to evaluate response to treatment, and the development of unwanted side effects;
- Dosing regimens for children can generally be estimated from their weight in kilograms, or from age, using the formula ((age + 4) × 2) if the child’s weight is unknown. In any case, the dose should not exceed the maximum adult dose;
- Treatment of orofacial infections entails collaborative efforts from all practitioners involved in the child’s healthcare.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Esposito, S.; Castellazzi, L.; Tagliabue, C.; Principi, N. Allergy to antibiotics in children: An overestimated problem. Int. J. Antimicrob. Agents 2016, 48, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Hurley, S.; Westgarth, D. When David met Sara Part 2. Br. Dent. J. 2015, 219, 477–478. [Google Scholar] [PubMed]
- Cars, O.; Molstad, S.; Melander, A. Variation in antibiotic use in the European Union. Lancet 2001, 357, 1851–1853. [Google Scholar] [CrossRef]
- Harte, H.; Palmer, N.O.; Martin, M.V. An investigation of therapeutic antibiotic prescribing for children referred for dental general anaesthesia in three community national health service trusts. Br. Dent. J. 2005, 198, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, S.S.; Hassan, M.; Pearson, J.M. A national survey of pediatric dentists on antibiotic use in children. Pediatr. Dent. 2013, 35, 546–549. [Google Scholar] [PubMed]
- Kouidhi, B.; Zmantar, T.; Hentati, H.; Najjari, F.; Mahdouni, K.; Bakhrouf, A. Molecular investigation of macrolide and Tetracycline resistances in oral bacteria isolated from Tunisian children. Arch. Oral Boil. 2011, 56, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Ready, D.; Bedi, R.; Spratt, D.A.; Mullany, P.; Wilson, M. Prevalence, proportions, and identities of antibiotic-resistant bacteria in the oral microflora of healthy children. Microb. Drug Resist. 2003, 9, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Abu-zineh, R.; Dar-Odeh, N.; Shehabi, A. Macrolide Resistance Genes and Virulence Factors of Common Viridans Streptococci Species Colonizing Oral Cavities of Patients in Jordan. Oral Health Dent. Manag. 2015, 14, 337–341. [Google Scholar]
- Dar-Odeh, N.S.; Al-Abdalla, M.; Al-Shayyab, M.H.; Obeidat, H.; Obeidat, L.; Abu Kar, M.; Abu-Hammad, O.A. Prescribing Antibiotics for pediatric dental patients in Jordan; knowledge and attitudes of dentists. Int. Arab. J. Antimicrob. Agents 2013, 3, 1–6. [Google Scholar]
- Nemoto, H.; Nomura, R.; Ooshima, T.; Nakano, K. Distribution of amoxicillin-resistant oral streptococci in dental plaque specimens obtained from Japanese children and adolescents at risk for infective endocarditis. J. Cardiol. 2013, 62, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Palmer, N.O.; Martin, M.V.; Pealing, R.; Ireland, R.S. Paediatric antibiotic prescribing by general dental practitioners in England. Int. J. Paediatr. Dent. 2001, 11, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Droste, J.H.; Wieringa, M.H.; Weyler, J.J.; Nelen, V.J.; Vermeire, P.A.; Van Bever, H.P. Does the use of antibiotics in early childhood increase the risk of asthma and allergic disease? Clin. Exp. Allergy 2000, 30, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Yallapragada, S.G.; Nash, C.B.; Robinson, D.T. Early-Life Exposure to Antibiotics, Alterations in the Intestinal Microbiome, and Risk of Metabolic Disease in Children and Adults. Pediatr. Ann. 2015, 44, e265–e269. [Google Scholar] [CrossRef] [PubMed]
- Al-Shayyab, M.H.; Abu-Hammad, O.A.; Al-Omiri, M.K.; Dar-Odeh, N.S. Antifungal prescribing pattern and attitude towards the treatment of oral candidiasis among dentists in Jordan. Int. Dent. J. 2015, 65, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.; McEwen, J.; Al-Ajmi, H.; Purkins, L.; Colman, P.J.; Willavize, S.A. A comparison of the photosensitizing potential of trovafloxacin with that of other quinolones in healthy subjects. J. Antimicrob. Chemother. 2000, 45, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Verdugo, F.; Laksmana, T.; Uribarri, A. Systemic antibiotics and the risk of superinfection in peri-implantitis. Arch. Oral Boil. 2016, 64, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Easton, J.; Noble, S.; Perry, C.M. Amoxicillin/clavulanic acid: A review of its use in the management of paediatric patients with acute otitis media. Drugs 2003, 63, 311–340. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Levy, S.M.; Warren, J.J.; Dawson, D.V.; Bergus, G.R.; Wefel, J.S. Association of amoxicillin use during early childhood with developmental tooth enamel defects. Arch. Pediatr. Adolesc. Med. 2005, 159, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Cherry, W.R.; Lee, J.Y.; Shugars, D.A.; White, R.P., Jr.; Vann, W.F., Jr. Antibiotic use for treating dental infections in children: A survey of dentists’ prescribing practices. J. Am. Dent. Assoc. 2012, 143, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Sterne, J.A.C. Chapter 8: Assessing risk of bias in included studies. In Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0; Higgins, J.P.T., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- The American Academy of Pediatric Dentistry-Useful Medications for Oral Conditions. Available online: http://www.aapd.org/media/policies_guidelines/rs_commonmeds.pdf (accessed on 9 March 2018).
- Palmer, N.O. Pharmaceutical prescribing for children. Part 3. Antibiotic prescribing for children with odontogenic infections. Prim. Dent. Care 2006, 13, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Scottish Dental Clinical Effectiveness Programme DPFD, Dental Clinical Guidance, Third Edition. Available online: http://www.sdcep.org.uk/wp-content/uploads/2016/03/SDCEP-Drug-Prescribing-for-Dentistry-3rd-edition.pdf (accessed on 25 December 2016).
- Haas, A.N.; de Castro, G.D.; Moreno, T.; Susin, C.; Albandar, J.M.; Oppermann, R.V.; Rosing, C.K. Azithromycin as an adjunctive treatment of aggressive periodontitis: 12-months randomized clinical trial. J. Clin. Periodontol. 2008, 35, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Muppa, R.; Nallanchakrava, S.; Chinta, M.; Manthena, R.T. Nonsyndromic localized aggressive periodontitis of primary dentition: A rare case report. Contemp. Clin. Dent. 2016, 7, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Beliveau, D.; Magnusson, I.; Bidwell, J.A.; Zapert, E.F.; Aukhil, I.; Wallet, S.M.; Shaddox, L.M. Benefits of early systemic antibiotics in localized aggressive periodontitis: A retrospective study. J. Clin. Periodontol. 2012, 39, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.N.; Vovk, A.; Kalash, D.; Hovencamp, N.; Aukhil, I.; Harrison, P.; Zapert, E.; Bidwell, J.; Varnado, P.; Shaddox, L.M. Localized aggressive periodontitis treatment response in primary and permanent dentitions. J. Periodontol. 2014, 85, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Seremidi, K.; Gizani, S.; Madianos, P. Therapeutic management of a case of generalised aggressive periodontitis in an 8-year old child: 18-month results. Eur. Arch. Paediatr. Dent. 2012, 13, 266–271. [Google Scholar] [CrossRef] [PubMed]
- GRADE-Working Group. Available online: http://training.cochrane.org/path/grade-approach-evaluating-quality-evidence-pathway (accessed on 9 March 2018).
- Sayegh, A.; Dini, E.L.; Holt, R.D.; Bedi, R. Oral cleanliness, gingivitis, dental caries and oral health behaviours in Jordanian children. J. Int. Acad. Periodontol. 2002, 4, 12–18. [Google Scholar] [PubMed]
- Finucane, D. Rationale for restoration of carious primary teeth: A review. Eur. Arch. Paediatr. Dent. 2012, 13, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; van Winkelhoff, A.J. Acute focal infections of dental origin. Periodontology 2000 2014, 65, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, B.; Pakla, P.; Wolek, W.; Jednakiewicz, M.; Nicpon, J. A fatal case of descending necrotizing mediastinitis as a complication of odontogenic infection. A case report. Kardiochirurgia i Torakochirurgia Polska = Pol. J. Cardio-Thorac. Surg. 2014, 11, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Dhariwal, D.K.; Patton, D.W.; Gregory, M.C. Epidural spinal abscess following dental extraction—A rare and potentially fatal complication. Br. J. Oral Maxillofac. Surg. 2003, 41, 56–58. [Google Scholar] [CrossRef]
- Zachariades, N.; Vairaktaris, E.; Mezitis, M.; Rallis, G.; Kokkinis, C.; Moschos, M. Orbital abscess: Visual loss following extraction of a tooth—Case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, e70–e73. [Google Scholar] [CrossRef] [PubMed]
- Colares, V.; Franca, C.; Ferreira, A.; Amorim Filho, H.A.; Oliveira, M.C. Dental anxiety and dental pain in 5- to 12-year-old children in Recife, Brazil. Eur. Arch. Paediatr. Dent. 2013, 14, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Coric, A.; Banozic, A.; Klaric, M.; Vukojevic, K.; Puljak, L. Dental fear and anxiety in older children: An association with parental dental anxiety and effective pain coping strategies. J. Pain Res. 2014, 7, 515–521. [Google Scholar] [PubMed]
- Long, S.S. Optimizing antimicrobial therapy in children. J. Infect. 2016, 72, S91–S97. [Google Scholar] [CrossRef] [PubMed]
- Chardin, H.; Yasukawa, K.; Nouacer, N.; Plainvert, C.; Aucouturier, P.; Ergani, A.; Descroix, V.; Toledo-Arenas, R.; Azerad, J.; Bouvet, A. Reduced susceptibility to amoxicillin of oral streptococci following amoxicillin exposure. J. Med. Microbiol. 2009, 58 Pt 8, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.J.; Pellecchia, R. Antimicrobial Therapy in Management of Odontogenic Infections in General Dentistry. Dent. Clin. N. Am. 2016, 60, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Segura-Egea, J.J.; Gould, K.; Sen, B.H.; Jonasson, P.; Cotti, E.; Mazzoni, A.; Sunay, H.; Tjaderhane, L.; Dummer, P.M. Antibiotics in Endodontics: A review. Int. Endod. J. 2016, 50, 1169–1184. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arambula, H.; Hidalgo-Hurtado, A.; Rodriguez-Flores, R.; Gonzalez-Amaro, A.M.; Garrocho-Rangel, A.; Pozos-Guillen, A. Moxifloxacin versus Clindamycin/Ceftriaxone in the management of odontogenic maxillofacial infectious processes: A preliminary, intrahospital, controlled clinical trial. J. Clin. Exp. Dent. 2015, 7, e634–e639. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, D.; Carbon, C.; Balkau, B.; Geslin, P.; Lecoeur, H.; Vauzelle-Kervroedan, F.; Bouvenot, G.; Eschwege, E. Low dosage and long treatment duration of beta-lactam: Risk factors for carriage of penicillin-resistant Streptococcus pneumoniae. JAMA 1998, 279, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Widen, C.; Holmer, H.; Coleman, M.; Tudor, M.; Ohlsson, O.; Sattlin, S.; Renvert, S.; Persson, G.R. Systemic inflammatory impact of periodontitis on acute coronary syndrome. J. Clin. Periodontol. 2016, 43, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Mesia, R.; Gholami, F.; Huang, H.; Clare-Salzler, M.; Aukhil, I.; Wallet, S.M.; Shaddox, L.M. Systemic inflammatory responses in patients with type 2 diabetes with chronic periodontitis. BMJ Open Diabetes Res. Care 2016, 4, e000260. [Google Scholar] [CrossRef] [PubMed]
- White, D.A.; Tsakos, G.; Pitts, N.B.; Fuller, E.; Douglas, G.V.; Murray, J.J.; Steele, J.G. Adult Dental Health Survey 2009: Common oral health conditions and their impact on the population. Br. Dent. J. 2012, 213, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Clerehugh, V.; Kindelan, S. Guidelines for periodontal screening and management of children and adolescents under 18 years of age. British Society of Periodontology and The British Society of Paediatric Dentistry. Available online: https://www.bsperio.org.uk/publications/downloads/53_085556_executive-summary-bsp_bspd-perio-guidelines-for-the-under-18s.pdf (accessed on 9 February 2018).
- Jenkins, W.M.; Papapanou, P.N. Epidemiology of periodontal disease in children and adolescents. Periodontology 2000 2001, 26, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Califano, J.V. Position paper: Periodontal diseases of children and adolescents. J. Periodontol. 2003, 74, 1696–1704. [Google Scholar] [PubMed]
- Demmer, R.T.; Papapanou, P.N.; Jacobs, D.R., Jr.; Desvarieux, M. Evaluating clinical periodontal measures as surrogates for bacterial exposure: The Oral Infections and Vascular Disease Epidemiology Study (INVEST). BMC Med. Res. Methodol. 2010, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, K.; Jepsen, S. Antibiotics/antimicrobials: Systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontology 2000 2016, 71, 82–112. [Google Scholar] [CrossRef] [PubMed]
- Clerehugh, V.; Tugnait, A. Periodontal diseases in children and adolescents: 2. Management. Dent. Update 2001, 28, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Albandar, J.M. Aggressive periodontitis: Case definition and diagnostic criteria. Periodontology 2000 2014, 65, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontal. 1999, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Elamin, A.M.; Skaug, N.; Ali, R.W.; Bakken, V.; Albandar, J.M. Ethnic disparities in the prevalence of periodontitis among high school students in Sudan. J. Periodontol. 2010, 81, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Roshna, T.; Nandakumar, K. Generalized aggressive periodontitis and its treatment options: Case reports and review of the literature. Case Rep. Med. 2012, 2012, 535321. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, C.C.; Feres, M.; Goncalves, C.; Figueiredo, L.C.; Faveri, M.; Tu, Y.K.; Chambrone, L. Systemic antibiotics in the treatment of aggressive periodontitis. A systematic review and a Bayesian Network meta-analysis. J. Clin. Periodontol. 2015, 42, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Slots, J.; Ting, M. Systemic antibiotics in the treatment of periodontal disease. Periodontology 2000 2002, 28, 106–176. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J. Systemic antibiotics in periodontal therapy. Aust. Dent. J. 2009, 54 (Suppl. 1), S96–S101. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Alonso, B.; Leon, R.; Roldan, S.; Sanz, M. Antimicrobial therapy in periodontitis: The use of systemic antimicrobials against the subgingival biofilm. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 45–66. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Alonso, B.; de Arriba, L.; Santa Cruz, I.; Serrano, C.; Sanz, M. Acute periodontal lesions. Periodontology 2000 2014, 65, 149–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albandar, J.M.; Tinoco, E.M. Global epidemiology of periodontal diseases in children and young persons. Periodontology 2000 2002, 29, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Arendorf, T.M.; Bredekamp, B.; Cloete, C.A.; Joshipura, K. Seasonal variation of acute necrotising ulcerative gingivitis in South Africans. Oral Diseases 2001, 7, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Feller, L.; Altini, M.; Chandran, R.; Khammissa, R.A.; Masipa, J.N.; Mohamed, A.; Lemmer, J. Noma (cancrum oris) in the South African context. J. Oral Pathol. Med. 2014, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dalla Torre, D.; Brunold, S.; Kisielewsky, I.; Kloss, F.R.; Burtscher, D. Life-threatening complications of deep neck space infections. Wiener Klinische Wochenschrift 2013, 125, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Gelbier, S. 125 years of developments in dentistry, 1880–2005. Part 4: Clinical dentistry. Br. Dent. J. 2005, 199, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Gelbier, S. 125 years of developments in dentistry, 1880–2005. Part 7: War and the dental profession. Br. Dent. J. 2005, 199, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, P.; Dvorkin, L. Promoting medication adherence in children. Am. Fam. Phys. 2006, 74, 793–798. [Google Scholar]
- Serisier, D.J. Risks of population antimicrobial resistance associated with chronic macrolide use for inflammatory airway diseases. Lancet Respir. Med. 2013, 1, 262–274. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, M.; Li, S.; Gao, Y.; Xue, S.; Huang, H.; Chen, K.; Liu, F.; Chen, L. Electrophysiologic Studies on the Risks and Potential Mechanism Underlying the Proarrhythmic Nature of Azithromycin. Cardiovasc. Toxicol. 2017, 17, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Thanaviratananich, S.; Laopaiboon, M.; Vatanasapt, P. Once or twice daily versus three times daily amoxicillin with or without clavulanate for the treatment of acute otitis media. Cochrane Database Syst. Rev. 2013, CD004975. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.; Calder, L.; Thomas, D.W. Antibiotic prescribing for dental conditions: General medical practitioners and dentists compared. Br. Dent. J. 2000, 188, 398–400. [Google Scholar] [PubMed]
- Bax, R. Development of a twice daily dosing regimen of amoxicillin/clavulanate. Int. J. Antimicrob. Agents 2007, 30 (Suppl. 2), S118–S121. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.L.; Larsen, C.G. Pediatric sialadenitis. Otolaryngol. Clin. N. Am. 2014, 47, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Jeong, H.S.; Ko, M.H.; Cho, H.J.; Ryu, N.G.; Cho, D.Y.; Son, Y.I.; Baek, C.H. Pediatric sialolithiasis: What is different from adult sialolithiasis? Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Yim, M.T.; Liu, Y.C.; Ongkasuwan, J. A review of acute postoperative sialadenitis in children. Int. J. Pediatr. Otorhinolaryngol. 2017, 92, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Stong, B.C.; Sipp, J.A.; Sobol, S.E. Pediatric parotitis: A 5-year review at a tertiary care pediatric institution. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 541–544. [Google Scholar] [CrossRef] [PubMed]
| Oral Infection | Author/s (Year) | Type of Study | Indicated Antibiotic Regime | Indicated Antibiotic Regime in Penicillin-Allergic Patients | Additional Measures | Comments | Quality of the Evidence |
|---|---|---|---|---|---|---|---|
| Acute odontogenic abscess associated with raised axillary temperature and diffuse swelling | Palmer (2006) [22] | Expert opinion | Amoxicillin (2–3 days, max 5 days): <12 months: 62.5 mg tds 1–5 years: 125 mg tds 6–12 years: 250 mg tds Phenoxymethyl penicillin (2–3 days, max 5 days): <12 months: 62.5 mg qds 1–5 years: 125 mg qds 6–12 years: 250 mg qds | Metronidazole (3 days): 1–3 years: 50 mg tds 3–7 years: 100 mg bid 7–10 years: 100 mg tds >10 years: 200 mg tds Erythromycin (2–3 days, max 5 days): 1 month–2 years: 125 mg qds 2–12 years: 250 mg qds Azithromycin (2–3 days): 6 months–3 years: 10 mg/kg od 3–7 years 200 mg od 8–11 years: 300 mg od 12–14 years: 400 mg od >14 years: 500 mg od | Remove cause Establish drainage Review 2–3 days | Author recommends the use of these antibiotics in descending order: amoxicillin, phenoxymethyl penicillin, metronidazole and lastly erythromycin. | Low 1 |
| Cellulitis | SDCEP [23] | Clinical guidelines | Amoxicillin (5 days): 6 months–1 year: 62.5 mg tds 1–5 years: 125 mg tds 6–18 years: 250 mg tds OR Phenoxymethyl penicillin (5 days): 6 months–1 year: 62.5 mg qds 1–6 years: 125 mg qds 6–12 years: 250 mg qds 13–18 years: 500 mg qds | Metronidazole Tabs, or Oral Suspension for 5 days: 1–3 years: 50 mg tds 4–7 years: 100 mg bid 8–10 years: 100 mg tds 11–18 years: 200 mg tds OR Clarithromycin (7 days): 1–5 years: 125 g bid 6–12 years: 187.5 mg bid 13–18 years: 250 mg bid | Low 1 | ||
| Generalized aggressive periodontitis and localized aggressive periodontitis | Haas et al. (2008) [24] | RCT | Azithromycin 500 mg coated tablet once daily for 3 days. | Phase 1 consisted of two sessions of supragingival scaling and oral hygiene instructions. At day 15, a clinical examination was performed, and phase 2 started consisting of nonsurgical periodontal therapy with subgingival hand scaling and root planing. Phase 2 was completed within a period of 14 days. The subjects were given azithromycin the first treatment session of phase 2. | Patients were ≥13 years; One year follow up significant improvement. | Very low 2 | |
| Localized aggressive periodontitis | Muppa et al. (2016) [25] | Case report | Amoxicillin (50 mg/kg/day) (body weight in three divided doses) AND metronidazole 30 mg/kg/day for 15 days. | Further topical application of metronidazole in chlorhexidine (Rexidin-M gel) base was advised for 2 weeks. Vitamin B complex syrup was also included. | Child was 5 years old; Regular checkups and motivation for oral hygiene were done for 1½ years. | Very low 3 | |
| Localized Aggressive periodontitis | Beliveau et al. (2012) [26] | Retrospective analysis of clinical trial | 500 mg of amoxicillin and 250 mg of metronidazole three times per day tds for 7 days. | Oral hygiene is mandatory. | Antibiotics were administered immediately after mechanical debridement. | Very low 2 | |
| Merchant et al. (2014) [27] | Clinical trial | Same as above | Dose modified for children less than 40 kg. | Very low 4 | |||
| Seremidi et al. (2012) [28] | Case report | Amoxycillin 50 mg/kg and metronidazole 30 mg/kg tds) for 2 weeks. | The oral health preventive program included oral hygiene instructions and more specifically toothbrushing twice daily with a fluoridated toothpaste, use of dental floss for interdental cleaning, and use of disclosing tablets to increase the effectiveness of plaque removal. Dietary instructions (decrease of sweets intake up to once per day) were also given. In office fluoride application was carried out every 3–4 mοnths. Prescription of 0.2% chlorohexidine mouthrinse for 10 days. | 8-year-old boy; Antibiotics were also administered at the end of the second visit of periodontal therapy which included full mouth scaling and root planing under local analgesia in two visits within a one-week interval. | Very low 3 | ||
| Ulcerative necrotizing periodontitis | SDCEP [23] | Clinical guidelines | 3-day regimen Amoxicillin: 6 months–1 year: 62.5 mg tds 2–5 years: 125 mg tds 6–18 years: 250 mg tds | 3-day regimen Metronidazole: 1–3 years: 50 mg tds 4–7 years: 100 mg bid 8–10 years: 100 mg tds 11–18 years: 200 mg td | Low 1 | ||
| Pericoronitis | SDCEP [23] | Clinical guidelines | 3-day regimen Amoxicillin: 6 months–1 year: 62.5 mg tds 2–5 years: 125 mg tds 6–18 years: 250 mg tds | 3-day regimen Metronidazole: 1–3 years: 50 mg tds 4–7 years: 100 mg bid 8–10 years: 100 mg tds 11–18 years: 200 mg td | Low 1 |
| Infection | Recommended Antibiotic Regimen | Recommended Antibiotic Regimen for Penicillin-Allergic Patient |
|---|---|---|
| Cellulitis | Amoxicillin (2–3 days, max 5 days): Children >3 months and <40 kg: 20–40 mg/kg/day in divided doses 8 hourly OR 25–45 mg/kg/day in divided doses 12 hourly Children >40 kg: 250–500 mg 8 hourly OR 500–875 mg 12 hourly OR Phenoxymethyl penicillin: (2–3 days, max 5 days) Children <12 years: 25–50 mg/kg/day in divided doses 6 hourly (max 3 g/day) Children ≥12 years: 250–500 mg 6 hourly | Metronidazole (3 days): Children: 30/mg/kg/day in divided doses 6 hourly (max 4 g/24 h) Adolescents and adults: 7.5 mg/kg 6 hourly (max 4 g/24 h) OR Azithromycin: Children >6 months up to 16 years: 5–12 mg/kg daily for 3 days (max 500 mg/day) OR 30 mg/kg as a single dose (max 1500 mg) OR Clarithromycin (7 days): 7.5 mg/kg 12 hourly 13–18 years: 250 mg 12 hourly |
| Aggressive periodontitis | Amoxicillin (50 mg/kg/day) AND Metronidazole 30 mg/kg/day 8 hourly for 7 days | Azithromycin (3 days): 10 mg/kg daily OR Metronidazole: 30 mg/kg/day 8 hourly for 7 days |
| Necrotizing ulcerative gingivitis | Amoxicillin (3 days): Children >3 months and <40 kg: 20–40 mg/kg/day in divided doses 8 hourly OR 25–45 mg/kg/day in divided doses 12 hourly Children >40 kg: 250–500 mg 8 hourly OR 500–875 mg 12 hourly | Metronidazole (3 days): Children: 30/mg/kg/day in divided doses 6 hourly (max 4 g/24 h) Adolescents: 250 mg 6 hourly OR 500 mg 8 hourly |
| Pericoronitis | Amoxicillin (3 days): Children >3 months and <40 kg: 20–40 mg/kg/day in divided doses 8 hourly OR 25–45 mg/kg/day in divided doses 12 hourly Children >40 kg: 250–500 mg 8 hourly OR 500–875 mg 12 hourly | Metronidazole (3 days): Children: 30/mg/kg/day in divided doses 6 hourly (max 4 g/24 h) Adolescents: 250 mg 6 hourly OR 500 mg 8 hourly |
| Domain | Support for Judgment | Authors’ Judgment |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Participants were randomly assigned by means of a draw | Low risk of bias |
| Allocation concealment | Medications were stored in opaque-coloured bottles identified only by the respective code of each participant | Low risk of bias |
| Performance bias | ||
| Blinding of participants | Participants were masked from medications types | Low risk of bias |
| Blinding of personnel | Both periodontists involved in the treatment and clinical examination were masked from the identity of participants | Low risk of bias |
| Detection bias | ||
| Blinding of outcome assessor | Blinding was ensured | Low risk of bias |
| Attrition bias | ||
| Incomplete outcome data | There was no drop out of participants | Low risk of bias |
| Reporting bias | ||
| Selective reporting | The article includes all expected outcomes, including those that were pre-specified | Low risk of bias |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dar-Odeh, N.; Fadel, H.T.; Abu-Hammad, S.; Abdeljawad, R.; Abu-Hammad, O.A. Antibiotic Prescribing for Oro-Facial Infections in the Paediatric Outpatient: A Review. Antibiotics 2018, 7, 38. https://doi.org/10.3390/antibiotics7020038
Dar-Odeh N, Fadel HT, Abu-Hammad S, Abdeljawad R, Abu-Hammad OA. Antibiotic Prescribing for Oro-Facial Infections in the Paediatric Outpatient: A Review. Antibiotics. 2018; 7(2):38. https://doi.org/10.3390/antibiotics7020038
Chicago/Turabian StyleDar-Odeh, Najla, Hani T. Fadel, Shaden Abu-Hammad, Rua’a Abdeljawad, and Osama A. Abu-Hammad. 2018. "Antibiotic Prescribing for Oro-Facial Infections in the Paediatric Outpatient: A Review" Antibiotics 7, no. 2: 38. https://doi.org/10.3390/antibiotics7020038
APA StyleDar-Odeh, N., Fadel, H. T., Abu-Hammad, S., Abdeljawad, R., & Abu-Hammad, O. A. (2018). Antibiotic Prescribing for Oro-Facial Infections in the Paediatric Outpatient: A Review. Antibiotics, 7(2), 38. https://doi.org/10.3390/antibiotics7020038






