Methicillin-Resistant Staphylococcus aureus: Risk for General Infection and Endocarditis Among Athletes
Abstract
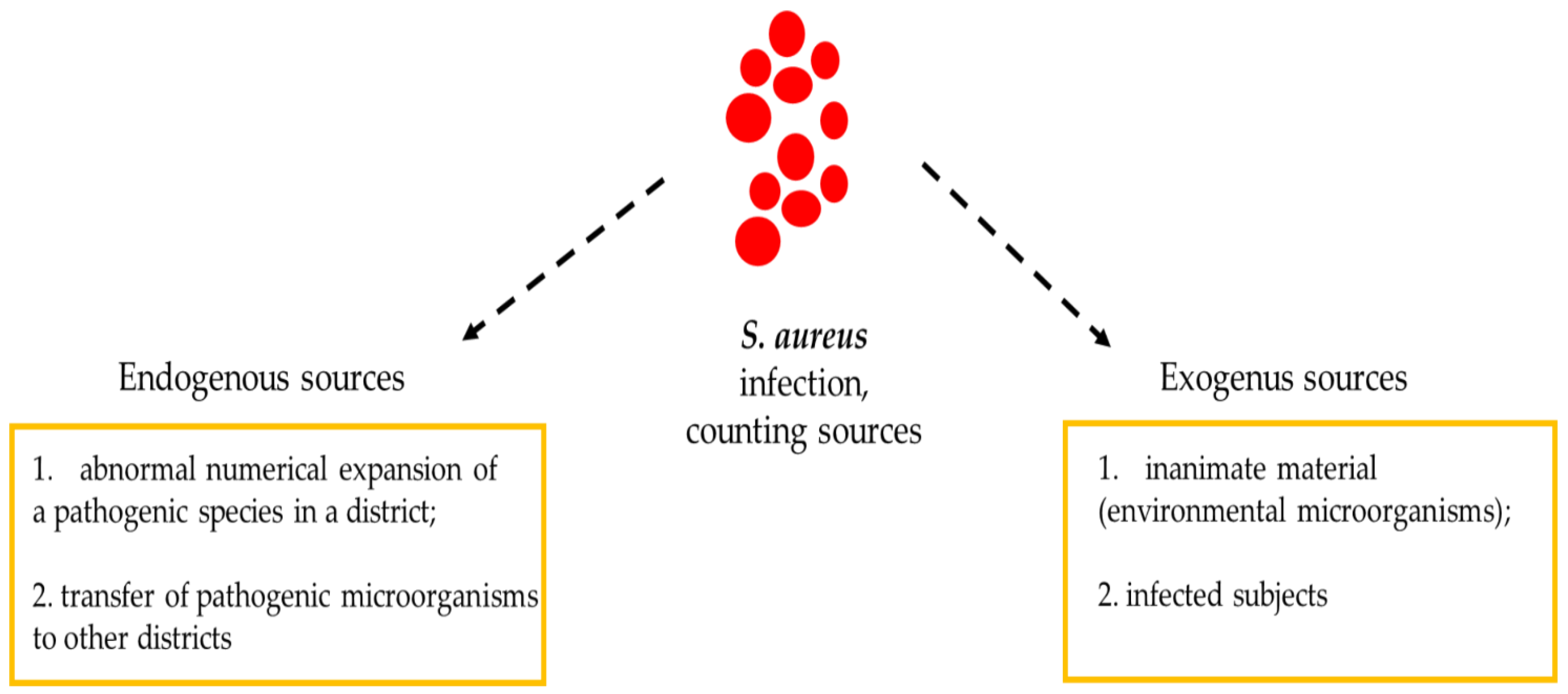
:1. Introduction
2. S. aureus outbreaks in Athletes
3. Importance of Fomites in S. aureus Infection Outbreaks
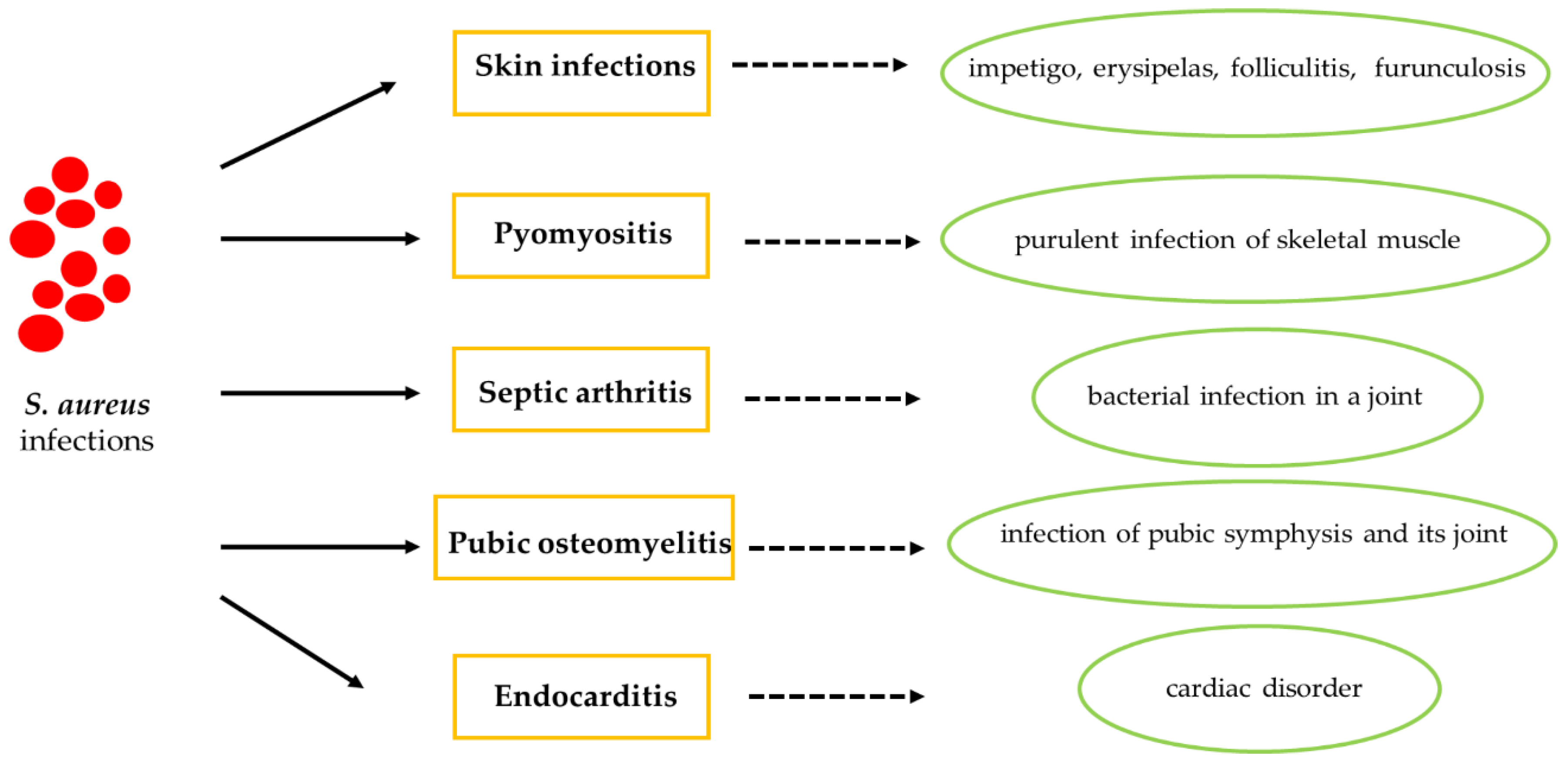
4. S. aureus Infection in Athletes
4.1. Cutaneous Tissue in Athletes with S. aureus Infection
4.2. Skin Disorders
4.3. Pyomyositis
4.4. Septic Arthritis
4.5. Pubic Osteomyelitis
4.6. Endocarditis
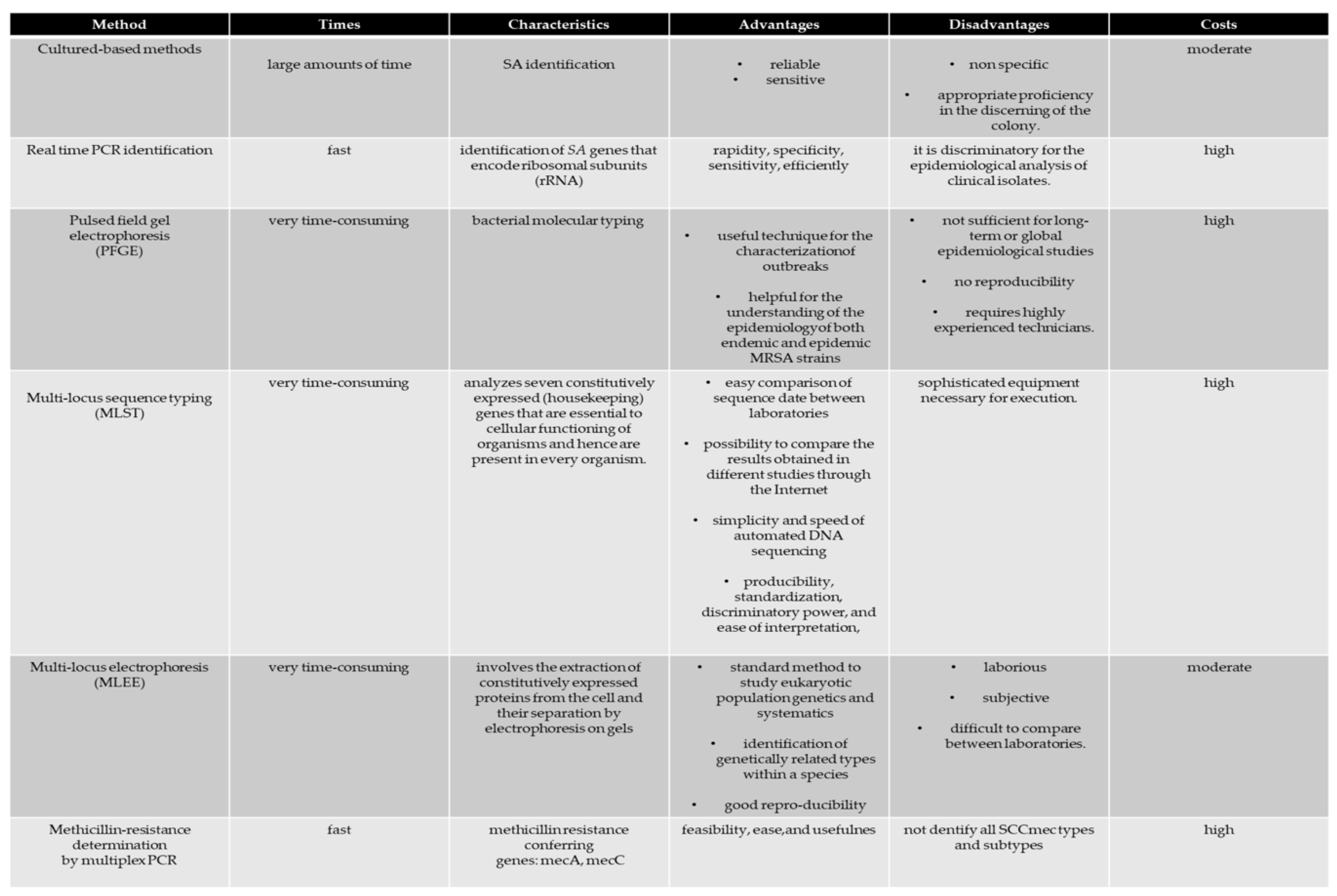
5. S. aureus Identification
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kazakova, S.V.; Hageman, J.C.; Matava, M.; Srinivasan, A.; Phelan, L.; Garfinkel, B.; Boo, T.; McAllister, S.; Anderson, J.; Jensen, B.; et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N. Engl. J. Med. 2005, 352, 468–475. [Google Scholar] [CrossRef]
- Oller, A.R.; Province, L.; Curless, B. Staphylococcus aureus recovery from environmental and human locations in 2 collegiate athletic teams. J. Athl. Train. 2009, 45, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Begier, E.M.; Frenette, K.; Barrett, N.L.; Mshar, P.; Petit, S.; Boxrud, D.J.; Watkins-Colwell, K.; Wheeler, S.; Cebelinski, E.A.; Glennen, A.; et al. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin. Infect. Dis. 2004, 39, 1446–1453. [Google Scholar] [CrossRef] [Green Version]
- Huijsdens, X.W.; Van Lier, A.M.; Van Kregten, E.; Verhoef, L.; Van Santen-Verheuvel, M.G.; Spalburg, E.; Wannet, W.J. Methicillin-resistant Staphylococcus aureus in Dutch soccer team. Emerg. Infect. Dis. 2006, 12, 1584–1586. [Google Scholar] [CrossRef]
- Scudiero, O.; Brancaccio, M.; Mennitti, C.; Laneri, S.; Lombardo, B.; Biasi, M.G.; Gregorio, E.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; et al. Human Defensins: A Novel Approach in the Fight against Skin Colonizing Staphylococcus aureus. Antibiotics 2020, 9, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, Y.H.; Kwon, Y.S.; Jung, M.K.; Kang, S.K.; Hwang, T.S.; Hong, Y.C. Helicobacter Pylori-Associated Iron-Deficiency Anemia in Adolescent Female Athletes. Athl. J. Pediatr. 2001, 139, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Angrisano, T.; Pero, R.; Brancaccio, M.; Coretti, L.; Florio, E.; Pezone, A.; Calabrò, V.; Falco, G.; Keller, S.; Lembo, F.; et al. Cyclical DNA Methylation and Histone Changes Are Induced by LPS to Activate COX-2 in Human Intestinal Epithelial Cells. PLoS ONE 2016, 11, e0156671. [Google Scholar] [CrossRef] [PubMed]
- Chiariotti, L.; Coretti, L.; Pero, R.; Lembo, F. Epigenetic Alterations Induced by Bacterial Lipopolysaccharides. Adv. Exp. Med. Biol. 2016, 879, 91–105. [Google Scholar] [PubMed]
- Kullander, J.; Forslund, O.; Dillner, J. Staphylococcus aureus and Squamous Cell Carcinoma of the Skin. Cancer Epidemiol. Biomark. Prev. 2009, 18, 472–478. [Google Scholar] [CrossRef] [Green Version]
- Coretti, L.; Cuomo, M.; Florio, E.; Palumbo, D.; Keller, S.; Pero, R.; Chiariotti, L.; Lembo, F.; Cafiero, C. Subgingival dysbiosis in smoker and non-smoker patients with chronic periodontitis. Mol. Med. Rep. 2017, 15, 2007–2014. [Google Scholar] [CrossRef] [Green Version]
- Coretti, L.; Natale, A.; Cuomo, M.; Florio, E.; Keller, S.; Lembo, F.; Chiariotti, L.; Pero, R. The Interplay between Defensins and Microbiota in Crohn’s Disease. Mediat. Inflamm. 2017, 2017, 8392523. [Google Scholar] [CrossRef] [PubMed]
- Pero, R.; Brancaccio, M.; Laneri, S.; De Biasi, M.G.; Lombardo, B.; Scudiero, O. A Novel View of Human Helicobacter pylori Infections: Interplay between Microbiota and Beta-Defensins. Biomolecules 2019, 9, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pero, R.; Angrisano, T.; Brancaccio, M.; Falanga, A.; Lombardi L6 Natale, F.; Laneri, S.; Lombardo, B.; Galdiero, S.; Scudiero, O. Beta-defensins and analogs in Helicobacter pylori infections: mRNA expression levels, DNA methylation, and antibacterial activity. PLoS ONE 2019, 14, e0222295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pero, R.; Coretti, L.; Nigro, E.; Lembo, F.; Laneri, S.; Lombardo, B.; Daniele, A.; Scudiero, O. β-Defensins in the Fight against. Helicobacter Pylori. Mol. 2017, 22, E424. [Google Scholar]
- Colavita, I.; Nigro, E.; Sarnataro, D.; Scudiero, O.; Granata, V.; Daniele, A.; Zagari, A.; Pessi, A.; Salvatore, F. Membrane protein 4F2/CD98 is a cell surface receptor involved in the internalization and trafficking of human β-Defensin 3 in epithelial cells. Chem. Biol. 2015, 2, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Nigro, E.; Colavita, I.; Sarnataro, D.; Scudiero, O.; Zambrano, G.; Granata, V.; Daniele, A.; Carotenuto, A.; Galdiero, S.; Folliero, V.; et al. An ancestral host defence peptide within human β-defensin 3 recapitulates the antibacterial and antiviral activity of the full-length molecule. Sci. Rep. 2015, 5, 18450. [Google Scholar] [CrossRef] [Green Version]
- Rackham, D.M.; Ray, S.M.; Franks, A.S.; Bielak, K.M.; Pinn, T.M. Community-associated methicillin-resistant Staphylococcus aureus nasal carriage in a college student athlete population. Clin. J. Sport Med. 2010, 20, 185–188. [Google Scholar] [CrossRef]
- Romano, R.; Lu, D.; Holtom, P. Outbreak of community-acquired methicillin-resistant Staphylococcus aureus skin infections among a collegiate football team. J. Athl. Train. 2006, 41, 141–145. [Google Scholar]
- Bartlett, P.C.; Martin, R.J.; Cahill, B.R. Furunculosis in a high school football team. Am. J. Sports Med. 1982, 10, 371–374. [Google Scholar] [CrossRef]
- Creech, C.B.; Saye, E.; McKenna, B.D.; Johnson, B.G.; Jimenez, N.; Talbot, T.R.; Bossung, T.; Gregory, A.; Edwards, K.M. One-year surveillance of methicillin-resistant Staphylococcus aureus nasal colonization and skin and soft tissue infections in collegiate athletes. Arch. Pediatr. Adolesc. Med. 2010, 164, 615–620. [Google Scholar] [CrossRef] [Green Version]
- Ellis, M.W.; Hospenthal, D.R.; Dooley, D.P.; Gray, P.J.; Murray, C.K. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin. Infect. Dis. 2004, 39, 971–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, D.M.; Mascola, L.; Brancoft, E. Recurring methicillin-resistant Staphylococcus aureus infections in a football team. Emerg. Infect. Dis. 2005, 11, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Methicillin-resistant Staphylococcus aureus infections among competitive sports participants -Colorado, Indiana, Pennsylvania, and Los Angeles County, 2000–2003. MMWR Morb. Mortal. Wkly. Rep. 2003, 2, 793–795. [Google Scholar]
- Haghverdian, B.; Patel, N.; Wang, L.; Cotter, J.C. The Sports Ball as a Fomite for Transmission of Staphylococcus aureus. J. Environ. Health 2018, 80, 8–13. [Google Scholar]
- Jiménez-Truque, N.; Elizabeth, J.; Saye, E.J.; Soper, N.; Saville, B.R.; Thomsen, I.; Edwards, K.M.; Creech, C.B. Association between Contact Sports and Colonization with Staphylococcus aureus in a Prospective Cohort of Collegiate Athletes. Sports Med. 2017, 5, 1011–1019. [Google Scholar] [CrossRef] [Green Version]
- Mascaro, V.; Capano, M.S.; Iona, T.; Nobile, C.G.A.; Ammendolia, A.; Pavia, M. Prevalence of Staphylococcus aureus Carriage and Pattern of Antibiotic Resistance, Including Methicillin Resistance, Among Contact Sport Athletes in Italy. Infect. Drug Resist. 2019, 7, 1161–1170. [Google Scholar] [CrossRef] [Green Version]
- Yokomori, R.; Tsurukiri, J.; Moriya, M.; Yamanaka, H.; Kobayashi, T.; Nakaminami, H.; Takadama, S.; Noguchi, N.; Matsumoto, T.; Arai, T. First Report of Fatal Infection Caused by Community-acquired Methicillin-resistant Staphylococcus aureus USA300 Clone in a Collegiate Athlete. JMA J. 2020, 3, 78–82. [Google Scholar]
- Suzuki, K.; Tagami, K. Role of nasal Staphylococcus aureus carriage in transmission among contact athletes. Int. J. Sports Med. 2015, 36, 1186–1191. [Google Scholar] [CrossRef] [Green Version]
- Zinder, S.M.; Basler, R.S.; Foley, J.; Scarlata, C.; Vasily, D.B. National athletic trainers’ association position statement: Skin diseases. J. Athl. Train. 2010, 45, 411–428. [Google Scholar] [CrossRef]
- Redziniak, D.E.; Diduch, D.R.; Turman, K.; Hart, J.; Grindstaff, T.L.; MacKnight, J.M.; Mistry, D.J. Methicillin-resistant Staphylococcus aureus (MRSA) in the athlete. Int. J. Sports Med. 2009, 30, 557–562. [Google Scholar] [CrossRef] [Green Version]
- Cordoro, K.M.; Ganz, J.E. Training room management of medical conditions: Sports dermatology. Clin. Sports Med. 2005, 24, 565–598. [Google Scholar] [CrossRef] [PubMed]
- Asgeirsson, H.; Thalme, A.; Weiland, O. Staphylococcus aureus bacteraemia and endocarditis—Epidemiology and outcome: A review. Infect. Dis. (Lond.) 2018, 3, 175–192. [Google Scholar] [CrossRef]
- Saeed, K.; Bal, A.M.; Gould, I.M.; David, M.Z.; Dryden, M.; Giannitsioti, E.; Hijazi, K.; Meisner, J.A.; Esposito, S.; Scaglione, F.; et al. An update on Staphylococcus aureus infective endocarditis from the International Society of Antimicrobial Chemotherapy (ISAC). Int. J. Antimicrob. Agents 2019, 53, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crosera, M.; Bovenzi, M.; Maina, G.; Adami, G.; Zanette, C.; Florio, C.; Filon Larese, F. Nanoparticle dermal absorption and toxicity: A review of the literature. Int. Arch. Occup. Environ. Health 2009, 82, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Kahanov, L.; Dannelly, K.; Lauber, C. CA-MRSA infection incidence and care in high school and intercollegiate athletics. Med. Sci. Sports Exerc. 2016, 48, 1530–1538. [Google Scholar] [CrossRef]
- Collins, C.J.; O’Connell, B. Infectious disease outbreaks in competitive sports, 2005–2010. J. Athl. Train. 2012, 47, 516–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Truque, N.; Saye, E.J.; Soper, N.; Saville, B.R.; Thomsen, I.; Edwards, K.M.; Creech, C.B. Longitudinal assessment of colonization with Staphylococcus aureus in healthy collegiateathletes. J. Pediatr. Infect. Dis. Soc. 2016, 5, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Korting, H.C.; Lukacs, A.; Vogt, N.; Urban, J.; Ehret, W.; Ruckdeschel, G. Influence of the pH-value on the growth of Staphylococcus epidermidis, Staphylococcus aureus and Propionibacterium acnes in continuous culture. Int. J. Hyg. Environ. Med. 1992, 193, 78–90. [Google Scholar]
- Weber, K. Community-Associated Methicillin-Resistant Staphylococcus aureus Infections in the Athlete. Sports Health 2009, 1, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Wertheim, H.F.; Melles, D.C.; Vos, M.C.; Van Leeuwen, W.; Van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- Kong, H.H.; Segre, J.A. Skin microbiome: Looking back to move forward. J. Investig. Dermatol. 2012, 132, 933–939. [Google Scholar] [CrossRef] [Green Version]
- Belkaid, Y.; Tamoutounour, S. The influence of skin microorganisms on cutaneous immunity. Nat. Rev. Immunol. 2016, 16, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Reagan, D.R.; Doebbeling, B.N.; Pfaller, M.A.; Sheetz, C.T.; Houston, A.K.; Hollis, R.J.; Wenzel, R.P. Elimination of coincident Staphylococcus aureus nasal and hand carriage with intranasal application of mupirocin calcium ointment. Ann. Intern. Med. 1991, 114, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Von Eiff, C.; Becker, K.; Machka, K.; Stammer, H.; Peters, G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study group. N. Engl. J. Med. 2001, 344, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.; Vos, M.C.; Ott, A.; Van Belkum, A.; Voss, A.; Kluytmans, J.A.; Van Keulen, P.H.; Vandenbroucke-Grauls, C.M.; Meester, M.H.; Verbrugh, H.A. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004, 364, 703–705. [Google Scholar] [CrossRef]
- Williams, R.E. Healthy carriage of Staphylococcus aureus: Its prevalence and importance. Bacteriol. Rev. 1963, 27, 56–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cespedes, C.; Said-Salim, B.; Miller, M.; Lo, S.H.; Kreiswirth, B.N.; Gordon, R.J.; Vavagiakis, P.; Klein, R.S.; Lowy, F.D. The clonality of Staphylococcus aureus nasal carriage. J. Infect. Dis. 2005, 191, 444–452. [Google Scholar] [CrossRef] [Green Version]
- Luke, A.; D’Hemecourt, P. Prevention of infectious diseases in athletes. Clin. Sports Med. 2007, 26, 321–344. [Google Scholar] [CrossRef]
- Eda, N.; Shimizu, K.; Suzuki, S.; Lee, E.; Akama, T. Effects of high-intensity endurance exerciseon epidermal barriers against microbial invasion. J. Sports Sci. Med. 2013, 12, 44–51. [Google Scholar]
- Freeman, M.J.; Bergfeld, W.F. Skin diseases of footballand wrestling participants. Cutis 1977, 20, 333–341. [Google Scholar]
- Bergfeld, W.F.; Taylor, J.S. Trauma, sports, and the skin. Am. J. Ind. Med. 1985, 8, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Powell, F.C. Sports dermatology. J. Eur. Acad. Dermatol. Venereol. 1994, 3, 1–15. [Google Scholar] [CrossRef]
- Sosin, D.M.; Gunn, R.A.; Ford, W.L.; Skaggs, J.W. An outbreak offurunculosis among high school athletes. Am. J. Sports Med. 1989, 17, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bhalla, A.; Singh, R.; Sharma, N.; Sharma, A.; Gautam, V.; Singh, S.; Varma, S. Primary pyomyositis in North India: A clinical, microbiological, and outcome study. Korean J. Intern. Med. 2018, 33, 417–431. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S.; Jain, S.; Varma, S.; Chauhan, S.S. Tropical pyomyositis (myositis tropicans): Current perspective. Postgrad. Med. J. 2004, 80, 267–270. [Google Scholar] [CrossRef] [Green Version]
- Crum, N.F. Bacterial pyomyositis in the United States. Am. J. Med. 2004, 117, 420–428. [Google Scholar] [CrossRef]
- Kiran, M.; Mohamed, S.; Newton, A.; George, H.; Garg, N.; Bruce, C. Pelvic pyomyositis in children: Changing trends in occurrence and management. Int. Orthop. 2018, 42, 1143–1147. [Google Scholar] [CrossRef]
- Drovandi, L.; Trapani, S.; Richichi, S.; Lasagni, D.; Resti, M. Primary pyomyositis as unusual cause of limp: Three cases in immunocompetent children and literature review. J. Pediatr. Infect. Dis. 2018, 13, 242–246. [Google Scholar]
- Xipell, M.; Ventura-Aguiar, P.; Revuelta, I.; Bodro, M.; Diekmann, F. Pyomyositis in a patient with IgA nephropathy and kidney transplant. Case Rep. Transplant. 2019, 2019, 7305683. [Google Scholar] [CrossRef]
- Ragozzino, E.; Brancaccio, M.; Di Costanzo, A.; Scalabrì, F.; Andolfi, G.; Wanderlingh, L.G.; Patriarca, E.J.; Minchiotti, G.; Altamura, S.; Varrone, F.; et al. 6-Bromoindirubin-3′-oxime intercepts GSK3 signaling to promote and enhance skeletal muscle differentiation affecting miR-206 expression in mice. Sci. Rep. 2019, 9, 18091. [Google Scholar] [CrossRef]
- Reinhold, I.; Dudler, J. Primary pyomyositis—A lifethreatening aetiology of febrile myalgia not to be discounted even in healthy individuals. Br. J. Gen. Pract. 2019, 2019, 5739714. [Google Scholar]
- Newman, J.H. Review of septic arthritis throughout the antibiotic era. Ann. Rheum. Dis. 1976, 35, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.; Su, E.Y. Therapy for septic arthritis. JAMA 1982, 247, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Ukwu, H.N.; Graham, B.S.; Latham, R.H. Acute pubic osteomyelitis in athletes. Clin. Infect. Dis. 1992, 15, 636–638. [Google Scholar] [CrossRef]
- Querques, F.; Cantilena, B.; Cozzolino, C.; Esposito, M.T.; Passaro, F.; Parisi, S.; Lombardo, B.; Russo, T.; Pastore, L. Angiotensin receptor I stimulates osteoprogenitor proliferation through TGFβ-mediated signaling. J. Cell. Physiol. 2015, 23, 1466–1474. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. ESC Scientific Document Group. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Kirkland, E.B.; Adams, B.B. Methicillin-resistant Staphylococcus aureus and athletes. J. Am. Acad. Dermatol. 2008, 59, 494–502. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- LaHaye, S.; Lincoln, J.; Garg, V. Genetics of Valvular Heart Disease. Curr. Cardiol. Rep. 2014, 16, 487. [Google Scholar] [CrossRef] [Green Version]
- Girolami, F.; Frisso, G.; Benelli, M.; Crotti, L.; Iascone, M.; Mango, R.; Mazzaccara, C.; Pilichou, K.; Arbustini, E.; Tomberli, B.; et al. Contemporary genetic testing in inherited cardiac disease: Tools, ethical issues, and clinical applications. J. Cardiovasc. Med. 2018, 19, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Detta, N.; Frisso, G.; Limongelli, G.; Marzullo, M.; Calabrò, R.; Salvatore, F. Genetic analysis in a family affected by sick sinus syndrome may reduce the sudden death risk in a young aspiring competitive athlete. Int. J. Cardiol. 2014, 170, e63–e65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zebisch, A.; Schulz, E.; Grosso, M.; Lombardo, B.; Acierno, G.; Sill, H.; Iolascon, A. Identification of a novel variant of epsilon-gamma-delta-beta thalassemia highlights limitations of next generation sequencing. Am. J. Hematol. 2015, 90, E52–E54. [Google Scholar] [CrossRef] [PubMed]
- Mohiyiddeen, G.; Brett, I.; Jude, E. Infective endocarditis caused by Staphylococcus aureus in a patient with atopic dermatitis: A case report. J. Med. Case Rep. 2008, 2, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahill, T.J.; Baddour, L.M.; Habib, G.; Hoen, B.; Salaun, E.; Pettersson, G.B.; Schäfers, H.J.; Prendergast, B.D. Challenges in Infective Endocarditis. J. Am. Coll. Cardiol. 2017, 69, 325–344. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, G.; Crisafulli, A.; Guccione, P.; Di Salvo, G.; Bassareo, P.P. Infective endocarditis triangle. Is it the time to revisit infective endocarditis susceptibility and indications for its antibiotic prophylaxis? Eur. J. Prev. Cardiol. 2019, 26, 1771–1774. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.Y.; MacDonald, B.B.; Peacock, J.E.; Musher, D.M.; Triplett, P.; Mylotte, J.M.; O’Donnell, A.; Wagener, M.M.; Yu, V.L. A prospective multicenter study of staphylococcus aureus bacteremia: Incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003, 82, 322–332. [Google Scholar] [CrossRef]
- Showler, A.; Burry, L.; Bai, A.D.; Steinberg, M.; Ricciuto, D.R.; Fernandes, T.; Chiu, A.; Raybardhan, S.; Science, M.; Fernando, E.; et al. Use of transthoracic echocardiography in the management of low-risk Staphylococcus aureus bacteremia: Results from a retrospective multicenter cohort study. JACC Cardiovasc. Imaging 2015, 8, 924–931. [Google Scholar] [CrossRef] [Green Version]
- Palraj, B.R.; Baddour, L.M.; Hess, E.P.; Steckelberg, J.M.; Wilson, W.R.; Lahr, B.D.; Sohail, M.R. Predicting Risk of Endocarditis Using a Clinical Tool (PREDICT): Scoring System to Guide Use of Echocardiography in the Management of Staphylococcus aureus Bacteremia. Clin. Infect. Dis. 2015, 61, 18–28. [Google Scholar] [CrossRef]
- May, C.L.; Hodde, J.P.; Badylak, S.F.; Smith, G.F. Infective endocarditis in a collegiate wrestler. J. Athl. Train. 1995, 30, 105–107. [Google Scholar]
- Fallon, K.E.; Horvath, P.K.; Hopkins, A. Subacute bacterial endocarditis in a footballer—Not a sports injury. Clin. J. Sport Med. 2002, 12, 41–42. [Google Scholar] [CrossRef]
- Hoerr, V.; Franz, M.; Pletz, M.W.; Diab, M.; Niemann, S.; Faber, C.; Doenst, T.; Schulze, P.C.; Deinhardt-Emmer, S.; Löffler, B. S. aureus endocarditis: Clinical aspects and experimental approaches. Int. J. Med. Microbiol. 2018, 308, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B.; Riley, L. Molecular Epidemiology: Focus on Infection. Am. J. Epidemiol. 2001, 153, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lin, D.; Xu, P.; Zhang, T.; Ou, Q.; Bai, C.; Yao, Z. Non-hospital environment contamination with Staphylococcus aureus and methicillin-resistant Staphylococcus aureus: Proportion meta-analysis and features of antibiotic resistance and molecular genetics. Environ. Res. 2016, 150, 528–540. [Google Scholar] [CrossRef]
- Aguadero, V.; Gonzalez Velasco, C.; Vindel, A.; Gonzalez Velasco, M.; Moreno, J.J. Evaluationof rep-PCR/DiversiLab versus PFGE and spa typing in genotyping methicillin-resistant Staphylococcus aureus (MRSA). Br. J. Biomed. Sci. 2015, 72, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Franco-Duarte, R.; Černáková, L.; Kadam, S.; Kaushik, K.S.; Salehi, B.; Bevilacqua, A.; Corbo, M.R.; Antolak, H.; Dybka-Stępień, K.; Leszczewicz, M.; et al. Advances in Chemical and Biological Methods to Identify Microorganisms—From Past to Present. Microorganisms 2019, 7, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katakweba, A.S.; Muhairwa, A.P.; Espinosa-Gongora, C.; Guardabassi, L.; Mtambo, M.M.; Olsen, J.E. Spa typing and antimicrobial resistance of Staphylococcus aureus from healthy humans, pigs and dogs in Tanzania. J. Infect. Dev. Ctries. 2016, 10, 143–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, J.R.; Meaney, W.J.; Hartigan, P.J.; Smyth, C.J.; Kapur, V. Fine-structure molecular epidemiological analysis of Staphylococcus aureus recovered from cows. Epidemiol. Infect. 1997, 119, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Price, J.R.; Didelot, X.; Crook, D.W.; Llewelyn, M.J.; Paul, J. Whole genome sequencing in the prevention and control of Staphylococcus aureus infection. J. Hosp. Infect. 2013, 83, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Harris, S.R.; Clarke, I.N.; Seth-Smith, H.M.; Solomon, A.W.; Cutcliffe, L.T.; Marsh, P.; Skilton, R.J.; Holland, M.J.; Mabey, D.; Peeling, R.W.; et al. Whole-genome analysis of diverse Chlamydia trachomatis strains identifies phylogenetic relationships masked by current clinical typing. Nat. Genet. 2012, 44, 413–419. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Tawada, Y.; Kato, M.; Hori, H.; Mamiya, N.; Hayashi, Y.; Nakano, M.; Fukushima, R.; Katai, A.; Tanaka, T.; et al. Development of a rapid strain differentiation method for methicillin-resistant Staphylococcus aureus isolated in Japan by detecting phage-derived open-reading frames. J. Appl. Microbiol. 2006, 101, 938–947. [Google Scholar] [CrossRef]
- Nada, T.; Yagi, T.; Ohkura, T.; Morishita, Y.; Baba, H.; Ohta, M.; Suzuki, M. Usefulness of phage open-reading frame typing method in an epidemiological study of an outbreak of methicillin-resistant Staphylococcus aureus infections. Jpn. J. Infect. Dis. 2009, 62, 386–389. [Google Scholar] [PubMed]
- O’Sullivan, M.V.; Kong, F.; Sintchenko, V.; Gilbert, G.L. Rapid identification of methicillin resistant Staphylococcus aureus transmission in hospitals by use of phage-derived open reading frame typing enhanced by multiplex PCR and reverse line blot assay. J. Clin. Microbiol. 2010, 48, 2741–2748. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brancaccio, M.; Mennitti, C.; Laneri, S.; Franco, A.; De Biasi, M.G.; Cesaro, A.; Fimiani, F.; Moscarella, E.; Gragnano, F.; Mazzaccara, C.; et al. Methicillin-Resistant Staphylococcus aureus: Risk for General Infection and Endocarditis Among Athletes. Antibiotics 2020, 9, 332. https://doi.org/10.3390/antibiotics9060332
Brancaccio M, Mennitti C, Laneri S, Franco A, De Biasi MG, Cesaro A, Fimiani F, Moscarella E, Gragnano F, Mazzaccara C, et al. Methicillin-Resistant Staphylococcus aureus: Risk for General Infection and Endocarditis Among Athletes. Antibiotics. 2020; 9(6):332. https://doi.org/10.3390/antibiotics9060332
Chicago/Turabian StyleBrancaccio, Mariarita, Cristina Mennitti, Sonia Laneri, Adelaide Franco, Margherita G. De Biasi, Arturo Cesaro, Fabio Fimiani, Elisabetta Moscarella, Felice Gragnano, Cristina Mazzaccara, and et al. 2020. "Methicillin-Resistant Staphylococcus aureus: Risk for General Infection and Endocarditis Among Athletes" Antibiotics 9, no. 6: 332. https://doi.org/10.3390/antibiotics9060332





