In Situ Fabrication of AlN Coating by Reactive Plasma Spraying of Al/AlN Powder
Abstract
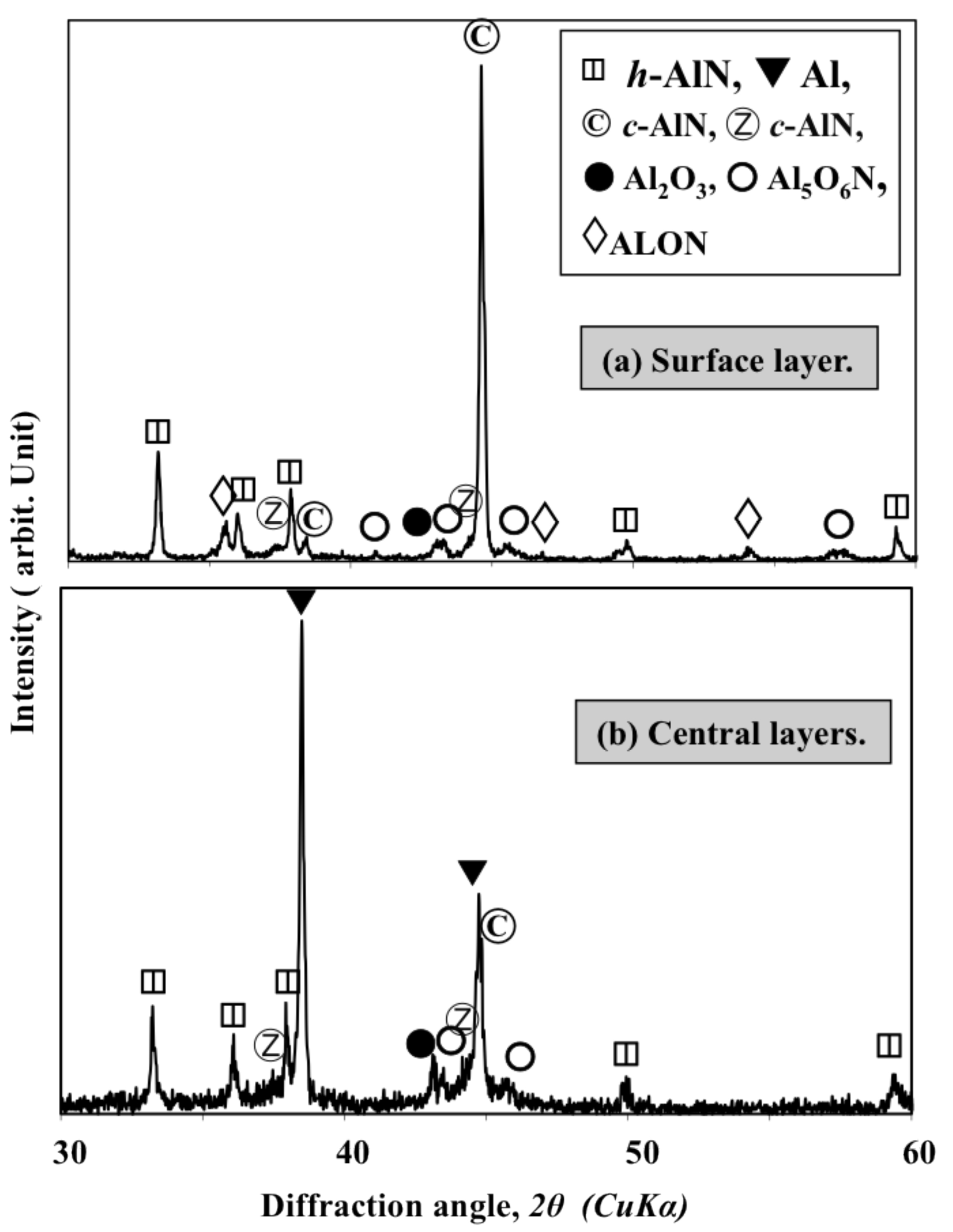
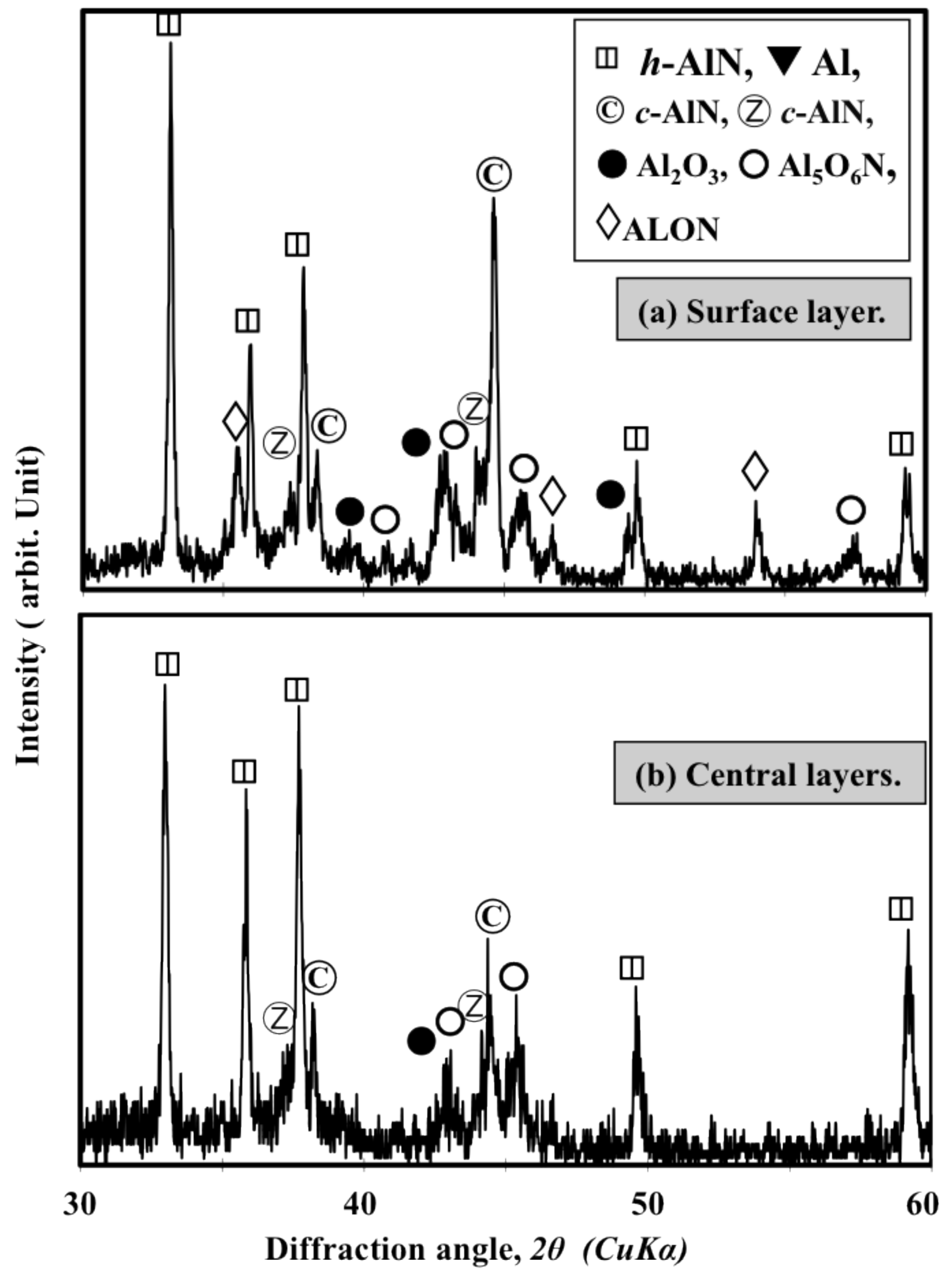
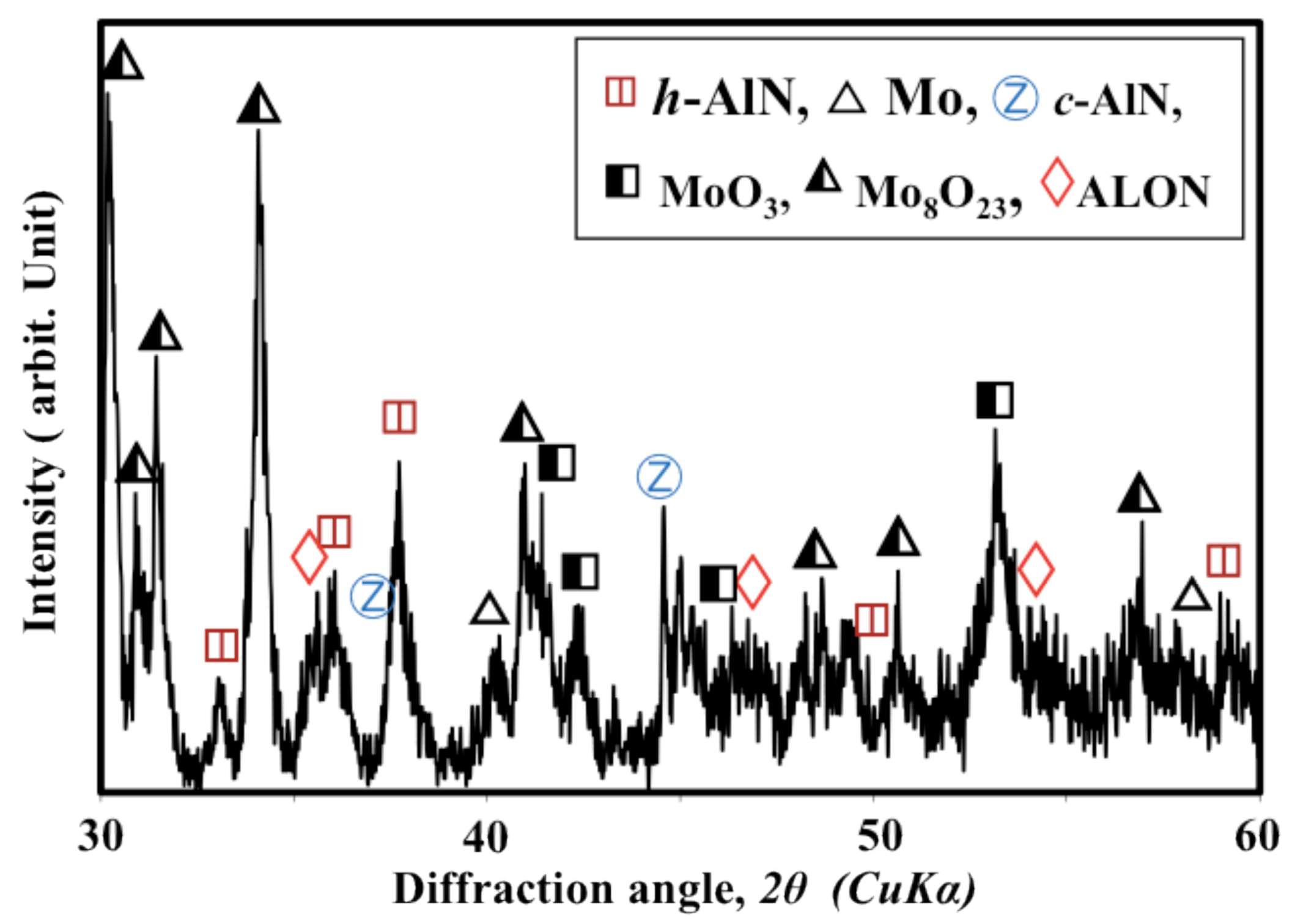
: Reactive plasma spraying is a promising technology for the in situ formation of aluminum nitride (AlN) coatings. Recently, it became possible to fabricate cubic-AlN-(c-AlN) based coatings through reactive plasma spraying of Al powder in an ambient atmosphere. However, it was difficult to fabricate a coating with high AlN content and suitable thickness due to the coalescence of the Al particles. In this study, the influence of using AlN additive (h-AlN) to increase the AlN content of the coating and improve the reaction process was investigated. The simple mixing of Al and AlN powders was not suitable for fabricating AlN coatings through reactive plasma spraying. However, it was possible to prepare a homogenously mixed, agglomerated and dispersed Al/AlN mixture (which enabled in-flight interaction between the powder and the surrounding plasma) by wet-mixing in a planetary mill. Increasing the AlN content in the mixture prevented coalescence and increased the nitride content gradually. Using 30 to 40 wt% AlN was sufficient to fabricate a thick (more than 200 μm) AlN coating with high hardness (approximately 1000 Hv). The AlN additive prevented the coalescence of Al metal and enhanced post-deposition nitriding through N2 plasma irradiation by allowing the nitriding species in the plasma to impinge on a larger Al surface area. Using AlN as a feedstock additive was found to be a suitable method for fabricating AlN coatings by reactive plasma spraying. Moreover, the fabricated coatings consist of hexagonal (h-AlN), c-AlN (rock-salt and zinc-blend phases) and certain oxides: aluminum oxynitride (Al5O6N), cubic sphalerite Al23O27N5 (ALON) and Al2O3. The zinc-blend c-AlN and ALON phases were attributed to the transformation of the h-AlN feedstock during the reactive plasma spraying. Thus, the zinc-blend c-AlN and ALON phases were not included in the feedstock and were not formed through nitriding of the Al.1. Introduction
2. Results and Discussion
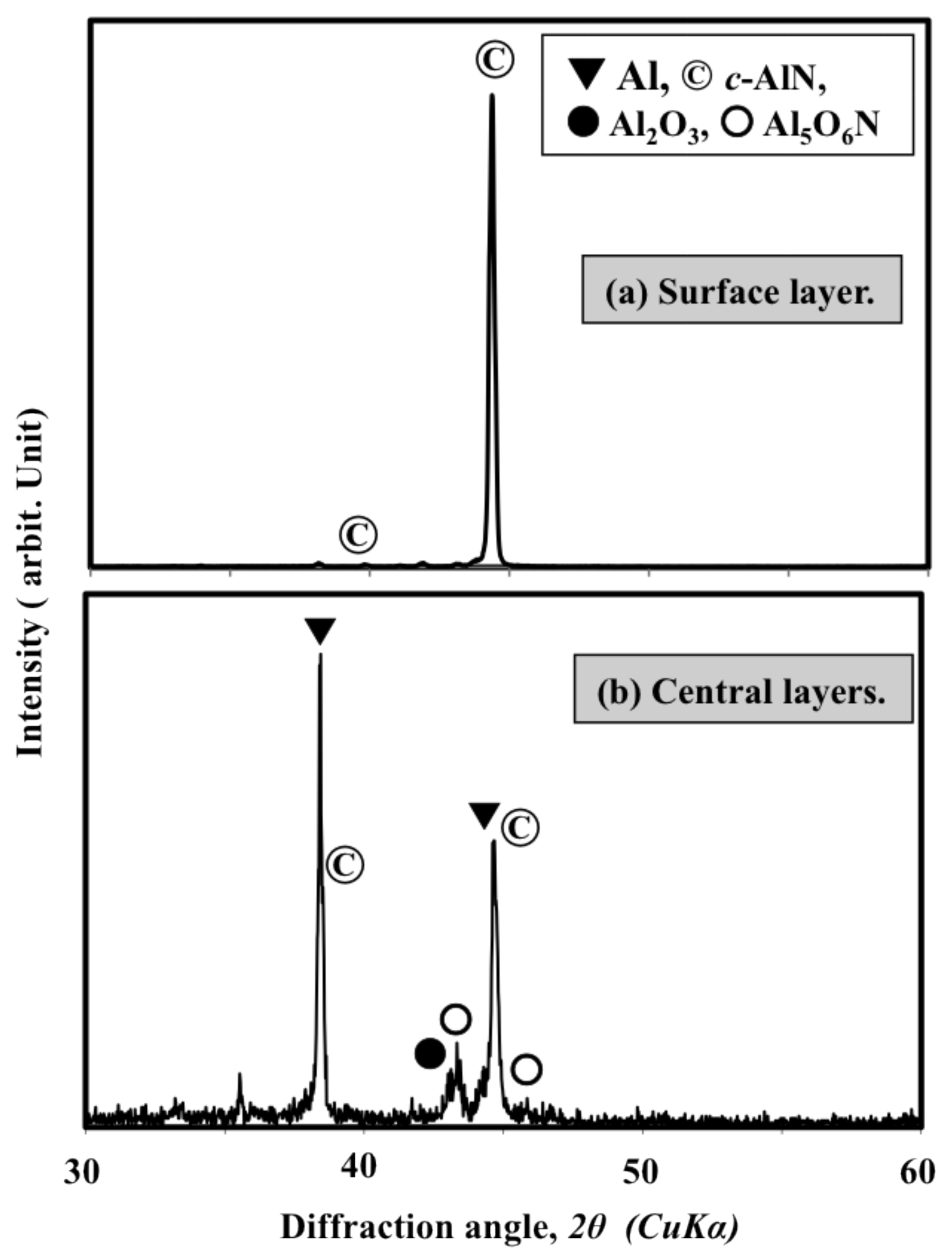
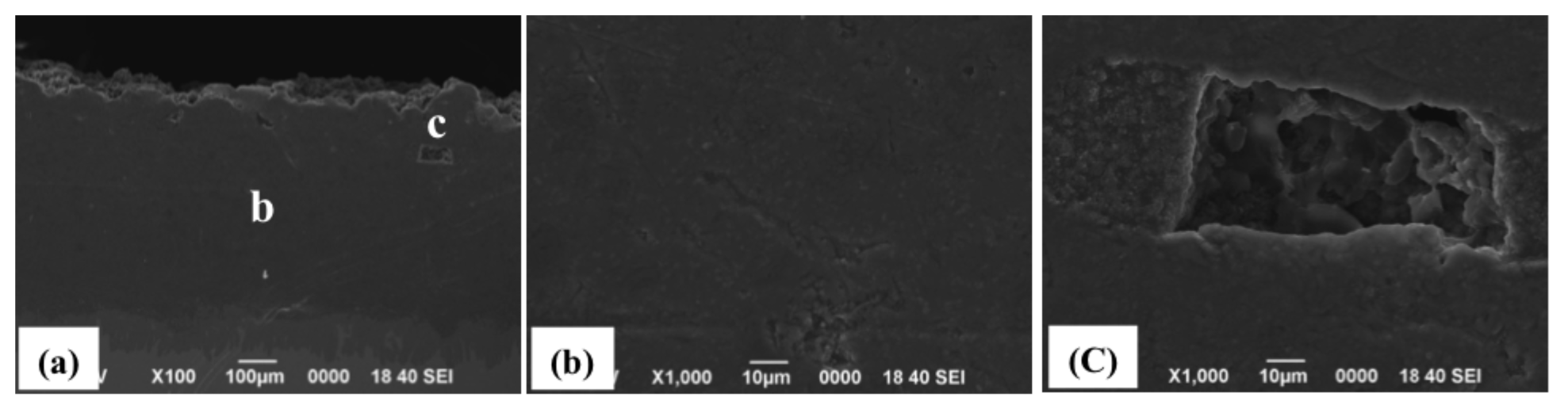
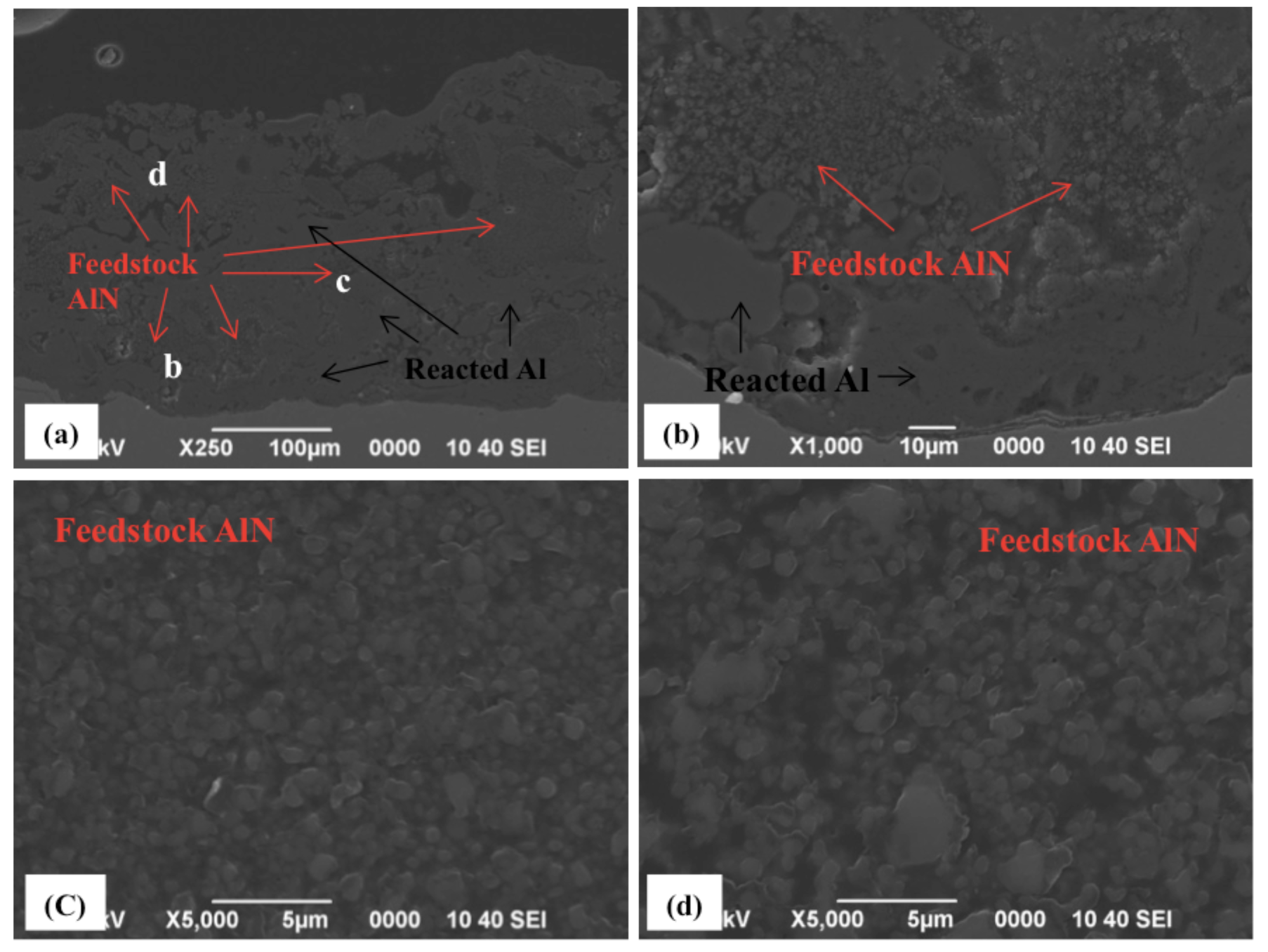
2.1. Spraying of Simply Mixed Al/AlN Powders
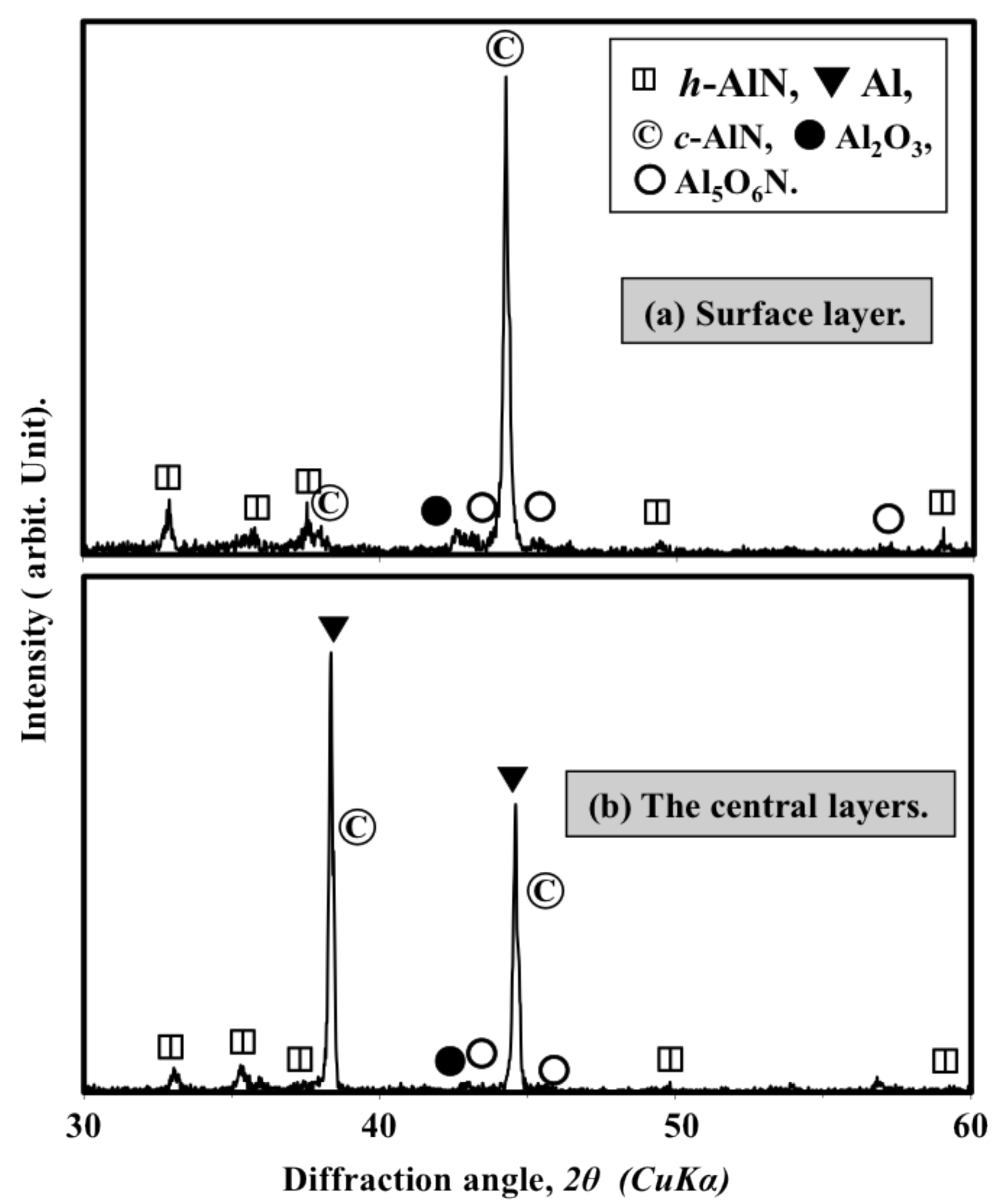
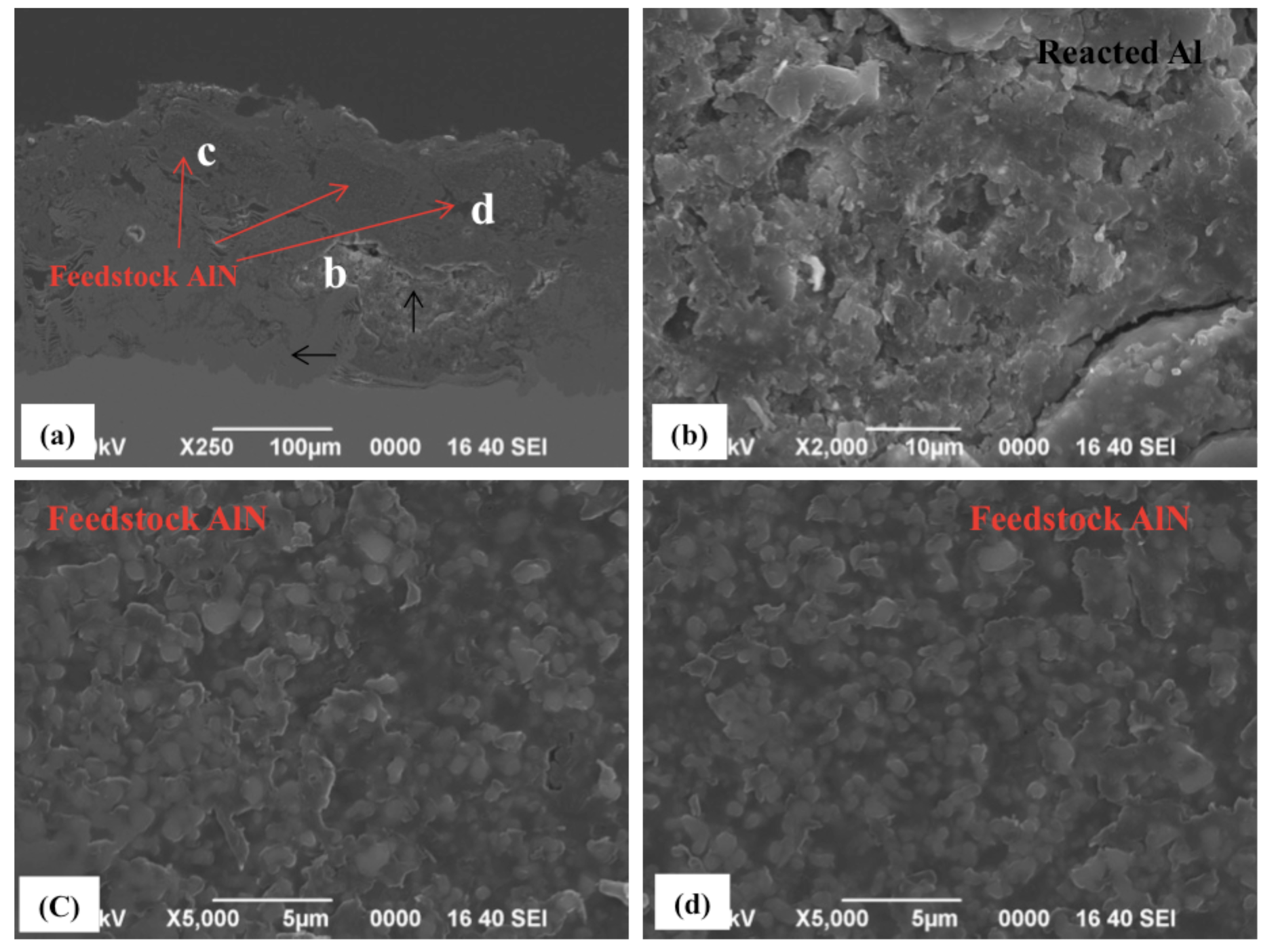
2.2. Spraying of Wet-Mixed Al/AlN Powders
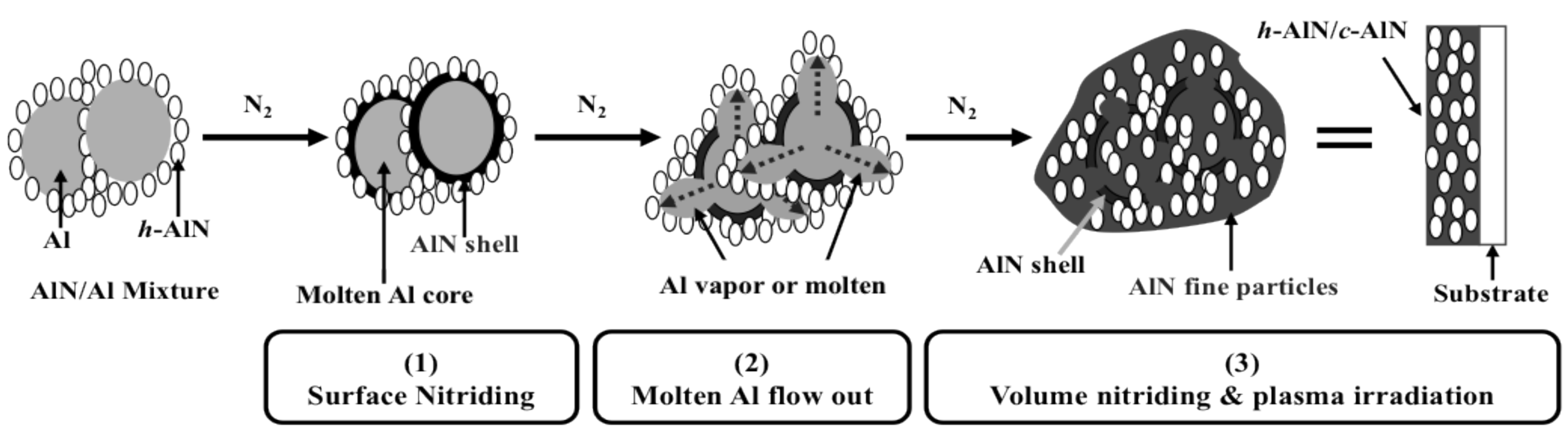
2.3. Discussion
3. Experimental Procedures
4. Conclusions
- Simple mixing is not suitable for preparing Al/AlN mixtures for reactive plasma spray processes. The AlN only weakly deposits when using simply mixed 30 wt% Al/AlN powders, and it has no significant effect on the nitriding of the Al powder.
- Wet mixing can be used to prepare a homogenously mixed, agglomerated and dispersed Al/AlN mixture suitable for reactive plasma spraying.
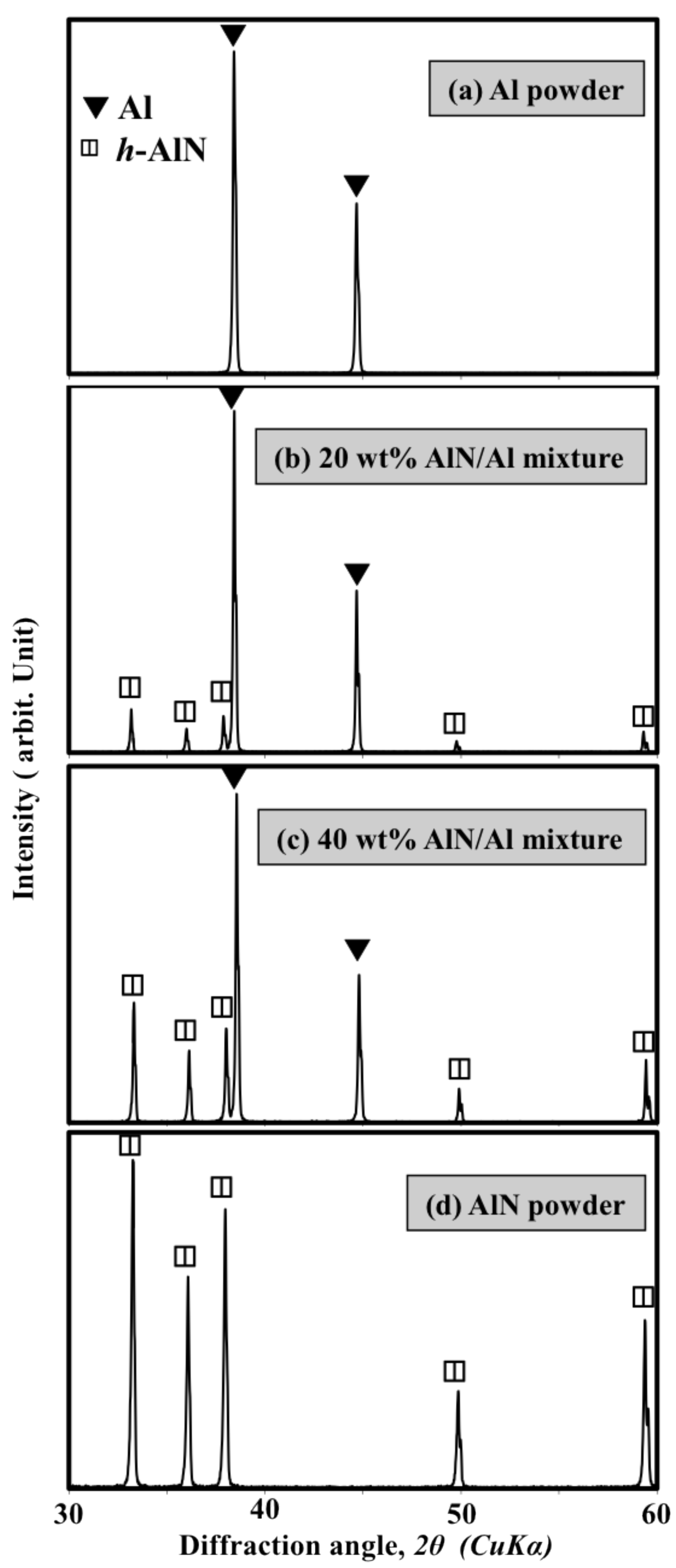
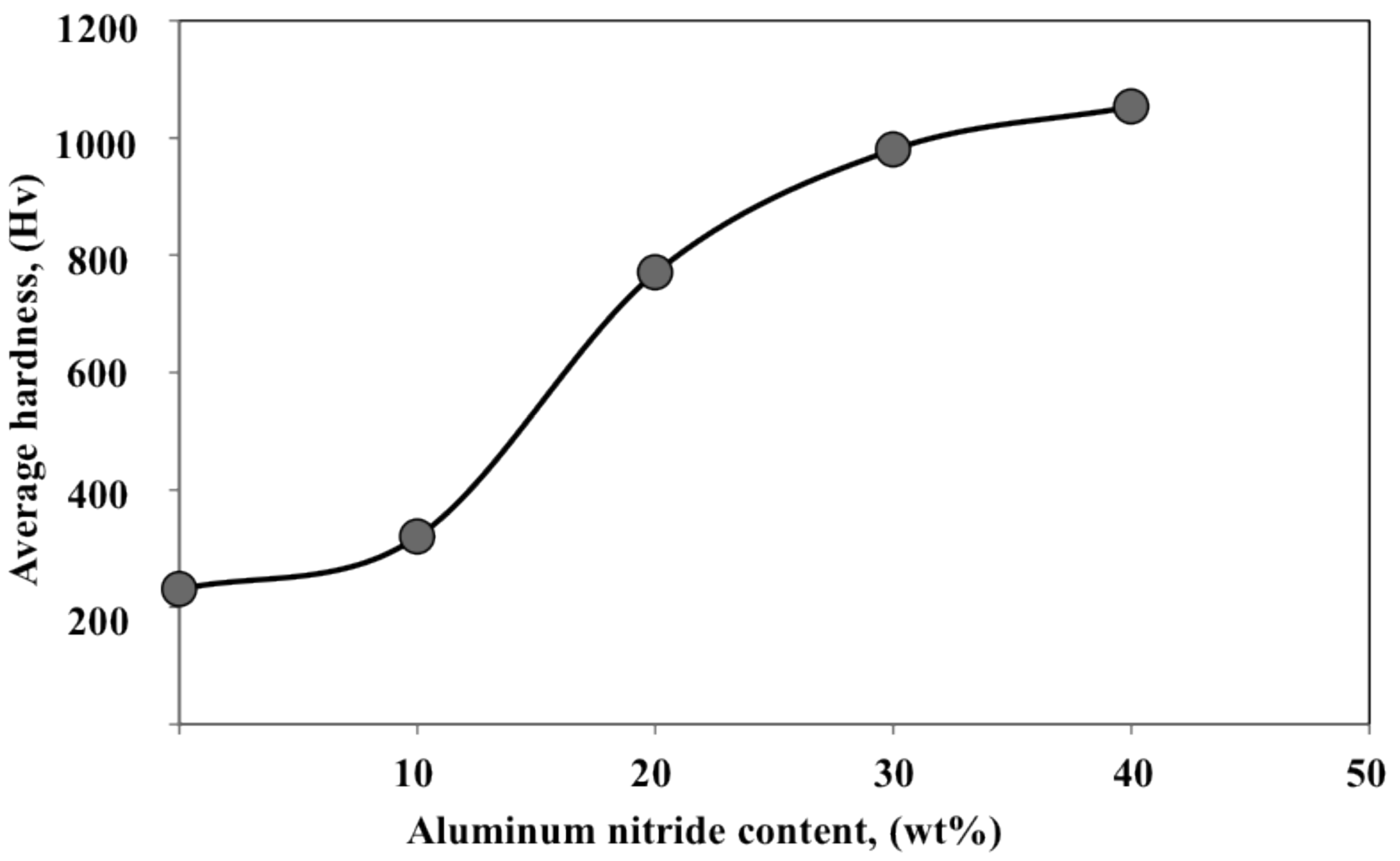
- The nitride content increased gradually with increasing AlN content in the feedstock powder. A high-nitride content coating more than 200 μm thick and with a hardness of approximately 1000 Hv was successfully fabricated by using 30∼40 wt% addition of AlN powder. The fabricated coating mainly consists of h-AlN and c-AlN (rock-salt and zinc-blend structures).
- The addition of AlN promoted the formation of the AlN phase in the reactive plasma spray process by preventing the coagulation and coalescence of the Al particles on the substrate. This promotes continuous nitriding through N2 plasma irradiation (post-deposition nitriding). Furthermore, using AlN in the feedstock powder increased the nitride content in the coating.
- The sprayed coatings contain cubic-AlN with zinc-blend structures, which was not used as the feedstock and was not formed during the nitriding of the Al. The formation of both the c-AlN zinc-blend phase and the cubic sphalerite ALON is attributed to transformation from the hexagonal AlN feedstock powder.
- AlN content of more than 40 wt% in the mixed powder is not suitable for supplying the mixture to the plasma stream.

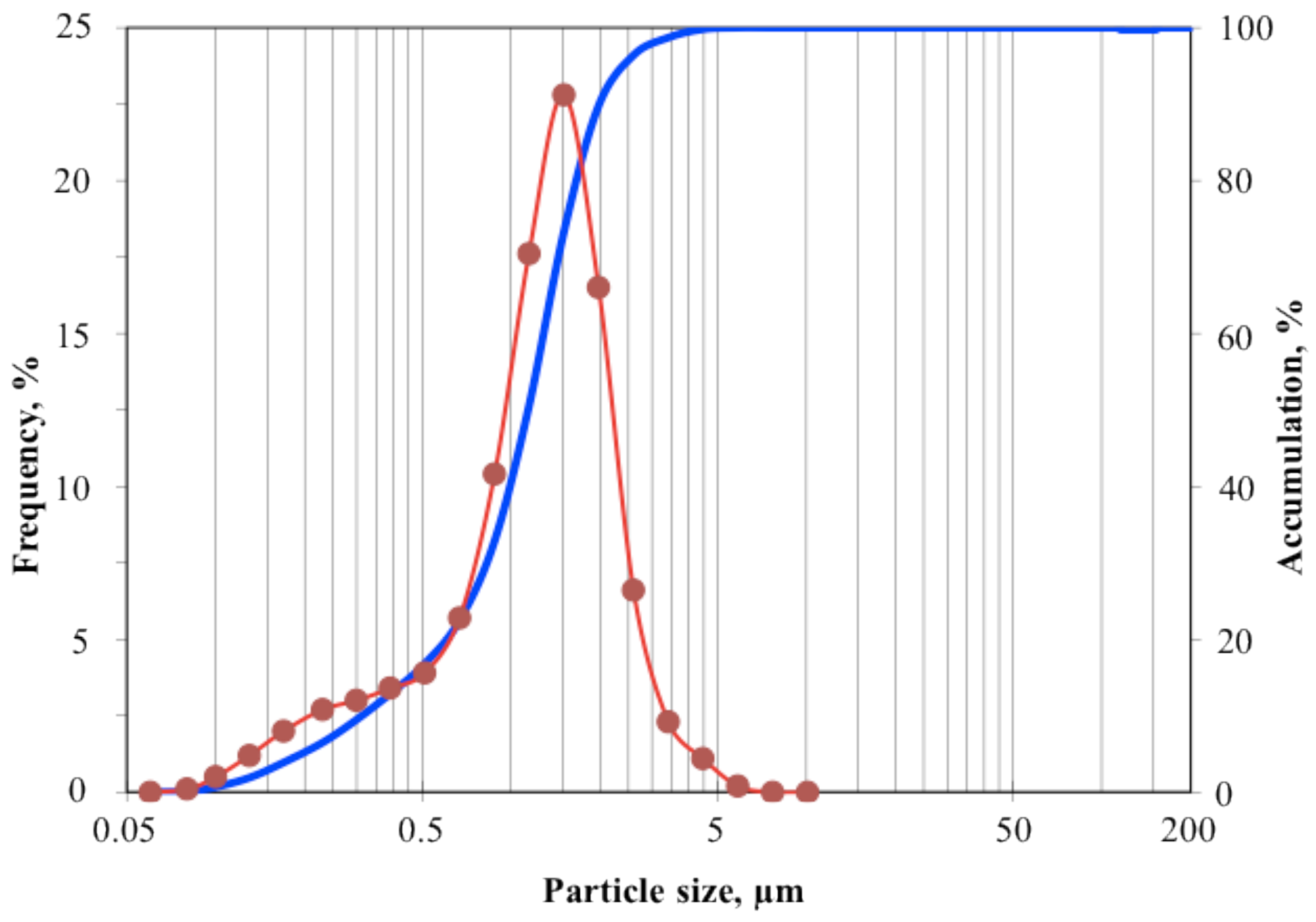
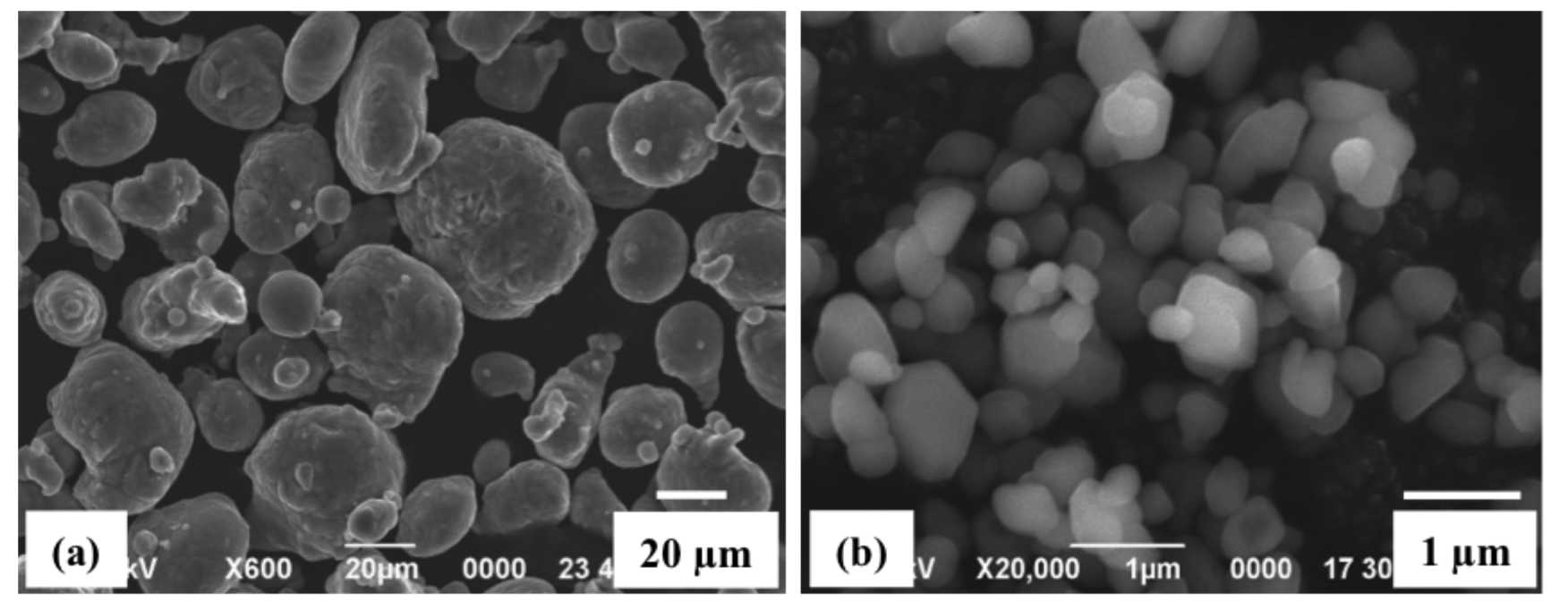
| First gas: Pressure, KPa | N2: 330.9 |
| Flow rate, l/min | 100 |
| Second gas: Pressure, KPa | H2: 330.9 |
| Flow rate, l/min | 5 |
| Arc current: A | 500 |
| Arc voltage: V | 70∼80 |
| Spray distance, mm | 150 |
| Carrier gas: flow rate, l/min | N2: 1 |
| Substrate material | SS400 |
| Aluminum powder | Aluminum nitride powder | |||||||
|---|---|---|---|---|---|---|---|---|
| Fe, mass% | Ti, mass% | Si, mass% | Ni, mass% | O, wt % | C, ppm | Ca, ppm | Si, ppm | Fe, ppm |
| 0.243 | 0.153 | 0.053 | 0.004 | 0.88 | 210 | 230 | 43 | 12 |
| Powder | Milling conditions | Ball | Solvent | ||||
|---|---|---|---|---|---|---|---|
| Al, wt% | AlN, wt% | Rotational Speed | Time, min | Repeat | Pause, min | ||
| 90∼50 | 10∼50 | 150 | 10 | 1 | 1 | Al2O3 | Ethanol |
Acknowledgments
References
- Pierson, H.O. Covalent nitrides: Properties and general characteristics. In Handbook of Refractory Carbides and Nitrides; Noyes Publications: Park Ridge, NJ, USA, 1996; pp. 237–239. [Google Scholar]
- Wemer, A.W. Carbide, Nitride and Boride Materials Synthesis and Processing, 1st ed.; Chapman & Hall: London, UK, 1997; pp. 6–68. [Google Scholar]
- Weimer, A.W.; Cochran, G.A.; Eisman, G.A.; Henley, J.P.; Hook, B.D.; Mills, L.K.; Guiton, T.A.; Knudsen, A.K.; Nicholas, N.R.; Volmering, J.E.; Moore, W.G. Rapid process for manufacturing aluminum nitride powder. J. Am. Ceram. Soc. 1994, 77, 3–18. [Google Scholar]
- Nakamura, S. The Roles of structural imperfections in InGaN-based blue light-emitting diodes and laser diodes. Science 1998, 281, 956–961. [Google Scholar]
- Krishna, L.R.; Sen, D.; Rao, Y.S.; Rao, G.V.N.; Sundararajan, G. Thermal spray coating of aluminum nitride utilizing the detonation spray technique. J. Mater. Res. 2002, 17, 2514–2523. [Google Scholar]
- Ohmori, A.; Wakamatsu, M.; Kamada, K. Synthesis of Al2O3-AlN coating by low pressure plasma spraying and nitriding. Trans. JWRI 1993, 22, 227–232. [Google Scholar]
- Kassabji, F.; Tourenne, F.; Derradji, A.; Fauchais, P. Aluminum and aluminum nitride deposition by low pressure nitrogen arc plasma spraying. Proc. 10th Inter. Therm. Spray Conf. 1983, 80, 82–84. [Google Scholar]
- Khor, K.A.; Boey, F.Y.C.; Zhao, X.L.; Cao, L.H. Aluminium nitride by plasma spraying of an Al2O3–C–Sm2O3 system. Mater. Sci. Eng. A 2001, 300, 203–210. [Google Scholar]
- Yamada, M.; Nakamura, H.; Yasui, T.; Fukumoto, M.; Takahashi, K. Influence of substrate materials upon fabrication of aluminum nitride coating by reactive RF plasma spraying. Mater. Trans. 2006, 47, 1671–1676. [Google Scholar]
- Yamada, M.; Yasui, T.; Fukumoto, M.; Takahashi, K. Nitridation of aluminum particles and formation process of aluminum nitride coatings by reactive RF plasma spraying. Thin Solid Films 2007, 515, 4166–4171. [Google Scholar]
- Ingo, G.M.; Kaciulis, S.; Mezzi, A.; Valente, T.; Casadei, F.; Gusmano, G. Characterization of composite titanium nitride coatings prepared by reactive plasma spraying. Electrochim. Acta 2005, 50, 4531–4537. [Google Scholar]
- Feng, W.; Yan, D.; He, J.; Li, X.; Dong, Y. Reactive Plasma Sprayed TiN Coating and its Tribological Properties. Wear 2005, 258, 806–811. [Google Scholar]
- Thiele, S.; Heimann, R.B.; Berger, L.M.; Herrmann, M.; Nebelung, M.; Schnick, T.; Wielage, B.; Vuoristo, P. Microstructure and properties of thermally sprayed silicon nitride-based coatings. J. Therm. Spray Technol. 2002, 11, 218–225. [Google Scholar]
- Yamada, M.; Inamoto, T.; Fukumoto, M.; Yasui, T. Fabrication of silicon nitride thick coatings by reactive RF plasma spraying. Mater. Trans. 2004, 45, 3304–3308. [Google Scholar]
- Shahien, M.; Yamada, M.; Yasui, T.; Fukumoto, M. Reactive atmospheric plasma spraying of AlN coatings: Influence of aluminum feedstock particle size. J. Therm. Spray Technol. 2011, 30, 580–589. [Google Scholar]
- Shahien, M.; Yamada, M.; Yasui, T.; Fukumoto, M. Influence of plasma gases on fabrication of AlN coatings through atmospheric plasma nitriding process. Ind. Appl. Plasma Process 2010, 3, 1–10. [Google Scholar]
- Shahien, M.; Yamada, M.; Yasui, T.; Fukumoto, M. Cubic aluminum nitride coating through atmospheric reactive plasma nitriding. J. Therm. Spray Technol. 2010, 19, 639–641. [Google Scholar]
- Shahien, M.; Yamada, M.; Yasui, T.; Fukumoto, M. Fabrication of AlN coatings by reactive atmospheric plasma spray nitriding of Al powders. Mater. Trans. 2010, 51, 957–961. [Google Scholar]
- Jung, W.S.; Ahn, S.K. Synthesis of aluminium nitride by the reaction of aluminium sulfide with ammonia. Mater. Lett. 2000, 43, 53–56. [Google Scholar]
- Qiu, Y.; Gao, L. Nitridation reaction of aluminium powder in flowing ammonia. J. Euro. Ceram. Soc. 2003, 23, 2015–2022. [Google Scholar]
- Hou, Q.; Mutharasan, R.; Koczak, M. Feasibility of aluminium nitride formation in aluminium alloys. Mater. Sci. Eng. A 1995, 195, 121–129. [Google Scholar]
- Jinxiang, L.; Xiuying, G.; Jianfeng, C.; Qun, W.; Yuhui, S.; Qin, G. Study of the kinetics of the nitridation reaction on Al–(Mg, Si) alloys by TG. Thermochimica Acta 1995, 253, 265–273. [Google Scholar]
- Kameshima, Y.; Irie, M.; Yasumori, A.; Okada, K. Low temperature synthesis of AlN by addition of various Li-salts. J. Euro. Ceram. Soc. 2004, 24, 3801–3806. [Google Scholar]
- Kameshima, Y.; Irie, M.; Yasumori, A.; Okada, K. Mechanochemical effect on low temperature synthesis of AlN by direct nitridation method. Solid State Ionics 2004, 172, 185–190. [Google Scholar]
- Juang, R.C.; Lee, C.J.; Chen, C.C. Combustion synthesis of hexagonal aluminum nitride powders under low nitrogen pressure. Mater. Sci. Eng. A 2003, 357, 219–227. [Google Scholar]
- Rosenband, V.; Gany, A. Activation of combustion synthesis of aluminium nitride powder. J. Mater. Process. Technol. 2004, 147, 179–203. [Google Scholar]
- Radwan, M.; Bahgat, M. A modified direct nitridation method for formation of nano-AlN wiskers. J. Mater. Process. Technol. 2007, 181, 99–105. [Google Scholar]
- Wang, J.; Wang, W.L.; Ding, P.D.; Yang, Y.X.; Fang, L.; Esteve, J.; Polo, M.C.; Sanchez, G. Synthesis of cubic aluminum nitride by carbothermal nitridation reaction. Diam. Rele. Mater. 1999, 8, 1342–1344. [Google Scholar]
- Boey, F.; Cao, L.; Khor, K.A.; Tok, A. Phase reaction and sintering behavior of a Al2O3–20wt%AlN–5wt%Y2O3 system. Acta Mater. 2001, 49, 3117–3127. [Google Scholar]
- Dolatabadi, A.; Mostaghimi, J.; Pershin, V. Effect of a cylindrical shroud on particle conditions in high velocity oxy-fuel (HVOF) spray process. J. Mater. Process. Technol. 2003, 137, 214–224. [Google Scholar]
- Kim, S.; Choi, S.; Kim, G-H.; Hong, S.H. Effects of shroud gas injection on material properties of tungsten layers coated by plasma spraying. Thin Solid Films 2010, 518, 6369–6372. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shahien, M.; Yamada, M.; Yasui, T.; Fukumoto, M. In Situ Fabrication of AlN Coating by Reactive Plasma Spraying of Al/AlN Powder. Coatings 2011, 1, 88-107. https://doi.org/10.3390/coatings1020088
Shahien M, Yamada M, Yasui T, Fukumoto M. In Situ Fabrication of AlN Coating by Reactive Plasma Spraying of Al/AlN Powder. Coatings. 2011; 1(2):88-107. https://doi.org/10.3390/coatings1020088
Chicago/Turabian StyleShahien, Mohammed, Motohiro Yamada, Toshiaki Yasui, and Masahiro Fukumoto. 2011. "In Situ Fabrication of AlN Coating by Reactive Plasma Spraying of Al/AlN Powder" Coatings 1, no. 2: 88-107. https://doi.org/10.3390/coatings1020088




