Surface Functionalization of a Polyurethane Surface via Radio-Frequency Cold Plasma Treatment Using Different Gases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasma Treatment of PU Films
2.3. Surface Wettability Measurements
2.4. Surface Chemistry Characterization
2.5. Surface Morphology Analysis
2.6. Peel Test
3. Results and Discussion
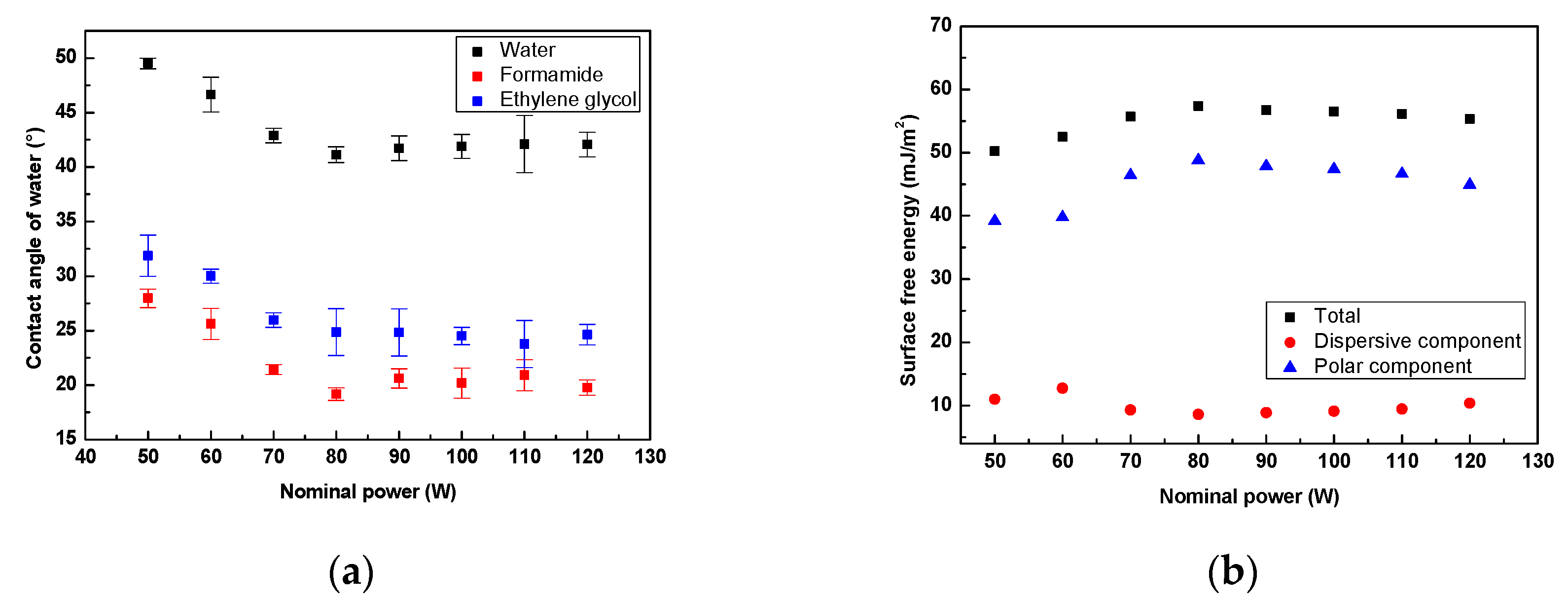
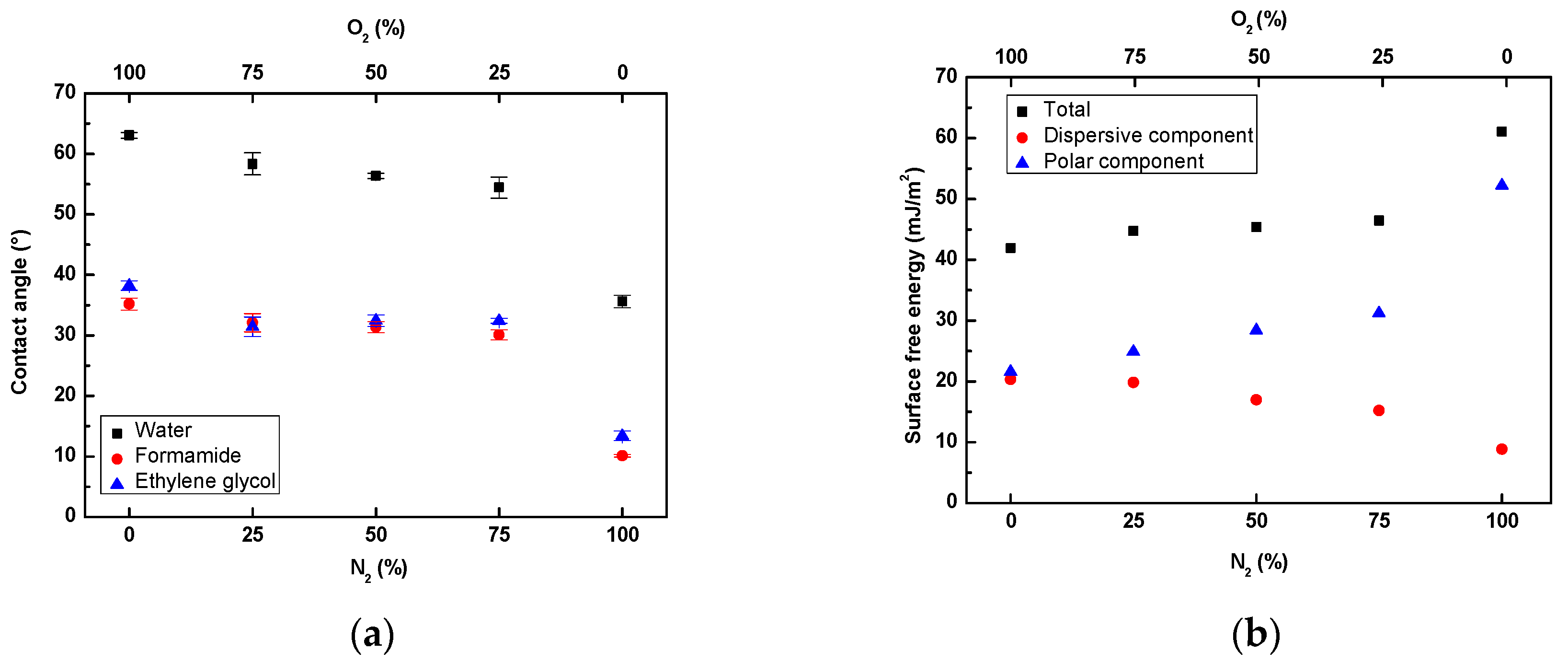
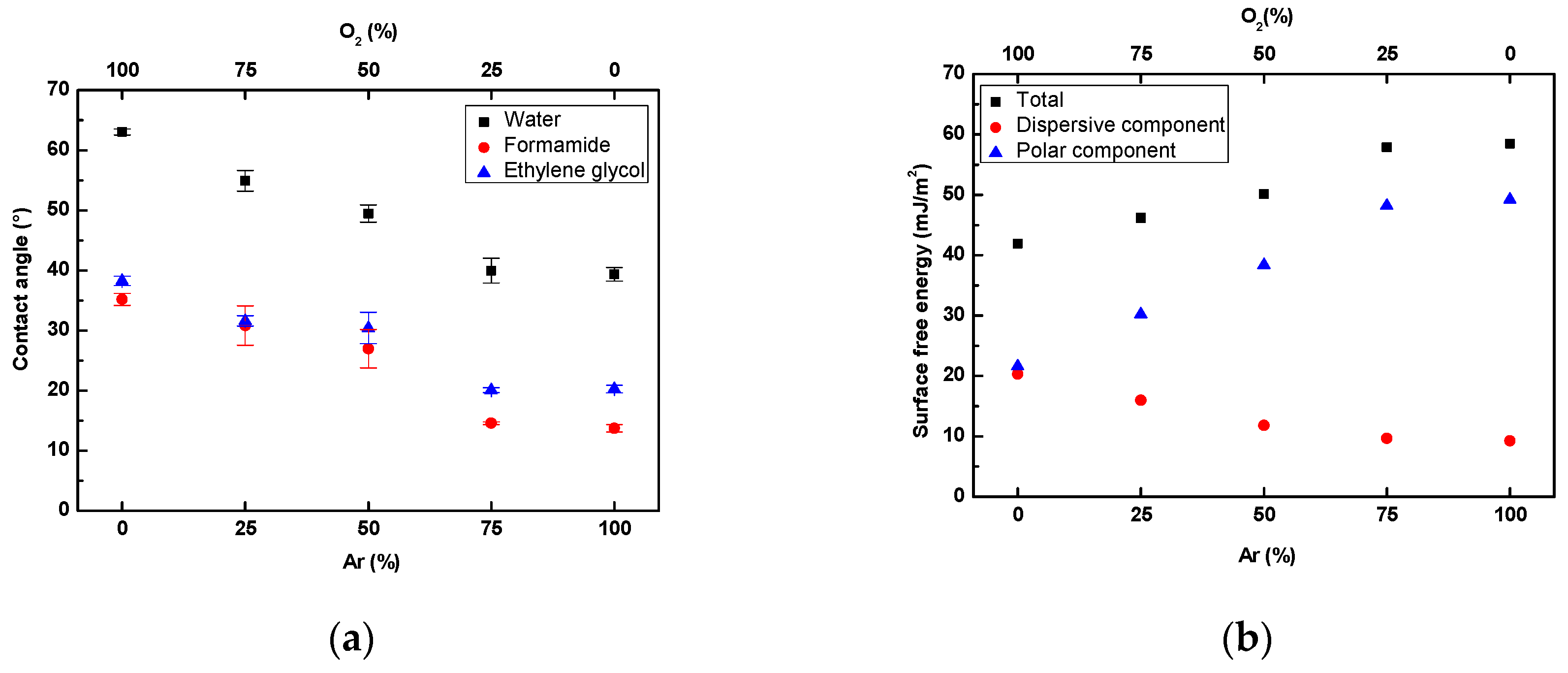
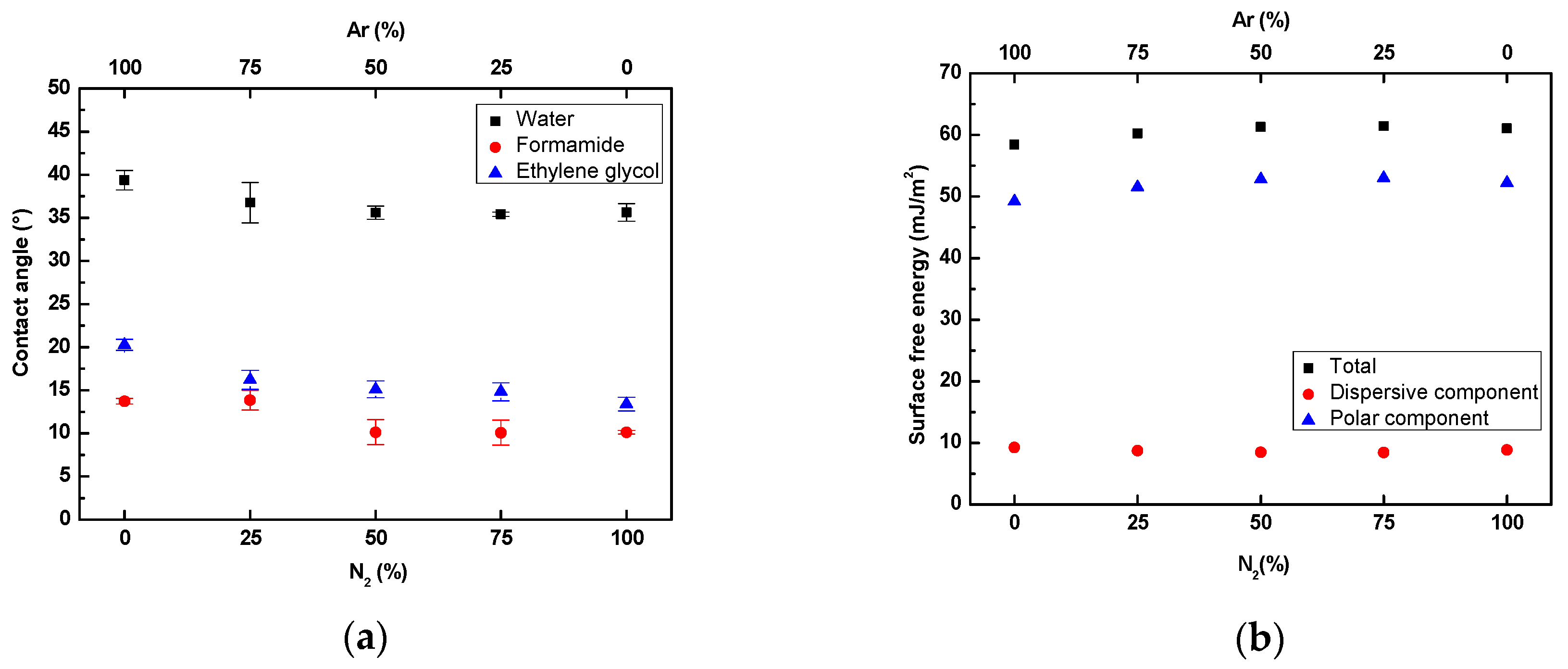
3.1. Surface Wettability Analysis
3.2. Adhesion Investigation
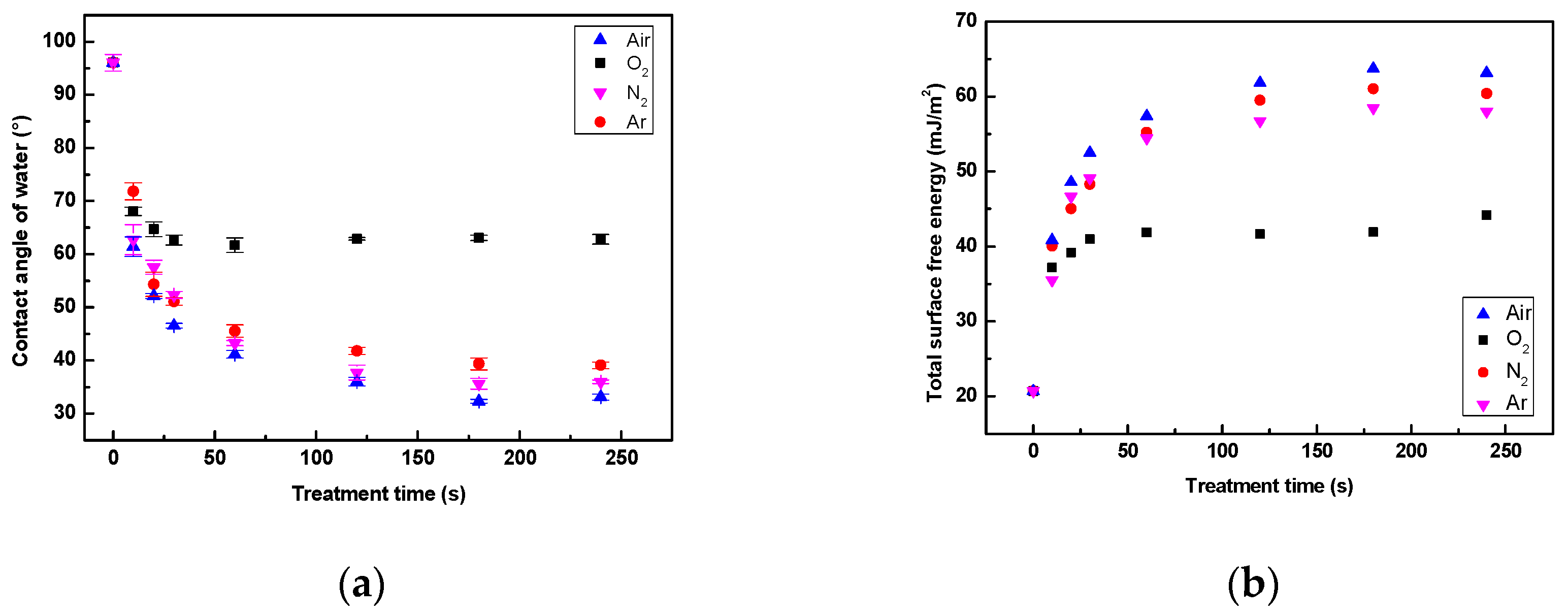
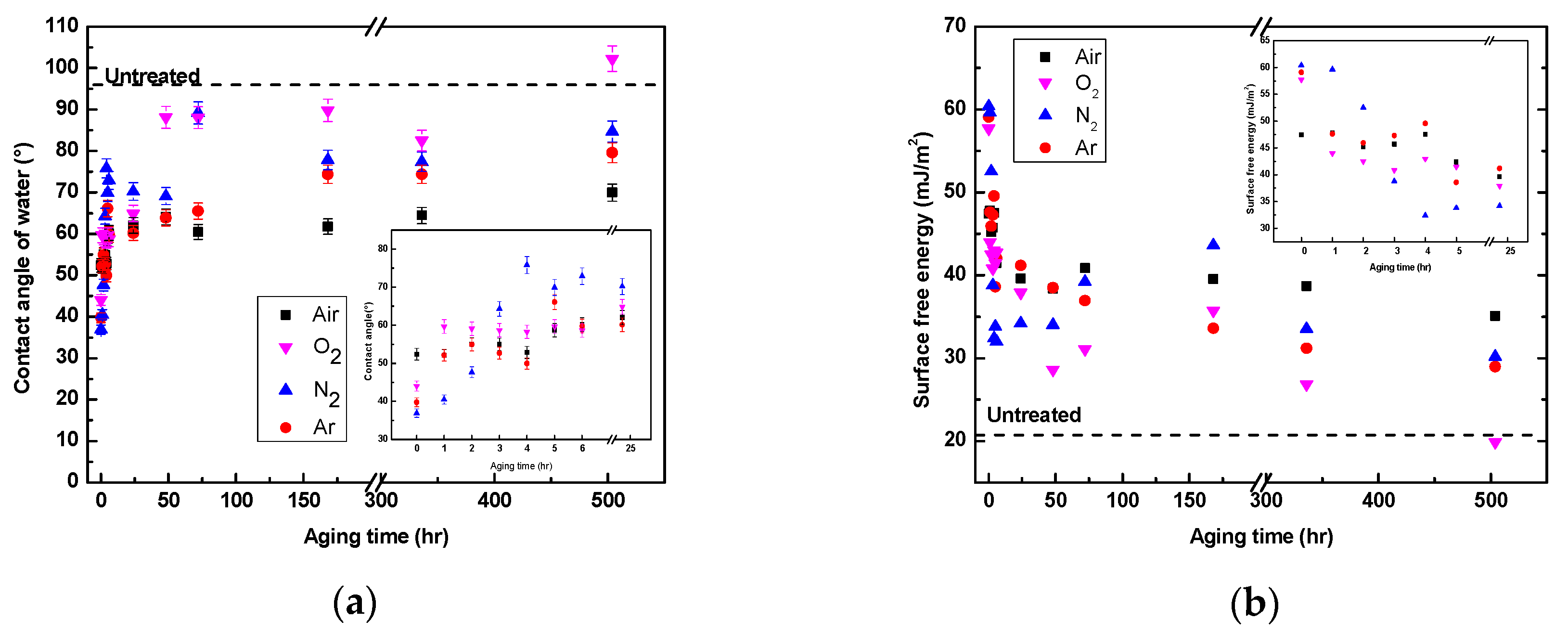
3.3. Hydrophobic Recovery Analysis
3.3.1. Wettability Aging
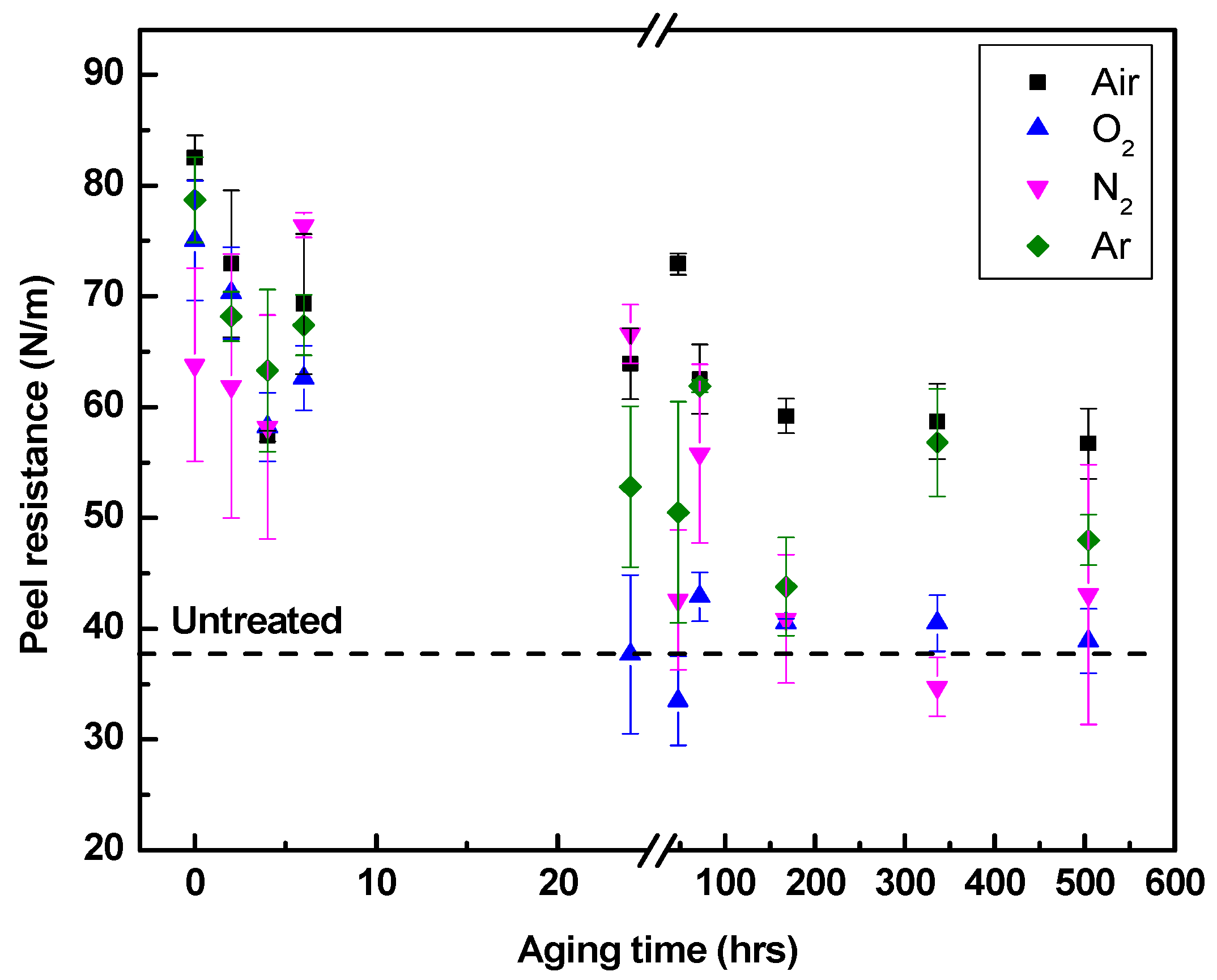
3.3.2. Adhesion Aging
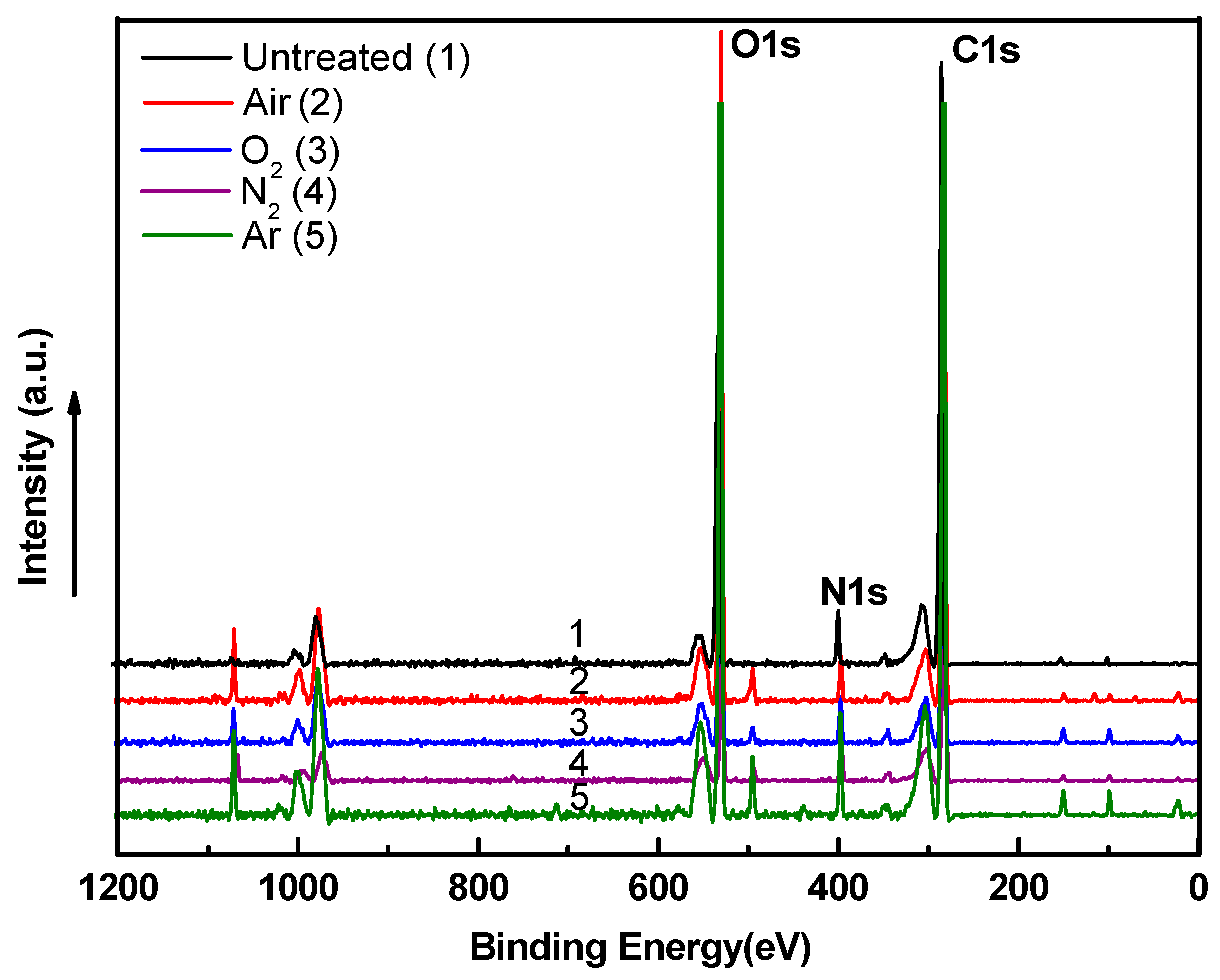
3.4. Surface Chemistry Analysis
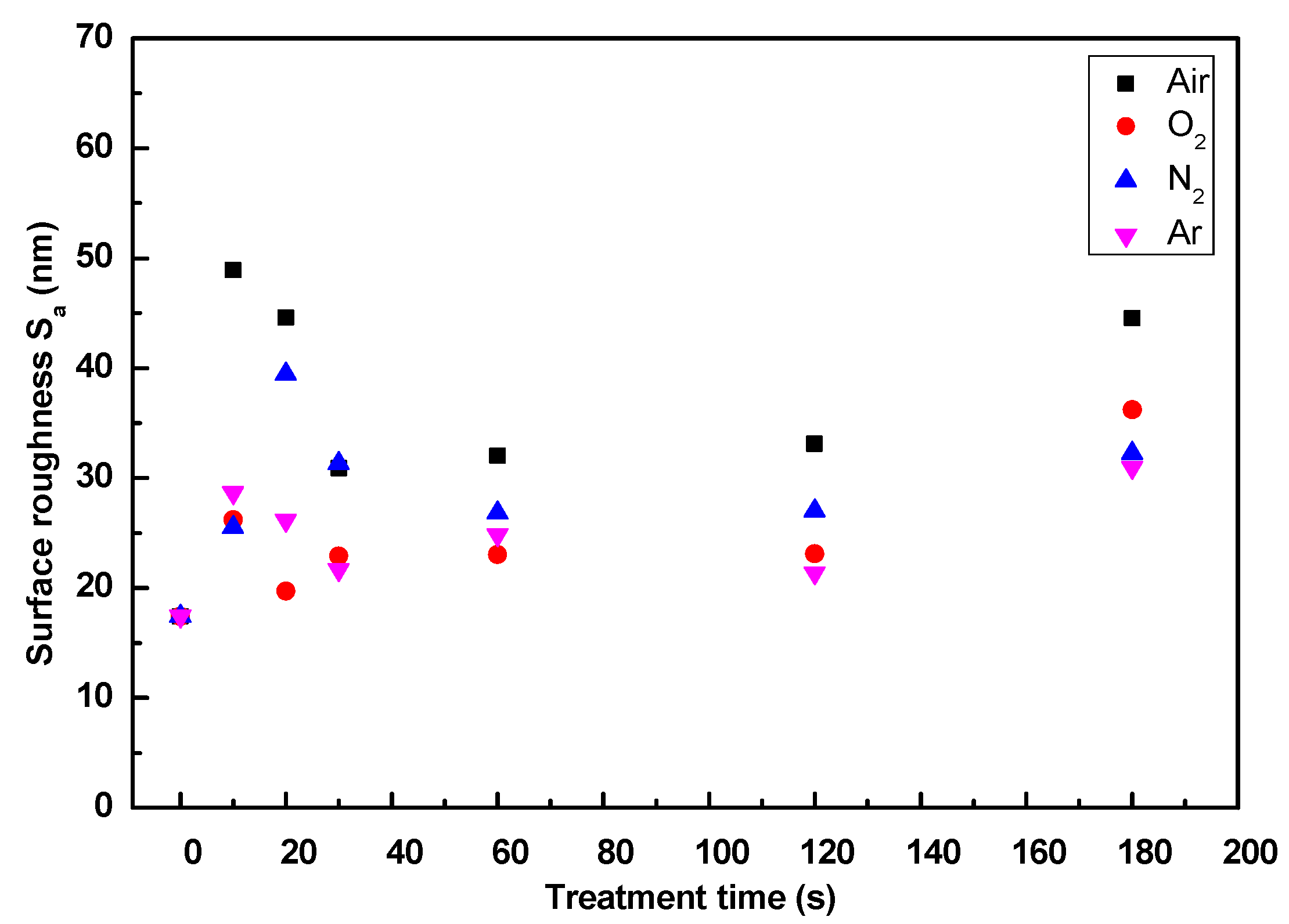
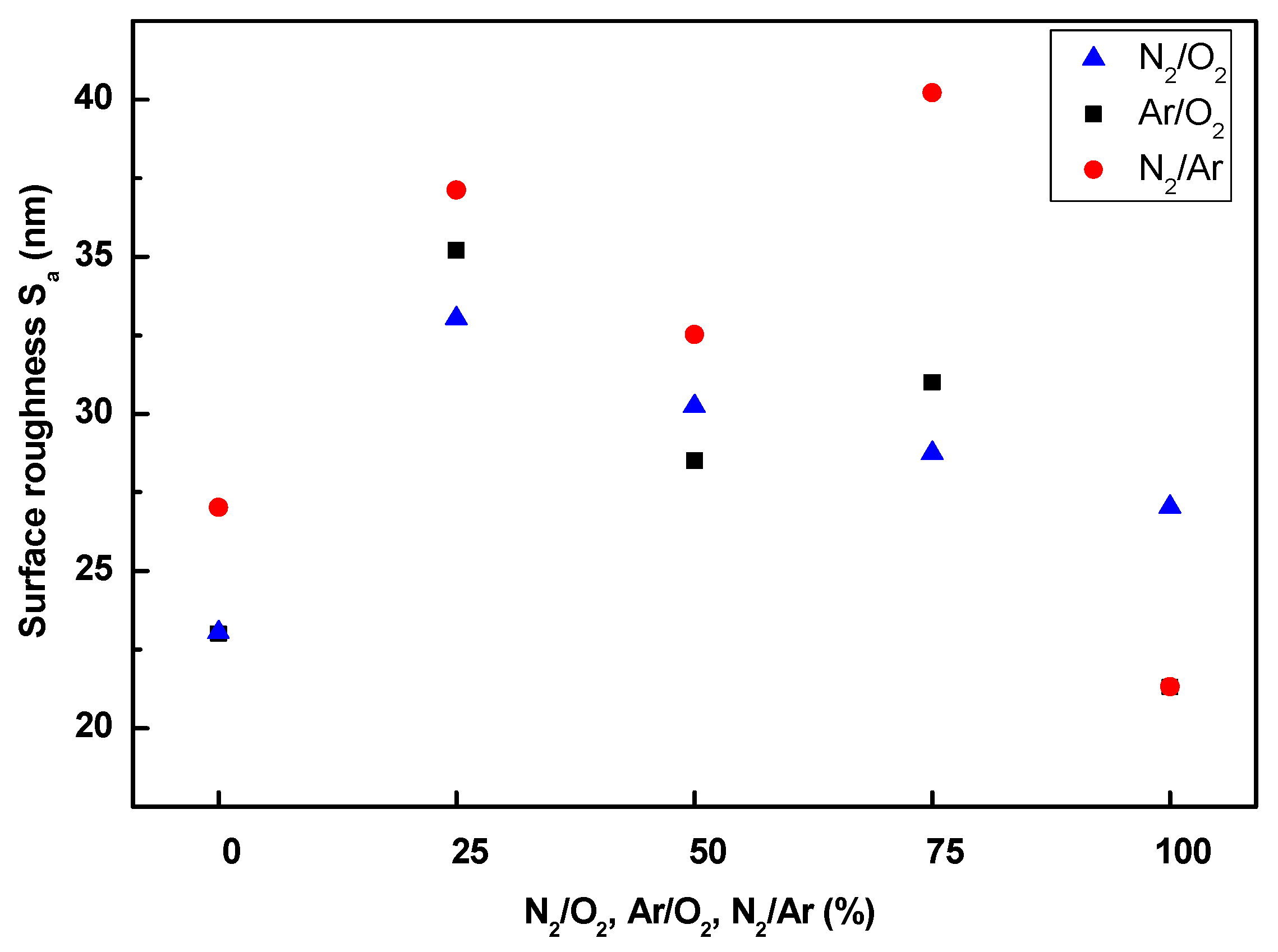
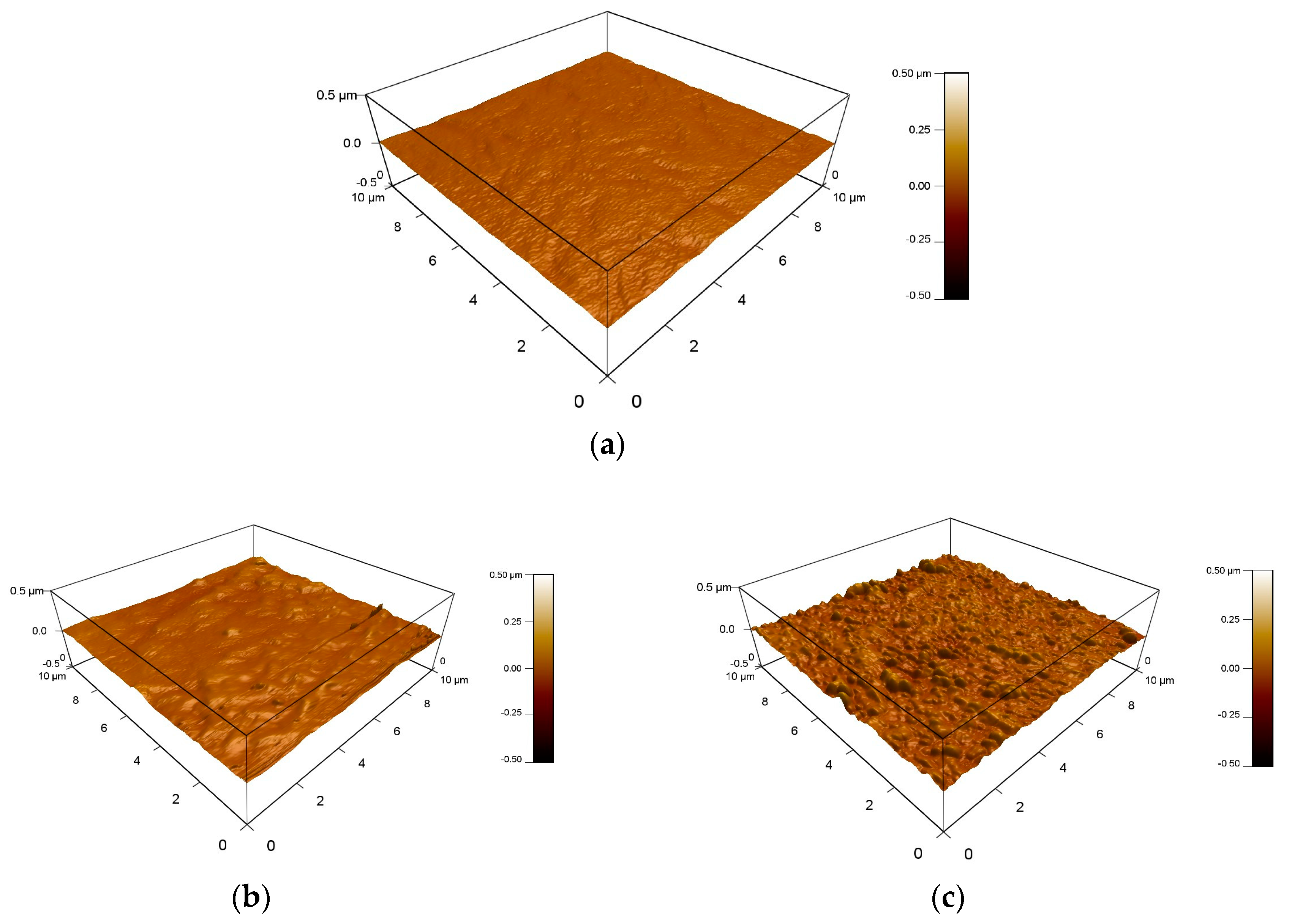
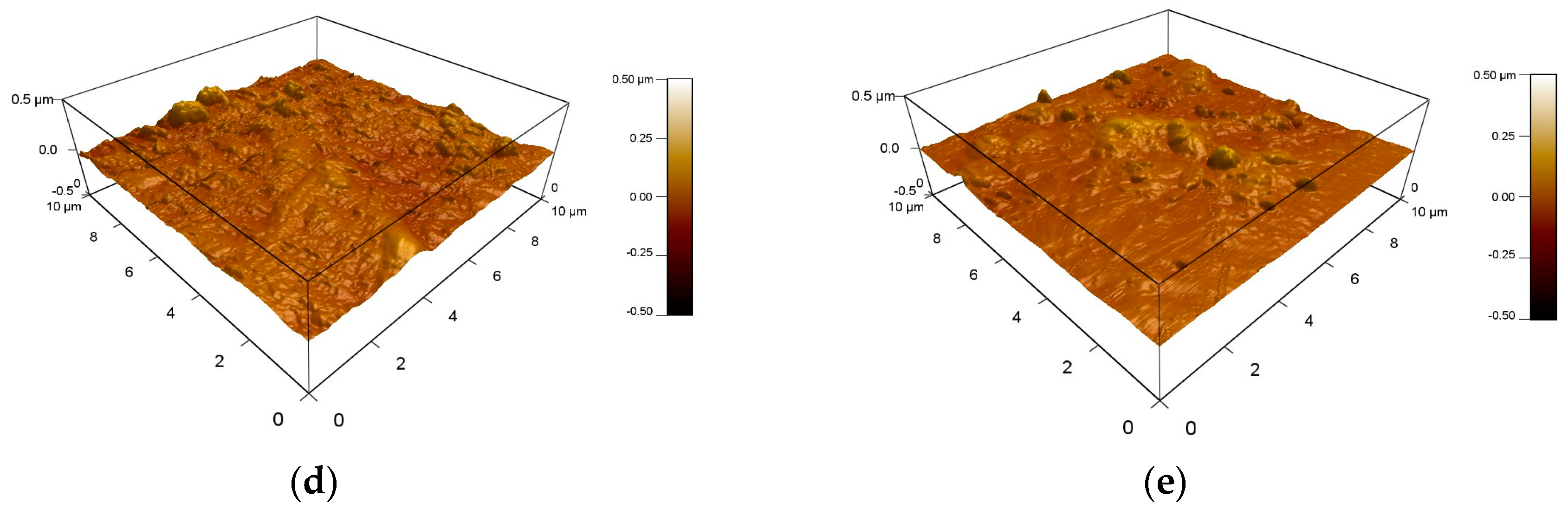
3.5. Surface Morphology Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fu, X.; Jenkins, M.J.; Sun, G.; Bertoti, I.; Dong, H. Characterization of active screen plasma modified polyurethane surfaces. Surf. Coat. Technol. 2012, 206, 4799–4807. [Google Scholar] [CrossRef]
- Thomson, T. Polyurethanes as Specialty Chemicals: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Bae, J.-S.; Seo, E.-J.; Kang, I.-K. Synthesis and characterization of heparinized polyurethanes using plasma glow discharge. Biomaterials 1999, 20, 529–537. [Google Scholar] [CrossRef]
- Lamba, N.K. Polyurethanes in Biomedical Applications; Routledge: Abingdon, UK, 2017. [Google Scholar]
- Chattopadhyay, D.K.; Raju, K. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Guelcher, S.A. Biodegradable polyurethanes: Synthesis and applications in regenerative medicine. Tissue Eng. Part B Rev. 2008, 14, 3–17. [Google Scholar] [CrossRef]
- Junkar, I.; Vesel, A.; Cvelbar, U.; Mozetič, M.; Strnad, S. Influence of oxygen and nitrogen plasma treatment on polyethylene terephthalate (PET) polymers. Vacuum 2009, 84, 83–85. [Google Scholar] [CrossRef]
- Hegemann, D.; Brunner, H.; Oehr, C. Plasma treatment of polymers for surface and adhesion improvement. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 208, 281–286. [Google Scholar] [CrossRef]
- Švorčík, V.; Kolářová, K.; Slepička, P.; Macková, A.; Novotná, M.; Hnatowicz, V. Modification of surface properties of high and low density polyethylene by Ar plasma discharge. Polym. Degrad. Stab. 2006, 91, 1219–1225. [Google Scholar] [CrossRef]
- Pandiyaraj, K.N.; Ferraria, A.M.; do Rego, A.M.B.; Deshmukh, R.R.; Su, P.-G.; Halleluyah, J.M.; Halim, A.S. Low-pressure plasma enhanced immobilization of chitosan on low-density polyethylene for bio-medical applications. Appl. Surf. Sci. 2015, 328, 1–12. [Google Scholar] [CrossRef]
- Lee, T.; Puligundla, P.; Mok, C. Inactivation of foodborne pathogens on the surfaces of different packaging materials using low-pressure air plasma. Food Control 2015, 51, 149–155. [Google Scholar] [CrossRef]
- Ghasemi, M.; Minier, M.J.; Tatoulian, M.l.; Chehimi, M.M.; Arefi-Khonsari, F. Ammonia plasma treated polyethylene films for adsorption or covalent immobilization of trypsin: Quantitative correlation between X-ray photoelectron spectroscopy data and enzyme activity. J. Phys. Chem. B 2011, 115, 10228–10238. [Google Scholar] [CrossRef]
- Park, S.C.; Koh, S.K.; Pae, K.D. Effects of surface modification by Ar+ irradiation on wettability of surfaces of poly (ethylene terephthalate) films. Polym. Eng. Sci. 1998, 38, 1185–1192. [Google Scholar] [CrossRef]
- Yamamoto, T.; Newsome, J.R.; Ensor, D.S. Modification of surface energy, dry etching, and organic film removal using atmospheric-pressure pulsed-corona plasma. IEEE Trans. Ind. Appl. 1995, 31, 494–499. [Google Scholar] [CrossRef]
- Egitto, F.D.; Matienzo, L.J. Plasma modification of polymer surfaces for adhesion improvement. IBM J. Res. Dev. 1994, 38, 423–439. [Google Scholar] [CrossRef]
- Kusano, Y. Atmospheric pressure plasma processing for polymer adhesion: A review. J. Adhes. 2014, 90, 755–777. [Google Scholar] [CrossRef]
- Chvátalová, L.; Čermák, R.; Mráček, A.; Grulich, O.; Vesel, A.; Ponížil, P.; Minařík, A.; Cvelbar, U.; Beníček, L.; Sajdl, P. The effect of plasma treatment on structure and properties of poly (1-butene) surface. Eur. Polym. J. 2012, 48, 866–874. [Google Scholar] [CrossRef]
- Ataeefard, M.; Moradian, S.; Mirabedini, M.; Ebrahimi, M.; Asiaban, S. Investigating the effect of power/time in the wettability of Ar and O2 gas plasma-treated low-density polyethylene. Prog. Org. Coat. 2009, 64, 482–488. [Google Scholar] [CrossRef]
- López-Santos, C.; Yubero, F.; Cotrino, J.; González-Elipe, A.R. Nitrogen plasma functionalization of low density polyethylene. Surf. Coat. Technol. 2011, 205, 3356–3364. [Google Scholar] [CrossRef]
- Chaivan, P.; Pasaja, N.; Boonyawan, D.; Suanpoot, P.; Vilaithong, T. Low-temperature plasma treatment for hydrophobicity improvement of silk. Surf. Coat. Technol. 2005, 193, 356–360. [Google Scholar] [CrossRef]
- Liston, E.; Martinu, L.; Wertheimer, M. Plasma surface modification of polymers for improved adhesion: A critical review. J. Adhes. Sci. Technol. 1993, 7, 1091–1127. [Google Scholar] [CrossRef]
- Tajima, S.; Komvopoulos, K. Surface modification of low-density polyethylene by inductively coupled argon plasma. J. Phys. Chem. B 2005, 109, 17623–17629. [Google Scholar] [CrossRef]
- Abusrafa, A.E.; Habib, S.; Krupa, I.; Ouederni, M.; Popelka, A. Modification of polyethylene by RF plasma in different/mixture gases. Coatings 2019, 9, 145. [Google Scholar] [CrossRef] [Green Version]
- Jordá-Vilaplana, A.; Fombuena, V.; García-García, D.; Samper, M.; Sánchez-Nácher, L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Bhat, N.; Upadhyay, D. Plasma-induced surface modification and adhesion enhancement of polypropylene surface. J. Appl. Polym. Sci. 2002, 86, 925–936. [Google Scholar] [CrossRef]
- Øiseth, S.K.; Krozer, A.; Kasemo, B.; Lausmaa, J. Surface modification of spin-coated high-density polyethylene films by argon and oxygen glow discharge plasma treatments. Appl. Surf. Sci. 2002, 202, 92–103. [Google Scholar] [CrossRef]
- Fozza, A.; Klemberg-Sapieha, J.; Wertheimer, M.R. Vacuum ultraviolet irradiation of polymers. Plasmas Polym. 1999, 4, 183–206. [Google Scholar] [CrossRef]
- Nakaoka, R.; Yamakoshi, Y.; Isama, K.; Tsuchiya, T. Effects of surface chemistry prepared by self-assembled monolayers on osteoblast behavior. J. Biomed. Mater. Res. Part A 2010, 94, 524–532. [Google Scholar] [CrossRef]





| Working Gas | C 1s | N 1s | O 1s |
|---|---|---|---|
| Untreated | 81.0% | 3.1% | 15.9% |
| Air | 73.0% | 2.3% | 24.7% |
| O2 | 75.0% | 3.0% | 22.0% |
| N2 | 79.8% | 3.6% | 16.6% |
| Ar | 75.4% | 2.8% | 21.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
E. Abusrafa, A.; Habib, S.; Popelka, A. Surface Functionalization of a Polyurethane Surface via Radio-Frequency Cold Plasma Treatment Using Different Gases. Coatings 2020, 10, 1067. https://doi.org/10.3390/coatings10111067
E. Abusrafa A, Habib S, Popelka A. Surface Functionalization of a Polyurethane Surface via Radio-Frequency Cold Plasma Treatment Using Different Gases. Coatings. 2020; 10(11):1067. https://doi.org/10.3390/coatings10111067
Chicago/Turabian StyleE. Abusrafa, Aya, Salma Habib, and Anton Popelka. 2020. "Surface Functionalization of a Polyurethane Surface via Radio-Frequency Cold Plasma Treatment Using Different Gases" Coatings 10, no. 11: 1067. https://doi.org/10.3390/coatings10111067





