Microstructure Evolution and Properties of Laser Cladding CoCrFeNiTiAlx High-Entropy Alloy Coatings
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
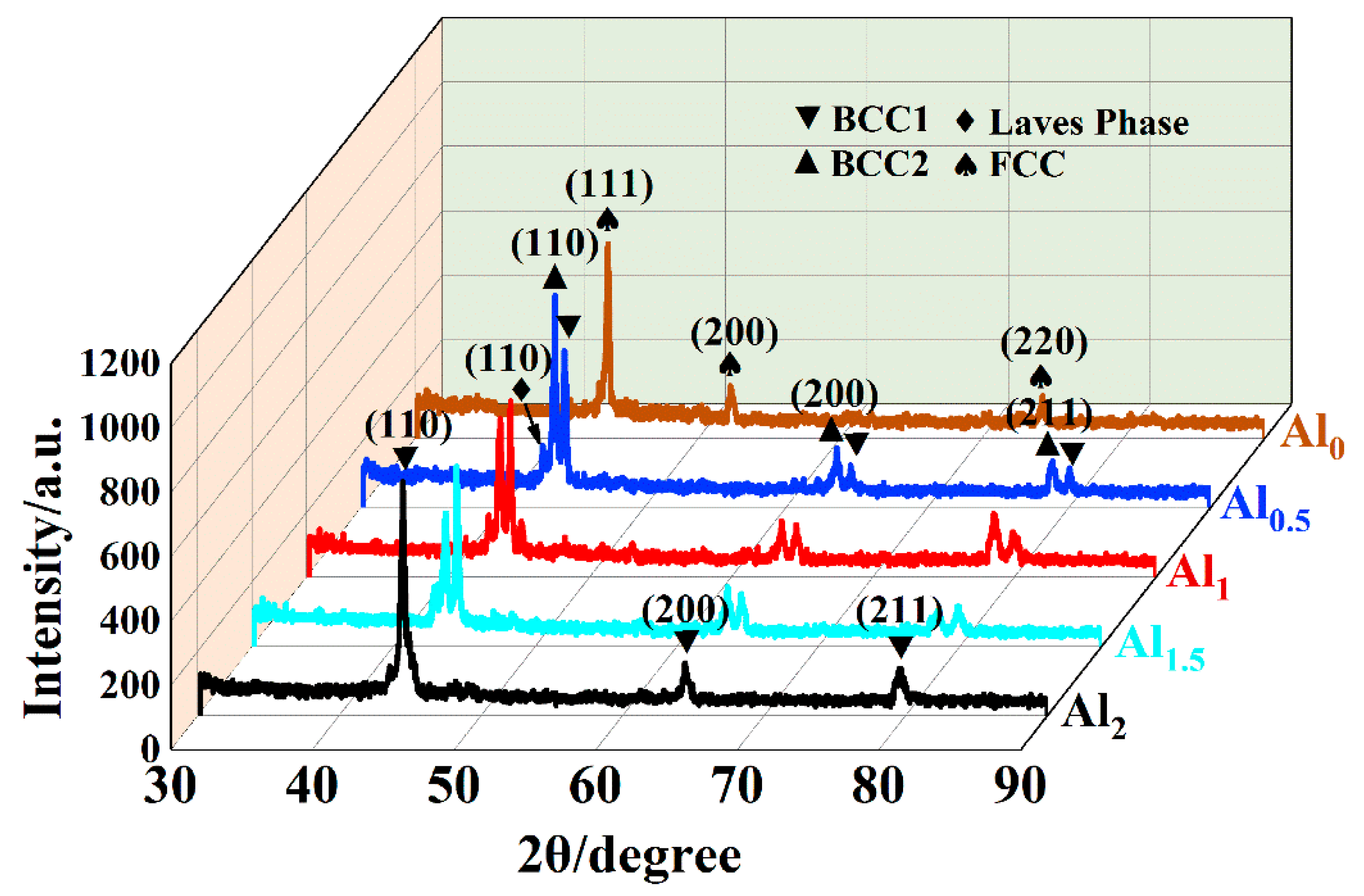
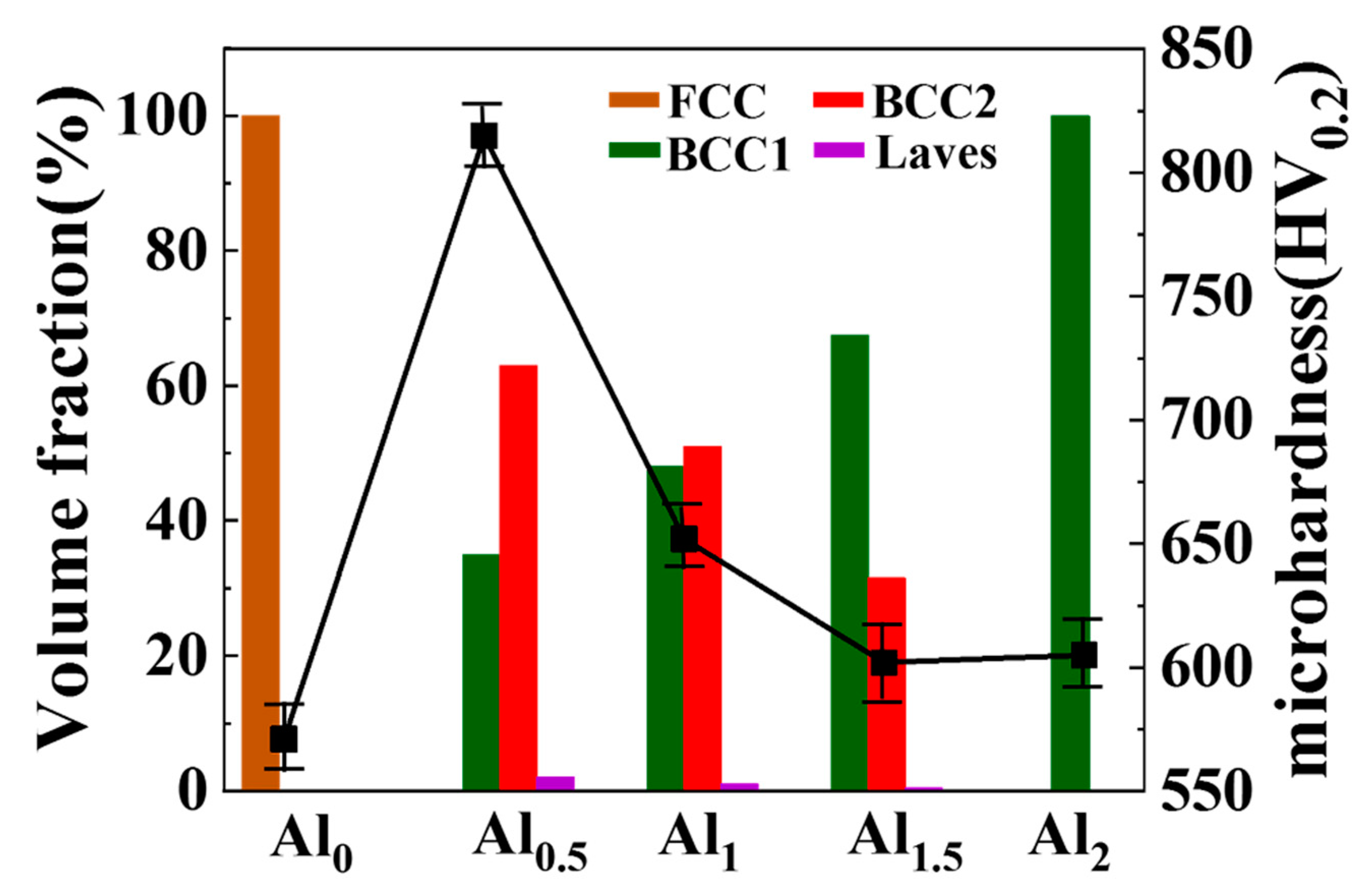
3.1. Phase Constitution
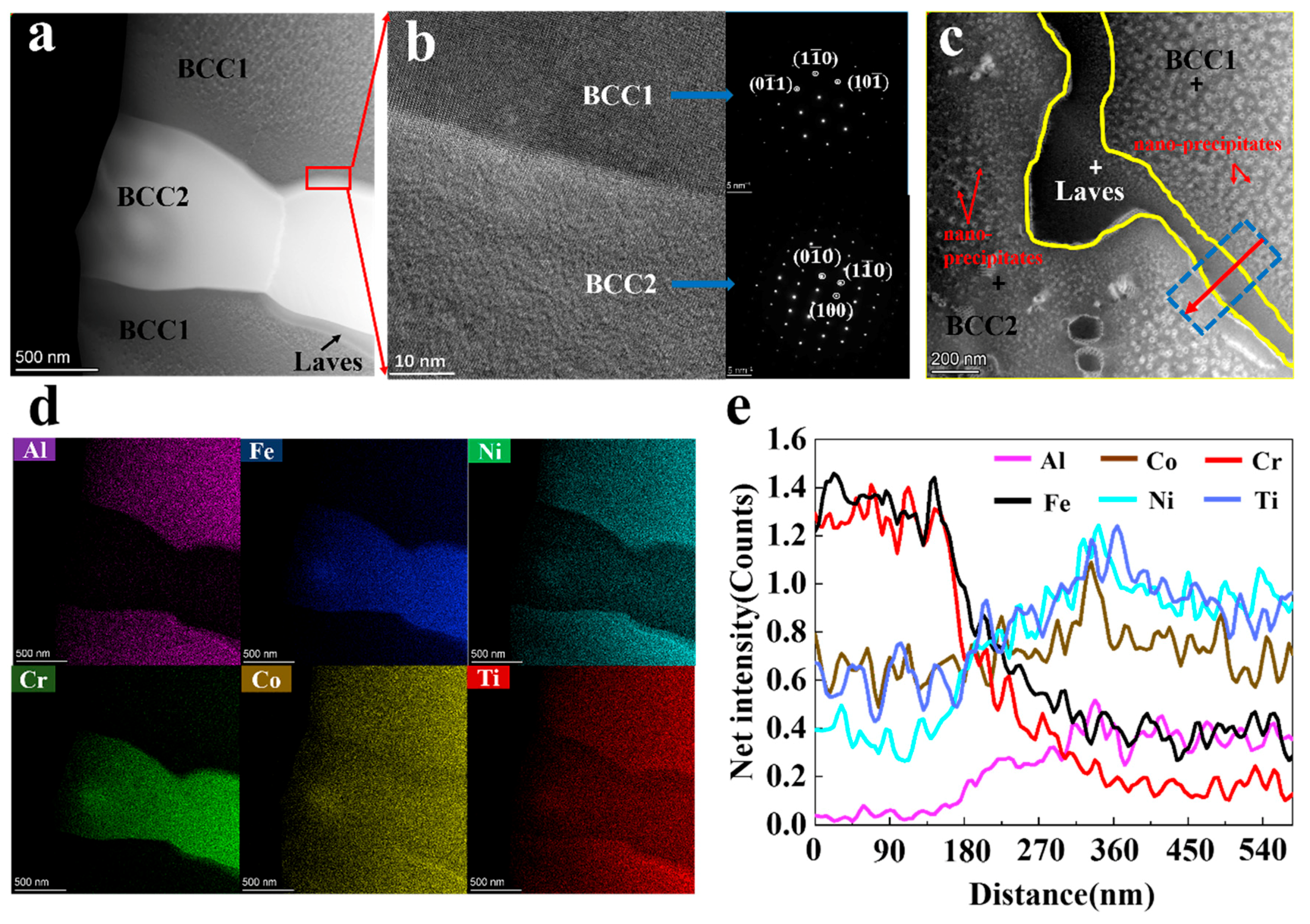
3.2. Microstructure Evolution
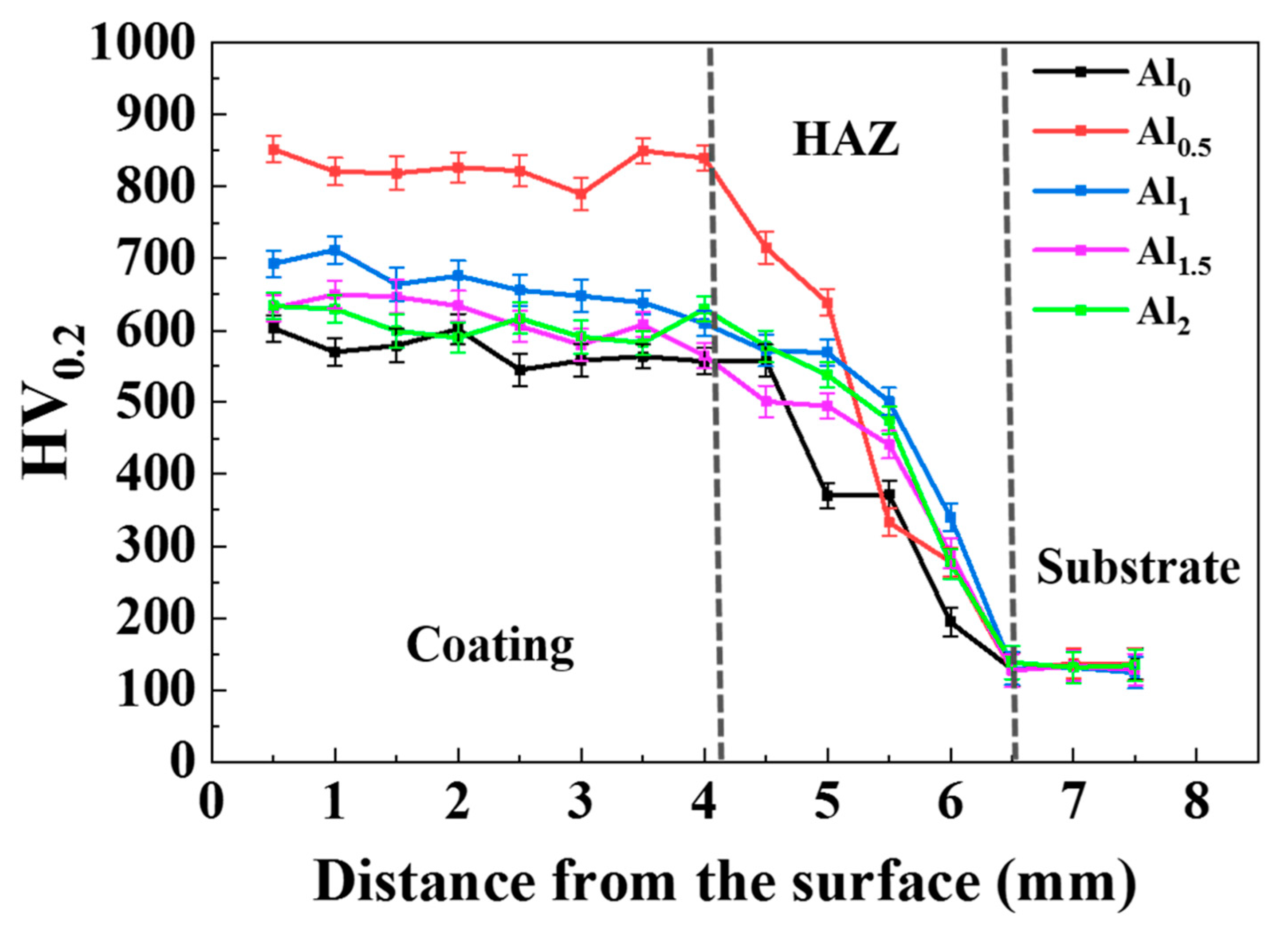
3.3. Microhardness
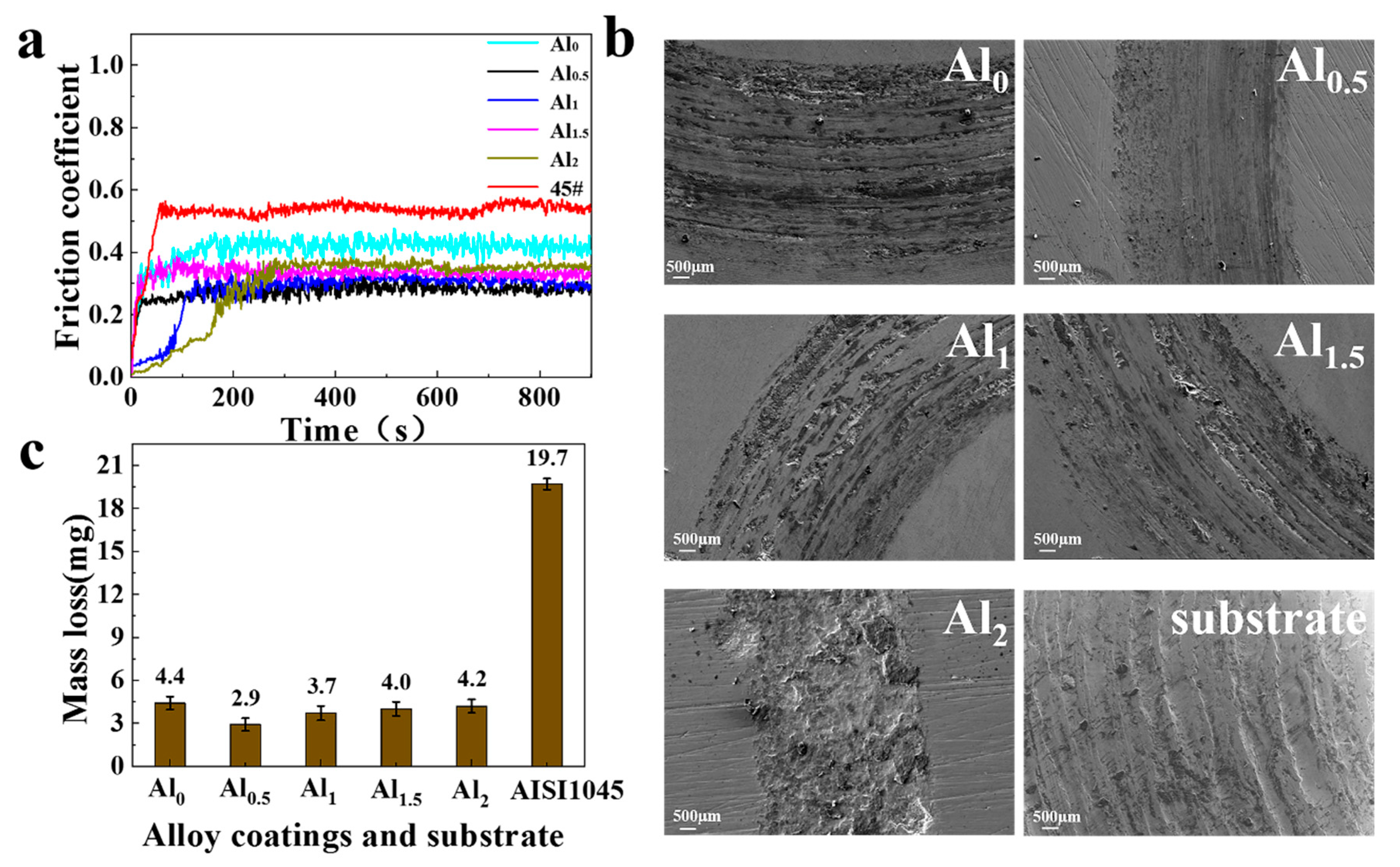
3.4. Friction and Wear
3.5. Corrosion Resistance
3.6. Discussion
4. Conclusion
- Under the conditions of high laser power and fast solidification rate, the increase in Al content promoted the transition from a single FCC phase to the BCC1 and BCC2 phases, and a small amount of Laves phase. Due to the larger atomic radius of Al, as the Al element increases, the lattice distortion energy increases, and the BCC2 phase gradually decreases to form a relatively stable BCC1 phase.
- As the Al content increases, the hardness and abrasion resistance of the coating first increase and then decrease. The crystallite size of each phase of the coating also changed, and the grains gradually refined. The grain size difference between the two phases of the Al0.5 coating was the smallest. The grain refinement resulted in the highest hardness of the Al0.5 coating, reaching 880 HV, 6.5 times that of the substrate. The abrasion mechanism was the transition from abrasive wear to slight adhesive wear, and finally to micro-fracture (flake) wear.
- The corrosion resistance of the CoCrFeNiTiAlx HEA coatings in a 3.5% NaCl solution increased first, then decreased with increasing Al content. The addition of a small amount of Al will form an Al2O3 passivation film, the self-corrosion potential of Al0.5 was the most positive, at −0.5766 V, while the self-corrosion current density was the smallest, at 9.13 × 10−7 A·cm−2. The Al0.5 coating showed good corrosion resistance. This also proves that when there are multiple phases, the performance of the alloy may be better when the crystallite size difference of each phase is small.
- The results show that adding a small amount of aluminum can refine the grains of the CoCrFeNiTiAlx coating and improve the corrosion resistance and mechanical properties of the coating. CoCrFeNiTiAl0.5 exhibits the best performance among the as-prepared coatings, can significantly improve the surface properties of AISI1045 steel.
Author Contributions
Funding
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K.; Zhang, Y. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Li, C.; Li, J.C.; Zhao, M.; Zhao, M.; Jiang, Q. Effect of alloying elements on microstructure and properties of multiprincipal elements high-entropy alloys. J. Alloys Compd. 2009, 475, 752–757. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Shang, C.; Axinte, E.; Ge, W.; Zhang, Z.; Wang, Y. High-entropy alloy coatings with excellent mechanical, corrosion resistance and magnetic properties prepared by mechanical alloying and hot pressing sintering. Surf. Interfaces 2017, 9, 36–43. [Google Scholar] [CrossRef]
- Chew, Y.; Bi, G.J.; Zhu, Z.G.; Ng, F.L.; Weng, F.; Liu, S.B.; Nai, S.M.L.; Lee, B.Y. Microstructure and enhanced strength of laser aided additive manufactured CoCrFeNiMn high entropy alloy. Mater. Sci. Eng. A 2019, 744, 137–144. [Google Scholar] [CrossRef]
- Liu, G.; Liu, L.; Liu, X.; Wang, Z.; Han, Z.; Zhang, G.; Kostka, A. Microstructure and mechanical properties of Al0.7CoCrFeNi high-entropy-alloy prepared by directional solidification. Intermetallics 2018, 93, 93–100. [Google Scholar] [CrossRef]
- Hong, S.; Wu, Y.; Li, G.; Wang, B.; Gao, W.; Ying, G. Microstructural characteristics of high-velocity oxygen-fuel (HVOF) sprayed nickel-based alloy coating. J. Alloys Compd. 2013, 581, 398–403. [Google Scholar] [CrossRef]
- Wang, C.; Yu, J.; Zhang, Y.; Yu, Y. Phase evolution and solidification cracking sensibility in laser remelting treatment of the plasma-sprayed CrMnFeCoNi high entropy alloy coating. Mater. Des. 2019, 182, 108040. [Google Scholar] [CrossRef]
- Anupam, A.; Kottada, R.S.; Kashyap, S.; Meghwal, A.; Murty, B.; Berndt, C.; Ang, A. Understanding the microstructural evolution of high entropy alloy coatings manufactured by atmospheric plasma spray processing. Appl. Surf. Sci. 2019, 505, 144117. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Chen, P.; Hao, J. Microstructural characterization and corrosion behaviour of AlCoCrFeNiTix high-entropy alloy coatings fabricated by laser cladding. Surf. Coat. Technol. 2019, 361, 63–74. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.L.; Zhang, C.H.; Guan, M.; Tan, J.Z. Laser surface alloying of FeCoCrAlNi high-entropy alloy on 304 stainless steel to enhance corrosion and cavitation erosion resistance. Opt. Laser Technol. 2016, 84, 23–31. [Google Scholar] [CrossRef]
- Matilainen, V.; Piili, H.; Salminen, A.; Syvänen, T.; Nyrhilä, O. Characterization of process efficiency improvement in laser additive manufacturing. Phys. Procedia 2014, 56, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Ocelík, V.; Janssen, N.; Smith, S.N.; De Hosson, J.T.M. Additive Manufacturing of High-Entropy Alloys by Laser Processing. JOM 2016, 68, 1810–1818. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Y.; Chen, Z.; Chen, Y.Z.; Shi, J.C.; Wang, Z.Y.; Wang, S.; Liu, F. Effect of Al content on high temperature oxidation resistance of AlxCoCrCuFeNi high entropy alloys (x = 0, 0.5, 1, 1.5, 2). Vacuum 2019, 169, 108837. [Google Scholar] [CrossRef]
- Zhang, K.; Fu, Z. Effects of annealing treatment on phase composition and microstructure of CoCrFeNiTiAlx high-entropy alloys. Intermetallics 2012, 22, 24–32. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, Z.; Chen, Y.; Shi, J.; Wang, Z.; Zhang, J. The effect of Al content on microstructures and comprehensive properties in AlxCoCrCuFeNi high entropy alloys. Vacuum 2019, 161, 143–149. [Google Scholar] [CrossRef]
- Jiang, S.; Lin, Z.; Xu, H.; Sun, Y. Studies on the microstructure and properties of AlxCoCrFeNiTi1−x high entropy alloys. J. Alloys Compd. 2018, 741, 826–833. [Google Scholar] [CrossRef]
- Kao, Y.F.; Lee, T.D.; Chen, S.K.; Chang, Y.S. Electrochemical passive properties of AlxCoCrFeNi (x = 0, 0.25, 0.50, 1.00) alloys in sulfuric acids. Corros. Sci. 2010, 52, 1026–1034. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Archard, J.F. Contact and Rubbing of Flat Surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.; Wang, F.J.; Chen, G.L. Phase transformation induced by lattice distortion in multiprincipal component CoCrFeNiCuXAl1−x solid-solution alloys. Appl. Physics. Lett. 2008, 92, 121–123. [Google Scholar] [CrossRef]
- Courtney, T. Mechanical Behavior of Materials; McGraw-Hill: New York, NY, USA, 1990; p. 173. [Google Scholar]
- Tong, C.J.; Chen, M.R.; Yeh, J.W.; Lin, S.J.; Chen, S.K.; Shun, T.T.; Chang, S.Y. Mechanical performance of the AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 1263–1271. [Google Scholar] [CrossRef]
- Sha, M.; Jia, C.; Qiao, J.; Feng, W.; Ai, X.; Jing, Y.-A.; Shen, M.; Li, S. Microstructure and Properties of High-Entropy AlxCoCrFe2.7MoNi Alloy Coatings Prepared by Laser Cladding. Metals 2019, 9, 1243. [Google Scholar] [CrossRef] [Green Version]
- Ye, F.; Jiao, Z.; Yan, S.; Guo, L.; Feng, L.; Yu, J. Microbeam plasma arc remanufacturing: Effects of Al on microstructure, wear resistance, corrosion resistance and high temperature oxidation resistance of AlxCoCrFeMnNi high-entropy alloy cladding layer. Vacuum 2020, 174, 109178. [Google Scholar] [CrossRef]




| Element | Co | Cr | Fe | Ni | Ti | Al |
|---|---|---|---|---|---|---|
| CoCrFeNiTi | 23.56 | 20.80 | 22.40 | 23.48 | 19.16 | 0 |
| CoCrFeNiTiAl0.5 | 23.56 | 20.80 | 22.40 | 23.48 | 19.16 | 5.40 |
| CoCrFeNiTiAl1 | 23.56 | 20.80 | 22.40 | 23.48 | 19.16 | 10.80 |
| CoCrFeNiTiAl1.5 | 23.56 | 20.80 | 22.40 | 23.48 | 19.16 | 16.20 |
| CoCrFeNiTiAl2 | 23.56 | 20.80 | 22.40 | 23.48 | 19.16 | 21.60 |
| Parameters | Data |
|---|---|
| Laser power | 2100 W |
| Scan speed | 9 mm/s |
| Laser beam diameter | 5 mm |
| Single layer thickness | 0.2 mm |
| Layers | 30 |
| Mass of feed powder | 8 g/min |
| Cladding area | 60 × 30 mm2 |
| Element | Co | Cr | Fe | Ni | Ti | Al |
|---|---|---|---|---|---|---|
| Co | - | −4 | −1 | 0 | −28 | −19 |
| Cr | −4 | - | −1 | −7 | −1 | −10 |
| Fe | −1 | −1 | - | −2 | −17 | −11 |
| Ni | 0 | −7 | −2 | - | −35 | −22 |
| Ti | −28 | −1 | −17 | −35 | - | −30 |
| Al | −19 | −10 | −11 | −22 | −30 | - |
| Sample No. | ba (mV) | bc (mV) | Ecorr/V | Icorr/(A·cm−2) |
|---|---|---|---|---|
| Al0 | 227.05 | 149.37 | −0.7382 | 5.60 × 10−6 |
| Al0.5 | 140.14 | 78.60 | −0.5766 | 9.13 × 10−7 |
| Al1 | 90.25 | 102.11 | −0.6791 | 5.43 × 10−6 |
| Al1.5 | 157.32 | 146.30 | −0.8591 | 8.03 × 10−6 |
| Al2 | 48.80 | 58.86 | −0.8802 | 9.14 × 10−6 |
| AISI1045 | 40.97 | 46.244 | −0.9701 | 4.71 × 10−5 |
| Element | Co | Cr | Fe | Ni | Ti | Al |
|---|---|---|---|---|---|---|
| BCC1 | 20.58 | 3.99 | 9.55 | 25.14 | 25.58 | 15.16 |
| BCC2 | 15.79 | 28.05 | 29.94 | 10.04 | 14.47 | 1.71 |
| Laves | 18.02 | 12.80 | 17.25 | 20.24 | 22.01 | 9.69 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Li, Z.; Liu, J.; Chen, Y.; Zhang, F.; Wu, L.; Hao, J.; Liu, L. Microstructure Evolution and Properties of Laser Cladding CoCrFeNiTiAlx High-Entropy Alloy Coatings. Coatings 2020, 10, 373. https://doi.org/10.3390/coatings10040373
Xu Y, Li Z, Liu J, Chen Y, Zhang F, Wu L, Hao J, Liu L. Microstructure Evolution and Properties of Laser Cladding CoCrFeNiTiAlx High-Entropy Alloy Coatings. Coatings. 2020; 10(4):373. https://doi.org/10.3390/coatings10040373
Chicago/Turabian StyleXu, Yiku, Zhiyuan Li, Jianru Liu, Yongnan Chen, Fengying Zhang, Lei Wu, Jianmin Hao, and Lin Liu. 2020. "Microstructure Evolution and Properties of Laser Cladding CoCrFeNiTiAlx High-Entropy Alloy Coatings" Coatings 10, no. 4: 373. https://doi.org/10.3390/coatings10040373




