Facile Fabrication of Methylcellulose/PLA Membrane with Improved Properties
Abstract
:1. Introduction
2. Experiment Section
2.1. Materials
2.2. Fabrication of MC/PLA Membrane
2.3. Characterization of Cellulose/PLA Membrane
2.4. Determination of Tensile Strength and Elongation at Break
2.5. Thermal Press Test of Cellulose/PLA Membrane
2.6. Cellulose/PLA Membrane Recovery
3. Results and Discussion
3.1. Preparation of MC/PLA Membrane
3.2. SEM, Solid-State 13C NMR, XRD and TGA Analysis
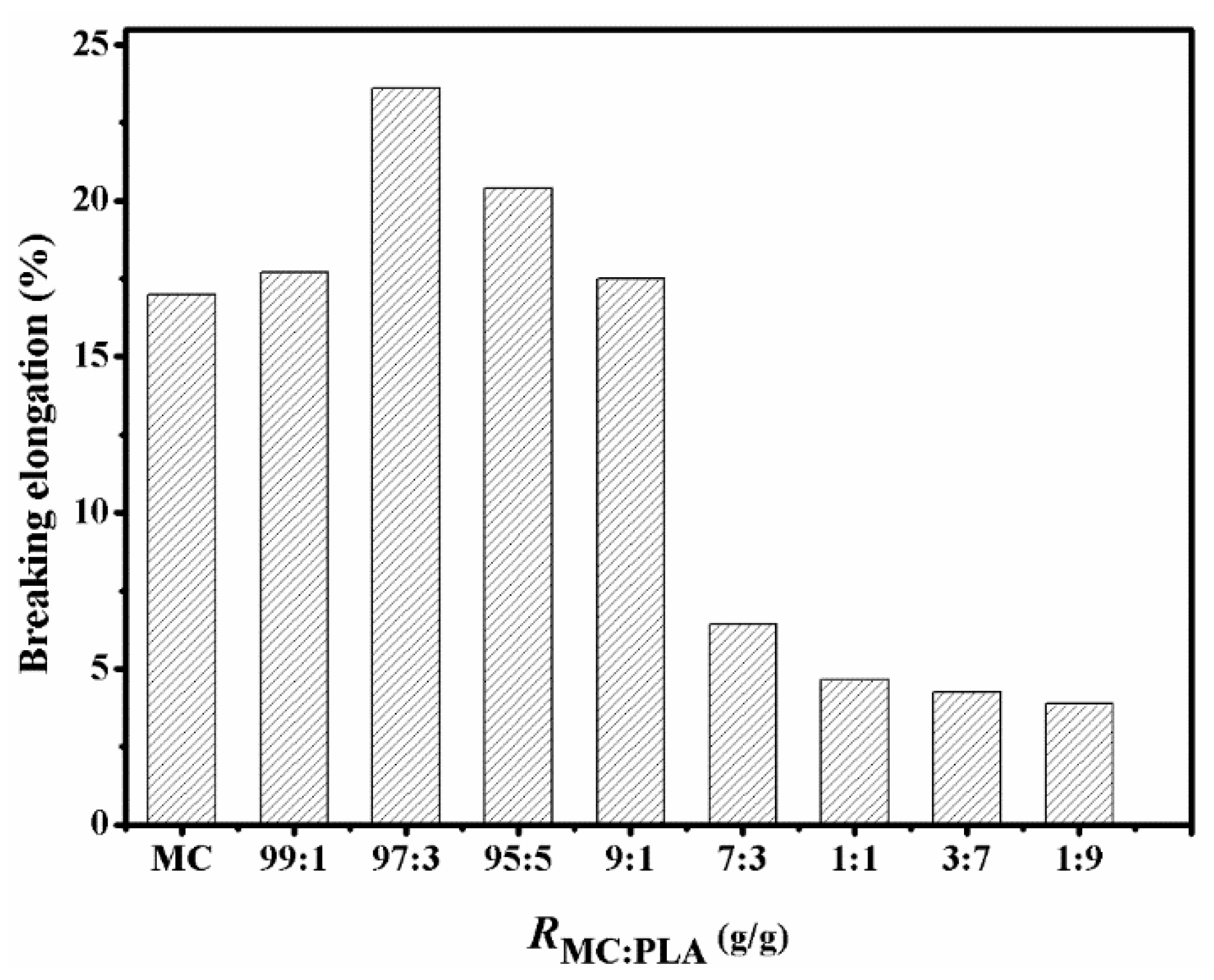
3.3. Analysis of Mechanical Properties
3.4. Thermocompression Effect on Mechanical Properties
3.5. Recovery of Cellulose/PLA Membrane
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sivanjineyulu, V.; Behera, K.; Chang, Y.-H.; Chiu, F.-C. Selective localization of carbon nanotube and organoclay in biodegradable poly(butylene succinate)/polylactide blend-based nanocomposites with enhanced rigidity, toughness and electrical conductivity. Compos. Part A Appl. Sci. Manuf. 2018, 114, 30–39. [Google Scholar] [CrossRef]
- Xu, A.; Chen, L.; Wang, J. Functionalized imidazalium carboxylates for enhancing practical applicability in cellulose processing. Macromolecules 2018, 51, 4158–4166. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, A.; Li, Z.; Wang, J.; Zhang, S. Influence of anionic structure on the dissolution of chitosan in 1-butyl-3-methylimidazolium-based ionic liquids. Green Chem. 2011, 13, 3446–3452. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kapoor, S.; Kundu, S.C. Silk fibroin/polyacrylamide semi-interpenetrating network hydrogels for controlled drug release. Biomaterials 2009, 30, 2826–2836. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Wang, J.; Luan, H.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. Dissolution behavior of maize starch in aqueous ionic liquids: Effect of anionic structure and water/ionic liquid ratio. ACS Omega 2019, 4, 14981–14986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, F.; Wang, J.; Yu, J.; Xiang, F.; Wang, S.; Wang, S.; Copeland, L. Dissolution of maize starch in aqueous ionic liquids: The role of alkyl chain length of cation and water: Ionic liquid ratio. ACS Sustain. Chem. Eng. 2019, 7, 6898–6905. [Google Scholar] [CrossRef]
- Sun, X.; Chi, Y.; Mu, T. Studies on staged precipitation of cellulose from an ionic liquid by compressed carbon dioxide. Green Chem. 2014, 16, 2736–2744. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on Polylactic Acid (PLA) based Sustainable Materials for Durable Applications: Focus on Toughness and Heat Resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Abdelrahman, M.S.; Nassar, S.H.; Mashaly, H.; Mahmoud, S.; Maamoun, D.; El-Sakhawy, M.; Khattab, T.A.; Kamel, S. Studies of Polylactic Acid and Metal Oxide Nanoparticles-Based Composites for Multifunctional Textile Prints. Coatings 2020, 10, 58. [Google Scholar] [CrossRef] [Green Version]
- Dascalu, C.-A.; Miculescu, F.; Mocanu, A.-C.; Constantinescu, A.E.; Butte, T.M.; Pandele, A.M.; Ciocoiu, R.-C.; Voicu, S.I.; Ciocan, L.T. Novel Synthesis of Core-Shell Biomaterials from Polymeric Filaments with a Bioceramic Coating for Biomedical Applications. Coatings 2020, 10, 283. [Google Scholar] [CrossRef] [Green Version]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.A.; Kenny, J.M. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef] [Green Version]
- Spiridon, I.; Paduraru, O.M.; Zaltariov, M.F.; Darie, R.N. Influence of Keratin on Polylactic Acid/Chitosan Composite Properties. Behavior upon Accelerated Weathering. Ind. Eng. Chem. Res. 2013, 52, 9822–9833. [Google Scholar] [CrossRef]
- Muiruri, J.K.; Liu, S.; Teo, W.S.; Kong, J.; He, C. Highly biodegradable and tough polylactic acid-cellulose nanocrystal composite. ACS Sustain. Chem. Eng. 2017, 5, 3929–3937. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Herrera, N.; Roch, H.; Salaberria, A.M.; Pino-Orellana, M.A.; Labidi, J.; Fernandes, S.C.M.; Radic, D.; Leiva, A.; Oksman, K. Functionalized blown films of plasticized polylactic acid/chitin nanocomposite: Preparation and characterization. Mater. Des. 2016, 92, 846–852. [Google Scholar] [CrossRef]
- Maharana, T.; Pattanaik, S.; Routaray, A.; Nath, N.; Sutar, A.K. Synthesis and characterization of poly(lactic acid) based graft copolymers. React. Funct. Polym. 2015, 93, 47–67. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic acid blends: The future of green, light and tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Alimuzzaman, S.; Gong, R.H.; Akonda, M. Biodegradability of Nonwoven Flax Fiber Reinforced Polylactic Acid Biocomposites. Polym. Compos. 2014, 35, 2094–2102. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Romero-Guzmán, D.; Fernández-Grajera, M.; González-Martín, M.L.; Gallardo-Moreno, A.M. Aging of Solvent-Casting PLA-Mg Hydrophobic Films: Impact on Bacterial Adhesion and Viability. Coatings 2019, 9, 814. [Google Scholar] [CrossRef] [Green Version]
- Sung, S.H.; Chang, Y.; Han, J. Development of polylactic acid nanocomposite films reinforced with cellulose nanocrystals derived from coffee silverskin. Carbohyd. Polym 2017, 169, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.A.; Geng, S.; Herrera, N.; Oksman, K. Aligned plasticized polylactic acid cellulose nanocomposite tapes: Effect of drawing conditions. Composites: Part A 2018, 104, 101–107. [Google Scholar] [CrossRef]
- Abdulkhani, A.; Hosseinzadeh, J.; Ashori, A.; Dadashi, S.; Takzare, Z. Preparation and characterization of modified cellulose nanofibers reinforced polylactic acid nanocomposite. Polym. Test 2014, 35, 73–79. [Google Scholar] [CrossRef]
- Hossain, K.M.Z.; Hasan, M.S.; Boyd, D.; Rudd, C.D.; Ahmed, I.; Thielemans, W. Effect of Cellulose Nanowhiskers on Surface Morphology, Mechanical Properties, and Cell Adhesion of Melt-Drawn Polylactic Acid Fibers. Biomacromolecules 2014, 15, 1498–1506. [Google Scholar] [CrossRef]
- Mukherjeen, T.; Sani, M.; Kao, N.; Gupta, R.K.; Quazi, N.; Bhattacharya, S. Improved dispersion of cellulose microcrystals in polylactic acid (PLA) based composites applying surface acetylation. Chem. Eng. Sci. 2013, 101, 655–662. [Google Scholar] [CrossRef]
- Murphy, C.A.; Collins, M.N. Microcrystalline cellulose reinforced polylactic acid biocomposite filaments for 3D printing. Polym. Composite. 2018, 39, 1311–1320. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Gao, J.; Wang, J. Facile fabrication of a homogeneous cellulose/polylactic acid composite film with improved biocompatibility, biodegradability and mechanical properties. Green Chem. 2019, 21, 4449–4456. [Google Scholar] [CrossRef]
- Matta, E.; Tavera-Quiroz, M.J.; Bertola, N. Active Edible Films of Methylcellulose with Extracts of Green Apple (Granny Smith) Skin. Int. J. Biol. Macromol. 2019, 124, 1292–1298. [Google Scholar] [CrossRef]
- Khan, R.A.; Salmieri, S.; Dussault, D.; Uribe-Calderon, J.; Kamal, M.R.; Safrany, A.; Lacroix, M. Production and Properties of Nanocellulose-Reinforced Methylcellulose-Based Biodegradable Films. J. Agric. Food Chem. 2010, 58, 7878–7885. [Google Scholar] [CrossRef]
- Das, B.; Basu, A.; Maji, S.; Dutta, K.; Dewan, M.; Adhikary, A.; Maiti, T.K.; Chattopadhyay, D. Nanotailored Hyaluronic Acid Modified Methylcellulose as an Injectable Scaffold with Enhanced Physico-Rheological and Biological Aspects. Carbohyd. Polym. 2020, 237, 116146. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Zhao, X.; Li, Q.; Dong, F.; Guo, Z. Preparation and Physicochemical Properties of Antioxidant Chitosan Ascorbate/Methylcellulose Composite Films. Int. J. Biol. Macromol. 2020, 146, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Jeong, L.; Cho, D.; Kwon, O.H.; Park, W.H. Effect of methylcellulose on the formation and drug release behavior of silk fibroin hydrogel. Carbohyd. Polym. 2013, 98, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Payne, C.; Dolan, E.B.; O’Sullivan, J.; Cryan, S.-A.; Kelly, H.M. A methylcellulose and collagen based temperature responsive hydrogel promotes encapsulated stem cell viability and proliferation in vitro. Drug Deliv. Transl. Res. 2017, 7, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Bain, M.K.; Bhowmik, M.; Ghosh, S.N.; Chattopadhyay, D. In situ fast gelling formulation of methyl cellulose for in vitro ophthalmic controlled delivery of ketorolac tromethamine. J. Appl. Polym. Sci. 2009, 113, 1241–1246. [Google Scholar] [CrossRef]
- Filho, G.R.; de Assuncao, R.M.N.; Vieira, J.G.; Meireles, C.d.S.; Cerqueira, D.A.; Barud, H.d.S.; Ribeiro, S.J.L.; Messaddeq, Y. Characterization of methylcellulose produced from sugar cane bagasse cellulose: Crystallinity and thermal properties. Polym. Degrad. Stabil. 2007, 92, 205–210. [Google Scholar] [CrossRef]
- Turhan, K.N.; Sahbaz, F.; Güner, A. A spectrophotometric study of hydrogen bonding in methylcellulose-based edible films plasticized by polyethylene glycol. J. Food Sci. 2001, 66, 59–62. [Google Scholar] [CrossRef]
- Velazquez, G.; Herrera-Gomez, A.; Martin-Polo, M.O. Identification of bound water through infrared spectroscopy in methylcellulose. J. Food Eng. 2003, 59, 79–84. [Google Scholar] [CrossRef]
- Pop, O.L.; Brandau, T.; Schwinn, J.; Vodnar, D.C.; Socaciu, C. The influence of different polymers on viability of Bifidobacterium lactis 300b during encapsulation, freeze-drying and storage. J. Food Sci. Technol. 2015, 52, 4146–4155. [Google Scholar] [CrossRef] [Green Version]
- Erdohan, Z.Ö.; Turhan, K.N. Barrier and mechanical properties of methylcellulose–whey protein films. Packag. Technol. Sci. 2005, 18, 295–302. [Google Scholar] [CrossRef]
- Turhan, K.N.; Erdohan Sancak, Z.Ö.; Ayana, B.; ErdoĞdu, F. Optimization of glycerol effect on the mechanical properties and water vapor permeability of whey protein-methylcellulose films. J. Food Process Eng. 2007, 30, 485–500. [Google Scholar] [CrossRef]
- Zuo, M.; Song, Y.; Zheng, Q. Preparation and properties of wheat gluten/methylcellulose binary blend film casting from aqueous ammonia: A comparison with compression molded composites. J. Food Eng. 2009, 91, 415–422. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Trofimchuk, E.S.; Dedushenko, S.K.; Fomin, A.S.; Davydova, G.A.; Selezneva, I.I.; Perfiliev, Y.D.; Barinov, S.M. Methylcellulose films partially crosslinked by iron compounds for medical applications. Mater. Today Commun. 2019, 18, 54–59. [Google Scholar] [CrossRef]
- Vargas, M.; Albors, A.; Chiralt, A.; González-Martínez, C. Water interactions and microstructure of chitosan-methylcellulose composite films as affected by ionic concentration. LWT-Food Sci. Technol. 2011, 44, 2290–2295. [Google Scholar] [CrossRef]
- Synytsya, A.; Grafová, M.; Slepicka, P.; Gedeon, O.; Synytsya, A. Modification of chitosan-methylcellulose composite films with meso-tetrakis(4-sulfonatophenyl)porphyrin. Biomacromolecules 2012, 13, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Guan, X. Fabrication of Poly-Lactic Acid (PLA) Composite Films and Their Degradation Properties. Master’s Thesis, University of Toledo, Toledo, OH, USA, 2012. [Google Scholar]
- Gupta, A.; Katiyar, V. Cellulose functionalized high molecular weight stereocomplex polylactic acid biocomposite films with improved gas barrier, thermomechanical properties. ACS Sustain. Chem. Eng. 2017, 5, 6835–6844. [Google Scholar] [CrossRef]
- Pinotti, A.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Study on microstructure and physical properties of composite films based on chitosan and methylcellulose. Food Hydrocolloid 2007, 21, 66–72. [Google Scholar] [CrossRef]
- Liu, H.; Liu, C.; Peng, S.; Pan, B.; Lu, C. Effect of polyethyleneimine modified graphene on the mechanical and water vapor barrier properties of methyl cellulose composite films. Carbohyd. Polym 2018, 182, 52–60. [Google Scholar] [CrossRef]
- Sangsuwan, J.; Rattanapanone, N.; Auras, R.A.; Harte, B.R.; Rachtanapun, P. Factors affecting migration of vanillin from chitosan/methyl cellulose films. J. Food. Sci. 2009, 74, 549–555. [Google Scholar] [CrossRef]
- Chen, C.; Li, D.; Hu, Q.; Wang, R. Properties of Polymethyl Methacrylate-Based Nanocomposites: Reinforced with Ultra-Long Chitin Nanofiber Extracted from Crab Shells. Mater. Des. 2014, 56, 1049–1056. [Google Scholar] [CrossRef]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, P.; Wang, F.; Duo, T.; Xiao, Z.; Xu, A.; Liu, R.; Jiang, C. Facile Fabrication of Methylcellulose/PLA Membrane with Improved Properties. Coatings 2020, 10, 499. https://doi.org/10.3390/coatings10050499
Guo P, Wang F, Duo T, Xiao Z, Xu A, Liu R, Jiang C. Facile Fabrication of Methylcellulose/PLA Membrane with Improved Properties. Coatings. 2020; 10(5):499. https://doi.org/10.3390/coatings10050499
Chicago/Turabian StyleGuo, Panjie, Fen Wang, Tongtong Duo, Zhihong Xiao, Airong Xu, Rukuan Liu, and Chaohui Jiang. 2020. "Facile Fabrication of Methylcellulose/PLA Membrane with Improved Properties" Coatings 10, no. 5: 499. https://doi.org/10.3390/coatings10050499



