Recent Advances in Mussel-Inspired Synthetic Polymers as Marine Antifouling Coatings
Abstract
:1. Introduction
- Anti-adhesive/foul-release coatings: such coating systems are usually based on hydrophobic/low surface energy polymers (e.g., silicones, fluoropolymers) which hinder the attachment or weaken the adhesive bond strength of biofouling species with their surface. However, it is the aforementioned hydrophobic and low surface energy nature of the polymer binder in these coatings that also makes the adhesion of the coating to the substrate (to be protected) problematic.
- Coatings based on poly(ethylene glycol)s, PEGs: PEGylation is a universal surface modification strategy across a number of industrial sectors, exploiting the hydrophilic nature of these polymers in order to prevent protein adsorption and subsequent fouling.
- Self-polishing non-biocidal coatings: the top layer of the paint on which biofouling occurs is hydrolysable and hence sacrificial, and is continuously depleted (along with the fouling organisms). In contrast to the self-polishing copolymer (SPC) biocidal coatings which rely on the release and action of a biocidal compound as the polymer binder hydrolyses and erodes from the coating, the self-polishing non-biocidal counterparts rely exclusively on the sacrificial surface layer to remove fouling.
2. Marine Biofouling: Mechanisms, Natural Defenses and Biomimicry
3. Mussel-Inspired Synthetic Polymers and Catechol-Mediated Adhesion
3.1. Mussel Biology, Biochemistry and Mechanics of Adhesion
3.2. l-DOPA and Catechol Chemistry: Adhesion, Cohesion, Self-Healing and Synthetic Mussel Foot Protein (MFP) Analogues
3.2.1. Adhesion: Metal Coordination and Catechol Oxidation
3.2.2. Cohesion: Covalent Interactions
3.2.3. Other Non-Covalent Interactions: Adhesion and Self-Healing
3.2.4. Chronology of Synthetic Mussel Protein Analogues and Mimics: Structures and Synthesis/Modification Strategies
- Reaction of catechol-bearing monomers (with protected hydroxyl groups, or not) by step-growth, chain-growth (free radical or controlled radical polymerisation methods) and ring-opening pathways to catechol-bearing homo- and co-polymers.
- Modification of an existing polymer or oligomer with a catechol-bearing reagent; either end-capping, or coupling with main-chain functional groups to yield pendant catechol chains.
- Solid-phase supported peptide synthesis.
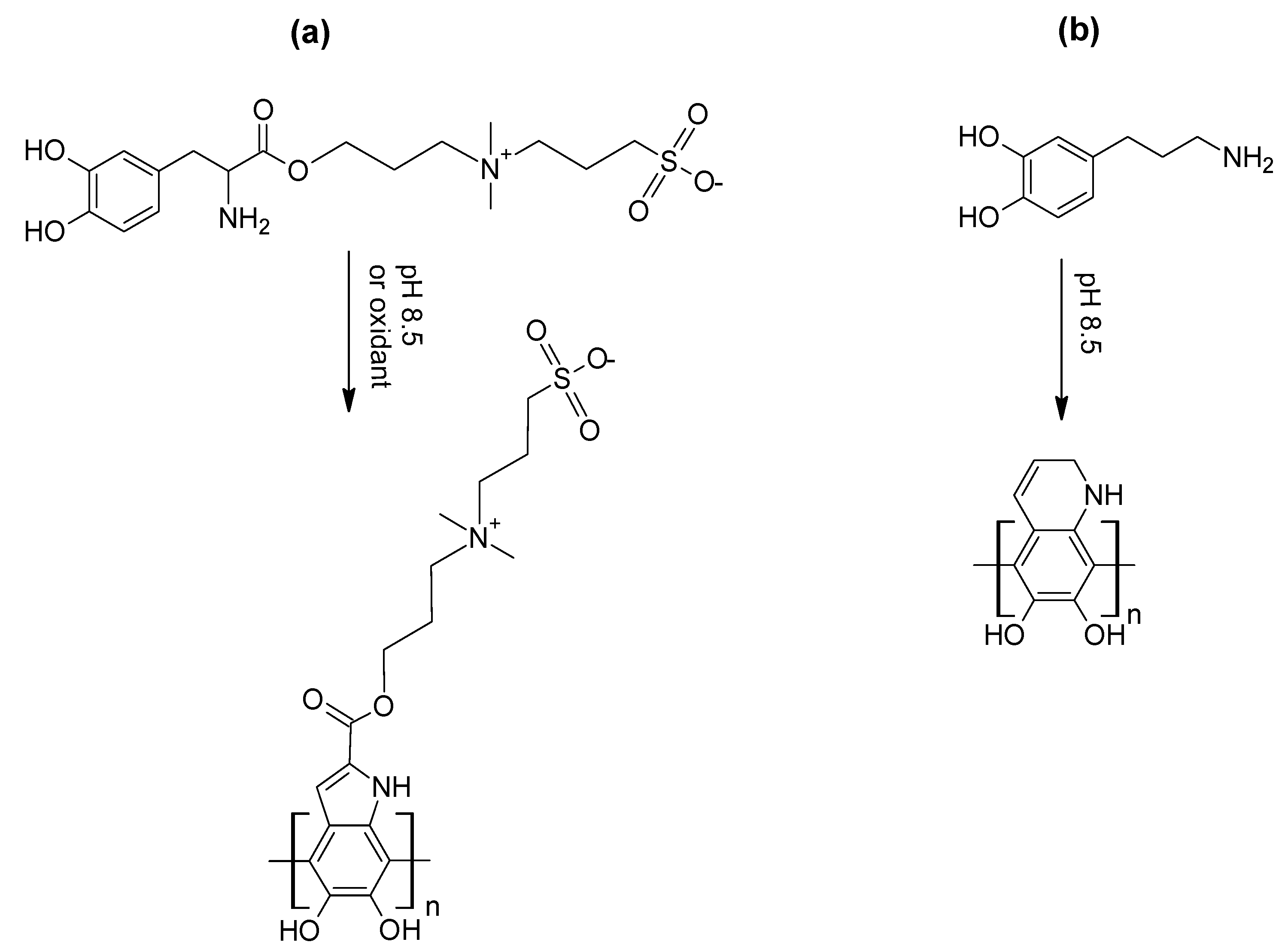
- Oxidative self-polymerisation of catecholamines: polydopamine and related materials.
4. Recent Advances in Mussel-Inspired Polymers as Marine Antifouling Coatings
4.1. Polydopamine-Type Materials
4.2. Poly(Ethylene Glycol) (PEG) Based Materials
4.3. Polysaccharide and Polypeptide Materials
4.4. Dopamine Methacrylamide Based Materials
5. Conclusions and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Marine Coating Market Estimated to Exceed $15 Billion by End of 2024. Available online: https://www.coatingsworld.com/contents/view_breaking-news/2017-03-01/marine-coating-market-estimated-to-exceed-15-billion-by-end-of-2024/# (accessed on 23 February 2020).
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Bannister, J.; Sievers, M.; Bush, F.; Nina Bloecher, N. Biofouling in marine aquaculture: A review of recent research and developments. Biofouling 2019, 35, 631–648. [Google Scholar] [CrossRef] [Green Version]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef] [Green Version]
- Anti-Fouling Systems. Available online: http://www.imo.org/en/OurWork/Environment/Anti-foulingSystems/Pages/Default.aspx?_sm_au_=iVVR7ZNMJnDMQS57 (accessed on 23 February 2020).
- Kirschner, C.M.; Brennan, A.B. Bio-Inspired Antifouling Strategies. Annu. Rev. Mater. Res. 2012, 42, 211–229. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012: Concerning the making available on the market and use of biocidal products. Off. J. Eur. Union. 27 June 2012. [Google Scholar]
- World Coatings Council Submits Paper on Cybutryne to IMO Subcommittee. Available online: https://www.paint.org/cybutryne/ (accessed on 21 March 2020).
- Ciriminna, R.; Bright, F.V.; Pagliaro, M. Ecofriendly Antifouling Marine Coatings. ACS Sustain. Chem. Eng. 2015, 3, 559–565. [Google Scholar] [CrossRef]
- Salta, M.; Wharton, J.A.; Stoodley, P.; Dennington, S.P.; Goodes, L.R.; Werwinski, S.; Mart, U.; Wood, R.J.K.; Stokes, K.R. Designing biomimetic antifouling surfaces. Phil. Trans. R. Soc. A 2010, 368, 4729–4754. [Google Scholar] [CrossRef] [Green Version]
- Damodaran, V.B.; Murthy, N.S. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20, 18–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Wang, J.-D.; Chen, H.-S.; Chen, D.-R. Progress of marine biofouling and antifouling technologies. Chin. Sci. Bull. 2011, 56, 598–612. [Google Scholar] [CrossRef] [Green Version]
- Magin, C.M.; Cooper, S.P.; Brennan, A.B. Non-Toxic Antifouling Strategies. Mater. Today 2010, 13, 36–44. [Google Scholar] [CrossRef]
- Waite, J.H.; Tanzer, M.L. Polyphenolic Substance of Mytilus edulis: Novel Adhesive Containing L-Dopa and Hydroxyproline. Science 1981, 212, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Meseguer Yebra, D.; Kiil, S.; Dam-Johansen, K. Antifouling technology—past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Salta, M.; Wharton, J.A.; Blache, Y.; Stokes, K.R.; Briand, J.-F. Marine biofilms on artificial surfaces: Structure and dynamics. Environ. Microbiol. 2013, 15, 2879–2893. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, G.H.; Vega, I.E.; Wahl, K.J.; Orihuela, B.; Beyley, V.; Rodriguez, E.N.; Everett, R.K.; Bonaventura, J.; Daniel Rittschof, D. Barnacle cement: A polymerization model based on evolutionary concepts. J. Exp. Biol. 2009, 212, 3499–3510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forooshani, P.K.; Lee, B.P. Recent Approaches in Designing Bioadhesive Materials Inspired by Mussel Adhesive Protein. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 9–33. [Google Scholar] [CrossRef]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-Inspired Adhesives and Coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Hwang, J.; Deming, T.J. Role of L-3, 4-Dihydroxyphenylalanine in Mussel Adhesive Proteins. J. Am. Chem. Soc. 1999, 121, 5825–5826. [Google Scholar] [CrossRef]
- Persson, F.; Svensson, R.; Nylund, G.M.; Fredriksson, N.J.; Pavia, H.; Hermansson, M. Ecological role of a seaweed secondary metabolite for a colonizing bacterial community. Biofouling 2011, 27, 579–588. [Google Scholar] [CrossRef]
- Barrios, C.A.; Xu, Q.; Cutright, T.; Zhang Newby, B. Incorporating zosteric acid into silicone coatings to achieve its slow release while reducing fresh water bacterial attachment. Colloids Surf. B Biointerfaces 2005, 41, 83–93.col. [Google Scholar] [CrossRef]
- Hao, E.; Fromont, J.; Jardine, D.; Karuso, P. Natural Products from Sponges of the Genus Agelas—on the Trail of a [2+2]-Photoaddition Enzyme. Molecules 2001, 6, 130–141. [Google Scholar] [CrossRef] [Green Version]
- Stowe, S.D.; Richards, J.J.; Tucker, A.T.; Thompson, R.; Melander, M.; Cavanagh, J. Anti-Biofilm Compounds Derived from Marine Sponges. Mar. Drugs 2011, 9, 2010–2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zidar, N.; Montalvão, S.; Hodnik, Ž.; Nawrot, D.A.; Žula, A.; Ilaš, J.; Kikelj, D.; Tammela, P.; Peterlin Mašič, L. Antimicrobial Activity of the Marine Alkaloids, Clathrodin and Oroidin, and their Synthetic Analogues. Mar. Drugs 2014, 12, 940–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef] [Green Version]
- Jun, J.-Y.; Jung, M.; Jeong, I.-H.; Yamazaki, K.; Kawai, Y.; Kim, B.-M. Antimicrobial and Antibiofilm Activities of Sulfated Polysaccharides from Marine Algae against Dental Plaque Bacteria. Mar. Drugs 2018, 16, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, T.; Cas, A.; Bracič, M.; Plohl, O.; Vesel, A.; Rupnik, M.; Zemljic, L.F.; Rebol, J. Highly Protein Repellent and Antiadhesive Polysaccharide Biomaterial Coating for Urinary Catheter Applications. ACS Biomater. Sci. Eng. 2019, 5, 5825–5832. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors. Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [Green Version]
- Sharklet® Adhesively Backed Film. Available online: https://www.sharklet.com/our-products/adhesively-backed-film/ (accessed on 10 April 2020).
- Bohn, H.F.; Federle, W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc. Natl. Acad. Sci. USA 2004, 101, 14138–14143. [Google Scholar] [CrossRef] [Green Version]
- Epstein, A.K.; Wong, T.-S.; Belisle, R.A.; Boggs, E.M.; Aizenberg, J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc. Natl. Acad. Sci. USA 2012, 109, 13182–13187. [Google Scholar] [CrossRef] [Green Version]
- MacCallum, N.; Howell, C.; Kim, P.; Sun, D.; Friedlander, R.; Ranisau, J.; Ahanotu, O.; Lin, J.J.; Vena, A.; Hatton, B.; et al. Liquid-Infused Silicone as a Biofouling-Free Medical Material. ACS Biomater. Sci. Eng. 2015, 1, 43–51. [Google Scholar] [CrossRef]
- Amini, S.; Kolle, S.; Petrone, L.; Ahanotu, O.; Sunny, S.; Sutanto, C.N.; Hoon, S.; Cohen, L.; Weaver, J.C.; Aizenberg, J.; et al. Preventing mussel adhesion using lubricant-infused materials. Science 2017, 357, 668–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SLIPS® Foul ProtectTM Bottom Paints from AST. Available online: https://slipsfoulprotect.com/ (accessed on 12 April 2020).
- Harrington, M.J.; Waite, J.H. Holdfast heroics: Comparing the molecular and mechanical properties of Mytilus californianus byssal threads. J. Exp. Biol. 2007, 210, 4307–4318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waite, J.H. Mussel adhesion – essential footwork. J. Exp. Biol. 2017, 220, 517–530. [Google Scholar] [CrossRef] [Green Version]
- Waite, J.H.; Qin, X.-X.; Coyne, K.J. The Peculiar Collagens of Mussel. Byssus. Matrix Biol. 1998, 17, 93–106. [Google Scholar] [CrossRef]
- Waite, J.H. The formation of mussel byssus: Anatomy of a natural manufacturing process. In Structure, Cellular Synthesis and Assembly of Biopolymers, 1st ed.; Case, S.T., Ed.; Springer: Berlin/Heidelberg, Germany, 1992; Volume 19, pp. 27–54. [Google Scholar]
- Waite, J.H. Evidence for a Repeating 3, 4-Dihydroxyphenylalanine and Hydroxyproline-containing Decapeptide in the Adhesive Protein of the Mussel, Mytilus edulis L. J. Biol. Chem. 1983, 258, 2911–2915. [Google Scholar] [PubMed]
- Zhao, Y.; Waite, J.H. Linking Adhesive and Structural Proteins in the Attachment Plaque of Mytilus californianus. J. Biol. Chem. 2006, 281, 26150–26158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Cohen Stuart, M.A.; Kamperman, M. Jack of all trades: Versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 2014, 43, 8271–8298. [Google Scholar] [CrossRef]
- Yu, J.; Wei, W.; Danner, E.; Ashley, R.K.; Israelachvili, J.N.; Waite, J.H. Mussel protein adhesion depends on interprotein thiol-mediated redox modulation. Nat. Chem. Biol. 2011, 7, 588–590. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.R.; Spahn, J.E.; Waite, J.H. The staying power of adhesion-associated antioxidant activity in Mytilus californianus. J. R. Soc. Interface 2015, 12, 614–621. [Google Scholar]
- Jameson, G.N.L.; Zhang, J.; Jameson, R.F.; Linert, W. Kinetic evidence that cysteine reacts with dopaminoquinone via reversible adduct formation to yield 5-cysteinyl-dopamine: An important precursor of neuromelanin. Org. Biomol. Chem. 2004, 2, 777–782. [Google Scholar] [CrossRef]
- Filippidi, E.; DeMartini, D.G.; Malo de Molina, P.; Danner, E.W.; Kim, J.; Helgeson, M.E.; Waite, J.H.; Valentine, M.T. The microscopic network structure of mussel (Mytilus) adhesive plaques. J. R. Soc. Interface 2015, 12, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H.; Vaccaro, E.; Sun, C.; Lucas, J.M. Elastomeric gradients: A hedge against stress concentration in marine holdfasts? Phil. Trans. R. Soc. Lond. B 2002, 357, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M.J.; Gupta, H.S.; Fratzl, P.; Waite, J.H. Collagen insulated from tensile damage by domains that unfold reversibly: In situ X-ray investigation of mechanical yield and damage repair in the mussel byssus. J. Struct. Biol. 2009, 167, 47–54. [Google Scholar] [CrossRef] [Green Version]
- McDowell, L.M.; Burzio, L.A.; Waite, H.J.; Schaefer, J. Rotational Echo Double Resonance Detection of Cross-links Formed in Mussel Byssus under High-Flow Stress. J. Biol. Chem. 1999, 274, 20293–20295. [Google Scholar] [CrossRef] [Green Version]
- Holten-Andersen, N.; Fantner, G.; Hohlbauch, S.; Waite, J.H.; Zok, F.W. Protective coatings on extensible biofibres. Nat. Mater. 2007, 6, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Holten-Andersen, N.; Waite, J.H. Mussel-designed Protective Coatings for Compliant Substrates. J. Dent. Res. 2008, 87, 701–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holten-Andersen, N.; Mates, T.E.; Toprak, M.S.; Stucky, G.D.; Zok, F.W.; Waite, J.H. Metals and the Integrity of a Biological Coating: The Cuticle of Mussel Byssus. Langmuir 2009, 25, 3323–3326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holten-Andersen, N.; Zhao, H.; Waite, J.H. Stiff Coatings on Compliant Biofibers: The Cuticle of Mytilus californianus Byssal Threads. Biochemistry 2009, 48, 2752–2759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrington, M.J.; Masic, A.; Holten-Andersen, N.; Waite, J.H.; Fratzl, P. Iron-Clad Fibers: A Metal-Based Biological Strategy for Hard Flexible Coatings. Science 2010, 328, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Meng, H.; Messersmith, P.B.; Lee, B.P.; Dalsin, J.L. Biomimetic Adhesives and Coatings Based on Mussel Adhesive Proteins. In Biological Adhesives, 2nd ed.; Smith, A.M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 345–378. [Google Scholar]
- Sever, M.J.; Wilker, J.J. Visible absorption spectra of metal–catecholate and metal–tironate complexes. Dalton Trans. 2004, 1061–1072. [Google Scholar] [CrossRef]
- Sever, M.J.; Wilker, J.J. Absorption spectroscopy and binding constants for first-row transition metal complexes of a DOPA-containing peptide. Dalton Trans. 2006, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Monahan, J.; Wilker, J.J. Specificity of metal ion cross-linking in marine mussel adhesives. Chem. Commun. 2003, 1672–1673. [Google Scholar] [CrossRef] [PubMed]
- Dalsin, J.L.; Lin, L.; Tosatti, S.; Voros, J.; Textor, M.; Messersmith, P.B. Protein Resistance of Titanium Oxide Surfaces Modified by Biologically Inspired mPEG-DOPA. Langmuir 2005, 21, 640–646. [Google Scholar] [CrossRef]
- Ribena, D.; Alekseev, A.; Van Asselen, O.; Mannie, G.J.; Hendrix, M.M.; van der Ven, L.G.; Sommerdijk, N.A.; de With, G. Significance of the Amide Functionality on DOPA-Based. Monolayers on Gold. Langmuir 2012, 28, 16900–16908. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holten-Andersen, N.; Harrington, M.J.; Birkedal, H.; Lee, B.P.; Messersmith, P.B.; Lee, K.Y.C.; Waite, J.H. pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. Proc. Natl. Acad. Sci. USA 2011, 108, 2651–2655. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry. For Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Fullenkamp, D.E.; Rivera, J.G.; Messersmith, P.B. pH responsive self-healing hydrogels formed by boronate–catechol complexation. Chem. Commun. 2011, 47, 7497–7499. [Google Scholar] [CrossRef] [Green Version]
- Narkar, A.R.; Barker, B.; Clisch, M.; Jiang, J.; Lee, B.P. pH Responsive and Oxidation Resistant Wet Adhesive based on Reversible Catechol−Boronate Complexation. Chem. Mater. 2016, 28, 5432–5439. [Google Scholar] [CrossRef]
- Springsteen, G.; Wang, B. A detailed examination of boronic acid-diol complexation. Tetrahedron 2000, 58, 5291–5300. [Google Scholar] [CrossRef]
- Yan, J.; Springsteen, G.; Deeter, S.; Wang, B. The relationship among pKa, pH, and binding constants in the. Interactions between boronic acids and diols—It is not as simple as it appears. Tetrahedron 2004, 60, 11205–11209. [Google Scholar] [CrossRef]
- Hofman, A.H.; van Hees, I.A.; Yang, J.; Kamperman, M. Bioinspired Underwater Adhesives by Using the. Supramolecular Toolbox. Adv. Mater. 2018, 30, 1704640–1704678. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yu, J.; Gebbie, M.A.; Tan, Y.; Nadine, R.; Martinez Rodriguez, N.R.; Israelachvili, J.N.; Waite, J.H. Bridging Adhesion of Mussel-Inspired Peptides: Role of Charge, Chain Length, and Surface Type. Langmuir 2015, 31, 1105–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Kan, Y.; Rappa, M.; Danner, E.; Wei, W.; Das, S.; Miller, D.S.; Chen, Y.; Waite, J.H.; Israelachvili, J.N. Adaptive hydrophobic and hydrophilic interactions of mussel foot proteins with organic thin films. Proc. Natl. Acad. Sci. USA 2013, 110, 15680–15685. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Danner, E.; Waite, J.H.; Israelachvili, J.N.; Zeng, H.; Hwang, D.S. Adhesion of mussel foot proteins to different substrate surfaces. J. R. Soc. Interface 2013, 10, 20120759–20120770. [Google Scholar] [CrossRef]
- Ahn, B.K.; Lee, D.W.; Israelachvili, J.N.; Waite, J.H. Surface-initiated self-healing of polymers in aqueous media. Nat. Mater. 2014, 13, 867–872. [Google Scholar] [CrossRef]
- Shannon, A.; Manolakis, I. A Facile Route to Bio-Inspired Supramolecular Oligo (Ethylene Glycol) Catecholates. Macromol. Chem. Phys. 2019, 220, 1800412–1800418. [Google Scholar] [CrossRef]
- Brown, C.J. The Crystal Structure of Catechol. Acta Cryst. 1966, 21, 170–174. [Google Scholar] [CrossRef]
- Yamamoto, H. Synthesis and Adhesive Studies of Marine Polypeptides. J. Chem. Soc. Perkin Trans I 1987. [Google Scholar] [CrossRef]
- Harwood, H.J.; Cassidy, H.G. Electron Exchange Polymers. IX. Synthesis of Polymers of 2, 5-Dihydroxyphenylalanine and of 3, 4-Dihydroxyphenylalanine (DOPA). J. Am. Chem. Soc. 1957, 79, 4360–4365. [Google Scholar] [CrossRef]
- Yamamoto, H.; Hayakawa, T. Sense of Helix of Poly-O, O’-dicarbobenzoxy-L-DOPA in Solution. Macromolecules 1976, 9, 532–534. [Google Scholar] [CrossRef]
- Yamamoto, H.; Hayakawa, T. Synthesis and conformational study of poly (l-β-3, 4-dihydroxyphenyl-α-alanine). Polymer 1977, 18, 979–983. [Google Scholar] [CrossRef]
- Fuller, W.D.; Verlander, M.S.; Goodman, M. DOPA-Containing Polypeptides. I. Improved Synthesis of High-Molecular-Weight Poly (L-DOPA) and Water-Soluble Copolypeptides. Biopolymers 1978, 17, 2939–2943. [Google Scholar] [CrossRef]
- Yu, M.; Deming, T.J. Synthetic Polypeptide Mimics of Marine Adhesives. Macromolecules 1998, 31, 4739–4745. [Google Scholar] [CrossRef] [PubMed]
- Statz, A.R.; Meagher, R.J.; Barron, A.E.; Messersmith, P.B. New Peptidomimetic Polymers for Antifouling Surfaces. J. Am. Chem. Soc. 2005, 127, 7972–7973. [Google Scholar] [CrossRef]
- Statz, A.R.; Barron, A.E.; Messersmith, P.B. Protein, cell and bacterial fouling resistance of polypeptoid modified surfaces: Effect of side-chain chemistry. Soft Matter 2008, 4, 131–139. [Google Scholar] [CrossRef]
- Statz, A.R.; Park, J.P.; Chongsiriwatana., N.P.; Barron, A.E.; Messersmith, P.B. Surface-immobilized antimicrobial peptoids. Biofouling 2008, 24, 439–448. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Lee, H.B.; Andrade, J.D. Blood Compatibility of Polyethylene Oxide Surfaces. Prog. Polym. Sci. 1995, 20, 1043–1079. [Google Scholar] [CrossRef]
- Schlenoff, J.B. Zwitteration: Coating Surfaces with Zwitterionic Functionality to Reduce Nonspecific Adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef]
- Lin, W.; Klein, J. Control of surface forces through hydrated boundary layers. Curr. Opin. Coll. Interf. Sci. 2019, 44, 94–106. [Google Scholar] [CrossRef]
- Huang, K.; Lee, B.P.; Ingram, D.I.; Messersmith, P.B. Synthesis and Characterization of Self-Assembling Block Copolymers Containing Bioadhesive End Groups. Biomacromolecules 2002, 3, 397–406. [Google Scholar] [CrossRef]
- Lee, B.P.; Dalsin, J.L.; Messersmith, P.B. Synthesis and Gelation of DOPA-Modified Poly (ethylene glycol) Hydrogels. Biomacromolecules 2002, 3, 1038–1047. [Google Scholar] [CrossRef]
- Brubaker, C.E.; Kissler, H.; Wang, L.-J.; Kaufman, D.B.; Messersmith, P.B. Biological performance of mussel-inspired adhesive in extrahepatic islet transplantation. Biomaterials 2010, 31, 420–427. [Google Scholar] [CrossRef] [Green Version]
- Wei Zhang, W.; Wang, R.; Sun, Z.M.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F.K.; Zhao, B.; Pinnaratip, R.; et al. Catechol-functionalized hydrogels: Biomimetic design, adhesion mechanism, and biomedical applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.D.; Pyo, K.B.; Park, S.Y.; Lee, H. Catechol-Grafted Poly (ethylene glycol) for PEGylation on Versatile Substrates. Langmuir 2010, 26, 3790–3793. [Google Scholar] [CrossRef]
- Khalila, F.; Franzmann, E.; Julian Ramcke, J.; Dakischew, O.; Lips, K.S.; Reinhardt, A.; Heisig, P.; Maison, W. Biomimetic PEG-catecholates for stabile antifouling coatings on metal surfaces: Applications on TiO2 and stainless steel. Colloids Surf. B Biointerfaces 2014, 117, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Manolakis, I.; Noordover, B.A.J.; Vendamme, R.; Eevers, W. Novel-DOPA-Derived Poly (ester amide) s: Monomers, Polymers, and the First L-DOPA-Functionalized Biobased Adhesive Tape. Macromol. Rapid Commun. 2014, 35, 71–76. [Google Scholar] [CrossRef]
- Sunder, A.; Hanselmann, R.; Frey, H.; Müllhaupt, R. Controlled Synthesis of Hyperbranched Polyglycerols by Ring-Opening Multibranching Polymerization. Macromolecules 1999, 32, 4240–4246. [Google Scholar] [CrossRef]
- Frey, H.; Haag, R. Dendritic polyglycerol: A new versatile biocompatible material. Rev. Mol. Biotechnol. 2002, 90, 257–267. [Google Scholar] [CrossRef]
- Wei, Q.; Krysiak, S.; Achazi, K.; Becherer, T.; Noeske, P.-L.M.; Paulus, F.; Liebe, H.; Grunwald, I.; Dernedde, J.; Hartwig, A.; et al. Multivalent anchored and crosslinked hyperbranched polyglycerol monolayers as antifouling coating for titanium oxide surfaces. Colloids Surf. B Biointerfaces 2014, 122, 684–692. [Google Scholar] [CrossRef]
- Wei, Q.; Achazi, K.; Liebe, H.; Schulz, A.; Noeske, P.-L.M.; Grunwald, I.; Haag, R. Mussel-Inspired Dendritic Polymers as Universal Multifunctional Coatings. Angew. Chem. Int. Ed. 2014, 53, 11650–11655. [Google Scholar] [CrossRef]
- Schlaich, C.; Li, M.; Cheng, C.; Donskyi, I.S.; Yu, L.; Song, G.; Osorio, E.; Wei, Q.; Haag, R. Mussel-Inspired Polymer-Based Universal Spray Coating for Surface Modification: Fast Fabrication of Antibacterial and Superhydrophobic Surface Coatings. Adv. Mater. Interfaces 2018, 5, 1701254–1701262. [Google Scholar] [CrossRef]
- Pranantyo, D.; Xu, L.Q.; Neoh, K.G.; Kang, E.-T.; Lay-Ming Teo, S. Antifouling Coatings via Tethering of Hyperbranched Polyglycerols on Biomimetic Anchors. Ind. Eng. Chem. Res. 2016, 55, 1890–1901. [Google Scholar] [CrossRef]
- Westwood, G.; Horton, T.N.; Wilker, J.J. Simplified Polymer Mimics of Cross-Linking Adhesive Proteins. Macromolecules 2007, 40, 3960–3964. [Google Scholar] [CrossRef]
- Matos-Pérez, C.R.; White, J.D.; Wilker, J.J. Polymer Composition and Substrate Influences on the Adhesive. Bonding of a Biomimetic, Cross-Linking Polymer. J. Am. Chem. Soc. 2012, 134, 9498–9505. [Google Scholar]
- White, J.D.; Wilker, J.J. Underwater Bonding with Charged Polymer Mimics of Marine. Mussel Adhesive Proteins. Macromolecules 2011, 44, 5085–5088. [Google Scholar] [CrossRef]
- Matos-Pérez, C.R.; Wilker, J.J. Ambivalent Adhesives: Combining Biomimetic Cross-Linking with.Antiadhesive Oligo (ethylene glycol). Macromolecules 2012, 45, 6634–6639. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.; Glass, P.; Pothen, J.M.; Sitti, M.; Washburn, N.R. Enhanced Adhesion of Dopamine Methacrylamide Elastomers via Viscoelasticity Tuning. Biomacromolecules 2011, 12, 342–347. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, T.; Newland, B.; Liu, W.; Wang, W.; Wang, W. Catechol functionalized hyperbranched polymers as biomedical materials. Prog. Pol. Sci. 2018, 78, 47–55. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, T.; Newland, B.; Duffy, P.; Ni Annaidh, A.; O’Cearbhaill, E.D.; Wang, W. On-demand and negative-thermo-swelling tissue adhesive based on highly branched ambivalent PEG–catechol copolymers. J. Mater. Chem. B 2015, 3, 6420–6428. [Google Scholar] [CrossRef]
- Breydo, L.; Newland, B.; Zhang, H.; Rosser, A.; Werner, C.; Uversky, V.N.; Wang, W. A Hyperbranched Dopamine-Containing PEG-based Polymer for the Inhibition of α-Synuclein Fibrillation. Biochem. Biophys. Res. Commun. 2016, 469, 830–835. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhang, L.; Wang, Y.; Long, Y.; Gao, H.; Zhang, X.; Zhao, N.; Cai, Y.; Xu, J. Mussel-Inspired Chemistry for Robust and Surface-Modifiable Multilayer Films. Langmuir 2011, 27, 13684–13691. [Google Scholar] [CrossRef]
- Solovskij, M.V.; Denisov, V.M.; Panarin, E.E.; Petukhova, N.A.; Purkina, A.V. Synthesis of water-soluble biologically active phenol (or catechol) containing copolymers of N-vinyl-2-pyrrolidone. Macromol. Chem. Phys. 1996, 197, 2035–2046. [Google Scholar] [CrossRef]
- Laulicht, B.; Mancini, A.; Geman, N.; Cho, D.; Estrellas, K.; Furtado, S.; Hopson, R.; Tripathi, A.; Mathiowitz, E. Bioinspired Bioadhesive Polymers: Dopa-Modified Poly(acrylic acid) Derivatives. Macromol. Biosci. 2012, 12, 1555–1565. [Google Scholar] [CrossRef]
- Zhou, J.; Defante, A.P.; Lin, F.; Xu, Y.; Yu, J.; Gao, Y.; Childers, E.; Dhinojwala, A.; Becker, M.L. Adhesion Properties of Catechol-Based Biodegradable Amino Acid-Based Poly(ester urea) Copolymers Inspired from Mussel Proteins. Biomacromolecules 2015, 16, 266–274. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Q.; Narayanan, A.; Jain, D.; Dhinojwala, A.; Joy, A. Mussel-Inspired Polyesters with Aliphatic Pendant Groups Demonstrate the Importance of Hydrophobicity in Underwater Adhesion. Adv. Mater. Interfaces 2017, 4, 1700506–1700512. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Weng, H.; Gyawali, D.; Tang, L.; Yang, J. Injectable citrate-based mussel-inspired tissue bioadhesives with high wet strength for sutureless wound closure. Biomaterials 2012, 33, 7972–7983. [Google Scholar] [CrossRef] [Green Version]
- Patil, N.; Jérôme, C.; Detrembleur, C. Recent advances in the synthesis of catechol-derived (bio) polymers for applications in energy storage and environment. Prog. Pol. Sci. 2018, 82, 34–91. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Z.; Shi, J.; Zhang, C.; Zhang, W.; Wu, H. Dopamine-Modified Alginate Beads Reinforced by Cross-Linking via Titanium Coordination or Self-Polymerization and Its Application in Enzyme Immobilization. Ind. Eng. Chem. Res. 2013, 52, 14828–14836. [Google Scholar] [CrossRef]
- Alegre-Requena, J.V.; Häring, M.; Herrera, R.P.; Diaz Diaz, D. Regulatory parameters of self-healing alginate hydrogel networks prepared via mussel-inspired dynamic chemistry. New J. Chem. 2016, 40, 8493–8501. [Google Scholar] [CrossRef] [Green Version]
- Hyun Ryu, J.; Lee, Y.; Kong, W.H.; Kim, T.G.; Tae Gwan Park, T.G.; Lee, H. Catechol-Functionalized Chitosan/Pluronic Hydrogels for Tissue Adhesives and Hemostatic Materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar]
- Kim, K.; Hyun Ryu, J.; Lee, D.Y.; Lee, H. Bio-inspired catechol conjugation converts water-insoluble chitosan into a highly water-soluble, adhesive chitosan derivative for hydrogels and LbL assembly. Biomater. Sci. 2013, 1, 783–790. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.; Hyun Ryu, J.; Lee, H. Chitosan-catechol: A polymer with long-lasting mucoadhesive properties. Biomaterials 2015, 52, 161–170. [Google Scholar] [CrossRef]
- Hyun Ryu, J.; Hong, S.; Lee, H. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomater. 2015, 27, 101–115. [Google Scholar]
- Ways, T.M.W.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Chung, H.J.; Yeo, S.; Ahn, C.-H.; Lee, H.; Messersmith, P.B.; Park, T.G. Thermo-sensitive, injectable, and tissue adhesive sol–gel transition hyaluronic acid/pluronic composite hydrogels prepared from bio-inspired catechol-thiol reaction. Soft Matter 2010, 6, 977–983. [Google Scholar] [CrossRef]
- Jeong, Y.; Thuy, L.T.; Ki, S.H.; Ko, S.; Kim, S.; Cho, K.W.; Choi, J.S.; Kang, S.M. Multipurpose Antifouling Coating of Solid Surfaces with the Marine-Derived Polymer Fucoidan. Macromol. Biosci. 2018, 18, 1800137–1800144. [Google Scholar] [CrossRef]
- Lee, M.; Kim, Y.; Hyun Ryu, J.; Kim, K.; Han, Y.-M.; Lee, H. Long-term, feeder-free maintenance of human embryonic stem cells by mussel-inspired adhesive heparin and collagen type I. Acta Biomater. 2016, 32, 138–148. [Google Scholar] [CrossRef]
- Kim, S.; Ko, S.; Kang, S.M. Adhesive Heparin Coating for Marine Antifouling Applications. Macromol. Res. 2016, 24, 645–649. [Google Scholar] [CrossRef]
- Hyun Ryu, J.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar]
- Lee, H.A.; Ma, Y.; Zhou, F.; Hong, S.; Lee, H. Material-Independent Surface Chemistry beyond Polydopamine Coating. Acc. Chem. Res. 2019, 52, 704–713. [Google Scholar] [CrossRef]
- Liebscher, J. Chemistry of Polydopamine—Scope, Variation, and Limitation. Eur. J. Org. Chem. 2019, 31, 4976–4994. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.-C.; He, F.; Peng, S.; Li, Y.; Shao, L.; Darling, S.B. Mussel-Inspired Surface Engineering.for Water-Remediation Materials. Matter 2019, 1, 115–155. [Google Scholar] [CrossRef] [Green Version]
- Xi, Z.-Y.; Xu, Y.-Y.; Zhu, L.-P.; Wang, Y.; Zhu, B.-K. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly (DOPA) and poly(dopamine). J. Membr. Sci. 2009, 327, 244–253. [Google Scholar] [CrossRef]
- Lu, Z.; Douek, A.M.; Rozario, A.M.; Tabor, R.F.; Kaslin, J.; Follink, B.; Teo, B.M. Bioinspired polynorepinephrine nanoparticles as an efficient vehicle for enhanced drug delivery. J. Mater. Chem. B 2020, 8, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Malollari, K.G.; Delparastan, P.; Sobek, C.; Vachhani, S.J.; Fink, T.D.; Zha, R.H.; Messersmith, P.B. Mechanical Enhancement of Bioinspired Polydopamine Nanocoatings. ACS Appl. Mater. Interfaces 2019, 11, 43599–43607. [Google Scholar] [CrossRef]
- Saiz-Poseu, J.; Sedó, J.; García, B.; Benaiges, C.; Parella, T.; Alibés, R.; Hernando, J.; Busqué, F.; Ruiz-Molina, D. Versatile Nanostructured Materials via Direct Reaction of Functionalized Catechols. Adv. Mater. 2013, 25, 2066–2070. [Google Scholar] [CrossRef] [Green Version]
- Yeon, D.K.; Ko, S.; Jeong, S.; Hong, S.-P.; Kang, S.M.; Cho, W.K. Oxidation-Mediated, Zwitterionic Polydopamine Coatings for Marine Antifouling Applications. Langmuir 2019, 35, 1227–1234. [Google Scholar] [CrossRef]
- Hong, J.; Jwa, D.G.; Ha, H.; Kwak, J.; Kim, M.; Kang, S.M. 4-(3-Aminopropyl)-benzene-1,2-diol: An Improved Material-Independent Surface-Coating Reagent Compared to Dopamine. Langmuir 2019, 35, 6898–6904. [Google Scholar] [CrossRef]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic materials for antifouling membrane surface construction. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef]
- Kim, S.; Gim, T.; Jeong, Y.; Ryu, J.H.; Kang, S.M. Facile Construction of Robust Multilayered PEG Films on Polydopamine-Coated Solid Substrates for Marine Antifouling Applications. ACS Appl. Mater. Interfaces 2018, 10, 7626–7631. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Banquy, X.; Heo, J.; Lim, C.; Lynd, N.A.; Lundberg, P.; Oh, D.X.; Lee, H.-K.; Hong, Y.-K.; Hwang, D.S.; et al. Mussel-Inspired Anchoring of Polymer Loops That Provide Superior Surface Lubrication and Antifouling Properties. ACS Nano 2016, 10, 930–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, P.; Dub, C.; He, X.; Zhang, C.; Yuan, C. Modification of a derived antimicrobial peptide on steel surface for marine bacterial resistance. Appl. Surf. Sci. 2020, 510, 145512–145520. [Google Scholar] [CrossRef]
- Wang, J.; He, C. Photopolymerized biomimetic self-adhesive Polydimethylsiloxane-based amphiphilic cross-linked coating for anti-biofouling. Appl. Surf. Sci. 2019, 463, 1097–1106. [Google Scholar] [CrossRef]
- Sun, Q.; Lia, H.; Xian, C.; Yang, Y.; Song, Y.; Cong, P. Mimetic marine antifouling films based on fluorine-containing polymethacrylates. App. Surf. Sci. 2015, 344, 17–26. [Google Scholar] [CrossRef]




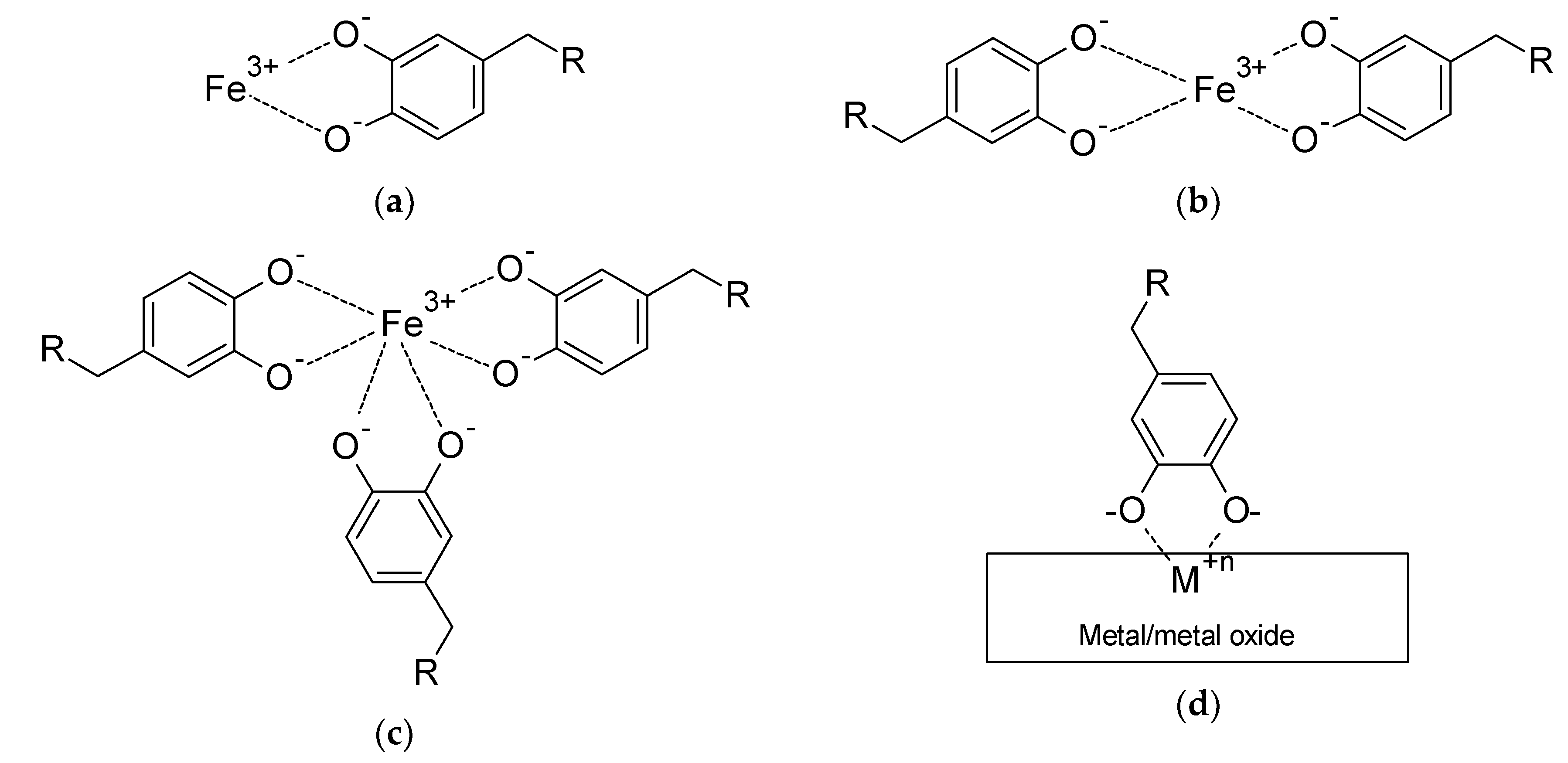
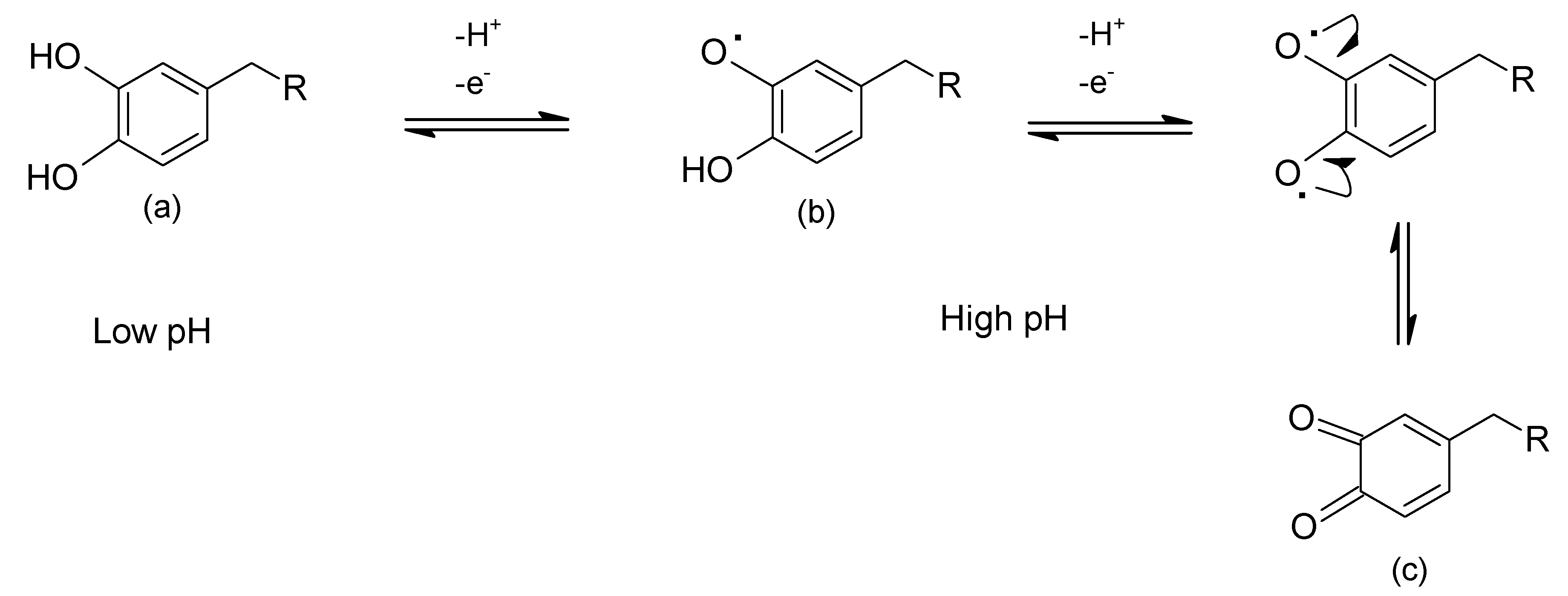





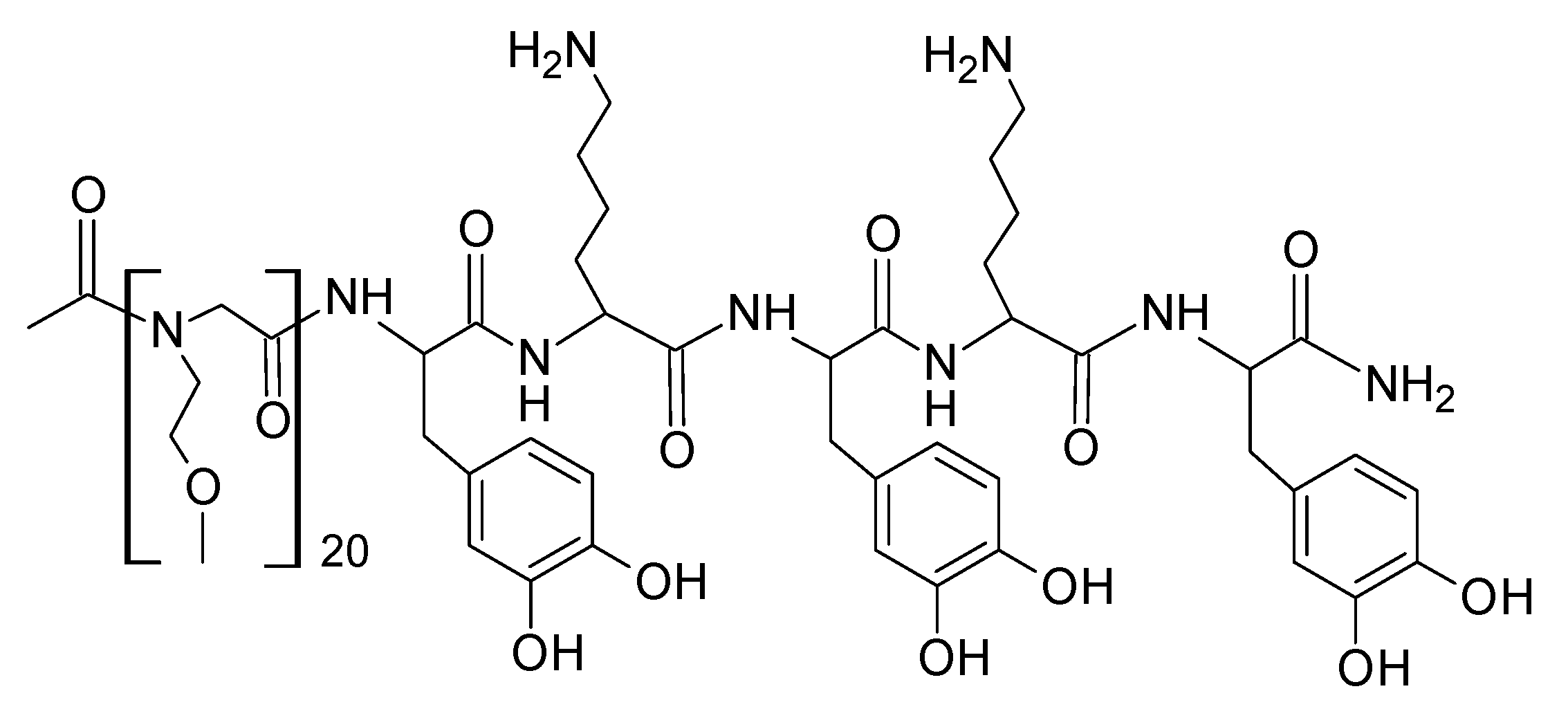


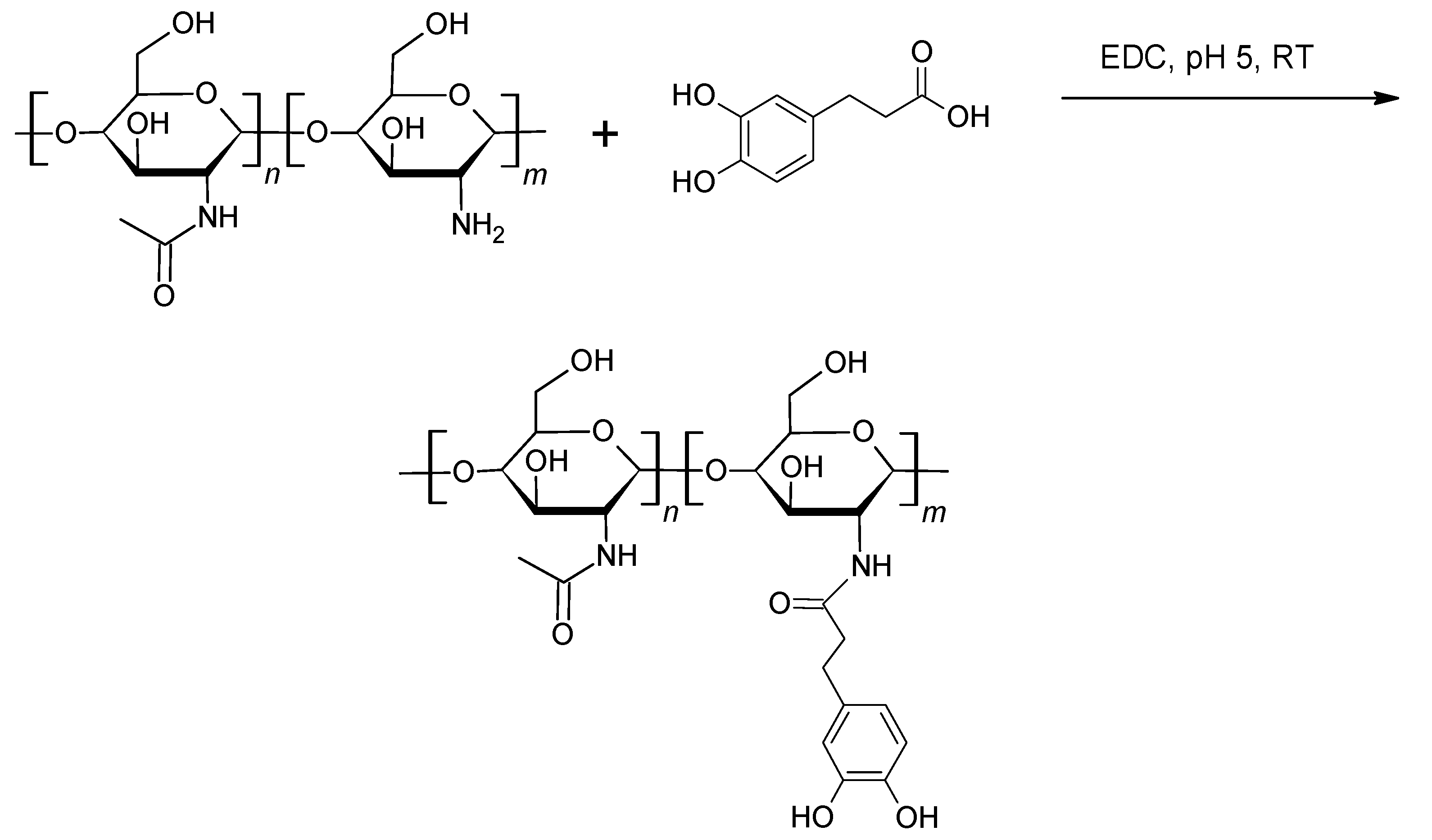





| Protein | Molar Mass (kDa) | l-DOPA Content (mol%) | Location in Byssus | Role |
|---|---|---|---|---|
| MFP-1 | 90–130 1 | 15 | Byssal thread cuticle | Main cuticle component |
| MFP-2 | 45 | 5 | Bulk of plaque | Main building block of bulk plaque |
| MFP-3 | 6 | 20 | Plaque-substrate interface | Plaque adhesive |
| MFP-4 | 90 | 2 | Proximal end of plaque | Linkage to preCols from byssal thread |
| MFP-5 | 9 | 30 | Plaque-substrate interface | Plaque adhesive |
| MFP-6 | 12 | 3–5 | Plaque-substrate interface | Sacrificial antioxidant to maintain MFP-3/MFP-5 adhesion potency |
| Sample | Diatom Adhesion Density (Diatoms per Image) | % Reduction 2 | Ref. |
|---|---|---|---|
| Bare Ti/TiO2 | 165 | - | [136] |
| Poly(ZW-DOPA) coated 3 | 125 | 24 | [136] |
| Poly(ZW-DOPA)/ammonium persulfate | 66 | 60 | [136] |
| Poly(ZW-DOPA)/sodium periodate | 10 | 94 | [136] |
| Bare Ti/TiO2 | 125 | - | [137] |
| Poly(CPA) coated 3 | 100 | 20 | [137] |
| PEG-grafted poly(CPA) | 10 | 92 | [137] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manolakis, I.; Azhar, U. Recent Advances in Mussel-Inspired Synthetic Polymers as Marine Antifouling Coatings. Coatings 2020, 10, 653. https://doi.org/10.3390/coatings10070653
Manolakis I, Azhar U. Recent Advances in Mussel-Inspired Synthetic Polymers as Marine Antifouling Coatings. Coatings. 2020; 10(7):653. https://doi.org/10.3390/coatings10070653
Chicago/Turabian StyleManolakis, Ioannis, and Usaid Azhar. 2020. "Recent Advances in Mussel-Inspired Synthetic Polymers as Marine Antifouling Coatings" Coatings 10, no. 7: 653. https://doi.org/10.3390/coatings10070653





