Al2O3 + Graphene Low-Friction Composite Coatings Prepared By Sol–Gel Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Sols
- (1)
- G1 graphene with average GNP thickness of 12 nm and average lateral size 4500 nm (AO-3, Graphene Laboratories Inc., New York, NY, USA);
- (2)
- G2 graphene with average GNP thickness of 1–5 nm and average lateral size less than 2000 nm (0540DX, SkySpring Nanomaterials, Inc., Houston, TX, USA).
2.2. Deposition of Coatings
2.3. Characterization of Coatings
2.4. Tribological Tests
3. Results and Discussion
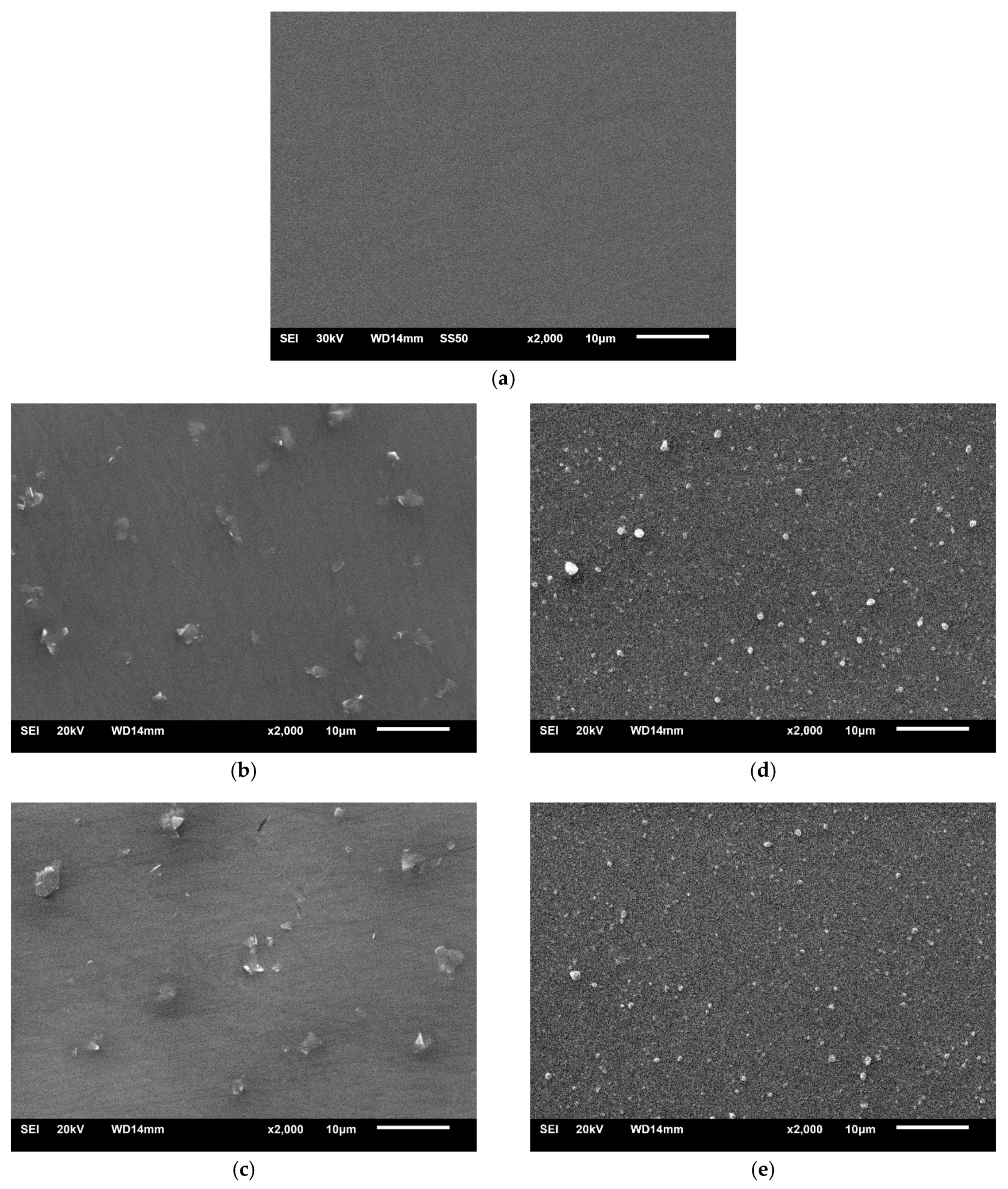
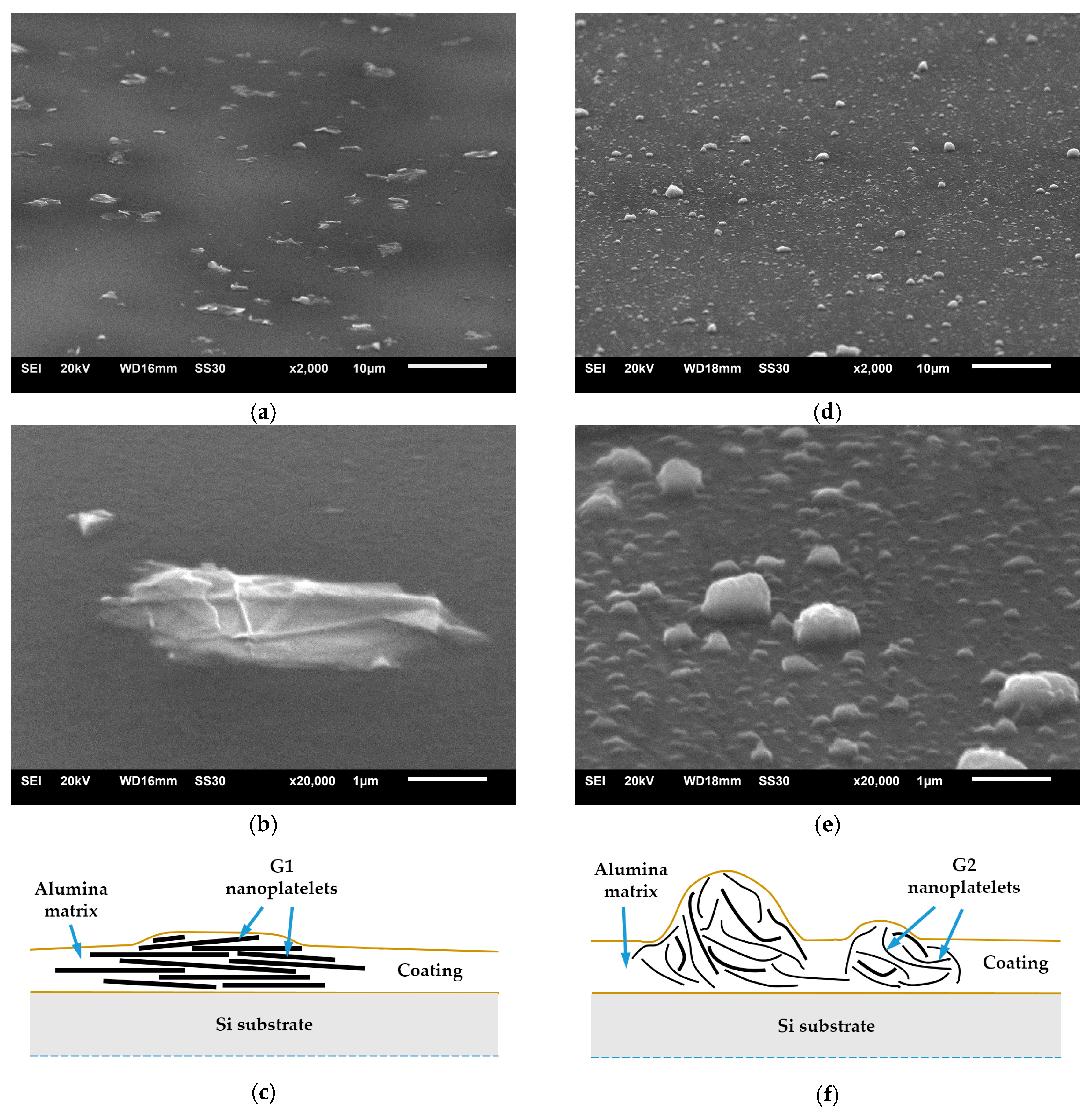
3.1. Morphology and Thickness of Coatings
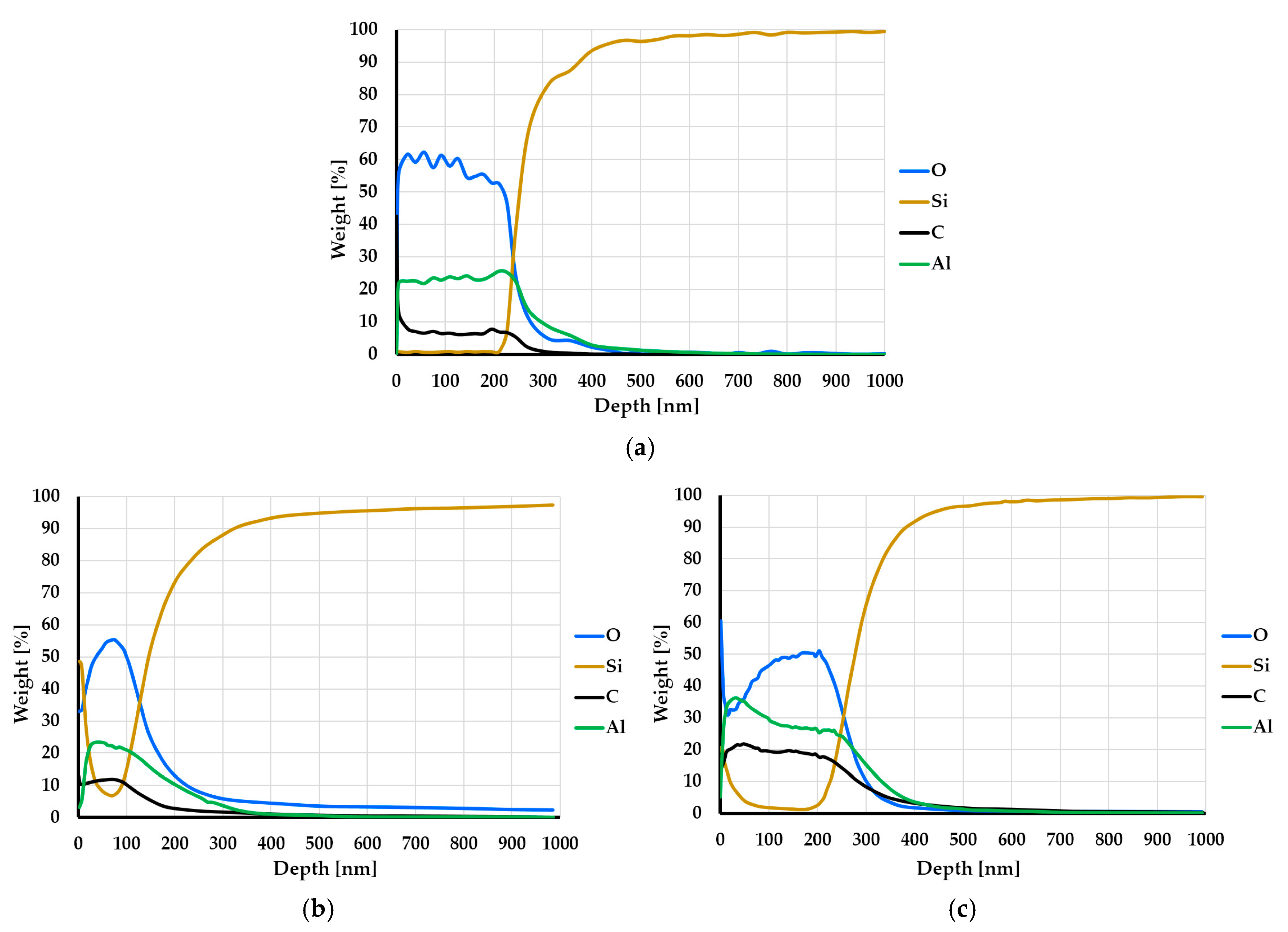
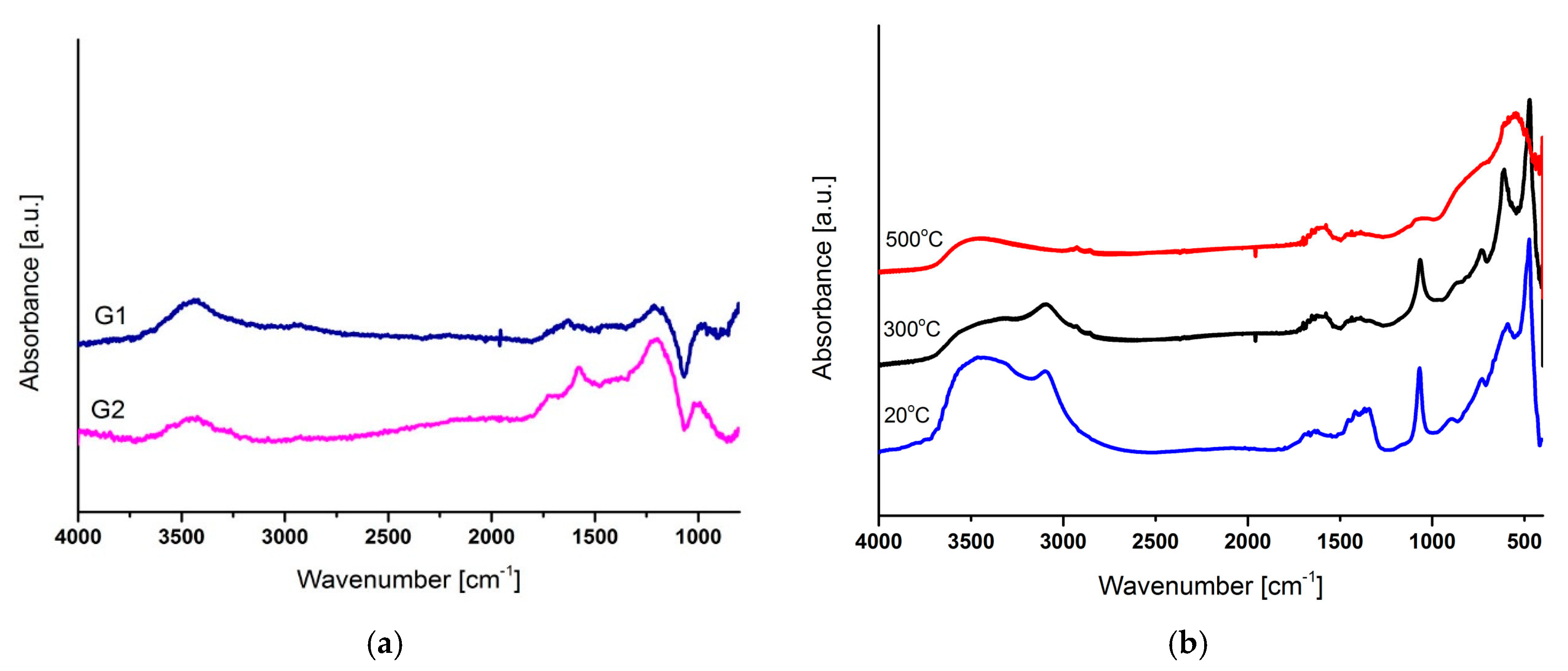
3.2. Chemical Structure
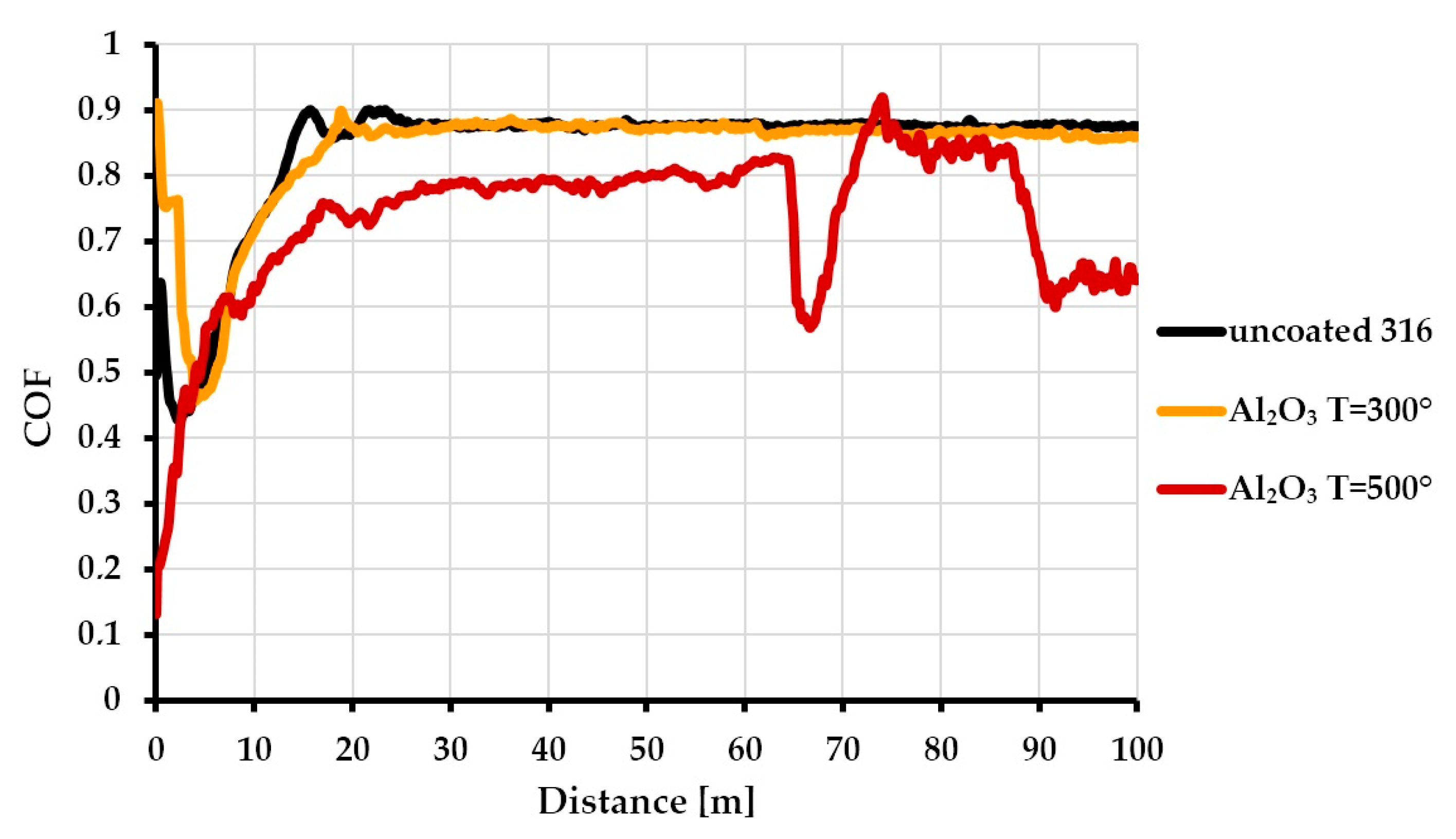
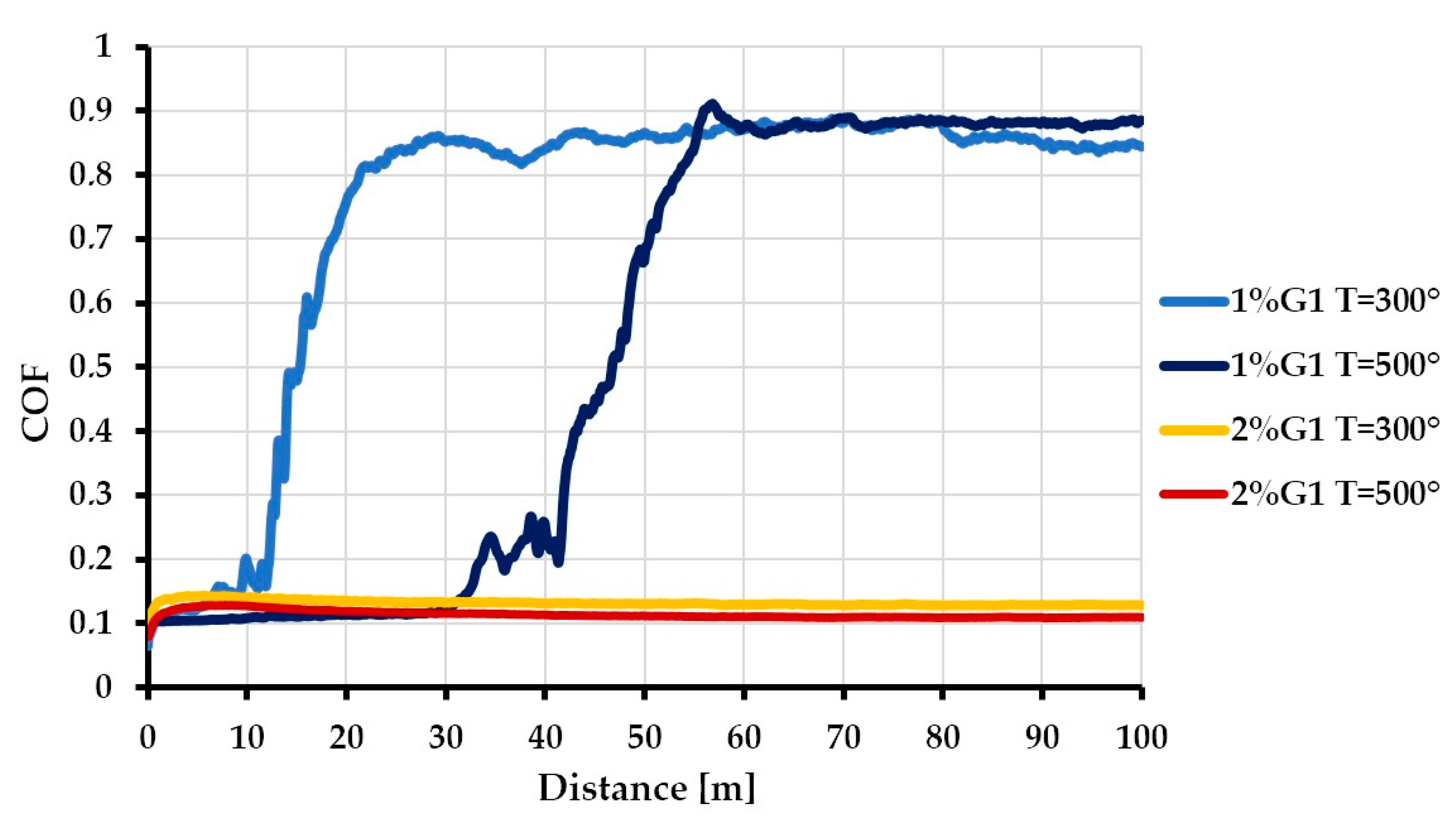
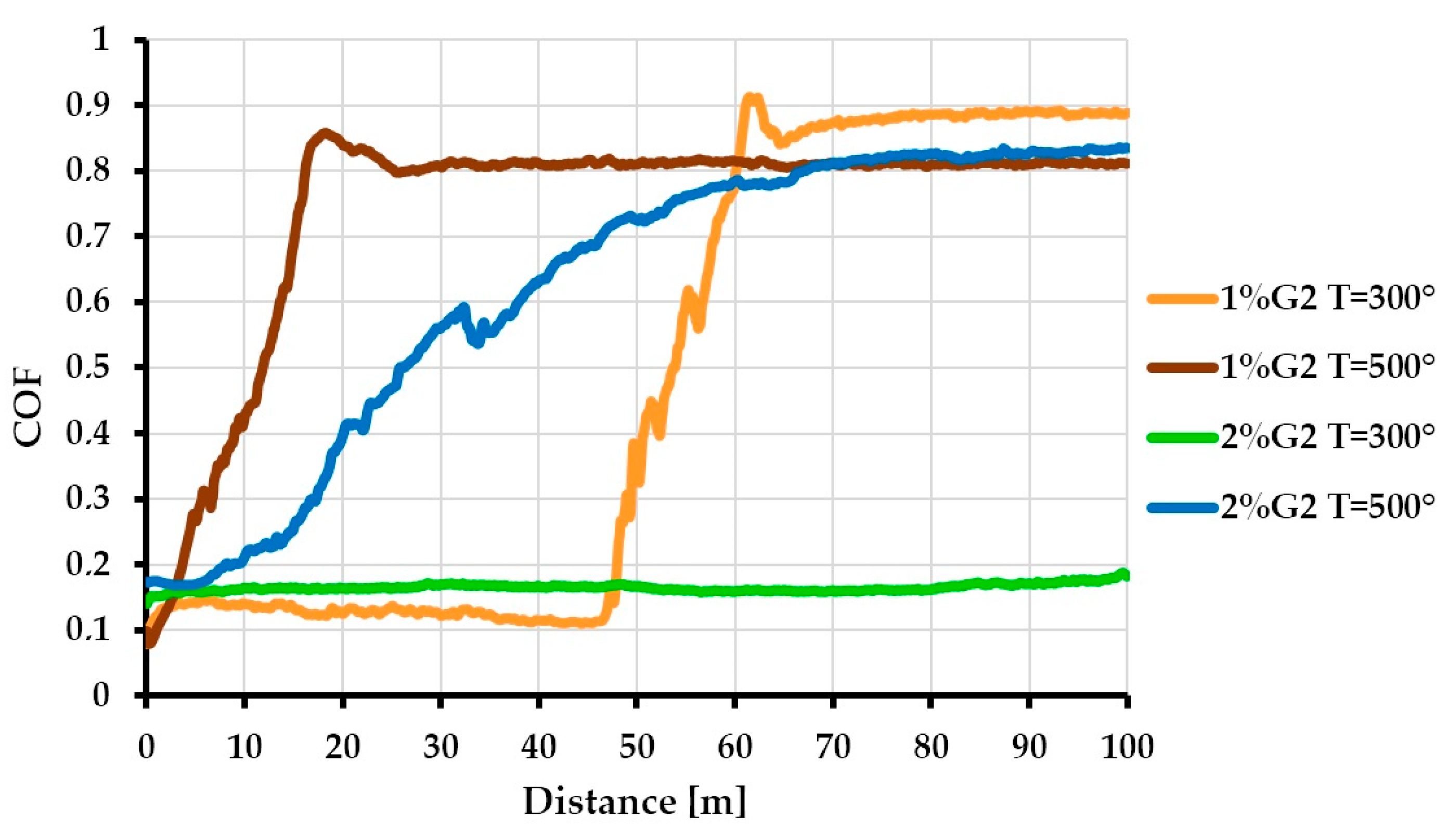
3.3. Tribological Properties
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heimann, R.B. Oxide Ceramics: Structure, Technology, and Applications. In Classic and Advanced Ceramics: From Fundamentals to Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weindheim, Germany, 2010; pp. 175–252. ISBN 978-3-527-63017-2. [Google Scholar]
- Said, S.; Mikhail, S.; Riad, M. Recent progress in preparations and applications of meso-porous alumina. Mater. Sci. Energy Technol. 2019, 2, 288–297. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yoo, S.-J.; Kwak, D.-H.; Jung, H.-J.; Kim, T.-Y.; Park, K.-H.; Lee, J.-W. Characterization and application of electrospun alumina nanofibers. Nanoscale Res. Lett. 2014, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakhova, I.; Mironov, E.; Azarmi, F.; Safonov, A. Thermo-electrical properties of the alumina coatings deposited by different thermal spraying technologies. Ceram. Int. 2017, 43, 15392–15401. [Google Scholar] [CrossRef]
- Ruan, M.; Wang, J.W.; Liu, Q.L.; Ma, F.M.; Yu, Z.L.; Feng, W.; Chen, Y. Superhydrophobic and anti-icing properties of sol–gel prepared alumina coatings. Russ. J. Non-Ferr. Met. 2016, 57, 638–645. [Google Scholar] [CrossRef]
- Luo, R.; Li, P.; Wei, H.; Chen, H.; Yang, K. Structure and electrical insulation characteristics of plasma-sprayed alumina coatings under pressure. Ceram. Int. 2018, 44, 6033–6036. [Google Scholar] [CrossRef]
- Denes, E.; Barrière, G.; Poli, E.; Lévêque, G. Alumina biocompatibility. J. Long-Term Eff. Med Implant. 2018, 28, 9–13. [Google Scholar] [CrossRef]
- Sequeira, S.; Fernandes, M.H.; Neves, N.; Almeida, M.M. Development and characterization of zirconia–alumina composites for orthopedic implants. Ceram. Int. 2017, 43, 693–703. [Google Scholar] [CrossRef]
- Niedźwiedź, M.; Skoneczny, W.; Bara, M. The Influence of Anodic Alumina Coating Nanostructure Produced on EN AW-5251 Alloy on Type of Tribological Wear Process. Coatings 2020, 10, 105. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Qiu, J.; Wei, X.; Sakai, E.; Jiang, G.; Wu, H.; Komiyama, T. Ultra-fast fabrication of porous alumina film with excellent wear and corrosion resistance via hard anodizing in etidronic acid. Surf. Coat. Technol. 2020, 393, 125767. [Google Scholar] [CrossRef]
- Deng, W.; Li, S.; Hou, G.; Liu, X.; Zhao, X.; An, Y.; Zhou, H.; Chen, J. Comparative study on wear behavior of plasma sprayed Al2O3 coatings sliding against different counterparts. Ceram. Int. 2017, 43, 6976–6986. [Google Scholar] [CrossRef]
- Hsain, Z.; Zeng, G.; Strandwitz, N.C.; Krick, B.A. Wear behavior of annealed atomic layer deposited alumina. Wear 2017, 372–373, 139–144. [Google Scholar] [CrossRef]
- Lazar, A.-M.; Yespica, W.P.; Marcelin, S.; Pébère, N.; Samélor, D.; Tendero, C.; Vahlas, C. Corrosion protection of 304L stainless steel by chemical vapor deposited alumina coatings. Corros. Sci. 2014, 81, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Lin, J. High rate reactive sputtering of Al2O3 coatings by HiPIMS. Surf. Coat. Technol. 2019, 357, 402–411. [Google Scholar] [CrossRef]
- Yushkov, Y.G.; Oks, E.M.; Tyunkov, A.V.; Zolotukhin, D.B. Alumina coating deposition by electron-beam evaporation of ceramic using a forevacuum plasma-cathode electron source. Ceram. Int. 2019, 45, 9782–9787. [Google Scholar] [CrossRef]
- Pietrzyk, B.; Miszczak, S.; Szymanowski, H.; Kucharski, D. Plasma enhanced aerosol–gel deposition of Al2O3 coatings. J. Eur. Ceram. Soc. 2013, 33, 2341–2346. [Google Scholar] [CrossRef]
- Tlili, B.; Barkaoui, A.; Walock, M. Tribology and wear resistance of the stainless steel. The sol–gel coating impact on the friction and damage. Tribol. Int. 2016, 102, 348–354. [Google Scholar] [CrossRef]
- Yu, Y.; Zuo, Y.; Zhang, Z.; Wu, L.; Ning, C.; Zuo, C. Al2O3 Coatings on Zinc for Anti-Corrosion in Alkaline Solution by Electrospinning. Coatings 2019, 9, 692. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, M.; Li, W.; Wang, Q.; Wang, Y.; You, C. Preparation, characteristics and corrosion properties of α-Al2O3 coatings on 10B21 carbon steel by micro-arc oxidation. Surf. Coat. Technol. 2019, 358, 637–645. [Google Scholar] [CrossRef]
- Lu, D.; Huang, Y.; Jiang, Q.; Zheng, M.; Duan, J.; Hou, B. An approach to fabricating protective coatings on a magnesium alloy utilising alumina. Surf. Coat. Technol. 2019, 367, 336–340. [Google Scholar] [CrossRef]
- Nofz, M.; Dörfel, I.; Sojref, R.; Wollschläger, N.; Mosquera-Feijoo, M.; Schulz, W.; Kranzmann, A. Thin Sol–Gel Alumina Coating as Protection of a 9% Cr Steel Against Flue Gas Corrosion at 650 °C. Oxid. Met. 2018, 89, 453–470. [Google Scholar] [CrossRef]
- Balcaen, Y.; Radutoiu, N.; Alexis, J.; Beguin, J.-D.; Lacroix, L.; Samélor, D.; Vahlas, C. Mechanical and barrier properties of MOCVD processed alumina coatings on TI6Al4V titanium alloy. Surf. Coat. Technol. 2011, 206, 1684–1690. [Google Scholar] [CrossRef] [Green Version]
- Hübert, T.; Schwarz, J.; Oertel, B. Sol-gel alumina coatings on stainless steel for wear protection. J. Sol-Gel Sci. Technol. 2006, 38, 179–184. [Google Scholar] [CrossRef]
- Lampke, T.; Meyer, D.; Alisch, G.; Wielage, B.; Pokhmurska, H.; Klapkiv, M.; Student, M. Corrosion and wear behavior of alumina coatings obtained by various methods. Mater. Sci. 2011, 46, 591–598. [Google Scholar] [CrossRef]
- Lorenzo-Martin, C.; Ajayi, O.O.; Hartman, K.; Bhattacharya, S.; Yacout, A. Effect of Al2O3 coating on fretting wear performance of Zr alloy. Wear 2019, 426–427, 219–227. [Google Scholar] [CrossRef]
- Singh, V.P.; Sil, A.; Jayaganthan, R. A study on sliding and erosive wear behaviour of atmospheric plasma sprayed conventional and nanostructured alumina coatings. Mater. Des. 2011, 32, 584–591. [Google Scholar] [CrossRef]
- Marsal, A.; Ansart, F.; Turq, V.; Bonino, J.P.; Sobrino, J.M.; Chen, Y.M.; Garcia, J. Mechanical properties and tribological behavior of a silica or/and alumina coating prepared by sol-gel route on stainless steel. Surf. Coat. Technol. 2013, 237, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Sarafoglou, C.I.; Pantelis, D.I.; Beauvais, S.; Jeandin, M. Study of Al2O3 coatings on AISI 316 stainless steel obtained by controlled atmosphere plasma spraying (CAPS). Surf. Coat. Technol. 2007, 202, 155–161. [Google Scholar] [CrossRef]
- Riedl, A.; Schalk, N.; Czettl, C.; Sartory, B.; Mitterer, C. Tribological properties of Al2O3 hard coatings modified by mechanical blasting and polishing post-treatment. Wear 2012, 289, 9–16. [Google Scholar] [CrossRef]
- Hübert, T.; Svoboda, S.; Oertel, B. Wear resistant alumina coatings produced by a sol–gel process. Surf. Coat. Technol. 2006, 201, 487–491. [Google Scholar] [CrossRef]
- Piwoński, I.; Soliwoda, K. The effect of ceramic nanoparticles on tribological properties of alumina sol–gel thin coatings. Ceram. Int. 2010, 36, 47–54. [Google Scholar] [CrossRef]
- Peng, Z.J.; Cheng, T.; Nie, X.Y. MoS2/Al2O3 Composite Coatings on A356 Alloy for Friction Reduction. Adv. Mater. Res. 2012, 496, 488–492. [Google Scholar] [CrossRef]
- Kim, S. Effects of Solid Lubricants on Tribological Behaviour of APSed Al2O3-ZrO2 Composite Coatings. Taehan-Kŭmsok-Hakhoe-chi J. Korean Inst. Met. Mater. 2014, 52, 263–270. [Google Scholar] [CrossRef]
- Pietrzyk, B.; Miszczak, S.; Kaczmarek, Ł.; Klich, M. Low friction nanocomposite aluminum oxide/MoS2 coatings prepared by sol-gel method. Ceram. Int. 2018, 44, 8534–8539. [Google Scholar] [CrossRef]
- Jambagi, S.C.; Bandyopadhyay, P.P. Improvement in Tribological Properties of Plasma-Sprayed Alumina Coating upon Carbon Nanotube Reinforcement. J. Mater. Eng. Perform. 2019, 28, 7347–7358. [Google Scholar] [CrossRef]
- Hentour, K.; Marsal, A.; Turq, V.; Weibel, A.; Ansart, F.; Sobrino, J.-M.; Chen, Y.M.; Garcia, J.; Cardey, P.-F.; Laurent, C. Carbon nanotube/alumina and graphite/alumina composite coatings on stainless steel for tribological applications. Mater. Today Commun. 2016, 8, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Keshri, A.K.; Huang, J.; Singh, V.; Choi, W.; Seal, S.; Agarwal, A. Synthesis of aluminum oxide coating with carbon nanotube reinforcement produced by chemical vapor deposition for improved fracture and wear resistance. Carbon 2010, 48, 431–442. [Google Scholar] [CrossRef]
- Hentour, K.; Turq, V.; Weibel, A.; Ansart, F.; Sobrino, J.-M.; Garcia, J.; Cardey, P.-F.; Laurent, C. Dispersion of graphite flakes into boehmite sols for the preparation of bi-layer-graphene/alumina coatings on stainless steel for tribological applications. J. Eur. Ceram. Soc. 2019, 39, 1304–1315. [Google Scholar] [CrossRef] [Green Version]
- Marcinauskas, L.; Mathew, J.S.; Milieška, M.; Aikas, M.; Kalin, M. Effect of graphite concentration on the tribological performance of alumina coatings. J. Alloy. Compd. 2020, 827, 154135. [Google Scholar] [CrossRef]
- Murray, J.W.; Rance, G.A.; Xu, F.; Hussain, T. Alumina-graphene nanocomposite coatings fabricated by suspension high velocity oxy-fuel thermal spraying for ultra-low-wear. J. Eur. Ceram. Soc. 2018, 38, 1819–1828. [Google Scholar] [CrossRef]
- Yin, H.; Dai, Q.; Hao, X.; Huang, W.; Wang, X. Preparation and tribological properties of graphene oxide doped alumina composite coatings. Surf. Coat. Technol. 2018, 352, 411–419. [Google Scholar] [CrossRef]
- Da, B.; Rongli, X.; Yongxin, G.; Yaxuan, L.; Aradhyula, T.V.; Yongwu, Z.; Yongguang, W. Tribological behavior of graphene reinforced chemically bonded ceramic coatings. Ceram. Int. 2020, 46, 4526–4531. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Yan, Y.; Gong, J.; Chen, J.; Zeng, Z.; Huang, W.; Pu, K.; Liu, J.; Chen, P. Recent Advances on Graphene Quantum Dots: From Chemistry and Physics to Applications. Adv. Mater. 2019, 31, 1808283. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.T. Graphene-based nano composites and their applications. A review. Biosens. Bioelectron. 2019, 141, 111384. [Google Scholar] [CrossRef] [PubMed]
- Nag, A.; Mitra, A.; Mukhopadhyay, S.C. Graphene and its sensor-based applications: A review. Sens. Actuators A Phys. 2018, 270, 177–194. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part. B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Penkov, O.; Kim, H.-J.; Kim, H.-J.; Kim, D.-E. Tribology of graphene: A review. Int. J. Precis. Eng. Manuf. 2014, 15, 577–585. [Google Scholar] [CrossRef]
- Kasar, A.K.; Menezes, P.L. Synthesis and recent advances in tribological applications of graphene. Int. J. Adv. Manuf. Technol. 2018, 97, 3999–4019. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol.-Gel Science: The Physics and Chemistry of Sol.-Gel Processing; Academic Press Inc.: San Diego, CA, USA, 1990; ISBN 978-0-12-134970-7. [Google Scholar]
- Levy, D.; Zayat, M. The Sol.-Gel Handbook: Synthesis, Characterization and Applications, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weindheim, Germany, 2015; ISBN 978-3-527-67084-0. [Google Scholar]
- Brinker, C.J.; Ashley, C.S.; Cairncross, R.A.; Chen, K.S.; Hurd, A.J.; Reed, S.T.; Samuel, J.; Schunk, P.R.; Schwartz, R.W.; Scotto, C.S. Sol—gel derived ceramic films—fundamentals and applications. In Metallurgical and Ceramic Protective Coatings; Stern, K.H., Ed.; Springer: Dordrecht, The Netherlands, 1996; pp. 112–151. ISBN 978-94-009-1501-5. [Google Scholar]
- Kobayashi, Y.; Ishizaka, T.; Kurokawa, Y. Preparation of alumina films by the sol-gel method. J. Mater. Sci. 2005, 40, 263–283. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.; Bahuguna, G. Thin Film Coating through Sol-Gel Technique. Res. J. Chem. Sci. 2016, 6, 65. [Google Scholar]
- Schneller, T.; Waser, R.; Kosec, M.; Payne, D. Chemical Solution Deposition of Functional Oxide Thin Films; Springer: Wien, Austria, 2013; p. 796. ISBN 978-3-211-99311-8. [Google Scholar]
- Manoratne, C.H.; Rosa, S.R.D.; Kottegoda, I.R.M. RD-HTA, UV Visible, FTIR and SEM Interpretation of Reduced Graphene Oxide Synthesized from High Purity Vein Graphite. Mater. Sci. Res. India 2017, 14, 19–30. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Warga, T.; Makowicz, M.; Kyzioł, K.; Bucholc, B.; Majchrzycki, Ł. The Influence of the Size and Oxidation Degree of Graphene Flakes on the Process of Creating 3D Structures during Its Cross-Linking. Materials 2020, 13, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, C.; Zhao, X.; Zhang, Y. Sol–gel fabrication of compact, crack-free alumina film. Mater. Res. Bull. 2007, 42, 600–608. [Google Scholar] [CrossRef]
- Cheng, Z.; Andy, N.; Arvind, A. Ultrathin graphene tribofilm formation during wear of Al2O3–graphene composites. Nanomater. Energy 2016, 5, 1–9. [Google Scholar]
- Kumar, P.; Wani, M. Synthesis and tribological properties of graphene: A review. J. Tribol. 2017, 13, 36–71. [Google Scholar]
- Berman, D.; Erdemir, A.; Sumant, A.V. Few layer graphene to reduce wear and friction on sliding steel surfaces. Carbon 2013, 54, 454–459. [Google Scholar] [CrossRef]
- Pu, J.; Wan, S.; Zhao, W.; Mo, Y.; Zhang, X.; Wang, L.; Xue, Q. Preparation and Tribological Study of Functionalized Graphene–IL Nanocomposite Ultrathin Lubrication Films on Si Substrates. J. Phys. Chem. C 2011, 115, 13275–13284. [Google Scholar] [CrossRef]
- Liang, H.; Bu, Y.; Zhang, J. Graphene Oxide Film as Solid Lubricant. ACS Appl. Mater. Interfaces 2013, 5, 6369–6375. [Google Scholar] [CrossRef]
- Feng, X.; Kwon, S.; Park, J.Y.; Salmeron, M. Superlubric Sliding of Graphene Nanoflakes on Graphene. ACS Nano 2013, 7, 1718–1724. [Google Scholar] [CrossRef]
- Liu, Y.; Grey, F.; Zheng, Q. The high-speed sliding friction of graphene and novel routes to persistent superlubricity. Sci. Rep. 2014, 4, 4875. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, J.; Luo, J. Superlubricity of Graphite Sliding against Graphene Nanoflake under Ultrahigh Contact Pressure. Adv. Sci. 2018, 5, 1800810. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, B.; Li, X.; Ye, Y.; Ma, J.; Liu, C.; Zhou, X.; Xie, X. Enhancing thermal oxidation and fire resistance of reduced graphene oxide by phosphorus and nitrogen co-doping, Mechanism and kinetic analysis. Carbon 2019, 146, 650–659. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Q.; Jing, P.; Zhai, W. High-Temperature Tribological Performance of TiAl Matrix Composites Reinforced by Multilayer Graphene. Tribol. Lett. 2015, 58, 3. [Google Scholar] [CrossRef]

| Type of Coating | Temperature of Heat Treatment (°C) | Concentration of Graphene in Suspension (%) | Thickness (nm) | n (Refractive Index) |
|---|---|---|---|---|
| Al2O3 + G1 | 300 | 1 | 134 ± 2 | 1.49 ± 0.005 |
| 2 | 130 ± 1 | 1.50 ± 0.01 | ||
| 500 | 1 | 125 ± 3 | 1.46 ± 0.03 | |
| 2 | 123 ± 2 | 1.43 ± 0.01 | ||
| Al2O3 + G2 | 300 | 1 | 184 ± 1 | 1.55 ± 0.01 |
| 2 | 171 ± 1 | 1.56 ± 0.01 | ||
| 500 | 1 | 152 ± 1 | 1.50 ± 0.01 | |
| 2 | 147 ± 1 | 1.55 ± 0.005 | ||
| Al2O3 | 300 | - | 163 ± 8 | 1.56 ± 0.06 |
| 500 | - | 152 ± 1 | 1.58 ± 0.005 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrzyk, B.; Miszczak, S.; Sun, Y.; Szymański, M. Al2O3 + Graphene Low-Friction Composite Coatings Prepared By Sol–Gel Method. Coatings 2020, 10, 858. https://doi.org/10.3390/coatings10090858
Pietrzyk B, Miszczak S, Sun Y, Szymański M. Al2O3 + Graphene Low-Friction Composite Coatings Prepared By Sol–Gel Method. Coatings. 2020; 10(9):858. https://doi.org/10.3390/coatings10090858
Chicago/Turabian StylePietrzyk, Bożena, Sebastian Miszczak, Ye Sun, and Marcin Szymański. 2020. "Al2O3 + Graphene Low-Friction Composite Coatings Prepared By Sol–Gel Method" Coatings 10, no. 9: 858. https://doi.org/10.3390/coatings10090858





