Artificial Superhydrophobic and Antifungal Surface on Goose Down by Cold Plasma Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thin Layer Deposition
2.3. Hydrophobicity Measurements
2.4. Molecular Structure Analysis
2.5. Surface Morphology Studies
2.6. Microbiological Testing
3. Results and Discussion
3.1. Superhydrophobicity
3.2. Surface Morphology
3.3. Molecular Structure
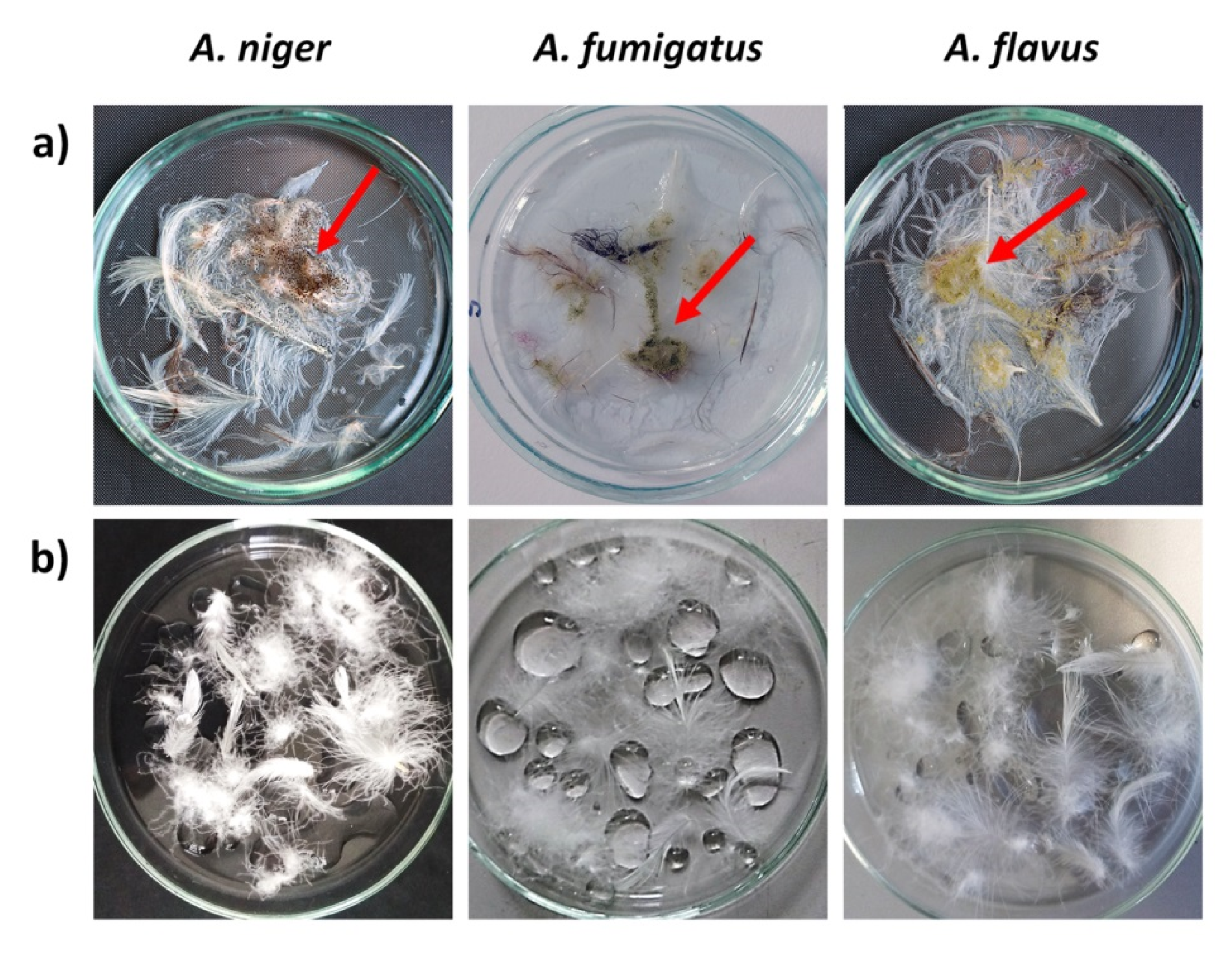
3.4. Antifungal Properties
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wrobel, A.M.; Wertheimer, M.R. Plasma-polymerized organosilicones and organometallics. In Plasma Deposition, Treatment, and Etching of Polymers; d’Agostino, R., Ed.; Academic Press: New York, NY, USA, 1990; pp. 163–268. [Google Scholar]
- Biederman, H. (Ed.) Plasma Polymer Films; Imperial College Press: London, UK, 2004. [Google Scholar]
- Liu, K.; Tian, Y.; Jiang, L. Bio-inspired superoleophobic and smart materials: Design, fabrication, and application. Prog. Mater. Sci. 2013, 58, 503–564. [Google Scholar] [CrossRef]
- Zajickova, L.; Bursikova, V.; Kucerova, Z.; Franta, D.; Dvorak, P.; Smid, R.; Perina, V.; Mackova, A. Deposition of protective coatings in rf organosilicon discharges. Plasma Sources Sci. Technol. 2007, 16, S123–S132. [Google Scholar] [CrossRef]
- Fracassi, F.; d’Agostino, R.; Palumbo, F.; Angelini, E.; Grassini, S.; Rosalbino, F. Application of plasma deposited organosilicon thin films for the corrosion protection of metals. Surf. Coat. Technol. 2003, 174–175, 107–111. [Google Scholar] [CrossRef]
- Behnisch, J.; Tyczkowski, J.; Pela, I.; Hollander, A.; Ledzion, R. Formation of hydrophobic layers on biologically degradable polymeric foils by plasma polymerization. Surf. Coat. Technol. 1998, 98, 872–874. [Google Scholar] [CrossRef]
- Martinu, L.; Poitras, D. Plasma deposition of optical films and coatings: A review. J. Vac. Sci. Technol. A 2000, 18, 2619–2645. [Google Scholar] [CrossRef]
- Favia, P.; d’Agostino, R. Plasma treatments and plasma deposition of polymers for biomedical applications. Surf. Coat. Technol. 1998, 98, 1102–1106. [Google Scholar] [CrossRef]
- Hirotsu, T.; Tagaki, C.; Partridge, A. Plasma copolymerization of acrylic acid with hexamethyldisilazane. Plasmas Polym. 2002, 7, 353–366. [Google Scholar] [CrossRef]
- Azioune, A.; Marcozzi, M.; Revello, V.; Pireaux, J.-J. Deposition of polysiloxane-like nanofilms onto an aluminium alloy by plasma polymerized hexamethyldisiloxane: Characterization by XPS and contact angle measurements. Surf. Interface Anal. 2007, 39, 615–623. [Google Scholar] [CrossRef]
- Twardowski, A.; Makowski, P.; Małachowski, A.; Hrynyk, R.; Pietrowski, P.; Tyczkowski, J. Plasma treatment of thermoactive membrane textiles for superhydrophobicity. Mater. Sci. Medzg. 2012, 18, 163–166. [Google Scholar] [CrossRef] [Green Version]
- Mahlberg, R.; Niemi, H.E.-M.; Denes, F.; Rowell, R.M. Effect of oxygen and hexamethyldisiloxane plasma on morphology, wettability and adhesion properties of polypropylene and lignocellulosic. Int. J. Adhes. Adhes. 1998, 18, 283–297. [Google Scholar] [CrossRef]
- Mobarakeh, L.F.; Jafari, R.; Farzaneh, M. The ice repellency of plasma polymerized hexamethyldisiloxane coating. Appl. Surf. Sci. 2013, 459–463. [Google Scholar] [CrossRef]
- Asadollahi, S.; Profili, J.; Farzaneh, M.; Stafford, L. Development of organosilicon-based superhydrophobic coatings through atmospheric pressure plasma polymerization of HMDSO in nitrogen plasma. Materials 2019, 12, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlos, C.M.; Harke-Bus, R.P.; Ward, K.; Owens, D.E.; Harwood, R.; O’Hare, T.; Ferguson, D.C. Method for Producing Improved Feathers and Improved Feather Thereto. Patent WO 2011/143488, 17 November 2011. [Google Scholar]
- Tyczkowski, J.; Kapica, R.; Markiewicz, J.; Malachowski, A.; Malachowski, B. Method for Producing Durable Water-Repellent Layer on the Surface of Natural Down. Patent PL 228924, 19 December 2017. [Google Scholar]
- Gao, J.; Yu, W.; Pan, N. Structures and properties of the goose down as a material for thermal insulation. Text. Res. J. 2007, 77, 617–626. [Google Scholar] [CrossRef]
- Bonser, R.H.C.; Dawson, C. The structural mechanical properties of down feathers and biomimicking natural insulation materials. J. Mater. Sci. Lett. 1999, 18, 1769–1770. [Google Scholar] [CrossRef]
- Yildiz, D.; Bozkur, E.U.; Akturks, S.H. Determination of goose feather morphology by using SEM. J. Anim. Vet. Adv. 2009, 8, 2650–2654. [Google Scholar] [CrossRef]
- Stettenheim, P.R. The integumentary morphology of modern birds: An overview. Amer. Zool. 2000, 40, 461–477. [Google Scholar] [CrossRef] [Green Version]
- Webb, D.R.; King, J.R. Effects of wetting on insulation of bird and mammal coats. J. Therm. Biol. 1984, 9, 189–191. [Google Scholar] [CrossRef]
- Bakken, G.S. Wind speed dependence of the overall thermal conductance of fur and feather insulation. J. Therm. Biol. 1991, 16, 121–126. [Google Scholar] [CrossRef]
- Woodcock, A.A.; Steel, N.; Moore, C.B.; Howard, S.J.; Custovic, A.; Denning, D.W. Fungal contamination of bedding. Allergy 2006, 61, 140–142. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Xin, J.H. Hydrophobic duck feathers and their simulation on textile substrates for water repellent treatment. Bioinsp. Biomim. 2008, 3, 046007. [Google Scholar] [CrossRef] [Green Version]
- Benkovicova, M.; Kisova, Z.; Buckova, M.; Majkova, E.; Siffalovic, P.; Pangallo, D. The antifungal properties of super-hydrophobic nanoparticles and essential oils on different material surfaces. Coatings 2019, 9, 176. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.; Seo, J.; Han, H.; Kang, S.; Kim, H.; Lee, T. Bio-inspired extreme wetting surfaces for biomedical applications. Materials 2016, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Hwang, W. Wettability modified aluminum surface for a potential antifungal surface. Mater. Lett. 2015, 161, 234–239. [Google Scholar] [CrossRef]
- Fan, H.; Guo, Z. Bioinspired surfaces with wettability: Biomolecule adhesion behaviors. Biomater. Sci. 2020, 8, 1502–1535. [Google Scholar] [CrossRef] [PubMed]
- Hydrophobic Shake Test in IDFB Testing Regulations 2020, Version June 2015, Part 18-A. Available online: http://www.cfd.com.cn/upload/contents/2020/08/Testing_Regulations_v202006.pdf (accessed on 10 September 2020).
- Wang, S.; Jiang, L. Definition of superhydrophobic states. Adv. Mater. 2007, 19, 3423–3424. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, F.; Niu, J.; Jiang, Y.; Wang, Z. Superhydrophobic surfaces: From structural control to functional application. J. Mater. Chem. 2008, 18, 621–633. [Google Scholar] [CrossRef]
- Dorrer, C.; Rühe, J. Some thoughts on superhydrophobic wetting. Soft Matter 2009, 5, 51–61. [Google Scholar] [CrossRef]
- Kurosawa, S.; Choi, B.G.; Park, J.W.; Aizawa, H.; Shim, K.B.; Yamamoto, K. Synthesis and characterization of plasma-polymerized hexamethyldisiloxane films. Thin Solid Films 2006, 506–507, 176–179. [Google Scholar] [CrossRef]
- Grimoldi, E.; Zanini, S.; Siliprandi, R.A.; Riccardi, C. AFM and contact angle investigation of growth and structure of pp-HMDSO thin films. Eur. Phys. J. D 2009, 54, 165–172. [Google Scholar] [CrossRef]
- Kraus, F.; Cruz, S.; Muller, J. Plasmapolymerized silicon organic thin films from HMDSN for capacitive humidity sensors. Sens. Actuators B Chem. 2003, 88, 300–311. [Google Scholar] [CrossRef]
- de Carvalho, A.T.; Carvalho, R.A.M.; da Silva, M.L.P.; Demarquette, N.R. Hydrophobic plasma polymerized hexamethyldisilazane thin films: Characterization and uses. Mater. Res. 2006, 9, 9–13. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Sui, T.; Li, A.; Chen, D. Investigation on hydrophobicity of lotus leaf: Experiment and theory. Plant. Sci. 2009, 176, 687–695. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Jung, Y.C.; Barthlott, W. Fabrication of artificial Lotus leaves and significance of hierarchical structure for superhydrophobicity and low adhesion. Soft Matter 2009, 5, 1386–1393. [Google Scholar] [CrossRef]
- Gengenbach, T.R.; Griesser, H.J. Post-deposition ageing reactions differ markedly between plasma polymers deposited from siloxane and silazane monomers. Polymer 1999, 40, 5079–5094. [Google Scholar] [CrossRef]
- Benitez, F.; Martinez, E.; Esteve, J. Improvement of hardness in plasma polymerized hexamethyldisiloxane coatings by silica-like surface modification. Thin Solid Films 2000, 377–378, 109–114. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Yoshida, Y.; Murayama, Y. Hybrid films formed from hexamethyldisiloxane and SiO by plasma process. Jpn. J. Appl. Phys. 1991, 30, 1803–1807. [Google Scholar] [CrossRef]
- Choudhury, A.J.; Chutia, J.; Kakati, H.; Barve, S.A.; Pal, A.R.; Sarma, N.S.; Chowdhury, D.; Patil, D.S. Studies of radiofrequency plasma deposition of hexamethyldisiloxane films and their thermal stability and corrosion resistance behavior. Vacuum 2010, 84, 1327–1333. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, N.; Kim, U.Y.; Hong, S.I.; Sasabe, H. Plasma polymerization of hexamethyldisilazane. Polym. J. 1990, 22, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Vassallo, E.; Cremona, A.; Ghezzi, F.; Dellera, F.; Laguardia, L.; Ambrosone, G.; Coscia, U. Structural and optical properties of amorphous hydrogenated silicon carbonitride films produced by PECVD. Appl. Surf. Sci. 2006, 252, 7993–8000. [Google Scholar] [CrossRef]
- Kim, M.T.; Lee, J. Characterization of amorphous SiC:H films deposited from hexamethyldisilazane. Thin Solid Films 1997, 303, 173–179. [Google Scholar] [CrossRef]
- Fracassi, F.; Lamendola, R. PECVD of SiOXNYCZHW thin films from hexamethyldisilazane containing feed. Investigation on chemical characteristics and aging behavior. Plasmas Polym. 1997, 2, 25–40. [Google Scholar] [CrossRef]
- Li, K.; Gabriel, O.; Meichsner, J. Fourier transform infrared spectroscopy study of molecular structure formation in thin films during hexamethyldisiloxane decomposition in low pressure rf discharge. J. Phys. D Appl. Phys. 2004, 37, 588–594. [Google Scholar] [CrossRef] [Green Version]
- Nouicer, I.; Sahli, S.; Kihel, M.; Ziari, Z. Superhydrophobic surface produced on polyimide and silicon by plasma enhanced chemical vapour deposition from hexamethyldisiloxane precursor. Int. J. Nanotechnol. 2015, 12, 597–607. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Chan, K.L.A. ATR-FTIR spectroscopic imaging: Recent advances and applications to biological systems. Analyst 2013, 138, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.R.; Short, R.D.; Jones, F.R.; Michaeli, W.; Blomfield, C.J. A study of HMDSO/O2 plasma deposits using a high-sensitivity and -energy resolution XPS instrument: Curve fitting of the Si 2p core level. Appl. Surf. Sci. 1999, 137, 179–183. [Google Scholar] [CrossRef]
- Chaiwong, C.; Rachtanapun, P.; Sarapirom, S.; Boonyawan, D. Plasma polymerization of hexamethyldisiloxane: Investigation of the effect of carrier gas related to the film properties. Surf. Coat. Technol. 2013, 229, 12–17. [Google Scholar] [CrossRef]
- Grüniger, A.; Bieder, A.; Sonnenfeld, A.; Von Rohr, P.R.; Muller, U.; Hauert, R. Influence of film structure and composition on diffusion barrier performance of SiOX thin films deposited by PECVD. Surf. Coat. Technol. 2006, 200, 4564–4571. [Google Scholar] [CrossRef]
- Uznanski, P.; Glebocki, B.; Walkiewicz-Pietrzykowska, A.; Zakrzewska, J.; Wrobel, A.M.; Balcerzak, J.; Tyczkowski, J. Surface modification of silicon ocycarbide films produced be remote hydrogen microwave plasma chemical vapour deposition from tetramethyldisiloxane precursor. Surf. Coat. Technol. 2019, 350, 686–698. [Google Scholar] [CrossRef]
- Sahoo, B.; Yoon, K.; Seo, J.; Lee, T. Chemical and physical pathways for fabricating flexible superamphiphobic surfaces with high transparency. Coatings 2018, 8, 47. [Google Scholar] [CrossRef] [Green Version]






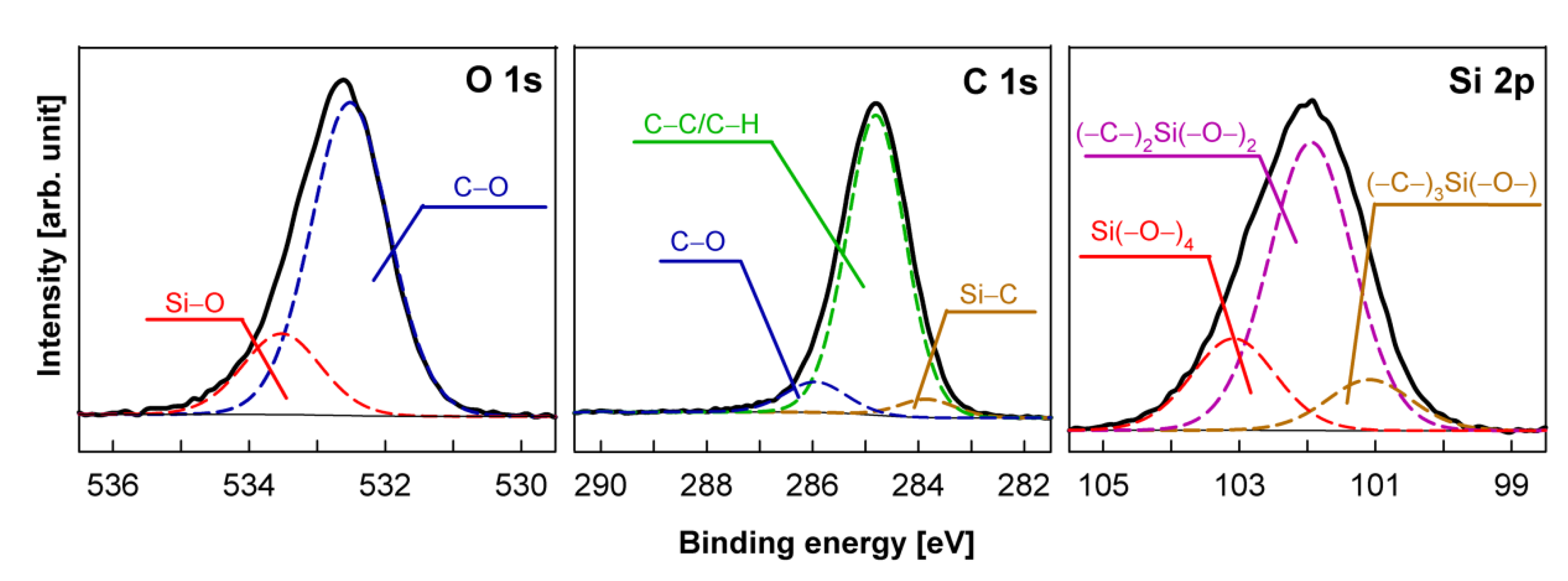


| Sample No. | Plasma Activation | Plasma Deposition | Contact Angle | ||
|---|---|---|---|---|---|
| Power | Time | Power | Time | ||
| (W) | (s) | (W) | (s) | (deg) | |
| pp-HMDSO | |||||
| 1 | 80 | 30 | 40 | 120 | 138 ± 1 |
| 2 | 80 | 60 | 40 | 120 | 133 ± 2 |
| 3 | 40 | 30 | 40 | 120 | 142 ± 2 |
| 4 | 40 | 60 | 40 | 120 | 139 ± 3 |
| 5 | 25 | 30 | 40 | 120 | 136 ± 4 |
| 6 | 25 | 60 | 40 | 120 | 138 ± 3 |
| 7 | 40 | 30 | 40 | 60 | 137 ± 5 |
| 8 | 40 | 30 | 40 | 240 | 143 ± 2 |
| 9 | 40 | 30 | 25 | 60 | 141 ± 4 |
| 10 | 40 | 30 | 25 | 120 | 143 ± 3 |
| 11 | 40 | 30 | 25 | 240 | 144 ± 5 |
| pp-HMDSN | |||||
| 12 | 40 | 30 | 25 | 60 | 143 ± 5 |
| 13 | 40 | 30 | 25 | 120 | 147 ± 1 |
| 14 | 40 | 30 | 25 | 240 | 152 ± 3 |
| Sample Name | Contact Angle (deg) | Tilt Angle (deg) | Globules (nm) |
|---|---|---|---|
| pellet pp-HMDSO No. 11 | 144 ± 5 | 9 ± 1 | 400 ± 100 |
| pellet pp-HMDSN No. 14 | 152 ± 3 | 6.5 ± 0.5 | 190 ± 70 |
| down pp-HMDSO | 150 ± 2 | ≈0 | 70 ± 20 |
| down pp-HMDSN | 161 ± 2 | ≈0 | 50 ± 20 |
| Si wafer pp-HMDSO | 100 ± 2 | 20 ± 1 | 0 |
| Si wafer pp-HMDSN | 95 ± 4 | 25 ± 1 | 0 |
| Layer | Band (cm−1) | Vibrational Mode | References |
|---|---|---|---|
| pp-HMDSO | 1399 | CH3 asymmetric bending in Si–(CH3)x, (x = 1, 2, 3) | [34,38,39] |
| 1350 | CH2 scissor vibration in Si–CH2–Si | [40] | |
| 1253 | CH3 symmetric bending in Si–(CH3)x, (x = 1, 2, 3) | [33,34,39,40,41,42] | |
| 1022 | Si–O–Si asymmetric stretching; Si–O–C stretching | [33,34,39,40,41,42] | |
| 896 | CH3 rocking in Si–(CH3)2 | [40] | |
| 834 | CH3 rocking in Si–(CH3)3 | [33,34,39,40,41] | |
| 781 | CH3 rocking in Si–(CH3)2, Si–O–Si bending | [33,34,39,40,41,42] | |
| 720 | CH3 rocking in Si–(CH3)3 | [40,41] | |
| pp-HMDSN | 1405 | CH3 asymmetric bending in Si–(CH3)x, (x = 1, 2, 3) | [39,43,44] |
| 1352 | CH2 asymmetric bending in Si–CH2–Si | [43] | |
| 1251 | CH3 symmetric bending in Si–(CH3)x, (x = 1, 2, 3) | [39,43,44,45,46] | |
| 1176 | N–H bending | [35,44] | |
| 1031 | Si–O–Si asymmetric stretching; Si–O–C stretching | [43,44,46] | |
| 908 | Si–N asymmetric stretching in Si–NH–Si | [39,43,44,45,46] | |
| 835 | CH3 rocking in Si–(CH3)3 | [39,43,44,45,46] | |
| 763 | Si-C stretching in Si–(CH3)x, (x = 1, 2, 3) | [43,44,45] |
| Sample Number | Surface Composition (at %) | O/(Si + C) | |||
|---|---|---|---|---|---|
| Si | C | N | O | ||
| Si wafer pp-HMDSO | 22.20 | 52.19 | 0 | 25.61 | 0.34 |
| Si wafer pp-HMDSN | 21.53 | 54.07 | 8.45 | 15.95 | 0.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapica, R.; Markiewicz, J.; Tyczkowska-Sieroń, E.; Fronczak, M.; Balcerzak, J.; Sielski, J.; Tyczkowski, J. Artificial Superhydrophobic and Antifungal Surface on Goose Down by Cold Plasma Treatment. Coatings 2020, 10, 904. https://doi.org/10.3390/coatings10090904
Kapica R, Markiewicz J, Tyczkowska-Sieroń E, Fronczak M, Balcerzak J, Sielski J, Tyczkowski J. Artificial Superhydrophobic and Antifungal Surface on Goose Down by Cold Plasma Treatment. Coatings. 2020; 10(9):904. https://doi.org/10.3390/coatings10090904
Chicago/Turabian StyleKapica, Ryszard, Justyna Markiewicz, Ewa Tyczkowska-Sieroń, Maciej Fronczak, Jacek Balcerzak, Jan Sielski, and Jacek Tyczkowski. 2020. "Artificial Superhydrophobic and Antifungal Surface on Goose Down by Cold Plasma Treatment" Coatings 10, no. 9: 904. https://doi.org/10.3390/coatings10090904







