Corrosion Behavior of AA2055 Aluminum-Lithium Alloys Anodized in the Presence of Sulfuric Acid Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Metallographic Preparation of Samples
2.2. Anodizing Treatment
- Pretreatment:
- ○
- Degreased and Pickling in a 50 wt.% HCl solution (analytical grade reagents (J.T. Baker, Nuevo León, México) for 5 s at 25 °C
- ○
- Rinsed in distilled water
- Anodizing treatment:
- ○
- Bath = 16 wt.% H2SO4 solution (analytical grade reagents (J.T. Baker))
- ○
- Current densities = 0.19 and 1.0 A·cm−2
- ○
- Electrolyte pH = 6.0
- ○
- Time = 45 min
- ○
- Temperature = 25 °C
- Sealing treatment:
- ○
- The anodized specimens were immersed in H2O or in 6 wt.% Na2Cr2O7 solution (analytical grade reagents (J.T. Baker)) at 95 °C for 25 min.
2.3. SEM Characterization
2.4. Electrochemical Impedance Spectroscopy
2.5. XPS Characterization
3. Results and Discussion
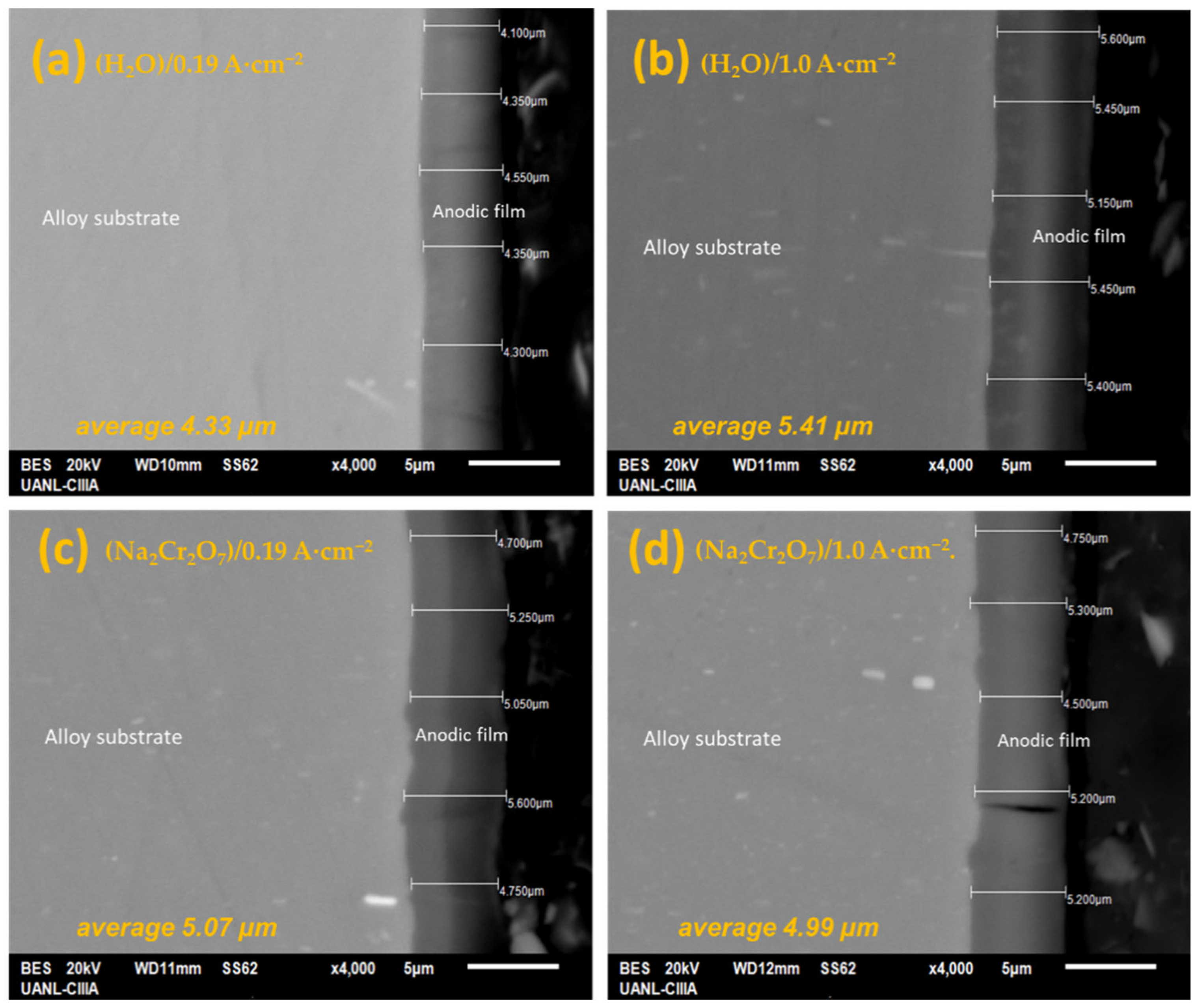
3.1. SEM Microstructural Analysis
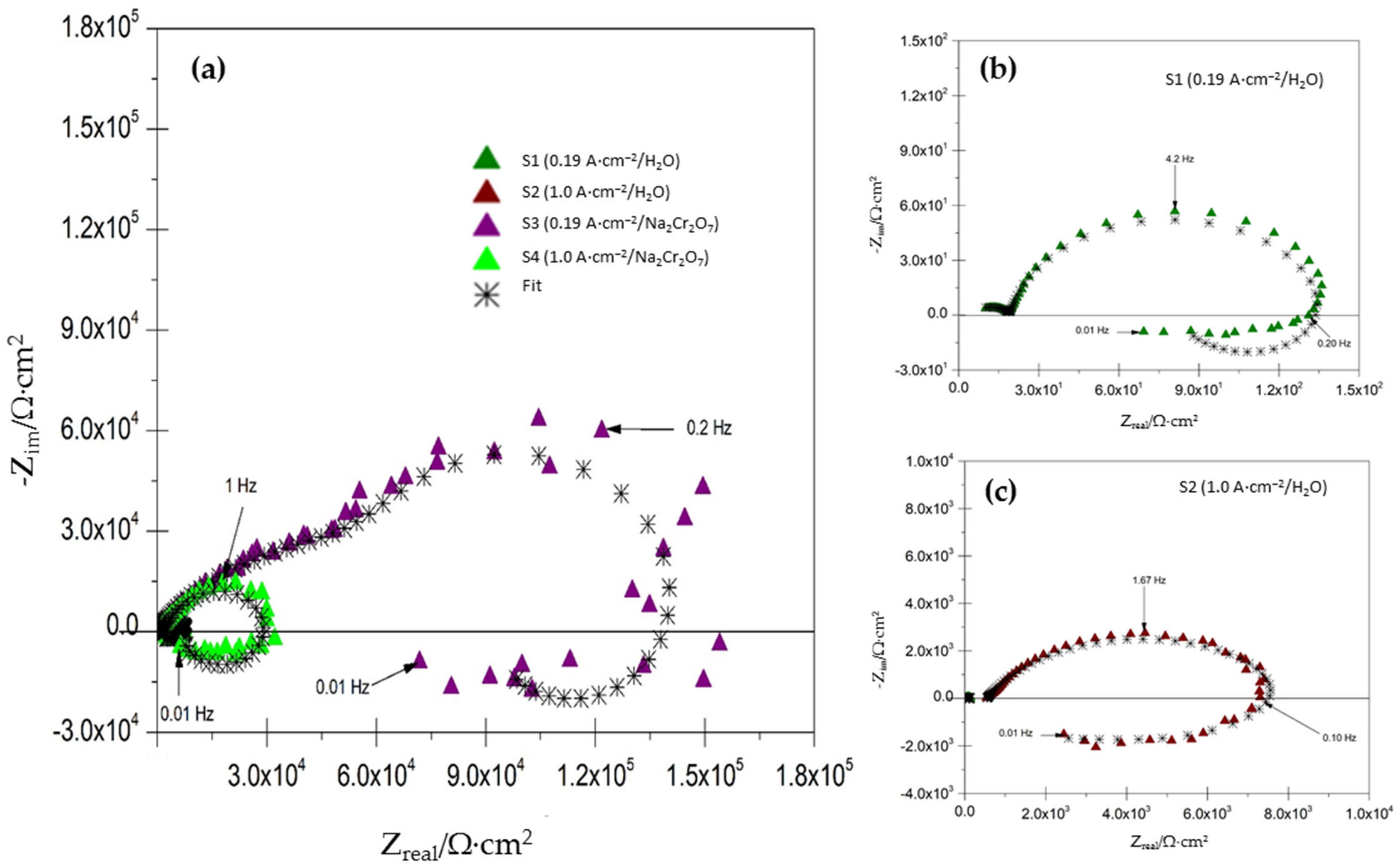
3.2. Electrochemical Impedance Spectroscopy Analysis
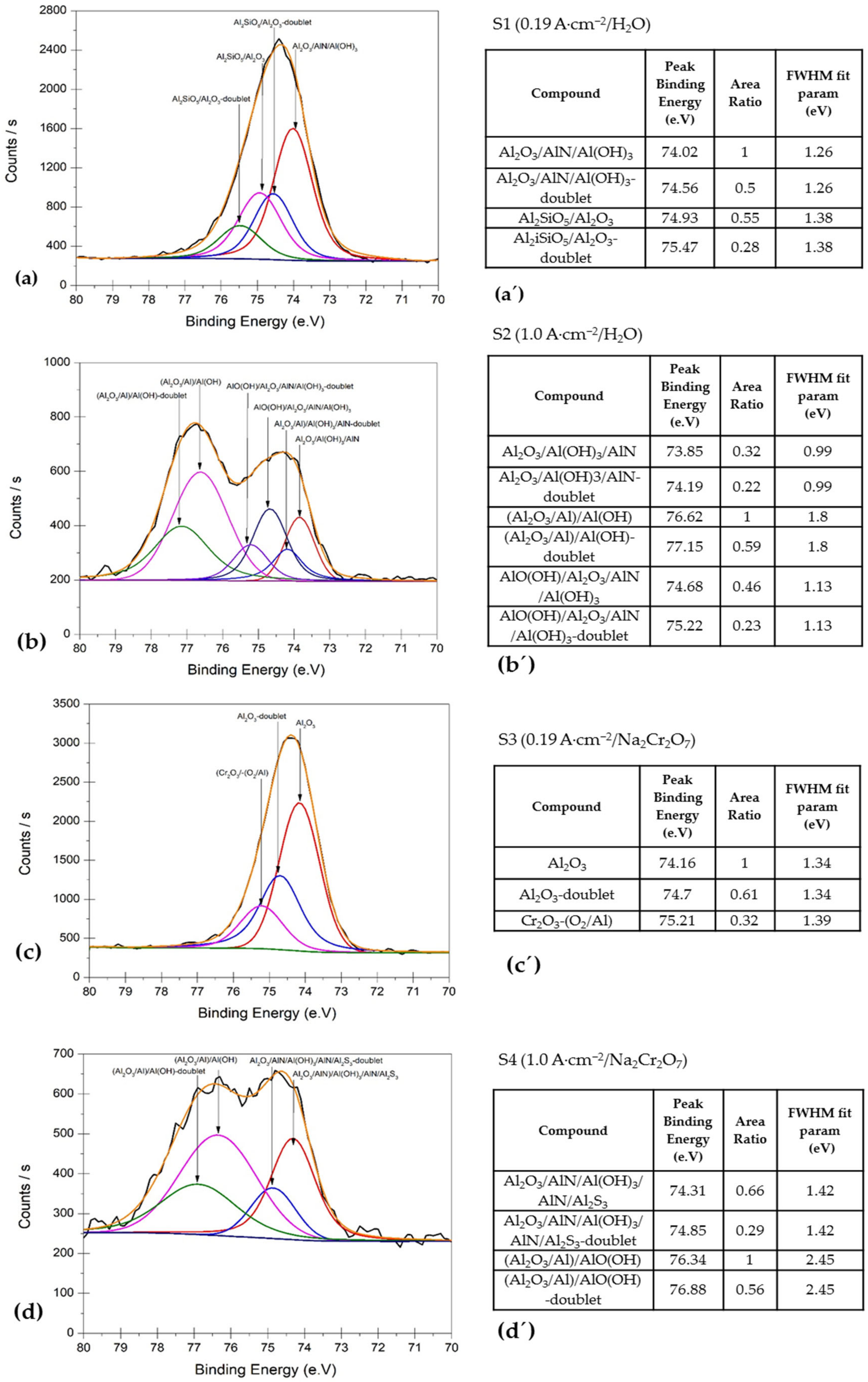
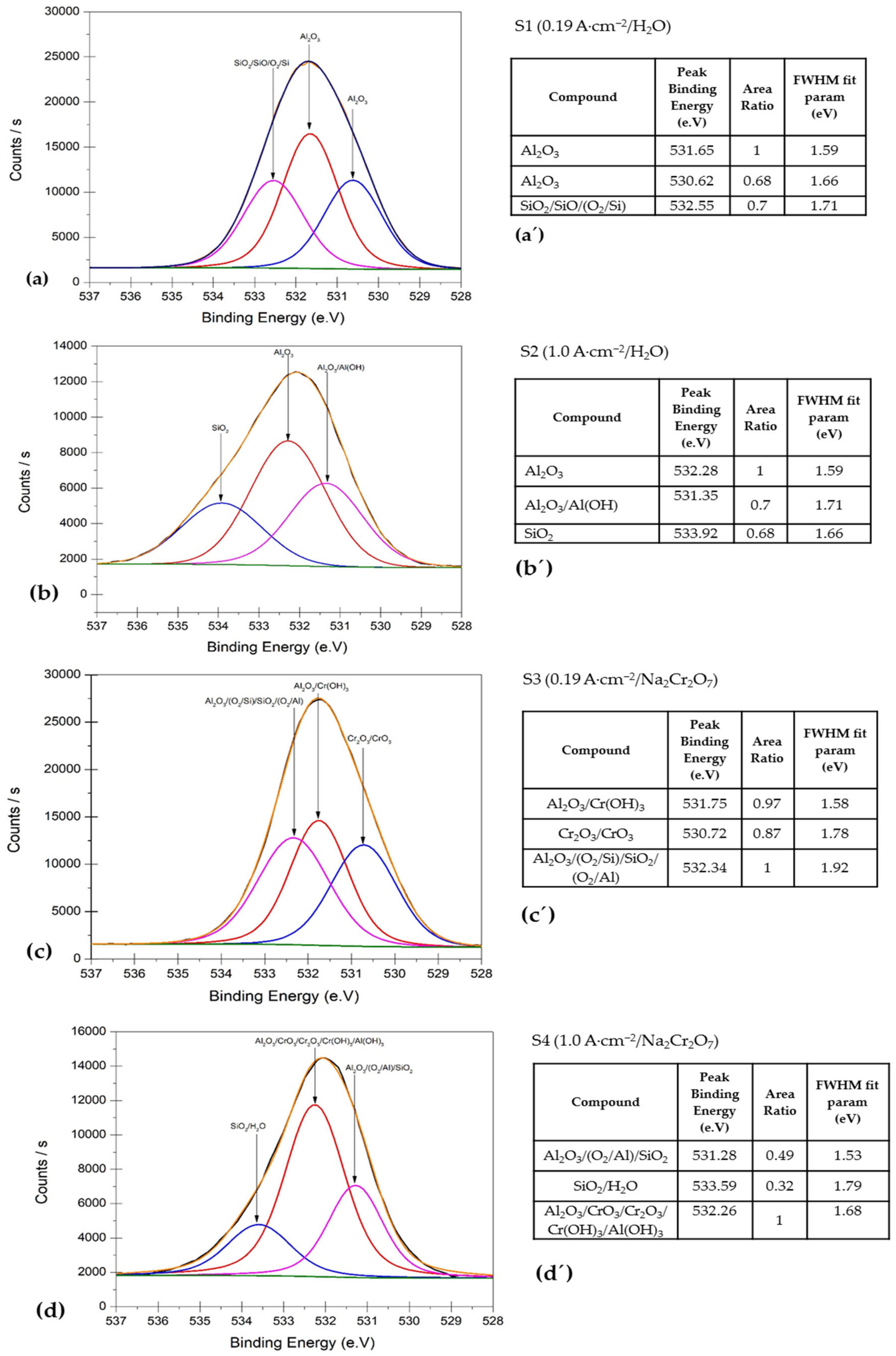
3.3. XPS Surface Analysis
4. Conclusions
- The results reveal that the EIS technique is a good tool for obtaining detailed information on the influence of sealing and the process on anodized aluminum.
- EIS results show that R2 increase for the sample S3 in presence of the Na2Cr2O7 sealing solution has a higher charge transfer resistance when it has a less homogeneous and less compact layer of anodic oxide.
- The inductive loop present in Nyquist diagrams for all samples indicates species on the surface decreases, adsorption and electrodissolution of the oxide on the AA2055 alloys anodized surface.
- The SEM and EIS results indicated that the formation of a more compact and homogeneous anodic oxide layer is due to an increase in charge transfer resistance (RCT), by preventing Cl− ions ingress into the anodized layer.
- SEM characterization indicated that, in both current densities, the thickness is homogeneous for the oxide films formed on anodized AA2055 aluminum-lithium alloy. The surface micrographs indicated that samples have a heterogeneous surface with bumps, some bright precipitations, and cavity. This may be due to varying the current density and the sealing solutions during the growth of the Al2O3 layer in the H2SO4 bath.
- XPS characterization verified that the surface film of AA2055 alloy in this study consisted of a mixture of chemical compounds, such as Al2O3 and AlO(OH), respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saffari, H.; Sohrabi, B.; Noori, M.R.; Bahrami, H.R.T. Optimal condition for fabricating superhydrophobic aluminum surfaces with controlled anodizing processes. Appl. Surf. Sci. 2018, 435, 1322–1328. [Google Scholar] [CrossRef]
- Jiang, B.; Yi, D.; Yi, X.; Zheng, F.; Wang, H.; Wang, B.; Hu, Z. Effect of trace amounts of added Sc on microstructure and mechanical properties of 2055 aluminum alloy. Mater. Charact. 2018, 141, 248–259. [Google Scholar] [CrossRef]
- Keller, F.; Hunter, M.S.; Robinson, D.L. Structural features of oxide coatings on aluminum. J. Electrochem. Soc. 1953, 100, 411–419. [Google Scholar] [CrossRef]
- Jaimes-Ramírez, R.; Covelo, A.; Rodil, S.E.; Corona-Lira, P.; Ramírez-Reivich, A.C.; Hernández, M. Development and characterization of hydrophobic anodized aluminum layer to act as a long-lasting protective film in corrosion. Surf. Interface Anal. 2018, 50, 1030–1035. [Google Scholar] [CrossRef]
- Etienne, M.; Rocca, E.; Chahboun, N.; Veys-Renaux, D. Local evolution of pH with time determined by shear force-based scanning electrochemical microscopy: Surface reactivity of anodized aluminium. Electroanalysis 2016, 28, 2466–2471. [Google Scholar] [CrossRef]
- Zhang, F.; Örnek, C.; Nilsson, J.-O.; Pan, J. Anodisation of aluminium alloy AA7075-influence of intermetallic particles on anodic oxide growth. Corros. Sci. 2020, 164, 108319. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, X.; Zhou, X.; Yi, Y.; Liao, Y.; Huang, W. Microstructural origin of localized corrosion in anodized AA2099-T8 aluminium-lithium alloy. Surf. Interface Anal. 2016, 48, 739–744. [Google Scholar] [CrossRef]
- Mouritz, P.A. Introduction to Aerospace Materials; Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar]
- Zhou, X.R.; Meng, X.M.; Huang, W.J.; Yi, L.; Chen, X.L.; Yi, Y.N.; Thompson, G.E. Influence of thermomechanical treatments on localized corrosion susceptibility and propagation mechanism of AA2099 Al–Li alloy. Trans. Nonferrous Met. Soc. 2016, 26, 1472–1481. [Google Scholar] [CrossRef]
- Gumbmann, E.; Lefebvre, W.; De Geuser, F.; Sigli, C.; Deschamps, A. The effect of minor solute additions on the precipitation path of an Al Cu Li alloy. Acta Mater. 2016, 115, 104–114. [Google Scholar] [CrossRef]
- Gumbmann, E.; De Geuser, F.; Deschamps, A.; Lefebvre, W.; Robaut, F.; Sigli, C. A combinatorial approach for studying the effect of Mg concentration on precipitation in an Al–Cu–Li alloy. Scr. Mater. 2016, 110, 44–47. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Zhou, X.; Huang, W.; Liao, Y.; Chen, X.; Zhang, X.; Thompson, G.E. Crystallographic defects induced localised corrosion in AA2099-T8 aluminium alloy. Corros. Eng. Sci. Technol. 2015, 50, 420–424. [Google Scholar] [CrossRef]
- Deschamps, A.; Garcia, M.; Chevy, J.; Davo, B.; De Geuser, F. Influence of Mg and Li content on the microstructure evolution of Al Cu Li alloys during long-term ageing. Acta Mater. 2017, 122, 32–46. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Bai, J.; Wu, X.; Huang, G.; Cao, L.; Huang, L. Investigation on formation mechanism of T1 precipitate in an Al-Cu-Li alloy. J. Alloys Compd. 2017, 723, 661–666. [Google Scholar] [CrossRef]
- Deschamps, A.; Decreus, B.; De Geuser, F.; Dorin, T.; Weyland, M. The influence of precipitation on plastic deformation of Al–Cu–Li alloys. Acta Mater. 2013, 61, 4010–4021. [Google Scholar] [CrossRef]
- Eswara, P.N.; Gokhale, A.A.; Wanhill, H.J.R. Aluminium-Lithium: Processing, Properties and Applications; Butterworth-Heinemann; Elsevier Inc.: Waltham, MA, USA, 2014. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Thompson, G.E.; Curioni, M.; Zhong, X.; Koroleva, E.; Fowles, M. Discontinuities in the porous anodic film formed on AA2099-T8 aluminium alloy. Corros. Sci. 2011, 53, 4141. [Google Scholar] [CrossRef]
- Vignoli Machado, T.; Atz Dick, P.; Knörnschild, G.H.; Dick, L.F.P. The effect of different carboxylic acids on the sulfuric acid anodizing of AA2024. Surf. Coat. Technol. 2020, 383, 125283. [Google Scholar] [CrossRef]
- Runge, J.M. The Metallurgy of Anodizing Aluminum; Springer: Chicago, IL, USA, 2018. [Google Scholar] [CrossRef]
- Varshney, D.; Kumar, K. Application and use of different aluminium alloys with respect to workability, strength and welding parameter optimization. Ain Shams Eng. J. 2021, 12, 1143–1152. [Google Scholar] [CrossRef]
- Ambor, M.; Nový, F.; Bokůvka, O.; Trško, L. The natural aging behavior of the AA 2055 Al-Cu-Li alloy. Transp. Res. Procedia 2019, 40, 42–45. [Google Scholar] [CrossRef]
- Mopon, M.L., Jr.; Garcia, J.S.; Manguerra, D.M.; Narisma, C.J.C. Corrosion behavior of AA 1100 anodized in gallic-sulfuric acid solution. Coatings 2021, 11, 405. [Google Scholar] [CrossRef]
- Raj, V.; Mumjitha, M. Comparative study of formation and corrosion performance of porous alumina and ceramic nanorods formed in different electrolytes by anodization. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2014, 179, 25–35. [Google Scholar] [CrossRef]
- Martínez-Viademonte, M.P.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A review on anodizing of aerospace aluminum alloys for corrosion protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.; Gaona-Tiburcio, C.; Estupinán-López, F.; Lara-Banda, M.; Zambrano-Robledo, P.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Corrosion resistance of hard coat anodized AA 6061 in citric–sulfuric solutions. Coatings 2020, 10, 601. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.A.; Gaona-Tiburcio, C.; Almeraya-Calderón, F.; Estupiñan-Lopez, H.F.; Pedraza-Basulto, G.; Poblano-Salas, C. Parameter studies on high-velocity oxy-fuel spraying of CoNiCrAlY coatings used in the aeronautical industry. Int. J. Corros. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhao, P.-H.; Zhao, J.-M. The influences of sealing methods on corrosion behavior of anodized aluminum alloys in NaCl solutions. Surf. Coat. Technol. 2003, 166, 237–242. [Google Scholar] [CrossRef]
- Ko, C.-L.; Kuo, Y.-L.; Chen, S.-H.; Chen, S.-Y.; Guo, J.-Y.; Wang, Y.-J. Formation of aluminum composite passive film on magnesium alloy by integrating sputtering and anodic aluminum oxidation processes. Thin Solid Films 2020, 709, 138151. [Google Scholar] [CrossRef]
- Gianni, L.; Cavallini, M.; Natali, S.; Adriaens, S. Wet and dry accelerated aging tests in a spray chamber to understand the effects of acid rain frequencies on bronze corrosion. Int. J. Electrochem. Sci. 2013, 8, 1822–1838. [Google Scholar]
- ASTM E3-95. Standard Practice for Preparation of Metallographic Specimens; ASTM International: West Conshohocken, PA, USA, 1995. [Google Scholar]
- Samaniego-Gámez, P.; Almeraya-Calderón, F.; Martin, U.; Ress, J.; Gaona-Tiburcio, C.; Silva-Vidaurri, L.; Cabral-Miramontes, J.; Bastidas, J.M.; Chacón-Nava, J.G.; Bastidas, D.M. Efecto del tratamiento de sellado en el comportamiento frente a corrosión de la aleación anodizada de aluminio-litio AA2099. Rev. Met. 2020, 56, e180. [Google Scholar] [CrossRef]
- ASTM G106-15. Standard Practice for Verification of Algorithm and Equipment for Electrochemical Impedance Measurements; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Macdonald, D.D. Review of mechanistic analysis by electrochemical impedance spectroscopy. Electrochim. Acta 1990, 35, 1509–1525. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, J.; Liu, S.; Wang, H.; Dong, P. Effect of NaAlO2 sealing on corrosion resistance of 2024 aluminum alloy anodized film. Mater. Corros. 2019, 70, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Cabral-Miramontes, J.; Bastidas, M.D.; Baltazar, M.A.; Zambrano-Robledo, P.; Bastidas, J.M.; Almeraya-Calderón, F.; Gaona-Tiburcio, C. Corrosion behavior of Zn-TiO2 and Zn-ZnO electrodeposited coatings in 3.5% NaCl solution. Int. J. Electrochem. Sci. 2019, 14, 4226–4239. [Google Scholar] [CrossRef]
- Gaona-Tiburcio, C.; Montoya-Rangel, M.; Cabral-Miramontes, J.A.; Estupiñan-López, F.; Zambrano-Robledo, P.; Orozco Cruz, R.; Chacón-Nava, J.G.; Baltazar-Zamora, M.A.; Almeraya-Calderón, F. Corrosion resistance of multilayer coatings deposited by PVD on Inconel 718 using electrochemical impedance spectroscopy technique. Coatings 2020, 10, 521. [Google Scholar] [CrossRef]
- Herrera-Hernandez, H.; Vargas-Garcia, J.R.; Hallen-López, J.M.; Mansfeld, F. Evaluation of different sealing methods for anodized aluminum-silicon carbide (Al/SiC) composites using EIS and SEM techniques. Mater. Corros. 2007, 58, 825–832. [Google Scholar] [CrossRef]
- Ma, T.; Wua, H.; Zhoub, X.; Lic, K.; Liaod, Y.; Lianga, Z.; Liua, L. Corrosion behavior of anodized Al-Cu-Li alloy: The role of intermetallic particle-introduced film defects. Corros. Sci. 2019, 58, 108110. [Google Scholar] [CrossRef]
- Wu, H.; Ma, Y.; Huang, W.; Zhou, X.; Li, K.; Liao, Y.; Wang, Z.; Liang, Z.; Liu, L. Effect of iron-containing intermetallic particles on film structure and corrosion resistance of anodized AA2099 alloy. J. Electrochem. Soc. 2018, 165, C573–C581. [Google Scholar] [CrossRef]
- Schneider, M.; Kremmer, K. Corrosion behavior of anodized AA-6060 depending on the anodizing bath aging. Mater. Corros. 2019, 70, 2041–2051. [Google Scholar] [CrossRef]
- Gao, Z.; Cao, J.; Chen, Y.; Muzammal, H.M.; Wang, W. Effect of Cu preferential orientation on the microstructure and property of anodized CuxO film. Eur. J. Inorg. Chem. 2020, 2020, 261–268. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, Z.; Bai, S.; Wang, J. Influence of sealing treatment on the corrosion resistance of PEO coated Al-Zn-Mg-Cu alloy in various environments. Coatings 2019, 9, 867. [Google Scholar] [CrossRef] [Green Version]
- Hua, L.; Liu, J.; Li, S.; Yu, M. Effect of adipic acid on deis characteristics during the aluminium anodizing process in sulfuric acid bath. Int. J. Electrochem. Sci. 2015, 10, 2194–2205. [Google Scholar]
- Brett, C.M.A. The application of electrochemical impedance techniques to aluminium corrosion in acidic chloride Solution. J. App. Electrochem. 1990, 20, 1000–1003. [Google Scholar] [CrossRef]
- Bastidas, D.M. Interpretation of impedance data for porous electrodes and diffusion processes. Corrosion 2007, 63, 515–521. [Google Scholar] [CrossRef]
- Jirón-Lazos, U.; Corvo, F.; De la Rosa-García, S.; García-Ochoa, E.; Bastidas, D. Localized corrosion of aluminum alloy 6061 in the presence of Asper gillus niger. Int. Biodeter. Boidegr. 2018, 133, 17–25. [Google Scholar] [CrossRef]
- Jinlong, L.; Tongxiang, L.; Chen, W.; Ting, G. The passive film characteristics of several plastic deformation 2099 Al–Li alloy. J. Alloys Compd. 2016, 662, 143–149. [Google Scholar] [CrossRef]
- Pivac, I.; Barbir, F. Inductive phenomena at low frequencies in impedance spectra of proton exchange membrane fuel cells–A review. J. Power Sources 2016, 326, 112–119. [Google Scholar] [CrossRef]
- Alexe-Ionescu, A.L.; Barbero, G.; Evangelista, L.R.; Lenzi, E.K. Current–voltage characteristics and impedance spectroscopy: Surface conduction and adsorption–desorption effects in electrolytic cells. J. Phys. Chem. C 2020, 124, 3150–3158. [Google Scholar] [CrossRef]
- Djellab, M.; Bentrah, H.; Chala, A.; Taoui, H. Synergistic effect of halide ions and gum arabic for the corrosion inhibition of API5L X70 pipeline steel in H2SO4. Mater. Corros. 2019, 70, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Bastidas, J.M.; Polo, J.; Torres, C.; Cano, E. A study on the stability of AISI 316L stainless steel pitting corrosion through its transfer function. Corros. Sci. 2001, 43, 269–281. [Google Scholar] [CrossRef] [Green Version]
- Klotz, D. Negative capacitance or inductive loop?—A general assessment of a common low frequency impedance feature. Electrochem. Commun. 2019, 98, 58–62. [Google Scholar] [CrossRef]
- Usman, B.J.; Scenini, F.; Curioni, M. Corrosion testing of anodized aerospace alloys: Comparison between immersion and salt spray testing using electrochemical impedance spectroscopy. J. Electrochem. Soc. 2020, 167, 041505. [Google Scholar] [CrossRef]
- Huang, Y.; Shih, H.; Huang, H.; Daugherty, J.; Wu, S.; Ramanathan, S.; Chang, C.; Mansfeld, F. Evaluation of the corrosion resistance of anodized aluminum 6061 using electrochemical impedance spectroscopy (EIS). Corros. Sci. 2008, 50, 3569–3575. [Google Scholar] [CrossRef]
- Zapata-Loría, A.D.; Pech-Canul, M.A. Corrosion inhibition of aluminum in 0.1 M HCL solution by glutamic acid. Chem. Eng. Commun. 2014, 201, 855–869. [Google Scholar] [CrossRef]
- Suay, J.; Giménez, E.; Rodriguez, T.; Habbib, K.; Saura, J. Characterization of anodized and sealed aluminium by EIS. Corros. Sci. 2003, 45, 611–624. [Google Scholar] [CrossRef]
- Wang, S.; Peng, H.; Shao, Z.; Zhao, Q.; Du, N. Sealing of anodized aluminum with phytic acid solution. Surf. Coat. Technol. 2015, 286, 155–164. [Google Scholar] [CrossRef]
- Saeedikhani, M.; Javidi, M.; Yazdani, A. Anodizing of 2024-T3 aluminum alloy in sulfuric-boric-phosphoric acids and its corrosion behavior. Trans. Nonferrous Met. Soc. China 2013, 23, 2551–2559. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Technical note: Concerning the conversion of the constant phase element parameter Y0 into a capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Scully, J.R.; Silverman, D.C.; Kendig, M.W. Electrochemical Impedance: Analysis and Interpretation STP 1188; ASTM International: Philadelphia, PA, USA, 1993. [Google Scholar] [CrossRef]
- Pérez, N. Electrochemistry and Corrosion Science; Springer: New York, NY, USA, 2004. [Google Scholar]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Evertsson, J.; Bertram, F.; Rullik, L.; Harlow, G.; Lundgren, E. Anodization of Al (100), Al (111) and Al Alloy 6063 studied in situ with X-ray reflectivity and electrochemical impedance spectroscopy. J. Electroanal. Chem. 2017, 799, 556–562. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.A.; Barceinas-Sánchez, J.D.O.; Poblano-Salas, C.A.; Pedraza-Basulto, G.K.; Nieves-Mendoza, D.; Zambrano-Robledo, P.C.; Almeraya-Calderón, F.; Chacón-Nava, J.G. Corrosion behavior of AISI 409Nb stainless steel manufactured by powder metallurgy exposed in H2SO4 and NaCl solutions. Int. J. Electrochem. Sci. 2013, 8, 564–577. [Google Scholar]
- Ayagou, M.D.D.; Tran, T.T.M.; Tribollet, B.; Kittel, J.; Sutter, E.; Ferrando, N.; Mendibide, C.; Duret-Thual, C. Electrochemical impedance spectroscopy of iron corrosion in H2S solutions. Electrochim. Acta 2018, 282, 775–783. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Jaquez, R.E.; Buelna-Rodríguez, J.E.; Barrios-Durstewitz, C.P.; Gaona-Tiburcio, C.; Almeraya-Calderón, F. Corrosion of modified concrete with sugar cane bagasse ash. Int. J. Corros. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.-H.; Zuo, Y.; Zhao, J.-M.; Xiong, J.-P.; Tang, Y.-M. A study on the self-sealing process of anodic films on aluminum by EIS. Surf. Coat. Technol. 2006, 200, 6846–6853. [Google Scholar] [CrossRef]
- Khadiri, M.; Elyaagoubi, M.; Idouhli, R.; Koumya, Y.; Zakir, O.; Benzakour, J.; Benyaich, A.; Abouelfida, A.; Outzourhit, A. Electrochemical study of anodized titanium in phosphoric acid. Adv. Mater. Sci. Eng. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Ono, S.; Asoh, H. Mechanism of hot water sealing of anodic films formed on aluminum. Corros. Sci. 2021, 181, 109221. [Google Scholar] [CrossRef]
- Martinez-Villafañe, A.; Chacon-Nava, J.; Gaona-Tiburcio, C.; Almeraya-Calderon, F.; Domínguez-Patiño, G.; Gonzalez-Rodríguez, J. Oxidation performance of a Fe–13Cr alloy with additions of rare earth elements. Mater. Sci. Eng. A 2003, 363, 15–19. [Google Scholar] [CrossRef]
- Boukamp, B.A. Interpretation of an ’inductive loop’ in the impedance of an oxygen ion conducting electrolyte/metal electrode system. Solid State Ion. 2001, 143, 47–55. [Google Scholar] [CrossRef]
- Corral, H.R.; Arredondo, R.S.P.; Neri, F.M.; Gómez, S.J.M.; Almeraya, C.F.; Castorena, G.J.H.; Almaral, S.J. Sulfate attack and reinforcement corrosion in concrete with recycled concrete aggregates and supplementary cementing materials. Int. J. Electrochem. Sci. 2011, 6, 613–621. [Google Scholar]
- Ofoegbu, S.U.; Fernandes, F.A.; Pereira, A.B. The sealing step in aluminum anodizing: A focus on sustainable strategies for enhancing both energy effciency and corrosion resistance. Coatings 2020, 10, 226. [Google Scholar] [CrossRef] [Green Version]
- Miramontes, J.A.C.; Sánchez, J.D.O.B.; Calderón, F.A.; Villafañe, A.M.; Nava, J.G.C. Effect of boron additions on sintering and densification of a ferritic stainless steel. J. Mater. Eng. Perform. 2010, 19, 880–884. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Sikora, E.; Engelhardt, G. Characterizing electrochemical systems in the frequency domain. Electrochim. Acta 1998, 43, 87–107. [Google Scholar] [CrossRef]
- Cao, C.-N. On the impedance plane displays for irreversible electrode reactions based on the stability conditions of the steady-state—II. Two state variables besides electrode potential. Electrochim. Acta 1990, 35, 837–844. [Google Scholar] [CrossRef]
- Clerc, C.; Landolt, D. AC impedance study of anodic films on nickel in LiCl. Electrochim. Acta 1988, 33, 859–871. [Google Scholar] [CrossRef]
- Elkin, V.V.; Marshakov, A.I.; Rybkina, A.A.; Maleeva, M. Interpretation of the impedance comprising negative capacitance and constant phase elements on iron electrode in weakly acidic media. Russ. J. Electrochem. 2011, 47, 136–146. [Google Scholar] [CrossRef]
- Strohmeier, B.R. An ESCA method for determining the oxide thickness on aluminum alloys. Surf. Interface Anal. 1990, 15, 51–56. [Google Scholar] [CrossRef]
- Pérez, O.E.L.; Sánchez, M.D.; Teijelo, M.L. Characterization of growth of anodic antimony oxide films by ellipsometry and XPS. J. Electroanal. Chem. 2010, 645, 143–148. [Google Scholar] [CrossRef]
- Alexander, M.R.; Thompson, G.E.; Beamson, G. Characterization of the oxide/hydroxide surface of aluminium using x-ray photoelectron spectroscopy: A procedure for curve fitting the O 1s core level. Surf. Interface Anal. 2000, 29, 468–477. [Google Scholar] [CrossRef]
- Feliu, S.; Bartolomé, M.J.; González, J.A.; Feliu, S. XPS characterization of porous and sealed anodic films on aluminum alloys. J. Electrochem. Soc. 2007, 154, C241–C248. [Google Scholar] [CrossRef]
- Caicedo-Martinez, C.E.; Koroleva, E.; Skeldon, P.; Thompson, G.E.; Hoellrigl, G.; Bailey, P.; Noakes, T.C.Q.; Habazaki, H.; Shimizu, K. Behavior of impurity and minor alloying elements during surface treatments of aluminum. J. Electrochem. Soc. 2002, 149, B139–B145. [Google Scholar] [CrossRef]
- Paez, A.M.; Bustos, O.; Thompson, E.G.; Skeldon, P.; Shimizu, K.; Wood, C.G. Porous anodic film formation on an Al-3.5 wt.% Cu Alloy. J. Electrochem. Soc. 2000, 147, 1015. [Google Scholar] [CrossRef]
- Romios, M.; Tiraschi, R.; Parrish, C.; Babel, H.W.; Ogren, J.R.; Es-Said, O.S. Design of multistep aging treatments of 2099 (C458) Al-Li Alloy. J. Mater. Eng. Perform. 2005, 14, 641–646. [Google Scholar] [CrossRef]
- Abrahami, S.; Hauffman, T.; De Kok, J.M.; Mol, A.; Terryn, H. XPS analysis of the surface chemistry and interfacial bonding of barrier-type Cr (VI)-free anodic oxides. J. Phys. Chem. C. 2015, 119, 19967–19975. [Google Scholar] [CrossRef]
- Skeldon, P.; Wang, H.; Thompson, G. Formation and characterization of self-lubricating MoS2 precursor films on anodized aluminium. Wear 1997, 206, 187–196. [Google Scholar] [CrossRef]
- Khan, M.F.; Kumar, A.M.; Ul-Hamid, A.; Al-Hems, L.M. Achieving non-adsorptive anodized film on Al-2024 alloy: Surface and electrochemical corrosion investigation. Surf. Interfaces 2019, 15, 78–88. [Google Scholar] [CrossRef]
- Ryu, S.-K.; Park, B.-J.; Park, S.-J. XPS analysis of carbon fiber surfaces—Anodized and interfacial effects in fiber–epoxy composites. J. Colloid Interface Sci. 1999, 215, 167–169. [Google Scholar] [CrossRef]
- Duong, L.V.; Wood, B.J.; Kloprogge, J.T. XPS study of basic aluminum sulphate and basic aluminium nitrate. Mater. Lett. 2005, 59, 1932–1936. [Google Scholar] [CrossRef] [Green Version]
- Zähr, J.; Oswald, S.; Türpe, M.; Ullrich, H.; Füssel, U. Characterisation of oxide and hydroxide layers on technical aluminum materials using XPS. Vacuum 2012, 86, 1216–1219. [Google Scholar] [CrossRef]
- Martínez-Villafañe, A.; Almeraya-Calderón, M.; Gaona-Tiburcio, C.; Gonzalez-Rodriguez, J.; Porcayo-Calderon, J. High-temperature degradation and protection of ferritic and austenitic steels in steam generators. J. Mater. Eng. Perform. 1998, 7, 108–113. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Guo, Y.; Chen, X.; Chen, L. Impurity element analysis of aluminum hydride using PIXE, XPS and elemental analyzer technique. Nucl. Instrum. Methods. Phys. Res. B Beam Interact. Mater. At. 2021, 488, 1–4. [Google Scholar] [CrossRef]
- Ramirez-Arteaga, A.M.; Gonzalez-Rodriguez, J.G.; Campillo, B.; Gaona-Tiburcio, C.; Dominguez-Patiño, G.; Leduc Lezama, L.; Chacon-Nava, J.G.; Neri-Flores, M.A.; Martinez-Villafañe, A. An electrochemical study of the corrosion behavior of a dual phase steel in 0.5 m H2SO4. Int. J. Electrochem. Sci. 2010, 5, 1786–1798. [Google Scholar]
- Vac, J. Practical guide for curve fitting in X-ray photoelectron spectroscopy. Sci. Technol. 2020, 38, 061203. [Google Scholar] [CrossRef]
- Sherwood, P.M.A. Introduction to studies of aluminum and its compounds by XPS. Surf. Sci. Spectra 1998, 5, 1–3. [Google Scholar] [CrossRef]
- Paparazzo, E. XPS and auger spectroscopy studies on mixtures of the oxides SiO2, Al2O3, Fe2O3 and Cr2O3. J. Electron Spectrosc. Relat. Phenom. 1987, 43, 97–112. [Google Scholar] [CrossRef]


| Alloy | Elements | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Li | Zn | Ag | Mg | Mn | Zr | Fe | Si | Al | |
| 2055 | 3.2–4.2 | 1.0–1.3 | 0.30–0.70 | 0.20–0.70 | 0.20–0.60 | 0.10–0.50 | 0.05–0.15 | 0.1 max | 0.07 max | Balance |
| Alloy | Nomenclature | Sealing Treatment | Anodizing Current Density (A·cm2) | ||
|---|---|---|---|---|---|
| Temperature (°C) | Time (min) | Solution | |||
| AA2055 | S1 | – | – | – H2O | 0.19 |
| S2 | 95 | – | 1.00 | ||
| S3 | – | 25 | – | 0.19 | |
| S4 | – | – | 6 wt.% Na2Cr2O7 | 1.00 | |
| Samples | Rs (Ω·cm2) | R1 (Ω·cm2) | Y1 (µsn1·cm−2) | n1 | R2 (Ω·cm2) | Y2 (µΩsn2·cm−2) | n2 | R3 (Ω·cm2) | Y3 (µΩsn3·cm−2) | n3 | X2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 4.6490 ± 0.23 | 14.78 ± 0.73 | 19.4996 ± 0.97 | 0.6637 | 128 ± 6.40 | 462.4946 ± 23.12 | 0.9032 | −66.77 ± 3.33 | −0.000275 ± 1.37 × 10−5 | 0.7724 | 9.49 × 10−3 |
| S2 | 520.9189 ± 26.04 | 1459 ± 72.95 | 22.1623 ± 1.10 | 0.6725 | 7680 ± 384 | 9.7601 ± 0.48 | 0.7725 | −7452 ± 372.6 | −319.4101 ± 15.97 | 0.7795 | 8.95 × 10−3 |
| S3 | 60.9330 ± 3.04 | 4121 ± 206.05 | 0.28 07 ± 0.01 | 0.7961 | 208,920 ± 10,446 | 3.2972 ± 0.16 | 0.5585 | −67,814 ± 3390.7 | −2.0652 ± 0.10 | 0.5788 | 1.65 × 10−2 |
| S4 | 20.6851 ± 1.03 | 1770 ± 88.50 | 4.1416 ± 0.20 | 0.6290 | 47,900 ± 2395 | 9.8849 ± 0.49 | 0.6653 | −35,940 ± 1797 | −0.4468 ± 0.02 | 0.5390 | 1.48 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samaniego-Gámez, P.O.; Almeraya-Calderon, F.; Maldonado-Bandala, E.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Olguin-Coca, J.; Lopez-Leon, L.D.; Silva Vidaurri, L.G.; Zambrano-Robledo, P.; Gaona-Tiburcio, C. Corrosion Behavior of AA2055 Aluminum-Lithium Alloys Anodized in the Presence of Sulfuric Acid Solution. Coatings 2021, 11, 1278. https://doi.org/10.3390/coatings11111278
Samaniego-Gámez PO, Almeraya-Calderon F, Maldonado-Bandala E, Cabral-Miramontes J, Nieves-Mendoza D, Olguin-Coca J, Lopez-Leon LD, Silva Vidaurri LG, Zambrano-Robledo P, Gaona-Tiburcio C. Corrosion Behavior of AA2055 Aluminum-Lithium Alloys Anodized in the Presence of Sulfuric Acid Solution. Coatings. 2021; 11(11):1278. https://doi.org/10.3390/coatings11111278
Chicago/Turabian StyleSamaniego-Gámez, Pedro Oliver, Facundo Almeraya-Calderon, Erick Maldonado-Bandala, Jose Cabral-Miramontes, Demetrio Nieves-Mendoza, Javier Olguin-Coca, Luis Daimir Lopez-Leon, Luis G. Silva Vidaurri, Patricia Zambrano-Robledo, and Citlalli Gaona-Tiburcio. 2021. "Corrosion Behavior of AA2055 Aluminum-Lithium Alloys Anodized in the Presence of Sulfuric Acid Solution" Coatings 11, no. 11: 1278. https://doi.org/10.3390/coatings11111278






