Effective Detoxification of Aflatoxin B1 and Ochratoxin A Using Magnetic Graphene Oxide Nanocomposite: Isotherm and Kinetic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Graphene Oxide (GO)
2.3. Preparation of Magnetic Graphene Oxide (MGO)
2.4. Characterization of Adsorbents
2.5. Analysis of Mycotoxins
2.6. Experimental Design
2.7. Statistical Analysis
2.8. Adsorption Studies
2.9. Experimental Analysis
2.9.1. Pseudo-First-Order
2.9.2. Pseudo-Second-Order
2.9.3. The Langmuir Isotherm
2.9.4. The Freundlich Isotherm
2.9.5. Thermodynamic Study
3. Results and Discussion
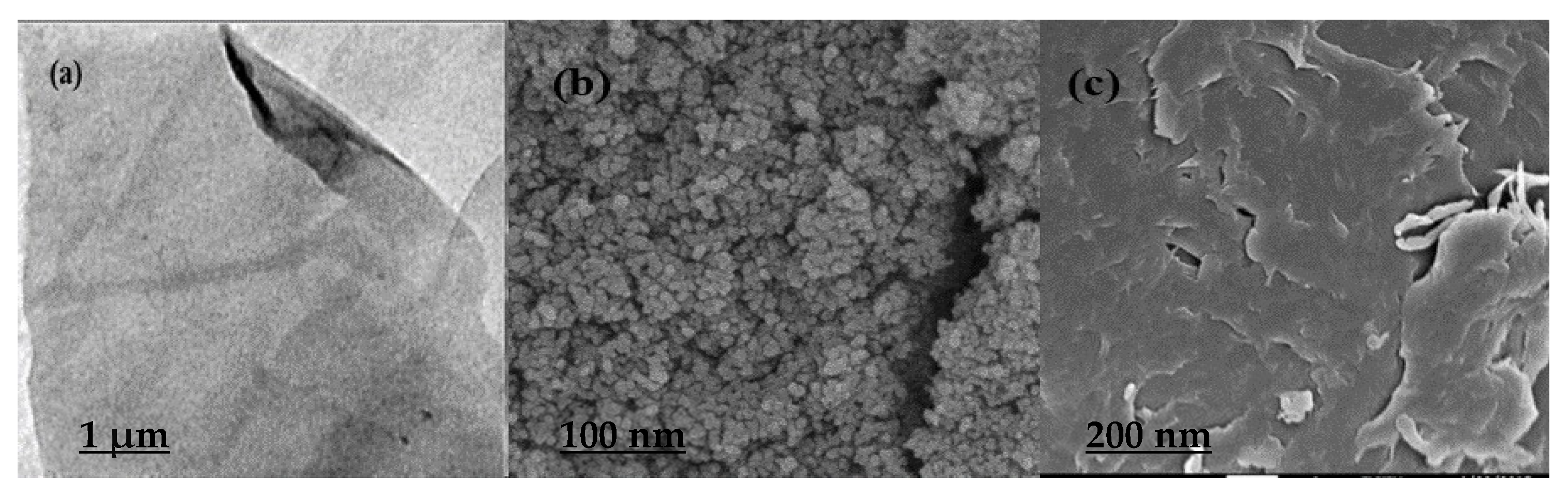
3.1. Characterization of Graphene Oxide and Magnetic Graphene Oxide
3.2. Fitting the Response Surface Models to Significant Independent Variables
3.3. Optimization and Verification Procedure
3.4. Adsorption Kinetics
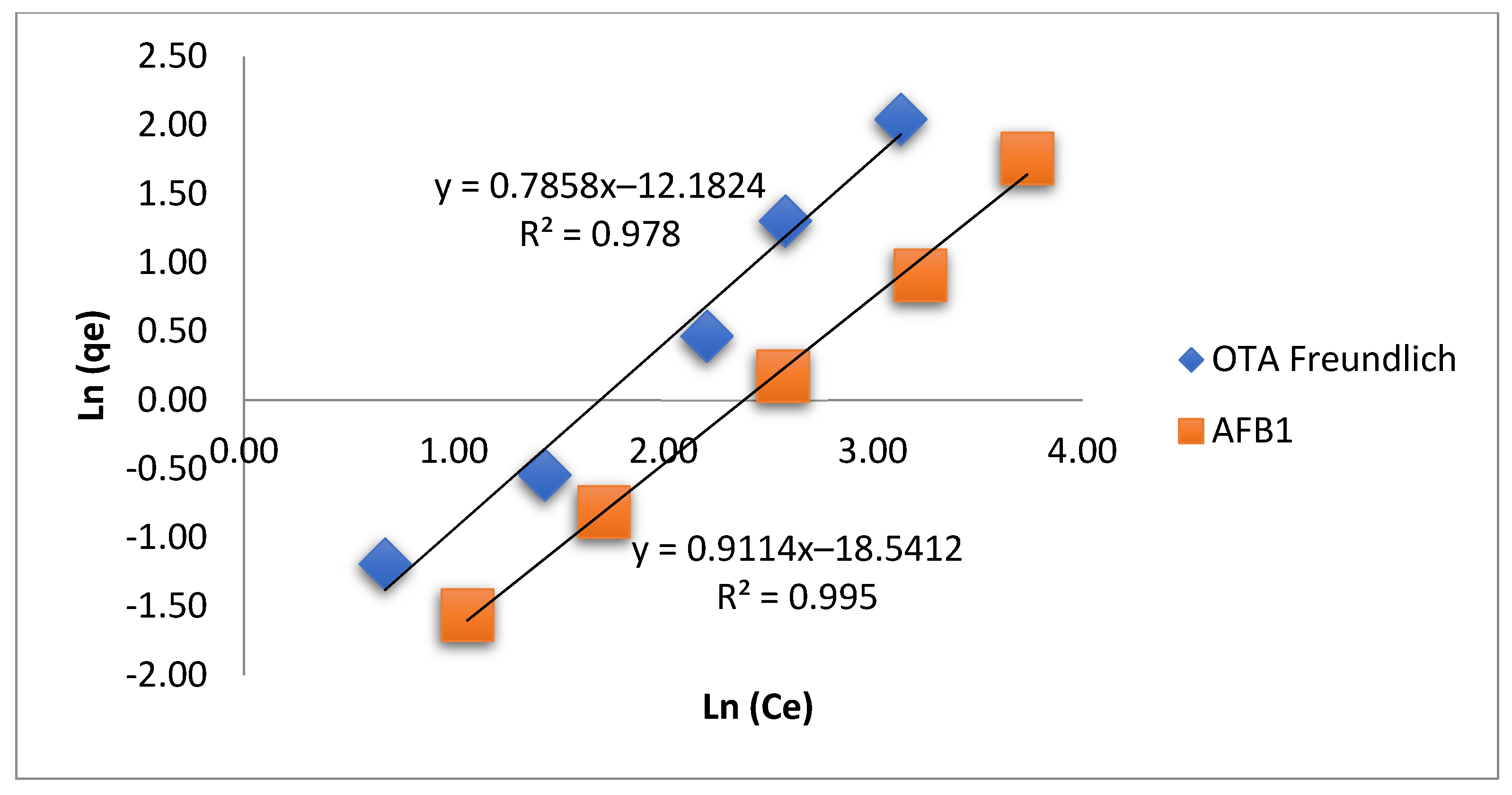
3.5. Adsorption Isotherm
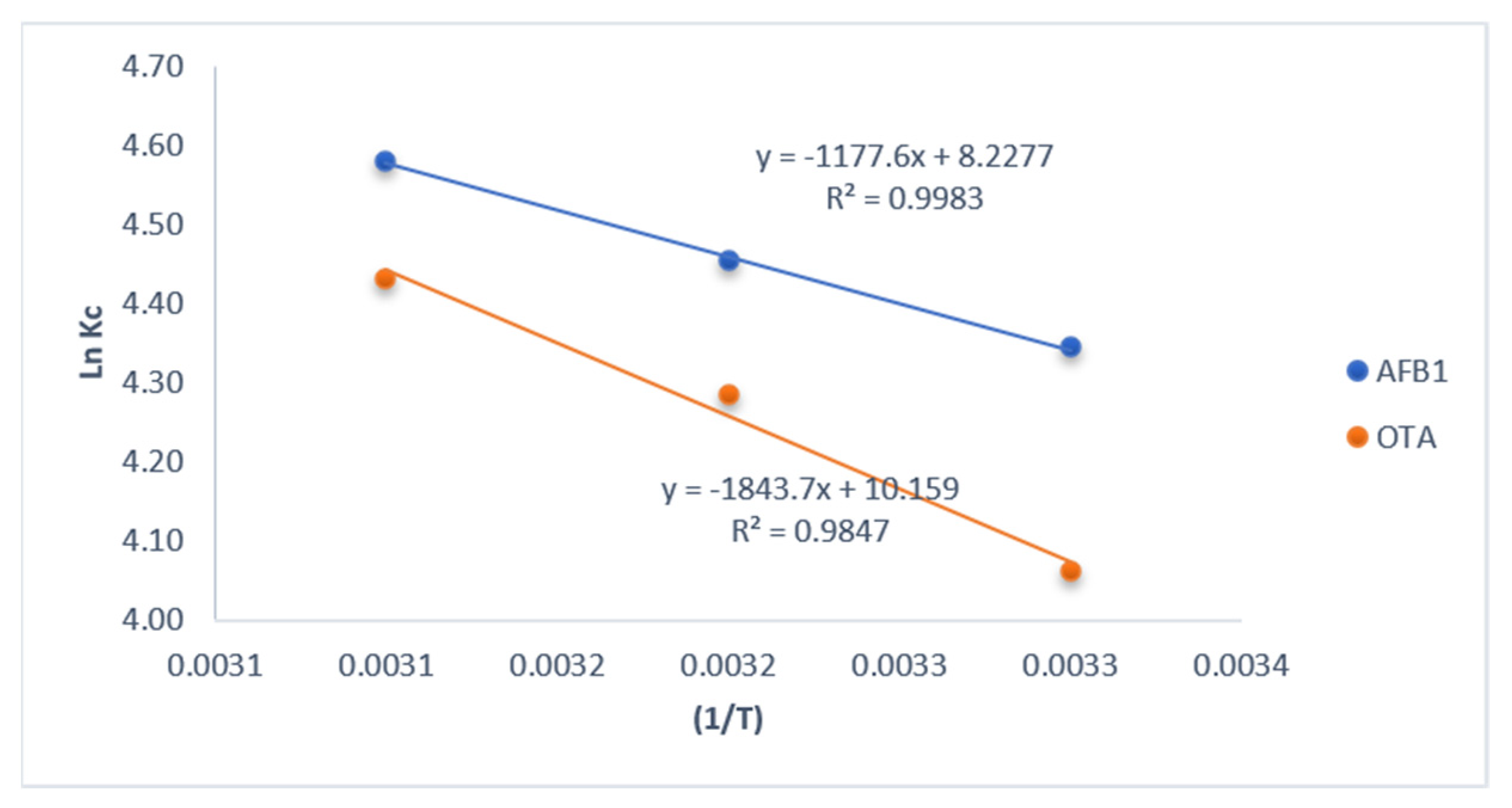
3.6. Thermodynamic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussein, H.S.; Brasel, J.M.J.T. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef] [Green Version]
- Bhat, R.; Rai, R.V.; Karim, A.A. Mycotoxins in food and feed: Present status and future concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Feng, Y.; Wei, J.-T.; Zhu, M.-X.; Zhang, L.; Zhang, J.-C.; Karrow, N.A.; Han, Y.-M.; Wu, Y.-Y.; Guo, Y.-M.J.T. Mitigation Effects of Bentonite and Yeast Cell Wall Binders on AFB1, DON, and OTA Induced Changes in Laying Hen Performance, Egg Quality, and Health. Toxins 2021, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, G.O.; Letuma, P. Strategies for the prevention and reduction of mycotoxins in developing countries. In Mycotoxin and Food Safety in Developing Countries; IntechOpen: London, UK, 2013. [Google Scholar]
- Luo, Y.; Liu, X.; Li, J. Updating techniques on controlling mycotoxins—A review. Food Control 2018, 89, 123–132. [Google Scholar] [CrossRef]
- Peng, W.-X.; Marchal, J.; van der Poel, A. Strategies to prevent and reduce mycotoxins for compound feed manufacturing. Anim. Feed Sci. Technol. 2018, 237, 129–153. [Google Scholar] [CrossRef]
- Zhu, Y.; Hassan, Y.I.; Watts, C.; Zhou, T. Innovative technologies for the mitigation of mycotoxins in animal feed and ingredients—A review of recent patents. Anim. Feed Sci. Technol. 2016, 216, 19–29. [Google Scholar] [CrossRef]
- Kolosova, A.; Stroka, J. Substances for reduction of the contamination of feed by mycotoxins: A review. World Mycotoxin J. 2011, 4, 225–256. [Google Scholar] [CrossRef]
- Manafi, M.; Umakantha, B.; Narayana Swamy, H.; Mohan, K. Evaluation of high-grade sodium bentonite on performance and immune status of broilers, fed ochratoxin and aflatoxin. World Mycotoxin J. 2009, 2, 435–440. [Google Scholar] [CrossRef]
- Sabater-Vilar, M.; Malekinejad, H.; Selman, M.; Van der Doelen, M.; Fink-Gremmels, J. In vitro assessment of adsorbents aiming to prevent deoxynivalenol and zearalenone mycotoxicoses. Mycopathologia 2007, 163, 81. [Google Scholar] [CrossRef] [Green Version]
- Sprynskyy, M.; Gadzała-Kopciuch, R.; Nowak, K.; Buszewski, B. Removal of zearalenone toxin from synthetics gastric and body fluids using talc and diatomite: A batch kinetic study. Colloids Surf. B Biointerfaces 2012, 94, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Boudergue, C.; Burel, C.; Dragacci, S.; FAVROT, M.C.; FREMY, J.M.; Massimi, C.; PRIGENT, P.; Debongnie, P.; Pussemier, L.; Boudra, H. Review of mycotoxin-detoxifying agents used as feed additives: Mode of action, efficacy and feed/food safety. EFSA Supporting Publ. 2009, 6, 22E. [Google Scholar] [CrossRef]
- Quintela, S.; Villarán, M.; De Armentia, I.L.; Elejalde, E. Ochratoxin A removal from red wine by several oenological fining agents: Bentonite, egg albumin, allergen-free adsorbents, chitin and chitosan. Food Addit. Contam. Part A 2012, 29, 1168–1174. [Google Scholar] [CrossRef]
- Liang, J.; Xu, Y.; Sui, D.; Zhang, L.; Huang, Y.; Ma, Y.; Li, F.; Chen, Y. Flexible, magnetic, and electrically conductive graphene/Fe3O4 paper and its application for magnetic-controlled switches. J. Phys. Chem. C 2010, 114, 17465–17471. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Chen, X.; Leng, L.; Wang, H.; Li, H.; Zeng, G. Facile synthesis of polypyrrole decorated reduced graphene oxide–Fe3O4 magnetic composites and its application for the Cr (VI) removal. Chem. Eng. J. 2015, 262, 597–606. [Google Scholar] [CrossRef]
- Sun, L.; Fugetsu, B. Mass production of graphene oxide from expanded graphite. Mater. Lett. 2013, 109, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Medina, A.; Casado-Carmona, F.A.; López-Lorente, Á.I.; Cárdenas, S.J.N. Magnetic graphene oxide composite for the microextraction and determination of benzophenones in water samples. Nanomaterials 2020, 10, 168. [Google Scholar] [CrossRef] [Green Version]
- Cui, G.; Lu, Y.; Zhou, W.; Lv, X.; Hu, J.; Zhang, G.; Gu, G.J.N. Excellent microwave absorption properties derived from the synthesis of hollow Fe3O4@reduced graphite oxide (RGO) nanocomposites. Nanomaterials 2019, 9, 141. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, A.; Solanki, P.R.; Ansari, A.A.; Ahmad, S.; Malhotra, B.D. Chitosan–iron oxide nanobiocomposite based immunosensor for ochratoxin-A. Electrochem. Commun. 2008, 10, 1364–1368. [Google Scholar] [CrossRef]
- Deng, J.-H.; Zhang, X.-R.; Zeng, G.-M.; Gong, J.-L.; Niu, Q.-Y.; Liang, J. Simultaneous removal of Cd (II) and ionic dyes from aqueous solution using magnetic graphene oxide nanocomposite as an adsorbent. Chem. Eng. J. 2013, 226, 189–200. [Google Scholar] [CrossRef]
- Purtov, K.; Shenderova, O.; Luo, M.; Brenner, D.; Bondar, V. The adsorption of aflatoxin B1 by detonation-synthesis nanodiamonds. In Proceedings of the Doklady Biochemistry and Biophysics, Krasnoyarsk, Russia, 22 May 2007; pp. 299–301. [Google Scholar]
- Soleimany, F.; Jinap, S.; Rahmani, A.; Khatib, A. Contaminants. Simultaneous detection of 12 mycotoxins in cereals using RP-HPLC-PDA-FLD with PHRED and a post-column derivatization system. Food Addit. Contam. Part A 2011, 28, 494–501. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Jinap, S.; Arzandeh, S.; Mirhosseini, H. Optimization of HPLC conditions for quantitative analysis of aflatoxins in contaminated peanut. Food Control 2011, 22, 381–388. [Google Scholar] [CrossRef]
- Ko, W.-C.; Chang, C.-K.; Wang, H.-J.; Wang, S.-J.; Hsieh, C.-W. Process optimization of microencapsulation of curcumin in γ-polyglutamic acid using response surface methodology. Food Chem. 2015, 172, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Mehrnoush, A.; Tan, C.P.; Hamed, M.; Aziz, N.A.; Ling, T.C. Optimisation of freeze drying conditions for purified serine protease from mango (Mangifera indica Cv. Chokanan) peel. Food Chem. 2011, 128, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Vea, M.; Corcuera, L.A.; Remiro, R.; Murillo-Arbizu, M.T.; González-Peñas, E.; Lizarraga, E. Validation of a UHPLC-FLD method for the simultaneous quantification of aflatoxins, ochratoxin A and zearalenone in barley. Food Chem. 2011, 127, 351–358. [Google Scholar] [CrossRef]
- Tran, L.; Wu, P.; Zhu, Y.; Liu, S.; Zhu, N. Comparative study of Hg (II) adsorption by thiol-and hydroxyl-containing bifunctional montmorillonite and vermiculite. Appl. Surf. Sci. 2015, 356, 91–101. [Google Scholar] [CrossRef]
- Robati, D. Pseudo-second-order kinetic equations for modeling adsorption systems for removal of lead ions using multi-walled carbon nanotube. J. Nanostructure Chem. 2013, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Li, Z.; Yuan, Y.; Yue, T. Bioadsorption of patulin from kiwi fruit juice onto a superior magnetic chitosan. J. Alloys Compd. 2016, 667, 101–108. [Google Scholar] [CrossRef]
- Azizian, S.; Haerifar, M.; Basiri-Parsa, J. Extended geometric method: A simple approach to derive adsorption rate constants of Langmuir–Freundlich kinetics. Chemosphere 2007, 68, 2040–2046. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Kilpatrick, M.; Baker, L., Jr.; McKinney, C., Jr. Studies of Fast Reactions Which Evolve Gases. the Reaction of Sodium-Potassium Alloy with Water in the Presence and Absence of Oxygen. Technical Report No. 6; Illinois Inst. of Tech.: Chicago, IL, USA, 1952. [Google Scholar]
- Barati, A.; Najafi, A.; Daryasafar, A.; Nadali, P.; Moslehi, H. Adsorption of a new nonionic surfactant on carbonate minerals in enhanced oil recovery: Experimental and modeling study. Chem. Eng. Res. Des. 2016, 105, 55–63. [Google Scholar] [CrossRef]
- Dada, A.O.; Ojediran, J.; Okunola, A.; Dada, F.E.; Lawal, A.; Isola, L.A.; Olalekan, A.P.; Dada, O.; Dada, J.O.O.; Ojediran, A.A.; et al. Modeling of biosorption of Pb (II) and Zn (II) ions onto PaMRH: Langmuir, Freundlich, Temkin, Dubinin-Raduskevich, Jovanovic, Flory-Huggins, Fowler-Guggenheim and Kiselev comparative isotherm studies. Int. J. Mech. Eng. Technol. 2019, 10, 1048–1058. [Google Scholar]
- Debnath, S.; Maity, A.; Pillay, K. Magnetic chitosan–GO nanocomposite: Synthesis, characterization and batch adsorber design for Cr (VI) removal. J. Environ. Chem. 2014, 2, 963–973. [Google Scholar] [CrossRef]
- Chuanyu, S.; Yu, W. Synthesis and characterization of graphene oxide composite with Fe3O4. Mater. Sci. 2015, 33, 488–490. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Guo, X.; Wei, Q.; Wang, Y.; Gao, L.; Yan, L.; Yan, T.; Du, B. Removal of mercury and methylene blue from aqueous solution by xanthate functionalized magnetic graphene oxide: Sorption kinetic and uptake mechanism. J. Colloid Interface Sci. 2015, 439, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Pirouz, A.; Selamat, J.; Iqbal, S.; Mirhosseini, H.; Karjiban, R.A.; Bakar, F.A. The use of innovative and efficient nanocomposite (magnetic graphene oxide) for the reduction on of Fusarium mycotoxins in palm kernel cake. Sci. Rep. 2017, 7, 12453. [Google Scholar] [CrossRef]
- Yang, J.H.; Ramaraj, B.; Yoon, K.R. Preparation and characterization of superparamagnetic graphene oxide nanohybrids anchored with Fe3O4 nanoparticles. J. Alloys Compd. 2014, 583, 128–133. [Google Scholar] [CrossRef]
- Pirouz, A.A.; Karjiban, R.A.; Bakar, F.A.; Selamat, J. A novel adsorbent magnetic graphene oxide modified with chitosan for the simultaneous reduction of mycotoxins. Toxins 2018, 10, 361. [Google Scholar] [CrossRef] [Green Version]
- Pirouz, A.A.; Selamat, J.; Iqbal, S.Z.; Samsudin, N.I.P. Efficient and simultaneous chitosan-mediated removal of 11 mycotoxins from palm kernel cake. Toxins 2020, 12, 115. [Google Scholar] [CrossRef] [Green Version]
- Mirhosseini, H.; Tan, C.P.; Taherian, A.R.; Boo, H.C. Modeling the physicochemical properties of orange beverage emulsion as function of main emulsion components using response surface methodology. Carbohydr. Polym. 2009, 75, 512–520. [Google Scholar] [CrossRef]
- Li, L.; Fan, L.; Sun, M.; Qiu, H.; Li, X.; Duan, H.; Luo, C. Adsorbent for chromium removal based on graphene oxide functionalized with magnetic cyclodextrin–chitosan. Colloids Surf. B Biointerfaces 2013, 107, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Hafshejani, L.D.; Nasab, S.B.; Gholami, R.M.; Moradzadeh, M.; Izadpanah, Z.; Hafshejani, S.B.; Bhatnagar, A. Removal of zinc and lead from aqueous solution by nanostructured cedar leaf ash as biosorbent. J. Mol. Liq. 2015, 211, 448–456. [Google Scholar] [CrossRef]
- Baccar, R.; Sarrà, M.; Bouzid, J.; Feki, M.; Blánquez, P. Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem. Eng. J. 2012, 211, 310–317. [Google Scholar] [CrossRef]
- Yibadatihan, S.; Jinap, S.; Mahyudin, N.A. Simultaneous determination of multi-mycotoxins in palm kernel cake (PKC) using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Food Addit. Contam. Part A 2014, 31, 2071–2079. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, N.; Yang, L.; Wang, J.; Song, S.; Nie, D.; Yang, X.; Hou, J.; Wu, A. Cross-linked chitosan polymers as generic adsorbents for simultaneous adsorption of multiple mycotoxins. Food Control 2015, 57, 362–369. [Google Scholar] [CrossRef]
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A.J. New mycotoxin adsorbents based on tri-octahedral bentonites for animal feed. Anim. Feed. Sci. Technol. 2019, 255, 114228. [Google Scholar] [CrossRef]
- Wu, H.; Salles, F.; Zajac, J. A critical review of solid materials for low-temperature thermochemical storage of solar energy based on solid-vapour adsorption in view of space heating uses. Molecules 2019, 24, 945. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Experimental Variables | Experimental Variables Level | ||
|---|---|---|---|
| Independent variables | −1 | 0 | +1 |
| pH (X1) | 3 | 5 | 7 |
| Time (X2) | 3 | 5 | 7 |
| Temperature (X3) | 30 | 40 | 50 |
| Response | pH | Time (h) | Temperature (°C) | Y0 | Yi | Y0 − Yi | Desirability |
|---|---|---|---|---|---|---|---|
| AFB1 | 5 | 5 | 40 | 94.09 ± 1.78 | 90.59 ± 1.05 | 3.5 ± 0.73 | 0.934 |
| OTA | 5 | 5 | 40 | 58.16 ± 2.06 | 59.96 ± 1.41 | −1.80 ± 0.65 | 0.934 |
| Mycotoxins | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| k1 (1/min) | qe | R2 | k2 (g/ng·min) | qe | R2 | |
| AFB1 | 0.004 | 2.36 | 0.935 | 0.003 | 4.08 | 0.965 |
| OTA | 0.005 | 2.02 | 0.925 | 0.004 | 3.18 | 0.981 |
| Mycotoxin | Langmuir Model | Freundlich Model | ||||||
|---|---|---|---|---|---|---|---|---|
| T (°C) | qmax (ng/g) | KL (L/mg) | R2 | KF (ng·L/g) | n | qmax (ng/g) | R2 | |
| AFB1 | 40 | 4.93 | 0.68 | 0.847 | 2.92 | 1.10 | 153 | 0.995 |
| OTA | 40 | 6.54 | 0.01 | 0.959 | 2.50 | 1.27 | 95 | 0.978 |
| Mycotoxin | C0 ng/g | qe | Kc | ln(Kc) | T | ΔG° (kj/mol·K) | ΔH° (kj/mol) | ΔS° (j/mol·K) |
|---|---|---|---|---|---|---|---|---|
| AFB1 | 100 | 7704 | 77.04 | 4.34 | 303 | −3.69 | −1.18 | 8.27 |
| 100 | 8594 | 85.94 | 4.45 | 313 | −3.77 | – | – | |
| 100 | 9750 | 97.50 | 4.58 | 323 | −3.85 | – | – | |
| OTA | 100 | 5806 | 58.06 | 4.06 | 303 | −4.92 | −1.84 | 10.16 |
| 100 | 7265 | 72.65 | 4.29 | 313 | −5.02 | – | – | |
| 100 | 8395 | 83.95 | 4.43 | 323 | −5.12 | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasi Pirouz, A.; Selamat, J.; Sukor, R.; Noorahya Jambari, N. Effective Detoxification of Aflatoxin B1 and Ochratoxin A Using Magnetic Graphene Oxide Nanocomposite: Isotherm and Kinetic Study. Coatings 2021, 11, 1346. https://doi.org/10.3390/coatings11111346
Abbasi Pirouz A, Selamat J, Sukor R, Noorahya Jambari N. Effective Detoxification of Aflatoxin B1 and Ochratoxin A Using Magnetic Graphene Oxide Nanocomposite: Isotherm and Kinetic Study. Coatings. 2021; 11(11):1346. https://doi.org/10.3390/coatings11111346
Chicago/Turabian StyleAbbasi Pirouz, Atena, Jinap Selamat, Rashidah Sukor, and Nuzul Noorahya Jambari. 2021. "Effective Detoxification of Aflatoxin B1 and Ochratoxin A Using Magnetic Graphene Oxide Nanocomposite: Isotherm and Kinetic Study" Coatings 11, no. 11: 1346. https://doi.org/10.3390/coatings11111346





