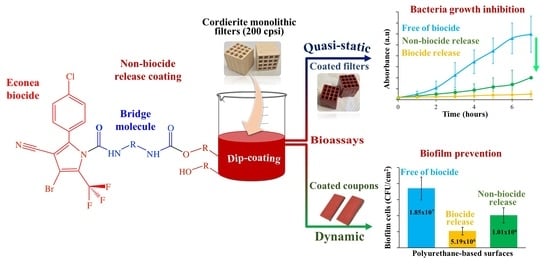
Antimicrobial Ceramic Filters for Water Bio-Decontamination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Monolithic Filters Coated with Biocide Non-Release Coatings
2.2. Structural Characterization and Antimicrobial Activity of Biocidal Agents
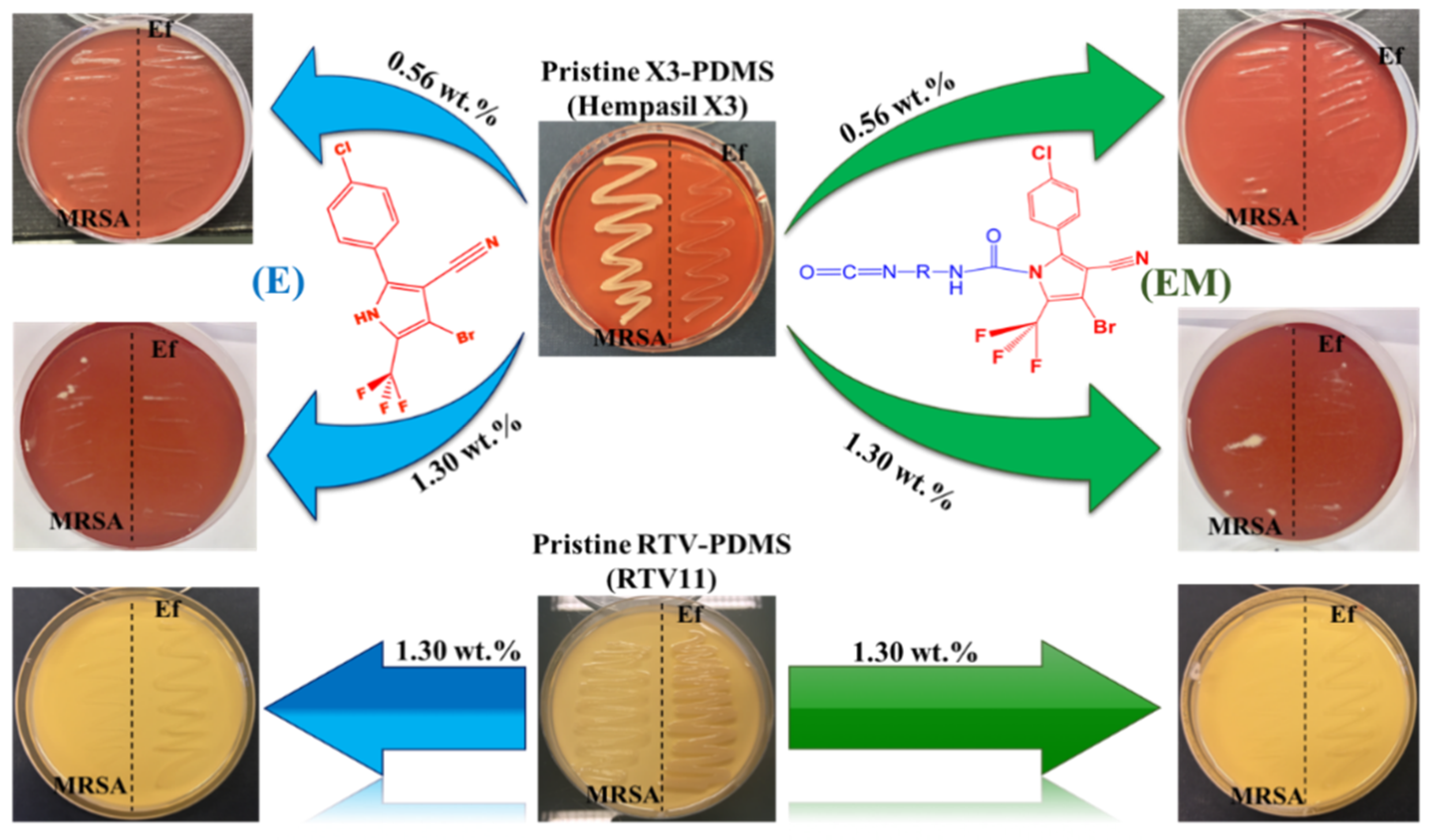
2.2.1. Antimicrobial Susceptibility by Direct Contact
2.2.2. Microdilution Method—Minimum Inhibitory Concentrations (MICs) and Minimum Bactericidal Concentrations (MBCs)
2.3. Antimicrobial Potential of Developed Biocidal Coatings
2.4. Biocide Grafting Efficacy on Coated Monolithic Filters
2.5. Water Bio-Decontamination Potential of Non-Biocide-Release-Coated Substrates
2.5.1. Antimicrobial Susceptibility of Coated Monolithic Filters
2.5.2. Biofilm Formation on Coated Substrates under Dynamic Conditions
3. Results and Discussion
3.1. Structural Characterization and Antimicrobial Activity of Biocidal Agents
3.2. Biocidal Coating Formulations
3.2.1. Antimicrobial Potential of Developed Biocidal Coatings
3.2.2. Biocide Grafting Efficacy on Coated Monolithic Filters
3.3. Potential of Non-Biocide-Release-Coated Filters for Waterborne Bio-Decontamination
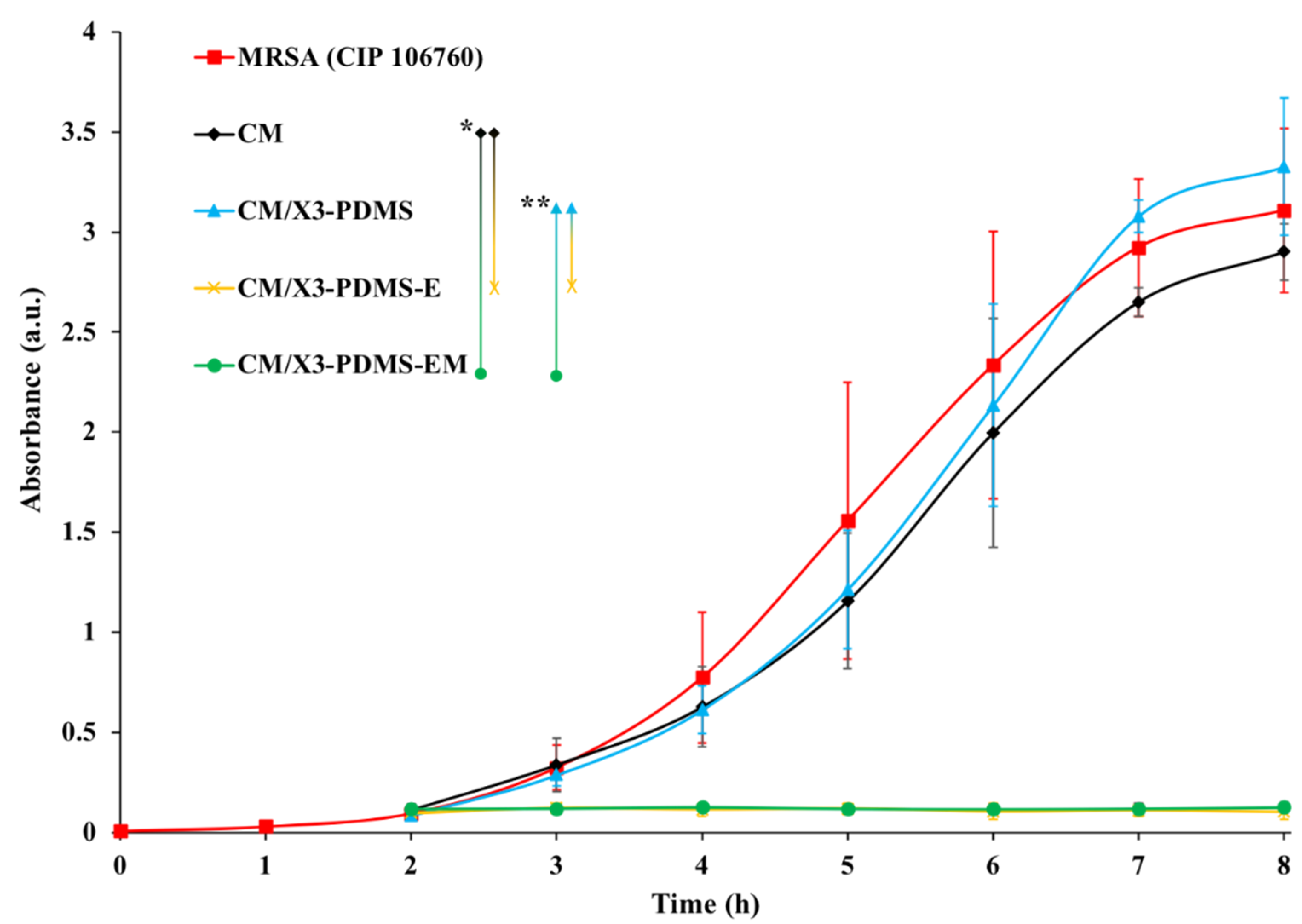
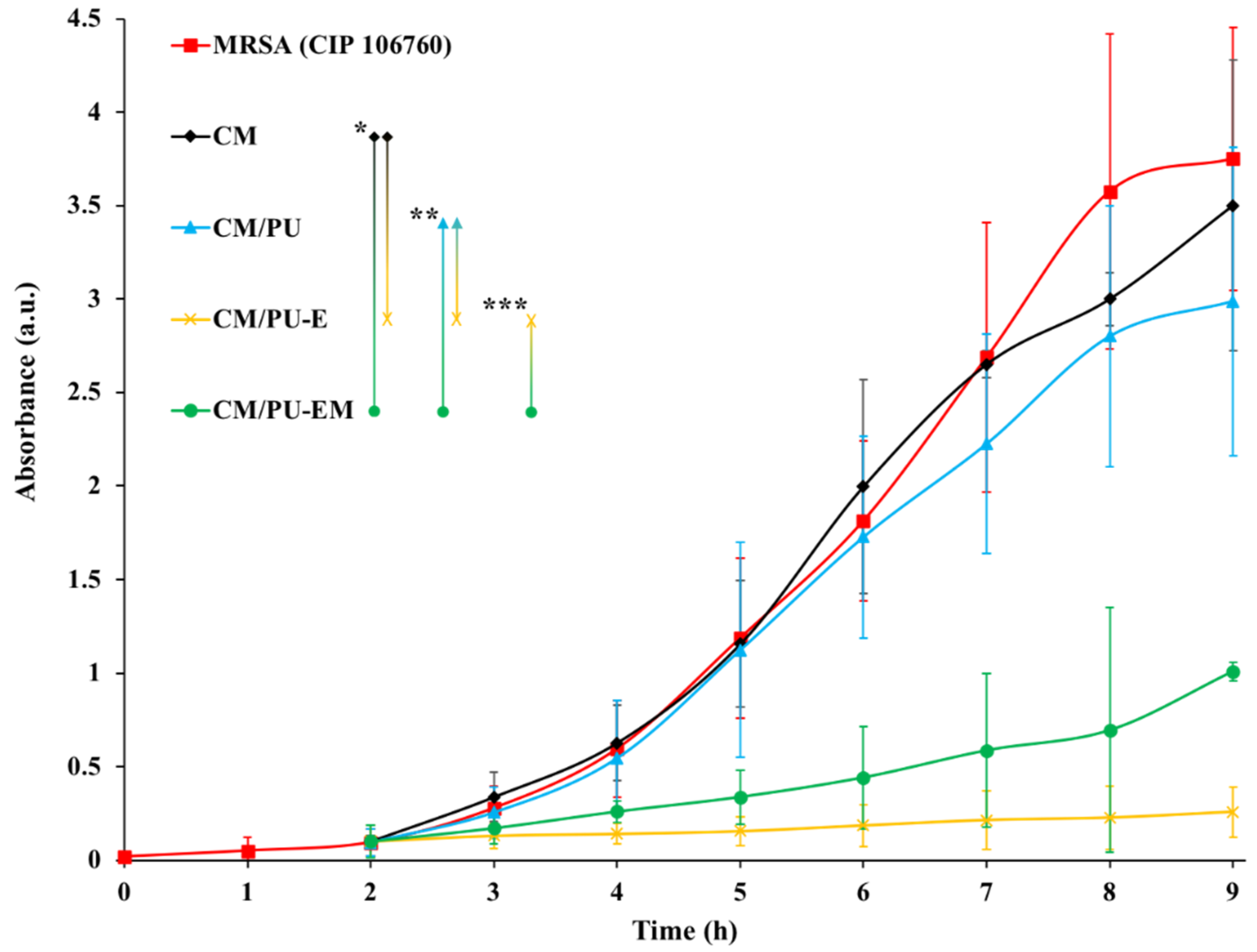
3.3.1. Antimicrobial Susceptibility of Coated Monolithic Filters
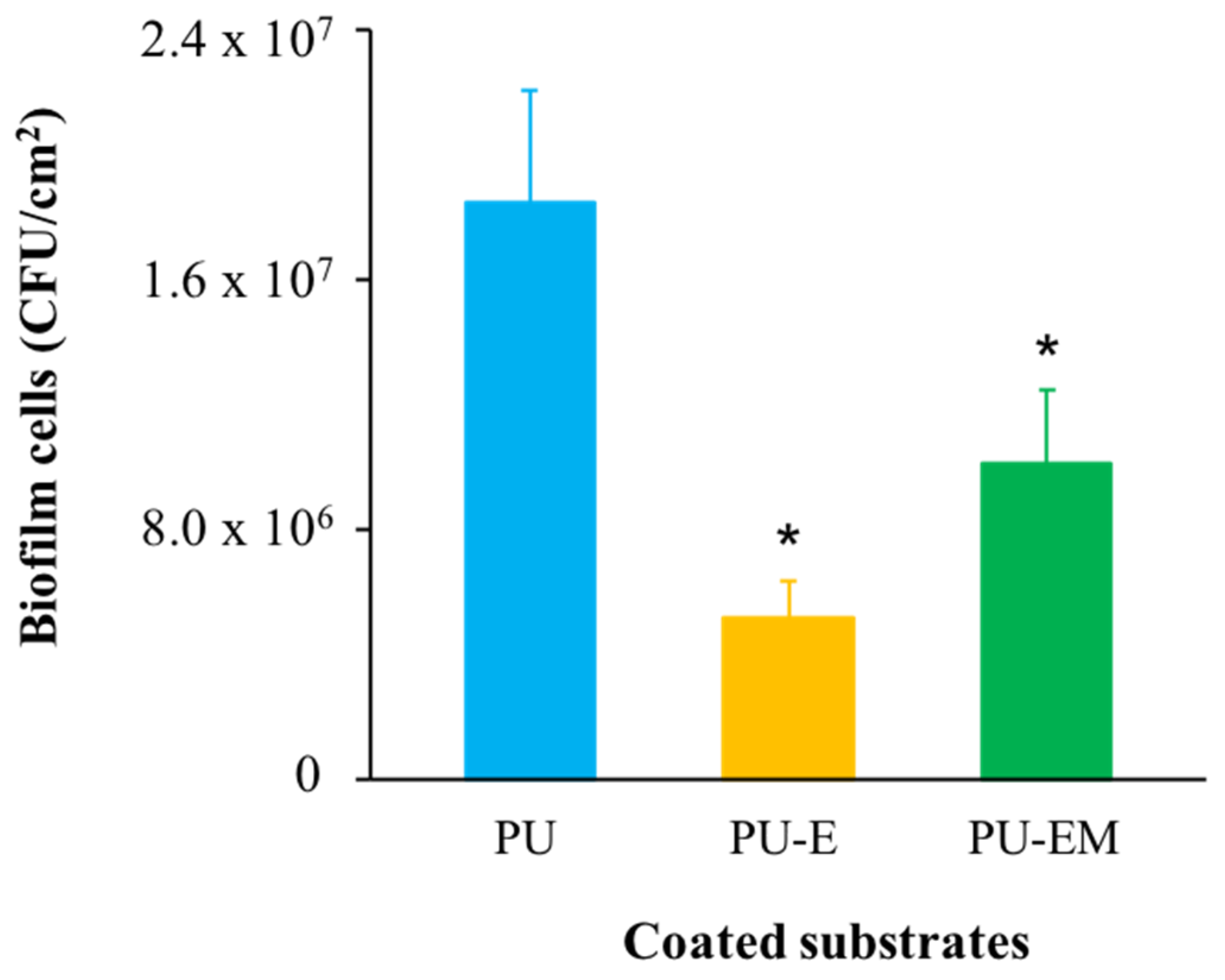
3.3.2. Biofilm Formation on Coated Substrates under Dynamic Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. MWH’s Water Treatment Principles and Design, 3rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Heydarifard, S.; Pan, Y.; Xiao, H.; Nazhad, M.; Shipin, O. Water-resistant cellulosic filter containing non-leaching antimicrobial starch for water purification and disinfection. Carbohydr. Polym. 2017, 163, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, G.M.; Rawlings, T.K.; Dobbs, F.C.; Drake, L.A.; Mullady, T.; Huq, A.; Colwell, R.R. Global spread of microorganisms by ships. Nature 2000, 408, 49–50. [Google Scholar] [CrossRef]
- Fish, K.E.; Osborn, A.M.; Boxall, J. Characterising and understanding the impact of microbial biofilms and the extracellular polymeric substance (EPS) matrix in drinking water distribution systems. Environ. Sci. Water Res. Technol. 2016, 2, 614–630. [Google Scholar] [CrossRef] [Green Version]
- Altug, G.; Gurun, S.; Cardak, M.; Ciftci, P.S.; Kalkan, S. The occurrence of pathogenic bacteria in some ships’ ballast water incoming from various marine regions to the Sea of Marmara, Turkey. Mar. Environ. Res. 2012, 81, 35–42. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Solon, K.; Volcke, E.I.P.; Spérandio, M.; Loosdrecht, M.C.M. Resource recovery and wastewater treatment modelling. Environ. Sci. Water Res. Technol. 2019, 5, 631–642. [Google Scholar] [CrossRef]
- Ali, A.; Jamil, M.I.; Jiang, J.; Shoaib, M.; Amin, B.U.; Luo, S.; Zhan, X.; Chen, F.; Zhang, Q. An overview of controlled-biocide-release coating based on polymeric resin for marine antifouling applications. J. Polym. Res. 2020, 27, 1–17. [Google Scholar] [CrossRef]
- Ng, C.; Chen, H.; Goh, S.G.; Haller, L.; Charles, F.R.; Wu, Z.; Trottet, A.; Gin, K.Y.H. Microbial water quality and the detection of multidrug resistant E. coli and antibiotic resistance genes in aquaculture sites of Singapore. Mar. Pollut. Bull. 2018, 135, 475–480. [Google Scholar] [CrossRef]
- Ng, C.; Goh, S.G.; Saeidi, N.; Gerhard, W.A.; Gunsch, C.K.; Gin, K.Y.H. Occurrence of Vibrio species, beta-lactam resistant Vibrio species, and indicator bacteria in ballast and port waters of a tropical harbour. Sci. Total Environ. 2018, 610, 651–656. [Google Scholar] [CrossRef]
- Maddah, H.; Chogle, A. Biofouling in reverse osmosis: Phenomena, monitoring, controlling and remediation. Appl. Water Sci. 2017, 7, 2637–2651. [Google Scholar] [CrossRef] [Green Version]
- Noori, M.T.; Ghangrekar, M.M.; Mukherjee, C.K.; Min, B. Biofouling effects on the performance of microbial fuel cells and recent advances in biotechnological and chemical strategies for mitigation. Biotechnol. Adv. 2019, 37, 107420. [Google Scholar] [CrossRef] [PubMed]
- Coetser, S.E.; Cloete, T.E. Biofouling and biocorrosion in industrial water systems. Crit. Rev. Microbiol. 2005, 31, 213–232. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.-D. Biofouling and prevention: Corrosion, biodeterioration and biodegradation of materials. In Handbook of Environmental Degradation of Materials; William Andrew Publishing: New York, NY, USA, 2005; pp. 179–206. [Google Scholar] [CrossRef]
- Nagaraj, V.; Skillman, L.; Li, D.; Ho, G. Review—Bacteria and their extracellular polymeric substances causing biofouling on seawater reverse osmosis desalination membranes. J. Environ. Manag. 2018, 223, 586–599. [Google Scholar] [CrossRef]
- Chan, J.; Wong, S. Biofouling: Types, impact and anti-fouling. In Pollution Science, Technology and Abatement, 1st ed.; Nova Science Publishers Inc.: New York, NY, USA, 2010; ISBN 978-1-60876-501-0. [Google Scholar]
- Lv, B.; Cui, Y.; Tian, W.; Wei, H.; Chen, Q.; Liu, B.; Zhang, D.; Xie, B. Vessel transport of antibiotic resistance genes across oceans and its implication for ballast water management. Chemosphere 2020, 253, 126697. [Google Scholar] [CrossRef]
- Flemming, H.C. Microbial biofouling: Unsolved problems, insufficient approaches, and possible solutions. In Biofilm Highlights; Flemming, H.C., Wingender, J., Szewzyk, U., Eds.; Springer Publishing: Berlin/Heidelberg, Germany, 2011; Volume 5, pp. 81–109. [Google Scholar] [CrossRef]
- Hammes, F.; Berney, M.; Wang, Y.; Vital, M.; Köster, O.; Egli, T. Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes. Water Res. 2008, 42, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Batista, W.R.; Fernandes, F.C.; Lopes, C.C.; Lopes, R.S.C.; Miller, W.; Ruiz, G. Which ballast water management system will you put aboard? Remnant anxieties: A mini-review. Environments 2017, 4, 54. [Google Scholar] [CrossRef] [Green Version]
- Lehaitre, M.; Compère, C. Biofouling and underwater measurement. In Real-Time Coastal Observing Systems for Marine Ecosystem Dynamics and Harmful Algal Blooms: Theory, Instrumentation and Modelling; Babin, M., Roesler, C.S., Cullen, J., Eds.; UNESCO Publishing: Paris, France, 2008; pp. 463–493. ISBN 978-92-3-104042-9. [Google Scholar]
- Silva, E.R.; Bordado, J.C.M.; Ferreira, O.R.V. Functionalization Process for Biocide Immobilization in Polymer Matrices. Granted Patent PT108096B (WO2016/093719A1), 12 July 2019. Available online: https://patents.google.com/patent/PT108096B/en (accessed on 2 March 2021).
- Silva, E.R.; Ferreira, O.; Ramalho, P.A.; Azevedo, N.F.; Bayón, R.; Igartua, A.; Bordado, J.C.; Calhorda, M.J. Eco-friendly non-biocide-release coatings for marine biofouling prevention. Sci. Total Environ. 2019, 650 Pt 2, 2499–2511. [Google Scholar] [CrossRef]
- MacCullum, N.; Howell, C.; Kim, P.; Sun, D.; Friedlander, R.; Ranisau, J.; Ahanotu, O.; Lin, J.J.; Vena, A.; Hatton, B.; et al. Liquid-Infused silicone as a biofouling-free medical material. ACS Biomater. Sci. Eng. 2015, 1, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Song, X.F.; Cui, L.Y.; Qi, Y.H. Synthesis of polydimethylsiloxane-modified polyurethane and the structure and properties of its antifouling coatings. Coatings 2018, 8, 157. [Google Scholar] [CrossRef] [Green Version]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling release coatings: A nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, J.; Chen, H.; Chen, D. Progress of marine biofouling and antifouling technologies. Chin. Sci. Bull. 2011, 56, 598–612. [Google Scholar] [CrossRef] [Green Version]
- Kriyzelr, S.; Kritzler, M. Biocidal Coating. PCT Patent Application WO 2013036996 A1, 21 March 2013. Available online: https://patents.google.com/patent/WO2013036996A1/un (accessed on 2 March 2021).
- Abe, Y.; Skali-Lami, S.; Block, J.C.; Francius, G. Cohesiveness and hydrodynamic properties of young drinking water biofilms. Water Res. 2012, 46, 1155–1166. [Google Scholar] [CrossRef]
- Moreira, J.M.R.; Fulgêncio, R.; Oliveira, F.; Machado, I.; Bialuch, I.; Melo, L.F.; Simões, M.; Mergulão, F.J. Evaluation of SICON® surfaces for biofouling mitigation in critical process areas. Food Bioprod. Process. 2016, 98, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Teodósio, J.S.; Silva, F.C.; Moreira, J.M.R.; Simões, M.; Melo, L.F.; Alves, M.A.; Mergulhão, F.J. Flow cells as quasi-ideal systems for biofouling simulation of industrial piping systems. Biofouling 2013, 29, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.M.R.; Fulgêncio, R.; Alves, P.; Machado, I.; Bialuch, I.; Melo, L.F.; Simões, M.; Mergulhão, F.J. Evaluation of SICAN performance for biofouling mitigation in the food industry. Food Control. 2016, 62, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Kunze, A.; Pei, L.; Elsässer, D.; Niessner, R.; Seidel, M. High performance concentration method for viruses in drinking water. J. Virol. Methods 2015, 222, 132–137. [Google Scholar] [CrossRef]
- Ferreira, O.; Rijo, P.; Gomes, J.F.; Santos, R.; Monteiro, S.; Vilas-Boas, C.; Correia-da-Silva, M.; Almada, S.; Alves, L.G.; Bordado, J.C.; et al. Biofouling inhibition with grafted Econea biocide: Toward a nonreleasing eco-friendly multiresistant antifouling coating. ACS Sustain. Chem. Eng. 2020, 8, 12–17. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fifth Informational Supplement; CLSI Document M100-S25; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2005; ISBN 1-56238-990-4. [Google Scholar]
- OECD. Test No. 313: Estimation of Emissions from Preservative—Treated Wood to the Environment: Laboratory Method for Wooden Commodities that are not Covered and are in Contact with Fresh Water or Seawater. In OECD Guidelines for the Testing of Chemicals, Section 3; OECD Publishing: Paris, France, 2007. [Google Scholar] [CrossRef] [Green Version]
- ISO 15181-1:2007. Paints and Varnishes-Determination of Release Rate of Biocides from Antifouling Paints. Part 1: General Method for Extraction of Biocides; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- Gomes, L.C.; Deschamps, J.; Briandet, R.; Mergulhão, F.J. Impact of modified diamond-like carbon coatings on the spatial organization and disinfection of mixed-biofilms composed of Escherichia coli and Pantoea agglomerans industrial isolates. Int. J. Food Microbiol. 2018, 277, 74–82. [Google Scholar] [CrossRef]
- Teodósio, J.S.; Simões, M.; Melo, L.F.; Mergulhão, F.J. Flow cell hydrodynamics and their effects on E. coli biofilm formation under different nutrient conditions and turbulent flow. Biofouling 2011, 27, 1–11. [Google Scholar] [CrossRef]
- Heath, R. Chapter 28—Isocyanate-Based Polymers: Polyurethanes, Polyureas, Polyisocyanurates, and their Copolymers. In Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 799–835. ISBN 978-0-323-35824-8. [Google Scholar] [CrossRef]
- French, G.L. Bactericidal agents in the treatment of MRSA infections—The potential role of daptomycin. J. Antimicrob. Chemother. 2006, 58, 1107–1117. [Google Scholar] [CrossRef] [Green Version]
- Downs, R.A.; Dean, J.R.; Downer, A.; Perry, J.J. Determination of the biocide Econea® in artificial seawater by solid phase extraction and high performance liquid chromatography mass spectrometry. Separations 2017, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Johnson, R.W.; Desai, T.A. XPS and AFM analysis of antifouling PEG interfaces for microfabricated silicon biosensors. Biosens. Bioelectron. 2004, 20, 227–239. [Google Scholar] [CrossRef]
- Zhang, X.; Brodus, D.S.; Hollimon, V.; Hu, H. A brief review of recent developments in the designs that prevent biofouling on silicon and silicon-based materials. Chem. Cent. J. 2017, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hempel Product Data HEMPASIL X3+ 87500; Hempel: Lyngby, Denmark, 2016.

| Coating Formulation 1 | Polymeric Matrix | Biocide Immobilization | Base Resin/Curing Agent Ratio (w/w) | Solvent/Biocide Ratio (w/w) 2 | Biocide Content in Wet Formulation (wt.%) |
|---|---|---|---|---|---|
| Polymeric coatings formulations | |||||
| X3-PDMS (control) | PDMS | - | 7.74 | - | - |
| X3-PDMS/E | PDMS | Incorporation | 7.77 | 1.19 | 0.58 ± 0.01 |
| X3-PDMS/EM | PDMS | Grafting | 7.36 | 1.72 | 0.60 ± 0.01 |
| PU (control) | PU | - | 8.61 | - | - |
| PU/E | PU | Incorporation | 9.23 | 1.34 | 2.06 ± 0.02 |
| PU/EM | PU | Grafting | 9.03 | 1.17 | 1.96 ± 0.02 |
| Polymeric coatings formulations for pre-screening bioassays | |||||
| X3-PDMS (control) | PDMS | - | 7.92 | - | - |
| X3-PDMS/E | PDMS | Incorporation | 7.92 8.94 | 2.28 2.09 | 0.57 ± 0.01 1.31 ± 0.01 |
| X3-PDMS/EM | PDMS | Grafting | 8.86 8.39 | 1.60 1.91 | 0.59 ± 0.01 1.24 ± 0.01 |
| PU (control) | PU | - | 9.31 | - | - |
| PU/EM | PU | Grafting | 9.27 9.21 | 2.79 2.79 | 1.96 ± 0.02 3.03 ± 0.02 |
| RTV-PDMS (control) | PDMS | - | 162.11 | - | - |
| RTV-PDMS/E | PDMS | Incorporation | 151.76 | 1.92 | 1.36 ± 0.01 |
| RTV-PDMS/EM | PDMS | Grafting | 190.97 | 2.27 | 1.32 ± 0.01 |
| Coated Monolith Filters (CM) 1 | Rt (min) | [M] | [M − H]−/[M + H]+ | Error (Δppm) | Formula | Proposed Compound |
|---|---|---|---|---|---|---|
| ESI negative mode | ||||||
| CM/X3-PDMS-E− | 11.6 | 323.9296 | 322.9220 | 2.6 | C12H6BrClN2O2 | BCCPCA |
| CM/X3-PDMS-EM− | 11.4 | 323.9296 | 322.9239 | −3.3 | C12H6BrClN2O2 | BCCPCA |
| CM/PU-E− | 11.3 | 323.9296 | 322.9232 | −1.1 | C12H6BrClN2O2 | BCCPCA |
| CM/PU-EM− 2 | - | - | - | - | - | - |
| ESI positive mode | ||||||
| CM/X3-PDMS-E+ | 11.2 | 323.9296 | 324.9376 | −0.6 | C12H6BrClN2O2 | BCCPCA |
| CM/X3-PDMS-EM+ 2 | - | - | - | - | - | - |
| CM/PU-E+ 2 | - | - | - | - | - | - |
| CM/PU-EM+ 2 | - | - | - | - | - | - |
| Coated Monolithic Filters 1 | Biocide Content (mg) | Concentration in Leaching Waters (45 days) | Econea Biocide Leached from Coated Filters (wt.%) | |
|---|---|---|---|---|
| BCCPCA mg/L | Econea 2 mg/L | |||
| CM/X3-PDMS | - | - | - | - |
| CM/X3-PDMS-E | 4.82 | 4.12 | 4.43 | 22.96 |
| CM/X3-PDMS-EM | 3.62 | 0.45 | 0.48 | 3.32 |
| CM/PU | - | - | - | - |
| CM/PU-E | 15.04 | 0.88 | 0.95 | 1.57 |
| CM/PU-EM | 13.79 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, O.; Rijo, P.; Gomes, J.; Santos, R.; Monteiro, S.; Guedes, R.; Serralheiro, M.L.; Gomes, M.; Gomes, L.C.; Mergulhão, F.J.; et al. Antimicrobial Ceramic Filters for Water Bio-Decontamination. Coatings 2021, 11, 323. https://doi.org/10.3390/coatings11030323
Ferreira O, Rijo P, Gomes J, Santos R, Monteiro S, Guedes R, Serralheiro ML, Gomes M, Gomes LC, Mergulhão FJ, et al. Antimicrobial Ceramic Filters for Water Bio-Decontamination. Coatings. 2021; 11(3):323. https://doi.org/10.3390/coatings11030323
Chicago/Turabian StyleFerreira, Olga, Patrícia Rijo, João Gomes, Ricardo Santos, Sílvia Monteiro, Rita Guedes, Maria Luísa Serralheiro, Marisa Gomes, Luciana C. Gomes, Filipe J. Mergulhão, and et al. 2021. "Antimicrobial Ceramic Filters for Water Bio-Decontamination" Coatings 11, no. 3: 323. https://doi.org/10.3390/coatings11030323