Tuning the Surface Characteristic of Al-Si Alloys and Its Impacts on the Formation of Micro Arc Oxidation Layers
Abstract
1. Introduction
2. Experiments
2.1. Layer Fabrication
2.2. Layer Characterization
3. Results and Discussions
3.1. Effects of Etching Time on the MAO of Al-12 Si Alloy
3.2. Effects of Etching on the MAO of Hypo- and Hyper-Eutectic Al-Si Alloys
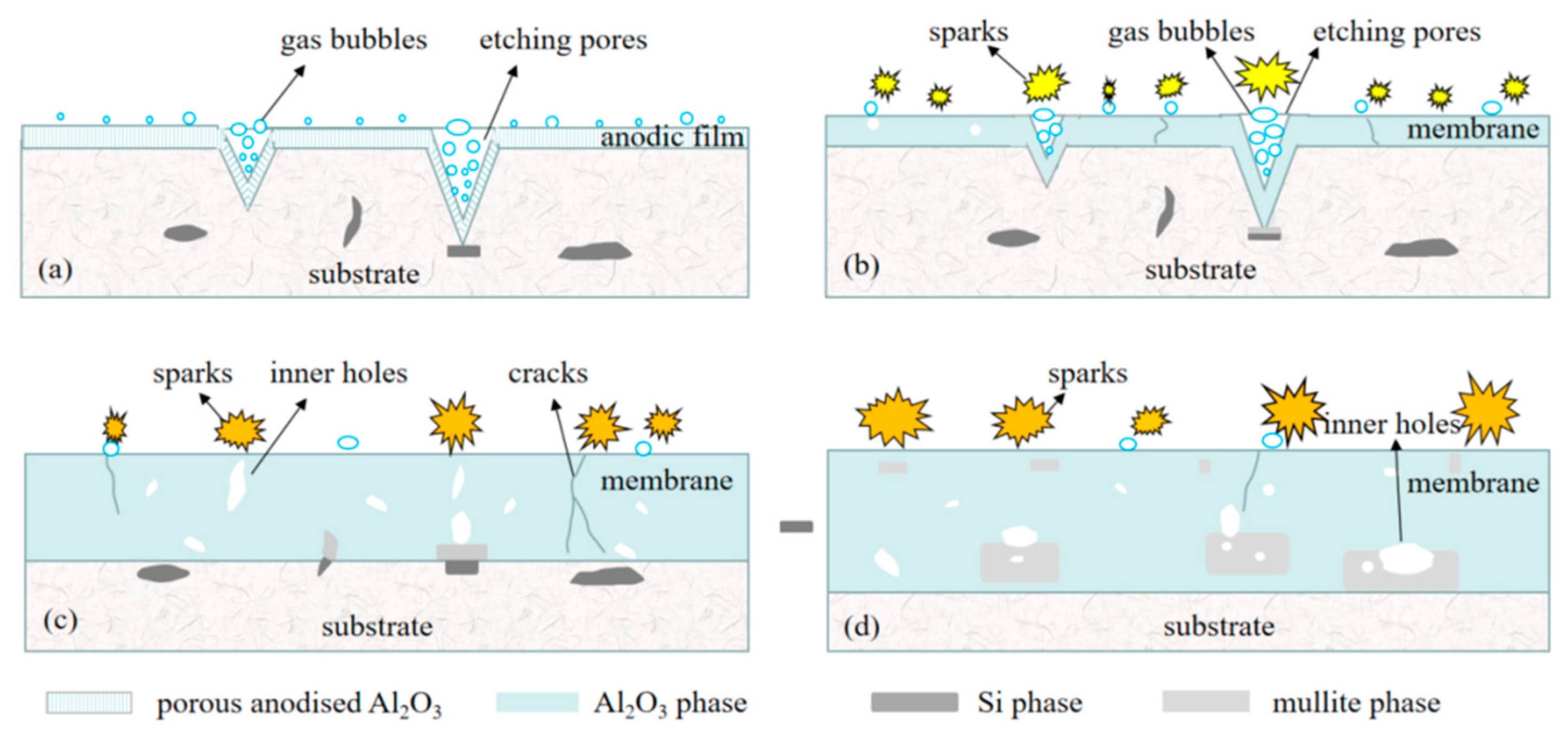
3.3. MAO Process and Discharge Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orłowicz, A.W.; Tupaj, M.; Mróz, M.; Trytek, A. Combustion engine cylinder liners made of Al-Si alloys. Arch. Metall. Mater. 2015, 15, 71–74. [Google Scholar] [CrossRef]
- Jin, L.; Liu, K.; Chen, X.G. Improved elevated temperature properties in Al-13%Si piston alloys by Mo addition. J. Mater. Eng. Perform. 2020, 29, 126–134. [Google Scholar] [CrossRef]
- Suryawanshi, J.; Prashanth, K.G.; Scudino, S.; Eckert, J.; Prakash, O.; Ramamurty, U. Simultaneous enhancements of strength and toughness in an Al-12Si alloy synthesized using selective laser melting. Acta Mater. 2016, 115, 285–294. [Google Scholar] [CrossRef]
- Abbas, M.K.; Mahmoud, A.K. Laser surface treatment of Al-12%Si alloy. Mater. Today Proc. 2017, 4, 9992–9996. [Google Scholar] [CrossRef]
- Rubben, T.; Revilla, R.I.; Graeve, I.D. Effect of heat treatments on the anodizing behavior of additive manufactured AlSi10Mg. J. Electrochem. Soc. 2019, 166, 42–48. [Google Scholar] [CrossRef]
- Pezzato, L.; Dabalà, M.; Gross, S.; Brunelli, K. Effect of microstructure and porosity of AlSi10Mg alloy produced by selective laser melting on the corrosion properties of plasma electrolytic oxidation coatings. Surf. Coat. Technol. 2020, 404, 126477. [Google Scholar] [CrossRef]
- Xue, W.B.; Shi, X.L.; Hua, M.; Li, Y.L. Preparation of anti-corrosion films by micro arc oxidation on an Al-Si alloy. Appl. Surf. Sci. 2007, 253, 6118–6124. [Google Scholar] [CrossRef]
- Li, X.; Nie, X.; Wang, L.; Northwood, D.O. Corrosion protection properties of anodic oxide coatings on an Al-Si alloy. Surf. Coat. Technol. 2005, 200, 1994–2000. [Google Scholar] [CrossRef]
- Zhu, B.W.; Seifeddine, S.; Persson, P.O.Å.; Jarfors, A.E.W.; Leisner, P.; Zanella, C. A study of formation and growth of the anodised surface layer on cast Al-Si alloys based on different analytical techniques. Mater. Des. 2016, 101, 254–262. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.P.; Guo, Y.C.; Yang, Z.; Wang, J.L. Ceramic coating formation on high Si containing Al alloy by PEO process. Surf. Eng. 2016, 32, 428–434. [Google Scholar] [CrossRef]
- Yu, H.J.; Dong, Q.; Chen, Y.; Chen, C.Z. Influence of silicon on growth mechanism of micro-arc oxidation coating on cast Al-Si alloy. R. Soc. Open sci. 2018, 5, 172428. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.O.; Nie, X.; Northwood, D.O.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (PEO) process. J. Phys. D Appl. Phys. 2010, 43, 105203. [Google Scholar] [CrossRef]
- Fu, J.G.; Li, M.; Liu, G.S.; Ma, S.L.; Zhu, X.H.; Ma, C.S.; Cheng, D.; Yan, Z.J. Robust ceramic based self-lubricating coating on Al-Si alloys prepared via PEO and spin-coating methods. Wear 2020, 203405, 458–459. [Google Scholar]
- He, J.; Cai, Q.Z.; Luo, H.H.; Yu, L.; Wei, B.K. Influence of silicon on growth process of plasma electrolytic oxidation coating on Al-Si alloy. J. Alloy. Compd. 2009, 471, 395–399. [Google Scholar] [CrossRef]
- Krishna, L.R.; Purnima, A.S.; Wasekar, N.P.; Sundararajan, G. Kinetics and properties of micro arc oxidation coatings deposited on commercial Al alloys. Metall. Mater. Trans. A 2007, 38, 370–378. [Google Scholar] [CrossRef]
- Gulec, A.E.; Gencer, Y.; Tarakci, M. The characterization of oxide based ceramic coating synthesized on Al-Si binary alloys by microarc oxidation. Surf. Coat. Technol. 2015, 269, 100–107. [Google Scholar] [CrossRef]
- Wang, L.; Nie, X. Silicon effects on formation of EPO oxide coatings on aluminum alloys. Thin Solid Films 2006, 494, 211–218. [Google Scholar] [CrossRef]
- Li, K.; Li, W.F.; Zhang, G.G.; Zhu, W.; Zheng, F.H.; Zhang, D.Q.; Wang, M. Effects of Si phase refinement on the plasma electrolytic oxidation of eutectic Al-Si alloy. J. Alloy. Compd. 2019, 790, 650–656. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E. Optimisation of the plasma electrolytic oxidation process efficiency on aluminum. Surf. Interface Anal. 2010, 42, 221–226. [Google Scholar] [CrossRef]
- Mohedano, M.; Matykina, E.; Arrabal, R.; Mingo, B.; Pardo, A. PEO of pre-anodized Al-Si alloys: Corrosion properties and influence of sealings. Appl. Surf. Sci. 2015, 346, 57–67. [Google Scholar] [CrossRef]
- Asquith, D.T.; Yerokhin, A.L.; Yates, J.R.; Matthews, A. The effect of combined shot-peening and PEO treatment on the corrosion performance of 2024 Al alloy. Thin Solid Films 2007, 516, 417–421. [Google Scholar] [CrossRef]
- Dejiu, S.; Jie, Z.; Lailei, W.; Fangfei, L.; Guolong, L.; Jingrui, C.; Donglei, H.; Haojie, M.; Guirong, J. Effect of high temperature oxidation prefab film on formation of micro-arc oxidation coatings on 6061aluminum alloy. Appl. Surf. Sci. 2013, 265, 431–437. [Google Scholar] [CrossRef]
- Alkharafi, F.M.; Badawy, W.A. Corrosion and passivation of Al and Al-Si alloys in nitric acid solutions II—effect of chloride ions. Electrochim. Acta 1995, 40, 1811–1817. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Leyland, A.; Matthews, A. Kinetic aspects of aluminium titanate layer formation on titanium alloys by plasma electrolytic oxidation. Appl. Surf. Sci. 2002, 200, 172–184. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Snizhko, L.O.; Gurevina, N.L.; Leyland, A.; Pilkington, A.; Matthews, A. Discharge characterization in plasma electrolytic oxidation of aluminium. J. Phys. D Appl. Phys. 2003, 36, 2110–2120. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Yerokhin, A.L.; Lyubimov, V.V.; Donley, M.S.; Zabinski, J.S. Characterization of wear protective Al-Si-O coatings formed on Al-based alloys by micro-arc discharge treatment. Surf. Coat. Technol. 1996, 86–87, 516–521. [Google Scholar] [CrossRef]
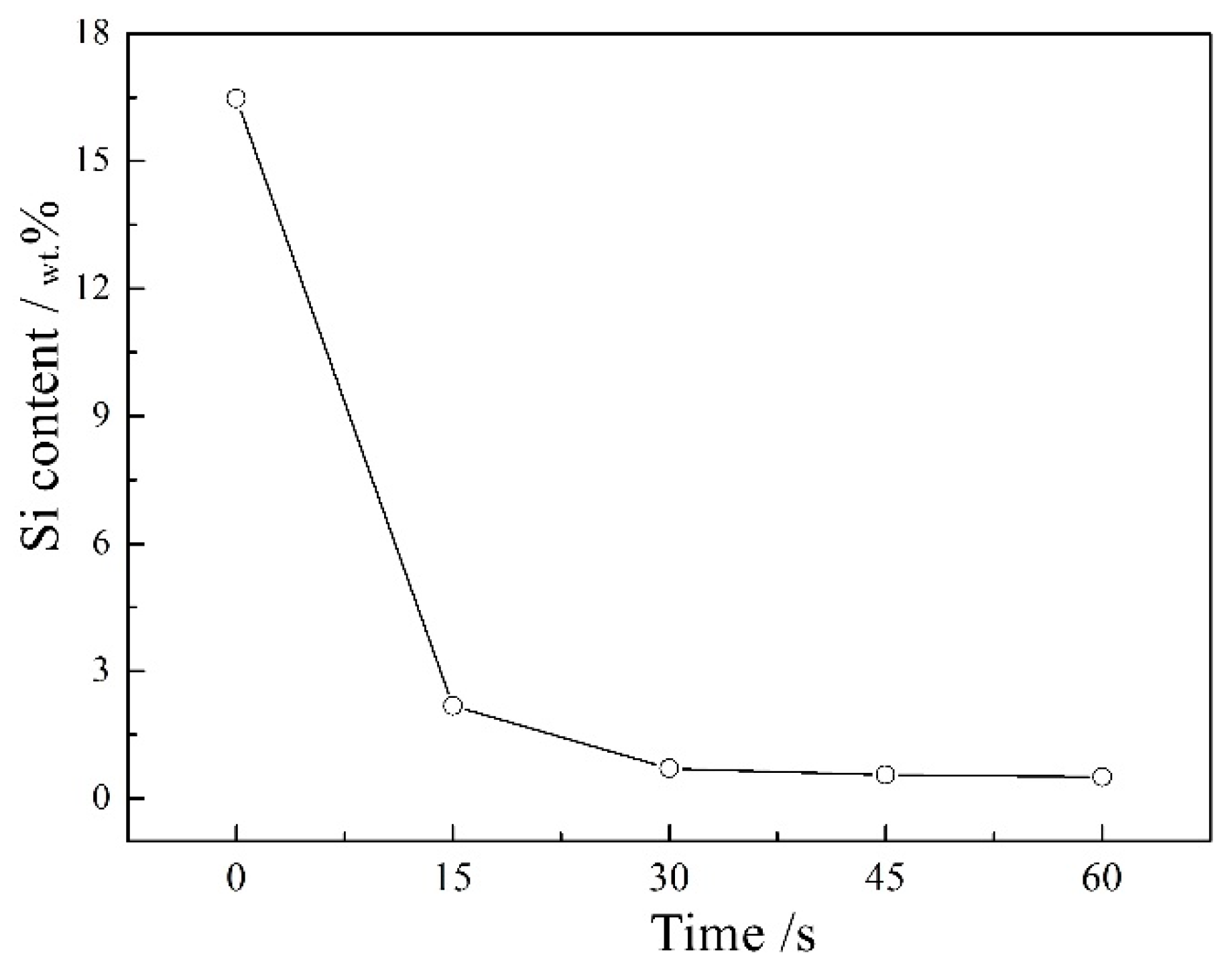

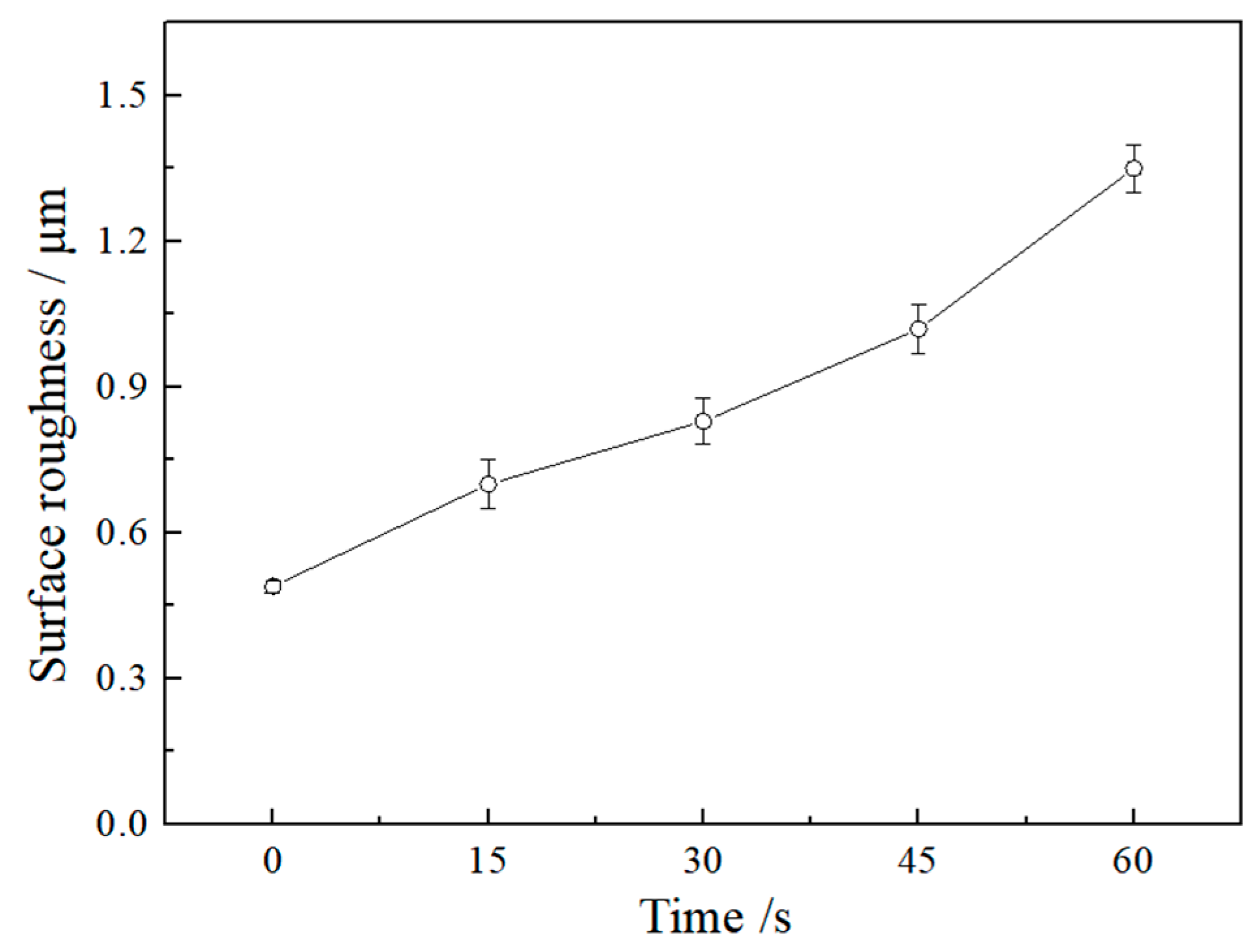

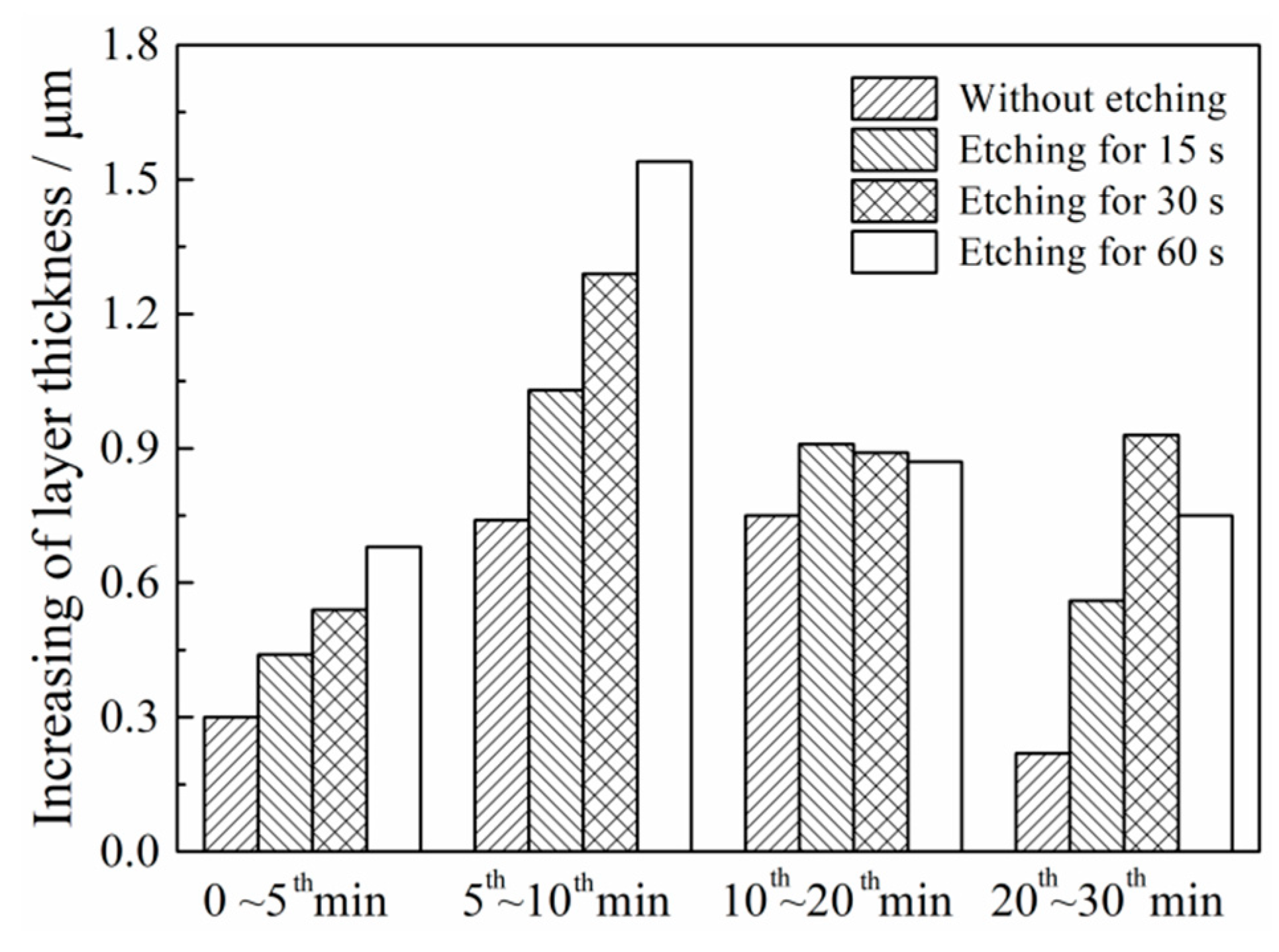
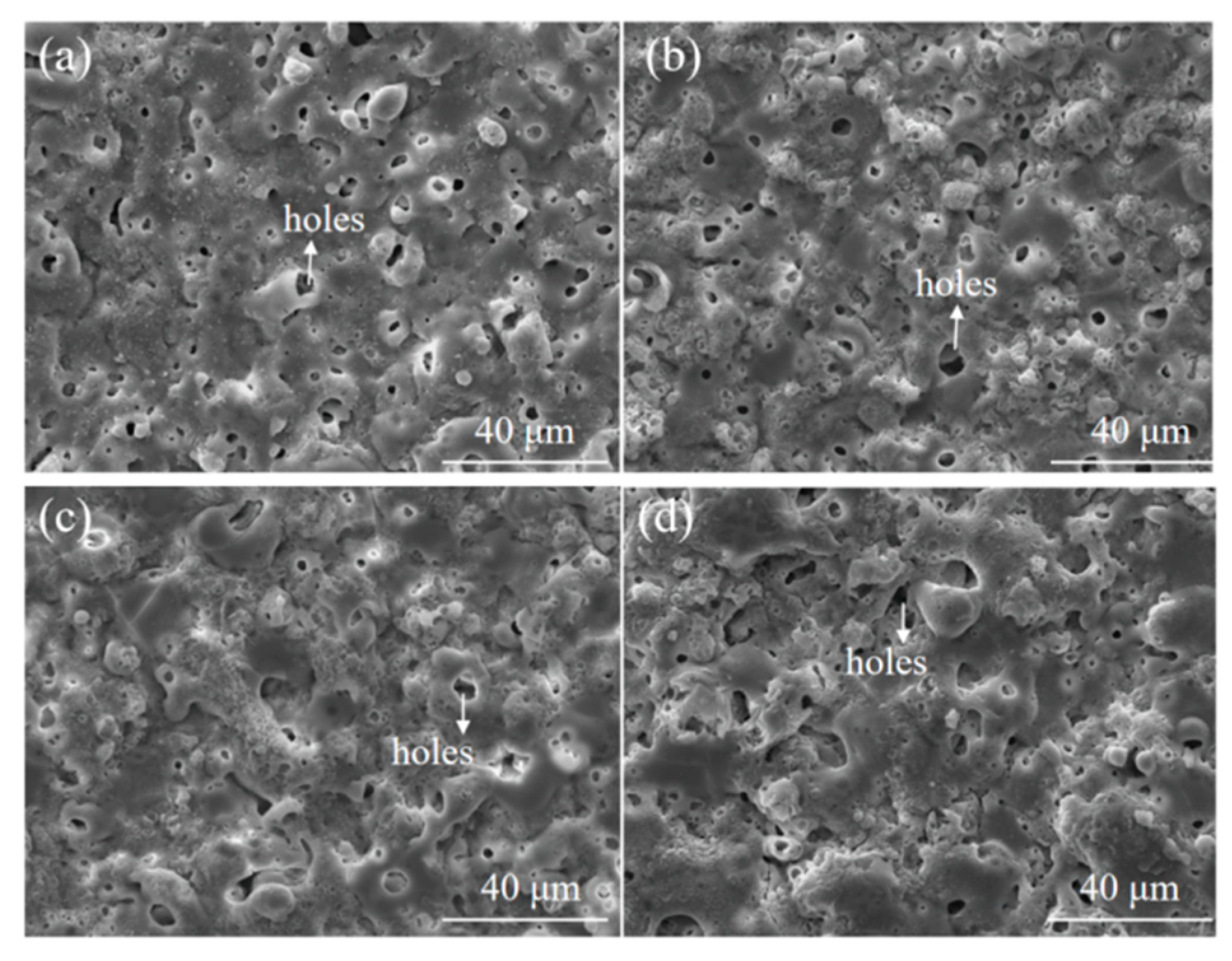
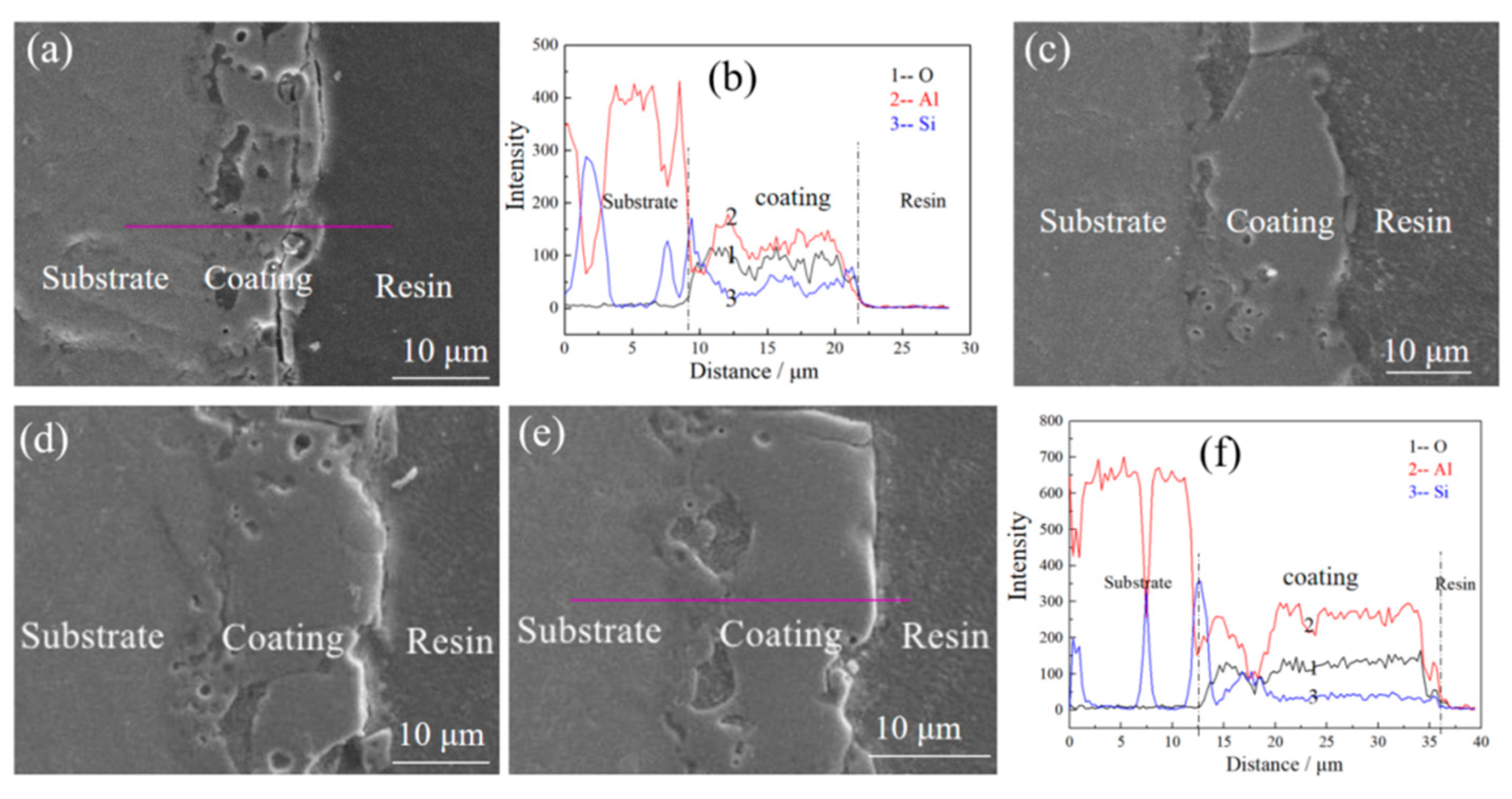


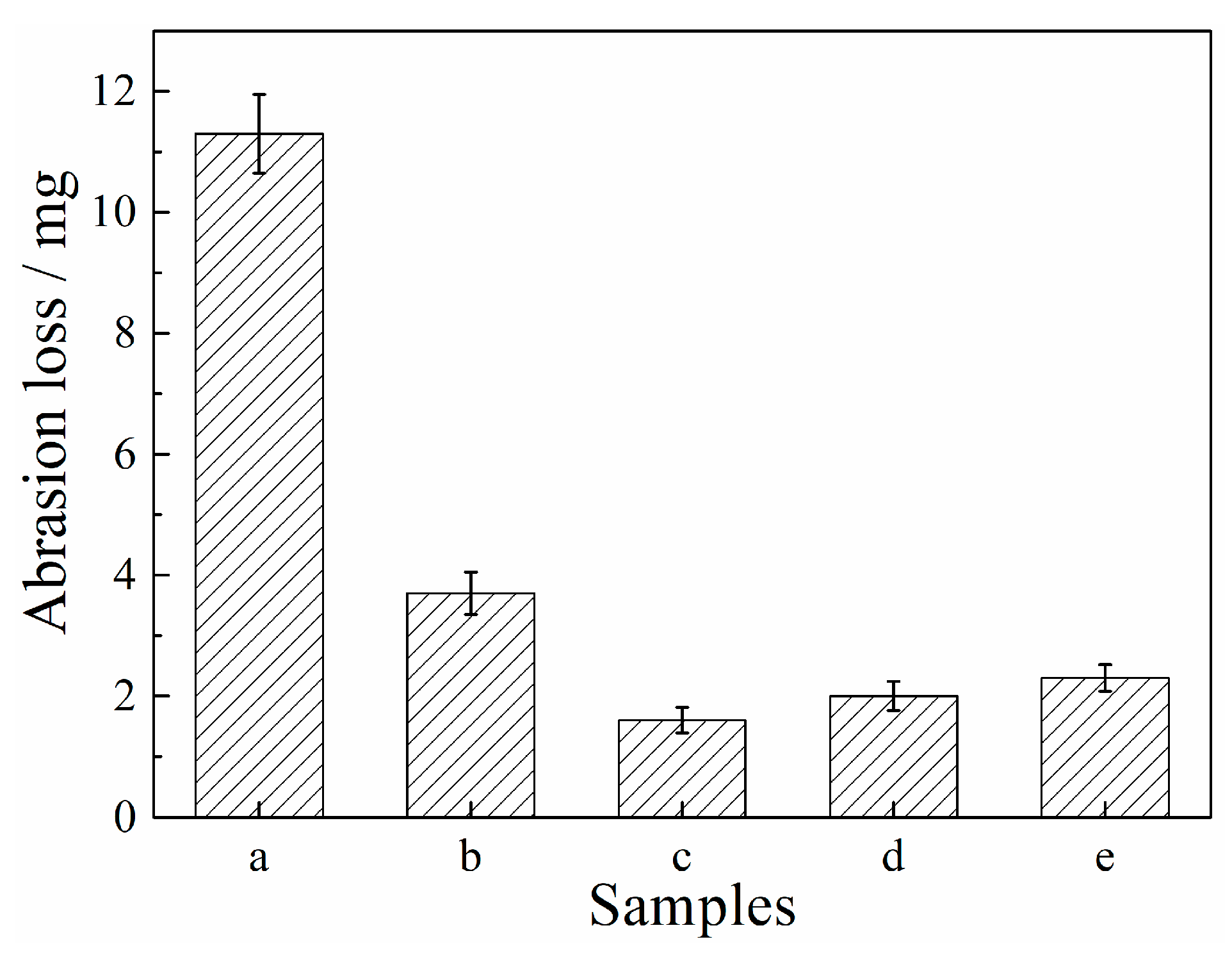
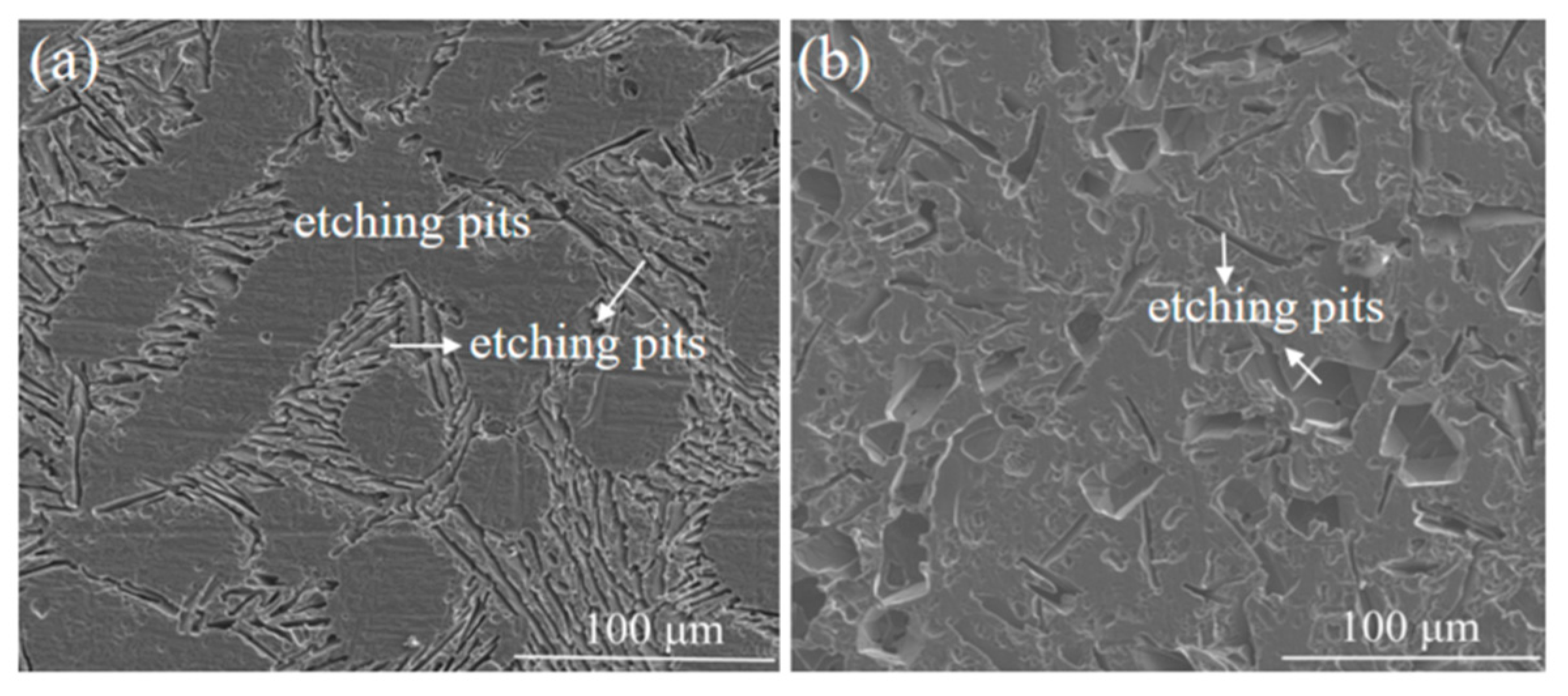
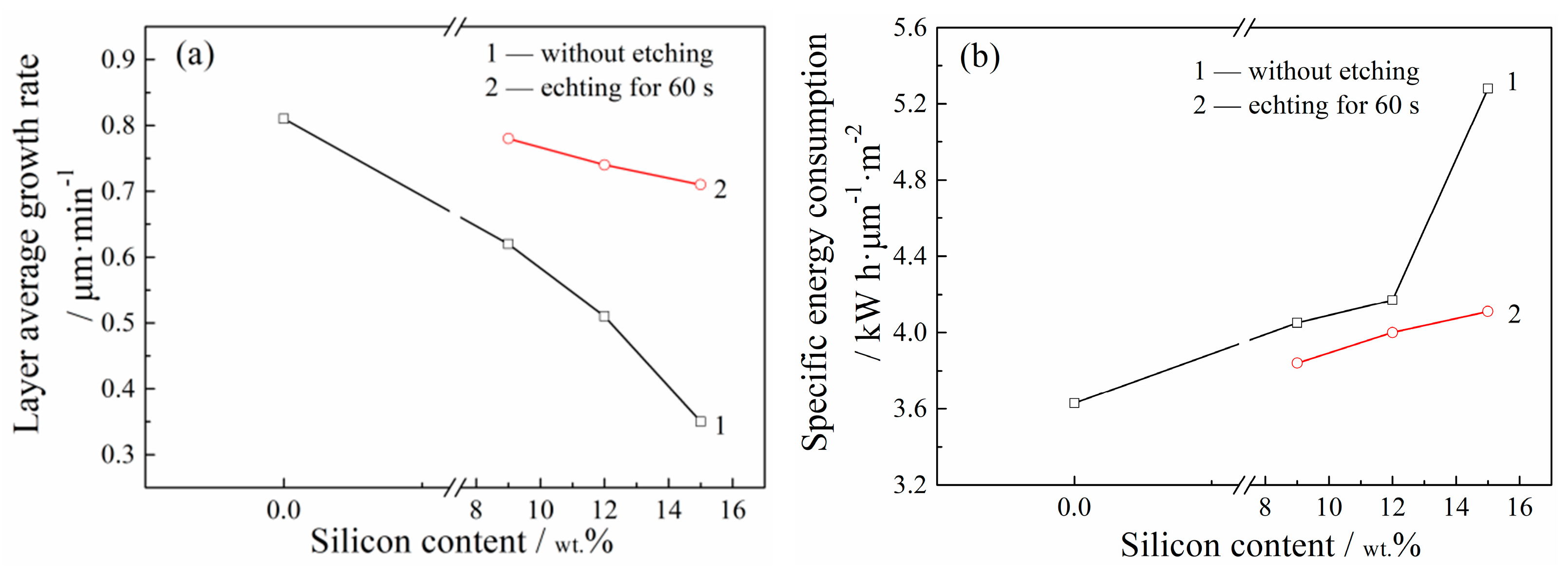
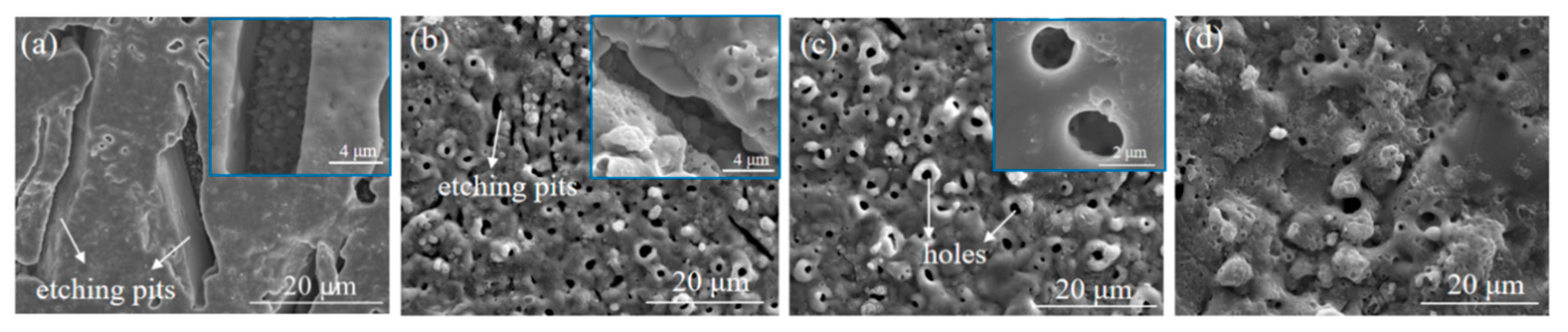
| Etching Time/s | 0 | 15 | 30 | 60 | ||||
|---|---|---|---|---|---|---|---|---|
| MAO time/min | 20 | 30 | 20 | 30 | 20 | 30 | 20 | 30 |
| Positive voltage/V | 434 | 456 | 466 | 475 | 462 | 470 | 457 | 467 |
| Layer thickness/μm | 12.7 | 14.9 | 14.7 | 22.8 | 15.9 | 25.2 | 17.1 | 25.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Li, W.; Yi, A.; Zhu, W.; Liao, Z.; Chen, K.; Li, W. Tuning the Surface Characteristic of Al-Si Alloys and Its Impacts on the Formation of Micro Arc Oxidation Layers. Coatings 2021, 11, 453. https://doi.org/10.3390/coatings11040453
Li K, Li W, Yi A, Zhu W, Liao Z, Chen K, Li W. Tuning the Surface Characteristic of Al-Si Alloys and Its Impacts on the Formation of Micro Arc Oxidation Layers. Coatings. 2021; 11(4):453. https://doi.org/10.3390/coatings11040453
Chicago/Turabian StyleLi, Kang, Wenfang Li, Aihua Yi, Wen Zhu, Zhongmiao Liao, Ken Chen, and Weimin Li. 2021. "Tuning the Surface Characteristic of Al-Si Alloys and Its Impacts on the Formation of Micro Arc Oxidation Layers" Coatings 11, no. 4: 453. https://doi.org/10.3390/coatings11040453
APA StyleLi, K., Li, W., Yi, A., Zhu, W., Liao, Z., Chen, K., & Li, W. (2021). Tuning the Surface Characteristic of Al-Si Alloys and Its Impacts on the Formation of Micro Arc Oxidation Layers. Coatings, 11(4), 453. https://doi.org/10.3390/coatings11040453





