Bioinspired Living Coating System in Service: Evaluation of the Wood Protected with Biofinish during One-Year Natural Weathering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Samples and Natural Weathering
2.2. Colour Measurement
2.3. Gloss Measurement
2.4. Wettability
2.5. Microscopic Observation and 3D Roughness Measurement
3. Results and Discussions
3.1. Surface Visual Appeal
3.2. Surface Colour
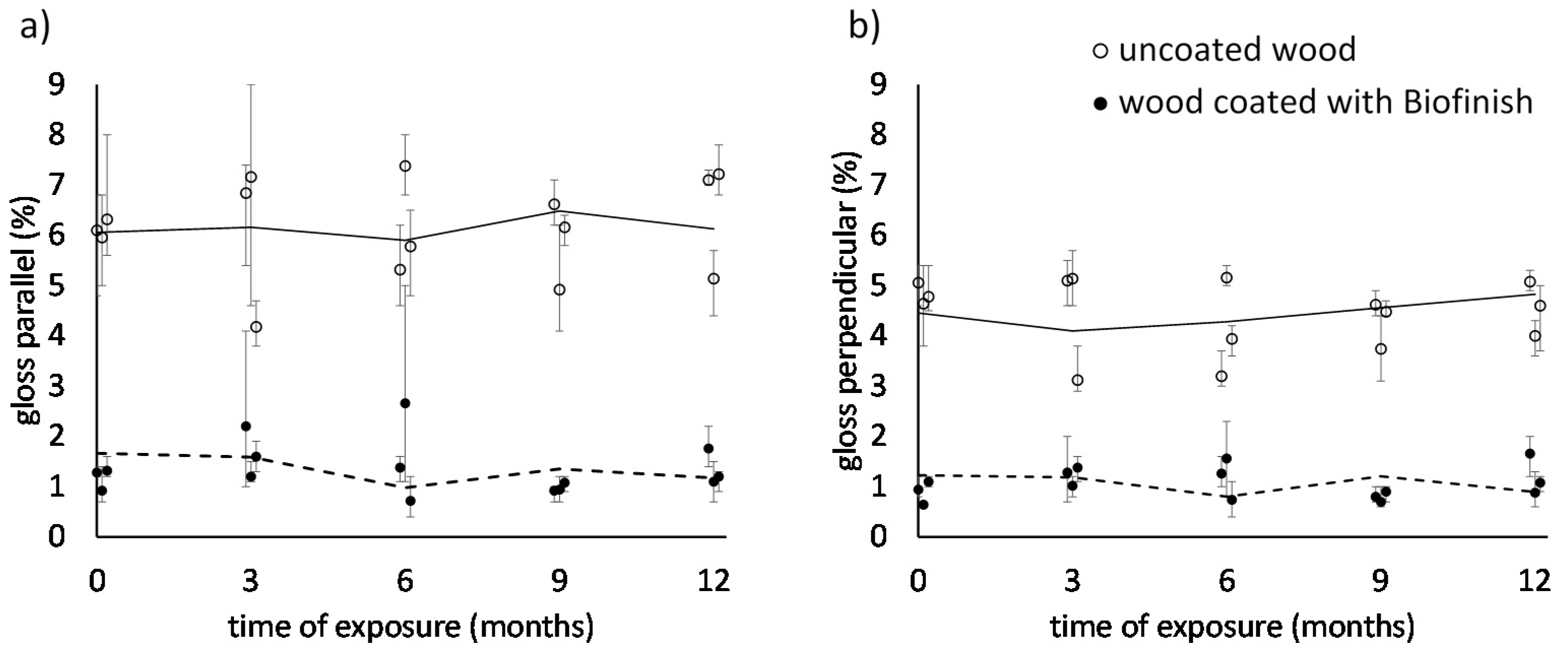
3.3. Gloss Evaluation
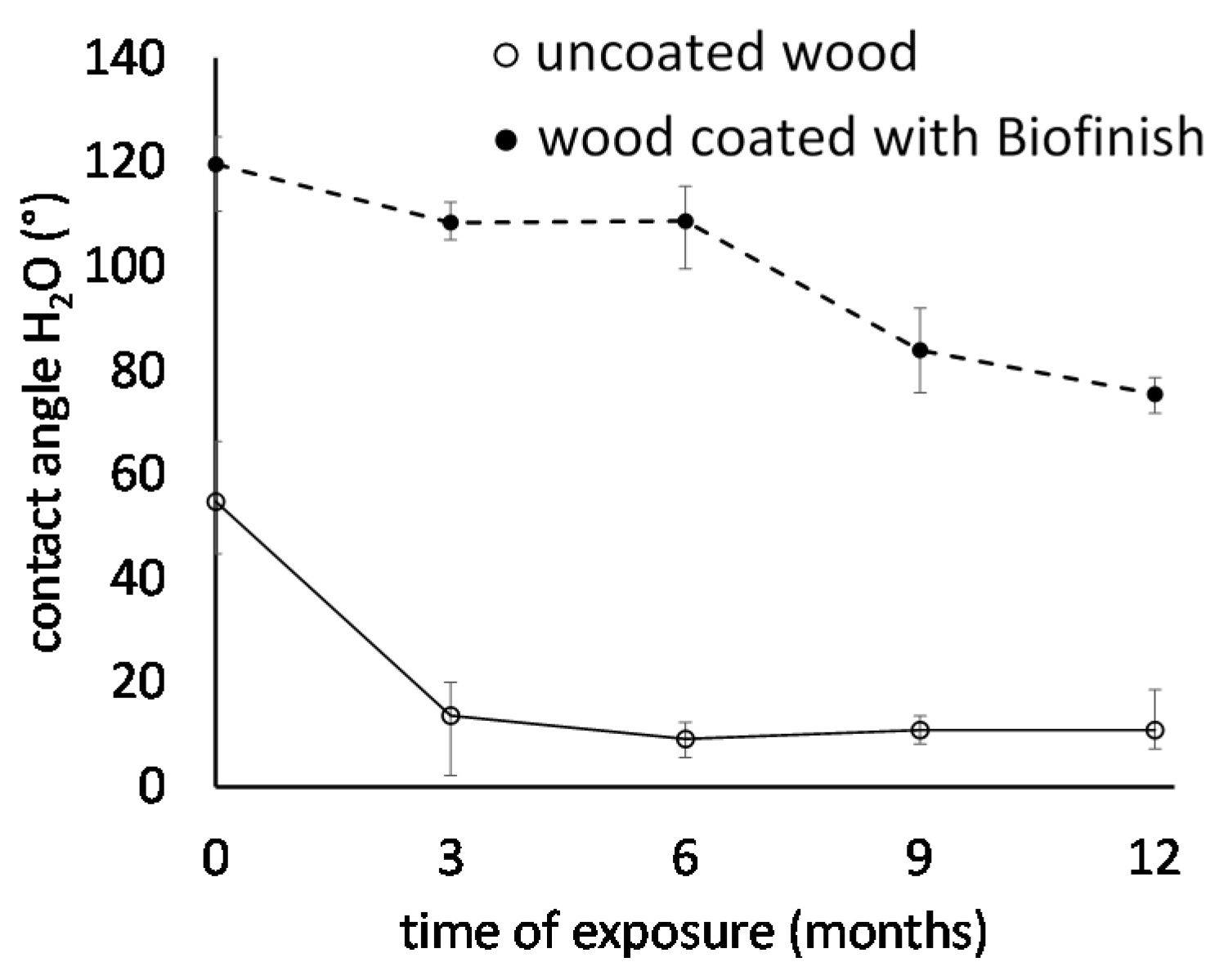
3.4. Wettability
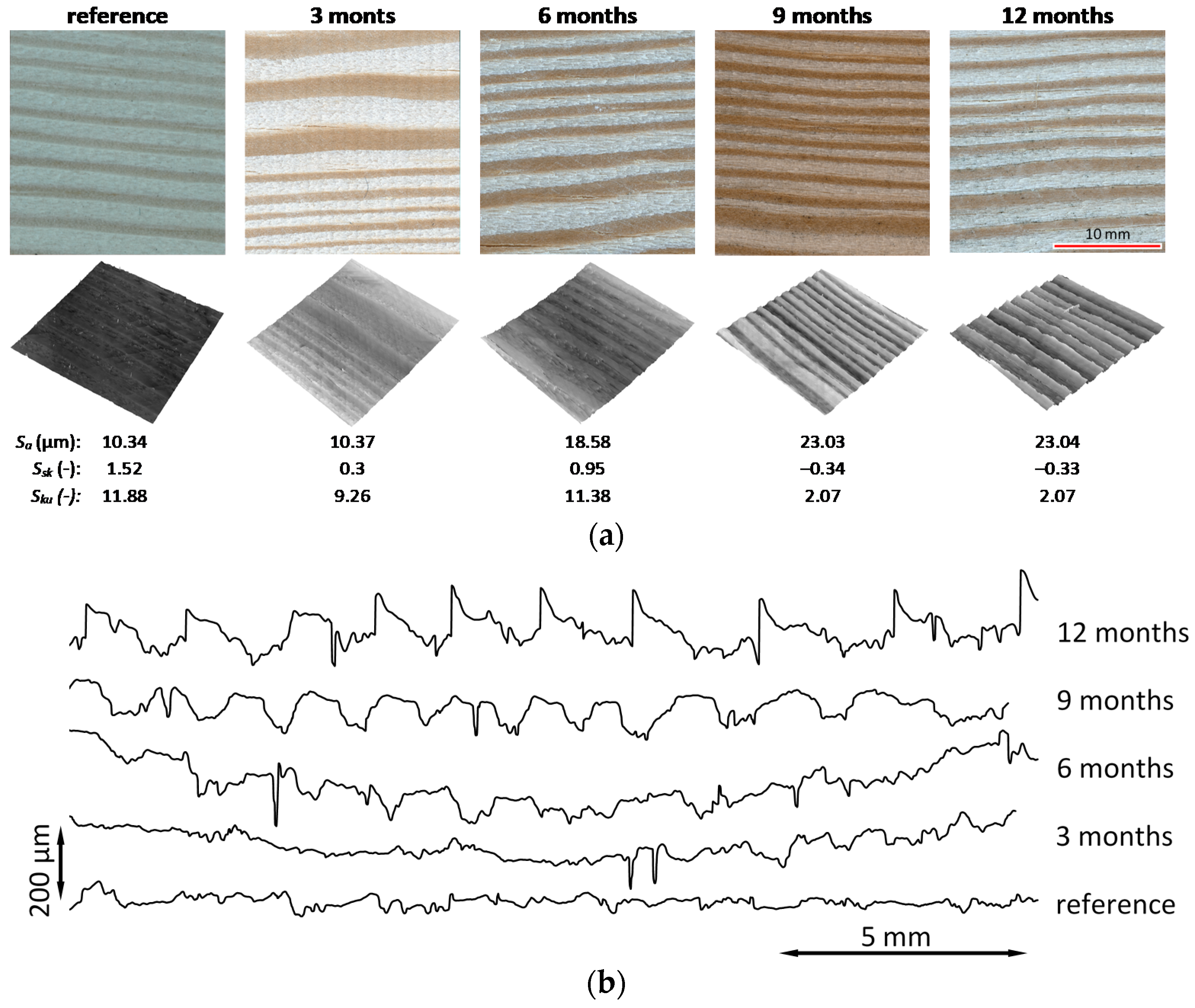
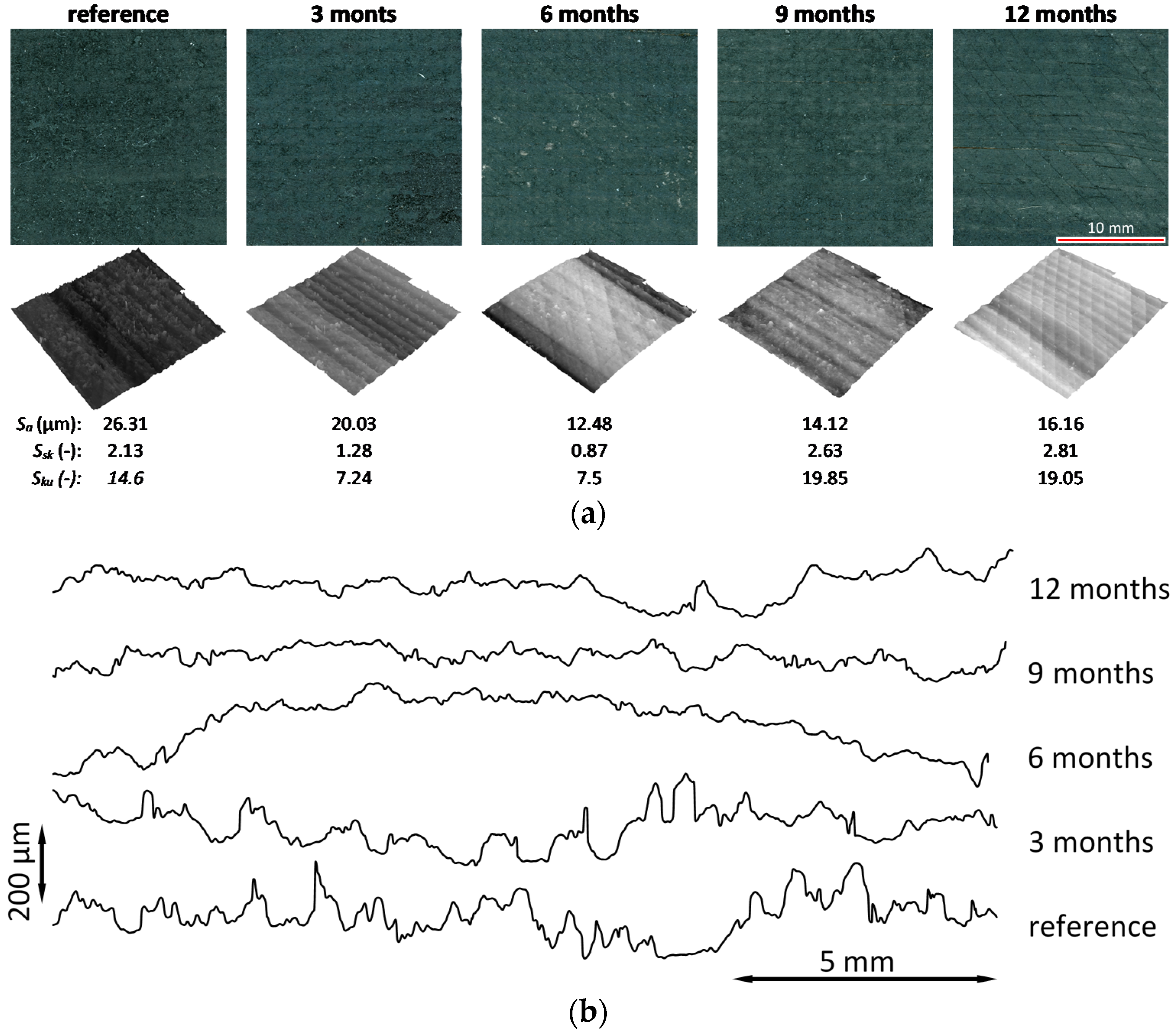
3.5. Surface Topography
3.6. Microscopic Observations
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feist, W.C.; Hon, D.N.-S. Chemistry of weathering and protection. Chem. Solid Wood 1984, 401–451. [Google Scholar] [CrossRef]
- Rowell, R.M. Handbook of Wood Chemistry and Wood Composites; CRC Press: New York, NY, USA, 2005; ISBN 0849315883. [Google Scholar]
- Evans, P.; Chowdhury, M.J.; Mathews, B.; Schmalzl, S.K.; Ayer, M.; Kataoka, K.Y.; Kutz, M. Handbook of Environmental Analysis; William Andrew Publishing, Norwich: Norwich, UK, 2005; ISBN 0815515006. [Google Scholar]
- Hayoz, P.; Peter, W.; Rogez, D. A new innovative stabilization method for the protection of natural wood. Prog. Org. Coat. 2003, 48, 297–309. [Google Scholar] [CrossRef]
- Šimkovic, I. Free radicals in wood chemistry. J. Macromol. Sci. Part C 1986, 26, 67–80. [Google Scholar] [CrossRef]
- Müller, U.; Rätzsch, M.; Schwanninger, M.; Steiner, M.; Zöbl, H. Yellowing and IR-changes of spruce wood as result of UV-irradiation. J. Photochem. Photobiol. B Biol. 2003, 69, 97–105. [Google Scholar] [CrossRef]
- Läszlo, T.; Faix, O. Artificial ageing of wood monitored by DRIFT spectroscopy and CIE L*a*b* color measurements. I. Effect of UV light. Holzforschung 1995, 49, 397–404. [Google Scholar] [CrossRef]
- Kataoka, Y.; Kiguchi, M.; Williams, R.S.; Evans, P.D. Violet light causes photodegradation of wood beyond the zone affected by ultraviolet radiation. Holzforschung 2007, 61, 23–27. [Google Scholar] [CrossRef]
- Sandak, J.; Sandak, A.; Cocchi, M. Multi-sensor data fusion and parallel factor analysis reveals kinetics of wood weathering. Talanta 2021, 225, 122024. [Google Scholar] [CrossRef] [PubMed]
- Sandak, A.; Sandak, J.; Noël, M.; Dimitriou, A. A method for accelerated natural weathering of wood subsurface and its multilevel characterization. Coatings 2021, 11, 126. [Google Scholar] [CrossRef]
- Pánek, M.; Reinprecht, L. Critical view on the possibility of color changes prediction in the surfaces of painted wood exposed outdoors using accelerated weathering in xenotest. J. Coat. Technol. Res. 2019, 16, 339–352. [Google Scholar] [CrossRef]
- Bobadilha, G.D.S.; Stokes, C.E.; Ohno, K.M.; Kirker, G.; Lopes, D.J.V.; Nejad, M. Physical, optical, and visual performance of coated cross-laminated timber during natural and artificial weathering. Coatings 2021, 11, 252. [Google Scholar] [CrossRef]
- Temiz, A.; Terziev, N.; Eikenes, M.; Hafren, J. Effect of accelerated weathering on surface chemistry of modified wood. Appl. Surf. Sci. 2007, 253, 5355–5362. [Google Scholar] [CrossRef]
- Ayadi, N.; Lejeune, F.; Charrier, F.; Charrier, B.; Merlin, A. Color stability of heat-treated wood during artificial weathering. Holz Als Roh-Und Werkst. 2003, 61, 221–226. [Google Scholar] [CrossRef]
- Colom, X.; Carrillo, F.; Nogués, F.; Garriga, P. Structural analysis of photodegraded wood by means of FTIR spectroscopy. Polym. Degrad. Stab. 2003, 80, 543–549. [Google Scholar] [CrossRef]
- Pandey, K.K. Study of the effect of photo-irradiation on the surface chemistry of wood. Polym. Degrad. Stab. 2005, 90, 9–20. [Google Scholar] [CrossRef]
- Ganne-Chédeville, C.; Jääskeläinen, A.S.; Froidevaux, J.; Hughes, M.; Navi, P. Natural and artificial ageing of spruce wood as observed by FTIR-ATR and UVRR spectroscopy. Holzforschung 2012, 66, 163–170. [Google Scholar] [CrossRef]
- Petrillo, M.; Sandak, J.; Grossi, P.; Sandak, A. Chemical and appearance changes of wood due to artificial weathering-dose-response model. J. Near Infrared Spectrosc. 2019. [Google Scholar] [CrossRef] [Green Version]
- Zanetti, M.; Rials, T.G.; Rammer, D. NIR-Monitoring of in-service wood structures. In Proceedings of the Structures Congress 2005, New York, NY, USA, 20–24 April 2005; American Society of Civil Engineers: New York, NY, USA, 2005; Volume 9. [Google Scholar]
- Sandak, J.; Sandak, A.; Riggio, M. Characterization and monitoring of surface weathering on exposed timber structures with a multi-sensor approach. Int. J. Archit. Herit. 2015, 9, 674–688. [Google Scholar] [CrossRef]
- Gobakken, L.R.; Vestøl, G.I. Surface mould and blue stain fungi on coated Norway spruce cladding. Int. Biodeterior. Biodegrad. 2012, 75, 181–186. [Google Scholar] [CrossRef]
- Jankowska, A.; Rybak, K.; Nowacka, M.; Boruszewski, P. Insight of weathering processes based on monitoring surface characteristic of tropical wood species. Coatings 2020, 10, 877. [Google Scholar] [CrossRef]
- Žlahtič, M.; Humar, M. Influence of artificial and natural weathering on the hydrophobicity and surface properties of wood. BioResources 2016, 11, 4964–4989. [Google Scholar] [CrossRef] [Green Version]
- Pánek, M.; Oberhofnerová, E.; Zeidler, A.; Šedivka, P. Efficacy of hydrophobic coatings in protecting oak wood surfaces during accelerated weathering. Coatings 2017, 7, 172. [Google Scholar] [CrossRef] [Green Version]
- Oberhofnerová, E.; Šimunková, K.; Dvořák, O.; Štěrbová, I.; Hiziroglu, S.; Šedivka, P.; Pánek, M. Comparison of exterior coatings applied to oak wood as a function of natural and artificial weathering exposure. Coatings 2019, 9, 864. [Google Scholar] [CrossRef] [Green Version]
- Salca, E.-A.; Krystofiak, T.; Lis, B.; Hiziroglu, S. Glossiness evaluation of coated wood surfaces as function of varnish type and exposure to different conditions. Coatings 2021, 11, 558. [Google Scholar] [CrossRef]
- Cogulet, A.; Blanchet, P.; Landry, V. The multifactorial aspect of wood weathering: A review based on a holistic approach of wood degradation protected by clear coating. BioResources 2018, 13, 2116–2138. [Google Scholar] [CrossRef] [Green Version]
- Pintus, V.; Wei, S.; Schreiner, M. Accelerated UV ageing studies of acrylic, alkyd, and polyvinyl acetate paints: Influence of inorganic pigments. Microchem. J. 2016, 124, 949–961. [Google Scholar] [CrossRef]
- Christensen, P.A.; Dilks, A.; Egerton, T.A.; Temperley, J. Infrared spectroscopic evaluation of the photodegradation of paint Part I the UV degradation of acrylic films pigmented with titanium dioxide. J. Mater. Sci. 1999, 34, 5689–5700. [Google Scholar] [CrossRef]
- Singh, R.P.; Tomer, N.S.; Bhadraiah, S.V. Photo-oxidation studies on polyurethane coating: Effect of additives on yellowing of polyurethane. Polym. Degrad. Stab. 2001, 73, 443–446. [Google Scholar] [CrossRef]
- Rosu, D.; Rosu, L.; Cascaval, C.N. IR-change and yellowing of polyurethane as a result of UV irradiation. Polym. Degrad. Stab. 2009, 94, 591–596. [Google Scholar] [CrossRef]
- Gobakken, L.R.; Westin, M. Surface mould growth on five modified wood substrates coated with three different coating systems when exposed outdoors. Int. Biodeterior. Biodegrad. 2008, 62, 397–402. [Google Scholar] [CrossRef]
- Schmidt, O. Wood and Tree Fungi; Springer: Berlin/Heidelberg Germany; Springer: New York, NY, USA, 2006; ISBN 9783540321385. [Google Scholar]
- Horvath, R.S.; Brent, M.M.; Cropper, D.G. Paint deterioration as a result of the growth of aureobasidium pullulans on wood. Appl. Environ. Microbiol. 1976, 32, 505–507. [Google Scholar] [CrossRef] [Green Version]
- Gesthuizen, J. Bio-Based Coatings Overview: Increasing Activities|European Coatings. Available online: https://www.european-coatings.com/articles/archiv/bio_based-coatings-overview-increasing-activities (accessed on 11 May 2021).
- Chemicals Strategy. Available online: https://ec.europa.eu/environment/strategy/chemicals-strategy_sl (accessed on 11 May 2021).
- López, M.; Rubio, R.; Martín, S.; Croxford, B. How plants inspire façades. From plants to architecture: Biomimetic principles for the development of adaptive architectural envelopes. Renew. Sustain. Energy Rev. 2017, 67, 692–703. [Google Scholar] [CrossRef]
- Lurie-Luke, E. Product and technology innovation: What can biomimicry inspire? Biotechnol. Adv. 2014, 32, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Imani, M.; Donn, M.; Balador, Z. Bio-Inspired Materials: Contribution of Biology to Energy Efficiency of Buildings. In Handbook of Ecomaterials; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 1–24. [Google Scholar]
- Vijay, K.; Murmu, M.; Deo, S.V. Bacteria based self healing concrete—A review. Constr. Build. Mater. 2017, 152, 1008–1014. [Google Scholar] [CrossRef]
- Sailer, M.F.; van Nieuwenhuijzen, E.J.; Knol, W. Forming of a functional biofilm on wood surfaces. Ecol. Eng. 2010, 36, 163–167. [Google Scholar] [CrossRef]
- Peeters, L.H.M.; Huinink, H.P.; Voogt, B.; Adan, O.C.G. Oil type and cross-linking influence growth of aureobasidium melanogenum on vegetable oils as a single carbon source. Microbiologyopen 2018, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xyhlo Biofinish|Sustainable and Eco-Friendly Wood Protection. Available online: https://www.xyhlo.com/en/ (accessed on 29 March 2021).
- Van Nieuwenhuijzen, E.J.; Houbraken, J.A.M.P.; Meijer, M.; Adan, O.C.G.; Samson, R.A. Aureobasidium melanogenum: A native of dark biofinishes on oil treated wood. Antonie Van Leeuwenhoek 2016, 109. [Google Scholar] [CrossRef] [Green Version]
- Van Nieuwenhuijzen, E.J.; Sailer, M.F.; van den Heuvel, E.R.; Rensink, S.; Adan, O.C.G.; Samson, R.A. Vegetable oils as carbon and energy source for aureobasidium melanogenum in batch cultivation. Microbiologyopen 2019, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Evans, P.D.; Michell, A.J.; Schmalzl, K.J. Studies of the degradation and protection of wood surfaces. Wood Sci. Technol. 1992, 26, 151–163. [Google Scholar] [CrossRef]
- Turkoglu, T.; Baysal, E.; Toker, H. The effects of natural weathering on color stability of impregnated and varnished wood materials. Adv. Mater. Sci. Eng. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Oberhofnerová, E.; Pánek, M.; García-Cimarras, A. The effect of natural weathering on untreated wood surface. Maderas Cienc. Y Tecnol. 2017, 19, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Hon, D.N.S.; Shiraishi, N. Wood and Cellulosic Chemistry, Revised, and Expanded, 2nd ed.; Marcel Dekker: New York, NY, USA, 2000; ISBN 0824700244. [Google Scholar]
- Ghosh, S.C.; Militz, H.; Mai, C. Natural weathering of scots pine (Pinus sylvestris L.) boards modified with functionalised commercial silicone emulsions. BioResources 2009, 4, 659–673. [Google Scholar] [CrossRef]
- Scheffer, T.C. Natural resistance of wood to microbial deterioration. Annu. Rev. Phytopathol. 1966, 4, 147–168. [Google Scholar] [CrossRef]
- Viitanen, H.A. Modelling the time factor in the development of mould fungi—The effect of critical humidity and temperature conditions on pine and spruce sapwood. Holzforschung 1997, 51, 6–14. [Google Scholar] [CrossRef]
- Butler, M.J.; Day, A.W. Fungal melanins: A review. Can. J. Microbiol. 1998, 44, 1115–1136. [Google Scholar] [CrossRef]
- El-Bialy, H.A.; El-Gamal, M.S.; Elsayed, M.A.; Saudi, H.A.; Khalifa, M.A. Microbial melanin physiology under stress conditions and gamma radiation protection studies. Radiat. Phys. Chem. 2019. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, N.N.; Liu, G.L.; Chi, Z.; Wang, J.M.; Zhang, L.L.; Chi, Z.M. Melanin production by a yeast strain XJ5-1 of aureobasidium melanogenum isolated from the Taklimakan desert and its role in the yeast survival in stress environments. Extremophiles 2016, 20, 567–577. [Google Scholar] [CrossRef]
- Kumar, C.G.; Mongolla, P.; Pombala, S.; Kamle, A.; Joseph, J. Physicochemical characterization and antioxidant activity of melanin from a novel strain of Aspergillus bridgeri ICTF-201. Lett. Appl. Microbiol. 2011, 53, 350–358. [Google Scholar] [CrossRef]
- Hernandez, V.A.; Evans, P.D. Technical note: Melanization of the wood-staining fungus aureobasidium pullulans in response to UV radiation. Wood Fiber Sci 2015, 47, 120–124. [Google Scholar]
- Gniewosz, M.; Duszkiewicz-Reinhard, W. Comparative studies on pullulan synthesis, melanin synthesis and morphology of white mutant aureobasidium pullulans B-1 and parent strain A.p.-3. Carbohydr. Polym. 2008, 72, 431–438. [Google Scholar] [CrossRef]
- Lingappa, Y.; Sussman, A.S.; Bernstein, I.A. Effect of light and media upon growth and melanin formation in aureobasidium pullulans (DE BY.) ARN. (=Pulluraria pullulans). Mycopathol. Mycol. Appl. 1960, 20, 109–128. [Google Scholar] [CrossRef] [Green Version]
- Kucuktuvek, M.; Baysal, E.; Turkoglu, T.; Peker, H.; Gunduz, A.; Toker, H. Surface characteristics of scots pine wood heated at high temperatures after weathering. Wood Res. 2017, 62, 905–917. [Google Scholar]
- Turkoglu, T.; Toker, H.; Baysal, E.; Kart, S.; Yuksel, M.; Ergun, M.E. Some surface properties of heat treated and natural weathered oriental beech. Wood Res. 2015, 60, 881–890. [Google Scholar]
- Oberhofnerová, E.; Pánek, M. Surface wetting of selected wood species by water during initial stages of weathering. Wood Res. 2016, 61, 545–552. [Google Scholar]
- Kalnins, M.A.; Feist, W.C. Increase in wettability of wood with weathering. For. Prod. J. 1993, 43, 55–57. [Google Scholar]
- Kishino, M.; Nakano, T. Artificial weathering of tropical woods. Part 2: Color change. Holzforschung 2004, 58, 558–565. [Google Scholar] [CrossRef]
- Gonzalezde, C.P.H.; Missio, A.L.; Mattos, B.D.; Gatto, D.A. Natural weathering performance of three fast-growing eucalypt woods. Maderas Cienc. Y Tecnol. 2016, 17, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.R. Weathering of Wood. In Handbook of Wood Chemistry and Wood Composites; Rowel, M.R., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 139–185. ISBN 0849315883. [Google Scholar]
- Goodell, B.; Winandy, J.E.; Morrell, J.J. Fungal degradation of wood: Emerging data, new insights and changing perceptions. Coatings 2020, 10, 1210. [Google Scholar] [CrossRef]
- Di Francesco, A.; Ugolini, L.; D’Aquino, S.; Pagnotta, E.; Mari, M. Biocontrol of monilinia laxa by aureobasidium pullulans strains: Insights on competition for nutrients and space. Int. J. Food Microbiol. 2017, 248, 32–38. [Google Scholar] [CrossRef]
- Bozoudi, D.; Tsaltas, D. The multiple and versatile roles of aureobasidium pullulans in the vitivinicultural sector. Fermentation 2018, 4, 85. [Google Scholar] [CrossRef] [Green Version]




| Exposure Month | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Temp (°C) | 12 | 14 | 19 | 24 | 25 | 25 | 17 | 14 | 7 | 1 | 4 | 4 |
| Days of Frost | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 5 | 24 | 16 | 10 |
| Total Rain (mm) | 32 | 108 | 50 | 36 | 27 | 17 | 6 | 1 | 28 | 3 | 1 | 7 |
| RH (%) | 49 | 45 | 51 | 51 | 50 | 52 | 68 | 62 | 69 | 74 | 82 | 55 |
| Mean Wind Speed (km/h) | 7 | 9 | 7 | 9 | 8 | 6 | 5 | 5 | 4 | 3 | 3 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poohphajai, F.; Sandak, J.; Sailer, M.; Rautkari, L.; Belt, T.; Sandak, A. Bioinspired Living Coating System in Service: Evaluation of the Wood Protected with Biofinish during One-Year Natural Weathering. Coatings 2021, 11, 701. https://doi.org/10.3390/coatings11060701
Poohphajai F, Sandak J, Sailer M, Rautkari L, Belt T, Sandak A. Bioinspired Living Coating System in Service: Evaluation of the Wood Protected with Biofinish during One-Year Natural Weathering. Coatings. 2021; 11(6):701. https://doi.org/10.3390/coatings11060701
Chicago/Turabian StylePoohphajai, Faksawat, Jakub Sandak, Michael Sailer, Lauri Rautkari, Tiina Belt, and Anna Sandak. 2021. "Bioinspired Living Coating System in Service: Evaluation of the Wood Protected with Biofinish during One-Year Natural Weathering" Coatings 11, no. 6: 701. https://doi.org/10.3390/coatings11060701






