A Synergistic Effect between Stearic Acid and (+)-α-Tocopherol as a Green Inhibitor on Ferritic Stainless Steel Corrosion Inhibition in 3.0% NaCl Solution
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Apparatus
- -
- Electrochemical measurements: Solartron 1287 Electrochemical Interface and Gamry 600™ potentiostat/galvanostat, Warminster, PA, USA controlled by an electrochemical program.
- -
- Processing and analysis of experimental data using the following software programs: CorrView, CorrWare, Zplot and ZView programs from Scribner Associates, Southern Pines, North Carolina, USA (all version 2.80).
- -
- ATR-FTIR: SHIMADZU-IRAffinity-1, Shimadzu Europa GmbH, Duisburg, F.R. Germany.
- -
- Scanning electron microscope (SEM): FEI Sirion 400 NC, Eindhoven, Netherlands.
- -
- Goniometer: Data Physics OCA 35, Filderstadt, Germany.
2.3. Pretreatment for Electrode
2.4. Electrochemical Measurement
2.5. Surface Characterisation
2.5.1. Contact Angle
2.5.2. ATR-FTIR Analysis
3. Results and Discussion
3.1. Wettability of a High-Level Hydrophobic Surface
Contact Angle Measurements
3.2. Corrosion Resistance of the As-Prepared Hydrophobic Layers—Electrochemical Results
3.2.1. Open Circuit Potential
3.2.2. Potentiodynamic Polarisation Test
3.2.3. Electrochemical Impedance Spectroscopy (EIS)
3.3. Surface Characterisation
3.3.1. ATR–FTIR Analysis
3.3.2. SEM EDAX
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kreysa, G.; Schűtze, M.; Fűrbeth, W. Corrosion News. Mater. Corros. 2018, 69, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. Npj Mater. Degrad. 2017, 1, 1–10. [Google Scholar] [CrossRef]
- Mazumder, M.A.J. Global Impact of Corrosion: Occurrence, Cost and Mitigation. Glob. J. Eng. Sci. 2020, 5, 4. [Google Scholar] [CrossRef]
- Abiola, O.K.; John, M.O.; Asekunowo, P.O.; Okafor, P.C.; James, O. 3-[(4-amino-2-methyl-5-pyrimidinyl) methyl]-5-(2-hydroxyethyl)-4-methyl thiazolium chloride hydrochloride as green corrosion inhibitor of copper in HNO3 solution and its adsorption characteristics. Green Chem. Lett. Rev. 2011, 4, 273–279. [Google Scholar] [CrossRef]
- Hoseizadeh, A.R.; Danaee, I.; Maddahy, M.H. Thermodynamic and Adsorption Behaviour of Vitamin B1 as a Corrosion Inhibitor for AISI 4130 Steel Alloy in HCl Solution. Zeitschrift für Physikalische Chemie 2013, 227, 403–418. [Google Scholar] [CrossRef]
- Solmaz, R. Investigation of corrosion inhibition mechanism and stability of Vitamin B1 on mild steel in 0.5M HCl solution. Corros. Sci. 2014, 81, 75–84. [Google Scholar] [CrossRef]
- Malhotra, S.; Singh, G. Vitamins: Potential inhibitors for nickel in acidic media. Surf. Eng. 2005, 21, 187–192. [Google Scholar] [CrossRef]
- Fuchs-Godec, R.; Pavlovic, M.G.; Tomic, M.V. The inhibitive effect of vitamin-C on the corrosive performance of steel in HCl solutions—Part II. Int. J. Electrochem. Sci. 2015, 10, 10502–10512. [Google Scholar]
- Sekine, I.; Nakahata, Y.; Tanabe, H. The corrosion inhibition of mild steel by ascorbic and folic acids. Corros. Sci. 1988, 28, 987–1001. [Google Scholar] [CrossRef]
- Fuchs-Godec, R.; Zerjav, G. Corrosion resistance of high-level-hydrophobic layers in combination with Vitamin E—(α-tocopherol) as green inhibitor. Corros. Sci. 2015, 97, 7–16. [Google Scholar] [CrossRef]
- Fuchs-Godec, R. Effect of α-tocopherol as a green inhibitor on chloride-induced corrosion of steel. Int. J. Electrochem. Sci. 2019, 14, 10396–10409. [Google Scholar] [CrossRef]
- Fuchs-Godec, R.; Pavlović, M.G.; Tomić, M.V. The inhibitive effect of vitamin-C on the corrosive performance of steel in HCL solutions. Int. J. Electrochem. Sci. 2013, 8, 1511–1519. [Google Scholar]
- Valek, L.; Martinez, S.; Serdar, M.; Stipanovic, I. Ascorbic Acid as Corrosion Inhibitor for Steel in Alkaline Media Containing Chloride Ions. Chem. Biochem. Eng. Q. 2007, 21, 65–70. [Google Scholar]
- Fuadi, A. Suhendrayatna Investigation of Ascorbic Acid as Environment-Friendly Corrosion Inhibitor of Low Carbon Steel in Marine Environment. IOP Conf. Ser. Mater. Sci. Eng. 2019, 536, 012108. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, L. Multifunctional Integration: From Biological to Bio-Inspired Materials. ACS Nano 2011, 5, 6786–6790. [Google Scholar] [CrossRef]
- Meuler, A.J.; Smith, J.D.; Varanasi, K.K.; Mabry, J.; McKinley, G.; Cohen, R.E. Relationships between Water Wettability and Ice Adhesion. ACS Appl. Mater. Interfaces 2010, 2, 3100–3110. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Z.; Yang, X.; Xu, X.; Zhang, L.; Zhao, N.; Xu, J. Antifogging and antireflective silica film and its application on solar modules. Surf. Coat. Technol. 2011, 206, 1490–1494. [Google Scholar] [CrossRef]
- Darmanin, T.; Tarrade, J.; Celia, E.; Bellanger, H.; Guittard, F. Superoleophobic Meshes with Relatively Low Hysteresis and Sliding Angles by Electropolymerization: Importance of Polymer-Growth Control. Chem. Plus. Chem. 2013, 79, 382–386. [Google Scholar] [CrossRef]
- Bäßler, R.; Uhlemann, M.; Mummert, K. Inhibiting effect of octadecylamine on pitting corrosion behaviour of stainless steel type 1.4541 up to 250 °C. Mater. Corros. 1999, 50, 146–153. [Google Scholar] [CrossRef]
- Xue, C.-H.; Jia, S.-T.; Zhang, J.; Ma, J.-Z. Large-area fabrication of superhydrophobic surfaces for practical applications: An overview. Sci. Technol. Adv. Mater. 2010, 11, 033002. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Khorsand, S.; Sokhanvar, S.; Kaboli, A. Science and Engineering of Superhydrophobic Surfaces: Review of Corrosion Resistance, Chemical and Mechanical Stability. Arab. J. Chem. 2020, 13, 1763–1802. [Google Scholar] [CrossRef]
- Wen, Q.; Guo, Z. Recent Advances in the Fabrication of Superhydrophobic Surfaces. Chem. Lett. 2016, 45, 1134–1149. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-X.; Zhang, X.-F. A non-particle and fluorine-free superhydrophobic surface based on one-step electrodeposition of dodecyltrimethoxysilane on mild steel for corrosion protection. Corros. Sci. 2019, 163, 108284. [Google Scholar] [CrossRef]
- Ma, Q.; Tong, Z.; Wang, W.; Dong, G. Fabricating robust and repairable superhydrophobic surface on carbon steel by nanosecond laser texturing for corrosion protection. Appl. Surf. Sci. 2018, 455, 748–757. [Google Scholar] [CrossRef]
- Qian, H.; Li, M.; Li, Z.; Lou, Y.; Huang, L.; Zhang, D.; Xu, D.; Du, C.; Lu, L.; Gao, J. Mussel-inspired superhydrophobic surfaces with enhanced corrosion resistance and dual-action antibacterial properties. Mater. Sci. Eng. C 2017, 80, 566–577. [Google Scholar] [CrossRef]
- Xiang, T.; Chen, D.; Lv, Z.; Yang, Z.; Yang, L.; Li, C. Robust superhydrophobic coating with superior corrosion resistance. J. Alloys Compd. 2019, 798, 320–325. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Chen, R.-J.; Hu, J.-M. Superhydrophobic surface constructed on electrodeposited silica films by two-step method for corrosion protection of mild steel. Corros. Sci. 2016, 104, 336–343. [Google Scholar] [CrossRef]
- Sebastian, D.; Yao, C.W.; Lian, I. Mechanical durability of engineered superhydrophobic surfaces for anti-corrosion. Coatings 2018, 8, 162. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Wang, L.; Yang, Z.; Li, S.; Wu, T.; Liu, G. Fabrication of polydimethylsiloxane-derived superhydrophobic surface on aluminium via chemical vapour deposition technique for corrosion protection. Corros. Sci. 2017, 128, 176–185. [Google Scholar] [CrossRef]
- Zhang, D.; Qian, H.; Wang, L.; Li, X. Comparison of barrier properties for a superhydrophobic epoxy coating under different simulated corrosion environments. Corros. Sci. 2016, 103, 230–241. [Google Scholar] [CrossRef]
- Ejenstam, L.; Swerin, A.; Pan, J.; Claesson, P.M. Corrosion protection by hydrophobic silica particle-polydimethylsiloxane composite coatings. Corros. Sci. 2015, 99, 89–97. [Google Scholar] [CrossRef]
- Liu, W.; Xu, Q.; Han, J.; Chen, X.; Min, Y. A novel combination approach for the preparation of superhydrophobic surface on copper and the consequent corrosion resistance. Corros. Sci. 2016, 110, 105–113. [Google Scholar] [CrossRef]
- Xu, W.; Hu, Y.; Bao, W.; Xie, X.; Liu, Y.; Song, A.; Hao, J. Superhydrophobic copper surfaces fabricated by fatty acid soaps in aqueous solution for excellent corrosion resistance. Appl. Surf. Sci. 2017, 399, 491–498. [Google Scholar] [CrossRef]
- Emelyanenko, A.M.; Pytskii, I.S.; Kaminsky, V.V.; Chulkova, E.V.; Domantovsky, A.G.; Emelyanenko, K.A.; Sobolev, V.D.; Aleshkin, A.V.; Boinovich, L.B. Superhydrophobic copper in biological liquids: Antibacterial activity and microbiologically induced or inhibited corrosion. Colloids Surf. B Biointerfaces 2019, 185, 110622. [Google Scholar] [CrossRef]
- Yuan, S.; Pehkonen, S.; Liang, B.; Ting, Y.; Neoh, K.G.; Kang, E.-T. Superhydrophobic fluoropolymer-modified copper surface via surface graft polymerisation for corrosion protection. Corros. Sci. 2011, 53, 2738–2747. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, S.; Xu, W.; Cao, K.; Li, J.; Zheng, Y. Controllable fabrication of superhydrophobic alloys surface on copper substrate for self-cleaning, anti-icing, anti-corrosion and anti-wear performance. Surf. Coat. Technol. 2018, 333, 61–70. [Google Scholar] [CrossRef]
- Huang, Y.; Sarkar, D.; Gallant, D.; Chen, X.-G. Corrosion resistance properties of superhydrophobic copper surfaces fabricated by one-step electrochemical modification process. Appl. Surf. Sci. 2013, 282, 689–694. [Google Scholar] [CrossRef]
- Khorsand, S.; Raeissi, K.; Ashrafizadeh, F. Corrosion resistance and long-term durability of super-hydrophobic nickel film prepared by electrodeposition process. Appl. Surf. Sci. 2014, 305, 498–505. [Google Scholar] [CrossRef]
- Qiu, R.; Zhang, D.; Wang, P. Superhydrophobic-carbon fibre growth on a zinc surface for corrosion inhibition. Corros. Sci. 2013, 66, 350–359. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Lei, J.; He, J.; Lv, R.; Li, N.; Pan, F. A facile approach to fabricate superhydrophobic Zn surface and its effect on corrosion resistance. Corros. Sci. 2014, 85, 174–182. [Google Scholar] [CrossRef]
- Zang, D.; Zhu, R.; Zhang, W.; Wu, J.; Yu, X.; Zhang, Y. Stearic acid modified aluminum surfaces with controlled wetting properties and corrosion resistance. Corros. Sci. 2014, 83, 86–93. [Google Scholar] [CrossRef]
- Malta, M.I.C.; Vieira, M.R.S.; Da Silva, R.G.C.; Da Silva, L.M.C.; De Araújo, E.G.; Maciel, S.H.D.O.; Filho, S.L.U. Superhydrophobic Surfaces on 5052 Aluminum Alloy Obtained from LDH Film Modified with Stearic Acid for Enhanced Corrosion Protection. Mater. Res. 2019, 22. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, H.; Wang, Z.; Liu, Y. Superhydrophobic aluminum alloy surface: Fabrication, structure, and corrosion resistance. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 319–325. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, W.; Zhu, Q.; Sun, Y.; Li, Y. Mechanically robust superhydrophobic porous anodized AA5083 for marine corrosion protection. Corros. Sci. 2019, 158, 108083. [Google Scholar] [CrossRef]
- Tang, H.; Sun, J.; Yan, X.; Wu, P. Electrochemical and adsorption behaviors of thiadiazole derivatives on the aluminum surface. RSC Adv. 2019, 9, 34617–34626. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, S.; Nouri, M.; Aghdam, A.S.R. A novel combined method for fabrication of stable corrosion resistance superhydrophobic surface on Al alloy. Corros. Sci. 2019, 159, 108144. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Q.; Gao, R.; Wang, J.; Yang, W.; Liu, L. One-step method for the fabrication of superhydrophobic surface on magnesium alloy and its corrosion protection, antifouling performance. Corros. Sci. 2014, 80, 177–183. [Google Scholar] [CrossRef]
- Ding, C.; Liu, Y.; Wang, M.; Wang, T.; Fu, J. Self-healing, superhydrophobic coating based on mechanized silica nanoparticles for reliable protection of magnesium alloys. J. Mater. Chem. A 2016, 4, 8041–8052. [Google Scholar] [CrossRef]
- Yeganeh, M.; Mohammadi, N. Superhydrophobic surface of Mg alloys: A review. J. Magnes. Alloys 2018, 6, 59–70. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, H. A Facile Method to Prepare a Superhydrophobic Magnesium Alloy Surface. Materials 2020, 13, 4007. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Pashinin, A.S.; Gnedenkov, S.V.; Egorkin, V.S.; Sinebryukhov, S.L. Mg alloy treatment for superhydrophobic anticorrosion coating formation. Surf. Innov. 2013, 1, 162–172. [Google Scholar] [CrossRef]
- Boisier, G.; Lamure, A.; Pébère, N.; Portail, N.; Villatte, M. Corrosion protection of AA2024 sealed anodic layers using the hydrophobic properties of carboxylic acids. Surf. Coat. Technol. 2009, 203, 3420–3426. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, S.P.M. Synergistic effect of Thiomalic acid and Zinc ions in Corrosion control of Carbon Steel in Aqueous Solution. Res. J. Chem. Sci. 2014, 4, 41. [Google Scholar]
- Wysocka, J.; Cieślik, M.; Krakowiak, S.; Ryl, J. Carboxylic acids as efficient corrosion inhibitors of aluminium alloys in alkaline media. Electrochim. Acta 2018, 289, 175–192. [Google Scholar] [CrossRef]
- Yoo, S.-H.; Kim, Y.-W.; Chung, K.; Kim, N.-K.; Kim, J.-S. Corrosion Inhibition Properties of Triazine Derivatives Containing Carboxylic Acid and Amine Groups in 1.0 M HCl Solution. Ind. Eng. Chem. Res. 2013, 52, 10880–10889. [Google Scholar] [CrossRef]
- Wang, S.; Feng, L.; Jiang, L. One-Step Solution-Immersion Process for the Fabrication of Stable Bionic Superhydrophobic Surfaces. Adv. Mater. 2006, 18, 767–770. [Google Scholar] [CrossRef]
- Cao, Y.; Jin, S.; Zheng, D.; Lin, C. Facile fabrication of ZnAl layered double hydroxide film co-intercalated with vanadates and laurates by one-step post modification. Colloid Interface Sci. Commun. 2020, 40, 100351. [Google Scholar] [CrossRef]
- Ke, X.; Guo, S.; Gou, B.; Wang, N.; Zhou, X.; Xiao, L.; Hao, G.; Jiang, W. Superhydrophobic Fluorine-Containing Protective Coating to Endow Al Nanoparticles with Long-Term Storage Stability and Self-Activation Reaction Capability. Adv. Mater. Interfaces 2019, 6, 1901025. [Google Scholar] [CrossRef]
- Ejenstam, L.; Tuominen, M.; Haapanen, J.; Mäkelä, J.M.; Pan, J.; Swerin, A.; Claesson, P.M. Long-term corrosion protection by a thin nano-composite coating. Appl. Surf. Sci. 2015, 357, 2333–2342. [Google Scholar] [CrossRef]
- Geuli, O.; Mandler, D. The synergistic effect of benzotriazole and trimethylsiloxysilicate towards corrosion protection of printed Cu-based electronics. Corros. Sci. 2018, 143, 329–336. [Google Scholar] [CrossRef]
- Ou, H.-H.; Tran, Q.T.P.; Lin, P.-H. A synergistic effect between gluconate and molybdate on corrosion inhibition of recirculating cooling water systems. Corros. Sci. 2018, 133, 231–239. [Google Scholar] [CrossRef]
- Zhang, C.; Duan, H.; Zhao, J. Synergistic inhibition effect of imidazoline derivative and l -cysteine on carbon steel corrosion in a CO 2 -saturated brine solution. Corros. Sci. 2016, 112, 160–169. [Google Scholar] [CrossRef]
- Fuchs-Godec, R.; Pavlović, M.G. Synergistic effect between non-ionic surfactant and halide ions in the forms of inorganic or organic salts for the corrosion inhibition of stainless-steel X4Cr13 in sulphuric acid. Corros. Sci. 2012, 58, 192–201. [Google Scholar] [CrossRef]
- Barthlott, W.; Mail, M.; Neinhuis, C. Superhydrophobic hierarchically structured surfaces in biology: Evolution, structural principles and biomimetic applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20160191. [Google Scholar] [CrossRef] [Green Version]
- Latthe, S.S.; Terashima, C.; Nakata, K.; Fujishima, A. Superhydrophobic Surfaces Developed by Mimicking Hierarchical Surface Morphology of Lotus Leaf. Molecules 2014, 19, 4256–4283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeevahan, J.; Chandrasekaran, M.; Joseph, G.B.; Raj, R.B.D.; Mageshwaran, G. Superhydrophobic surfaces: A review on fundamentals, applications, and challenges. J. Coat. Technol. Res. 2018, 15, 231–250. [Google Scholar] [CrossRef]
- Ellingson, L.; Shedlosky, T.; Bierwagen, G.; de la Rie, E.; Brostoff, L. The Use of Electrochemical Impedance Spectroscopy in the Evaluation of Coatings for Outdoor Bronze. Stud. Conserv. 2004, 49, 53–62. [Google Scholar] [CrossRef]
- Ates, M. Review study of electrochemical impedance spectroscopy and equivalent electrical circuits of conducting polymers on carbon surfaces. Prog. Org. Coat. 2011, 71, 1–10. [Google Scholar] [CrossRef]
- Cesiulis, H.; Tsyntsaru, N.; Ramanavicius, A.; Ragoisha, G. Nanostructures and Thin Films for Multifunctional Applications; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-30197-6. [Google Scholar]
- Bonastre, J.; Garces, P.; Galvan, J.C.; Cases, F. Characterisation and corrosion studies of steel electrodes covered by polypyrrole/phosphotungstate using Electrochemical Impedance Spectroscopy. Prog. Org. Coat. 2009, 66, 235–241. [Google Scholar] [CrossRef]
- Amin, M.A.; Ibrahim, M.M. Corrosion and corrosion control of mild steel in concentrated H2SO4 solutions by a newly synthesized glycine derivative. Corros. Sci. 2011, 53, 873–885. [Google Scholar] [CrossRef]
- Sacco, A. Electrochemical impedance spectroscopy: Fundamentals and application in dye-sensitized solar cells. Renew. Sustain. Energy Rev. 2017, 79, 814–829. [Google Scholar] [CrossRef]
- Nady, H.; El-Rabiei, M.; Samy, M. Corrosion behavior and electrochemical properties of carbon steel, commercial pure titanium, copper and copper–aluminum–nickel alloy in 3.5% sodium chloride containing sulfide ions. Egypt. J. Pet. 2017, 26, 79–94. [Google Scholar] [CrossRef] [Green Version]
- Zheludkevich, M.; Serra, R.; Montemor, F.; Yasakau, K.; Salvado, I.; Ferreira, M. Nanostructured sol–gel coatings doped with cerium nitrate as pre-treatments for AA2024-T3: Corrosion protection performance. Electrochim. Acta 2005, 51, 208–217. [Google Scholar] [CrossRef]
- Mahato, N.; Singh, M.M. Investigation of Passive Film Properties and Pitting Resistance of AISI 316 in Aqueous Ethanoic Acid Containing Chloride Ions using Electrochemical Impedance Spectroscopy(EIS). Port. Electrochim. Acta 2011, 29, 233–251. [Google Scholar] [CrossRef]
- Jo, B.E. Electrochemical Impedance Methods to Assess Coatings for Corrosion Protection. Technical Service Center, Materials and Corrosion Laboratory, U.S., PO Box 25007, Denver CO 80225-0007, 2019. Available online: https://www.usbr.gov/tsc/techreferences/mands/mands-pdfs/ElectrochemicalImpedanceMethods_8540-2019-03_508.pdf (accessed on 13 August 2021).
- Nezhad, A.N.; Davoodi, A.; Zahrani, E.M.; Arefinia, R. The effects of an inorganic corrosion inhibitor on the electrochemical behavior of superhydrophobic micro-nano structured Ni films in 3.5% NaCl solution. Surf. Coat. Technol. 2020, 395, 125946. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.C.; He, H.; Ouyang, L.; Yuan, S. A stearic Acid/CeO2 bilayer coating on AZ31B magnesium alloy with superhydrophobic and self-cleaning properties for corrosion inhibition. J. Alloys Compd. 2020, 834, 155210. [Google Scholar] [CrossRef]
- Porcayo-Calderon, J.; Rivera-Muñoz, E.; Peza-Ledesma, C.; Díaz, M.C.; de la Escalera, L.M.; Canto, J.; Martinez-Gomez, L. Sustainable Development of Palm Oil: Synthesis and Electrochemical Performance of Corrosion Inhibitors. J. Electrochem. Sci. Technol. 2017, 8, 133–145. [Google Scholar] [CrossRef]
- Abouzari, M.R.S.; Berkemeier, F.; Schmitz, G.; Wilmer, D. On the physical interpretation of constant phase elements. Solid State Ionics 2009, 180, 922–927. [Google Scholar] [CrossRef]
- Sowa, M.; Simka, W. Electrochemical Impedance and Polarization Corrosion Studies of Tantalum Surface Modified by DC Plasma Electrolytic Oxidation. Materials 2018, 11, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, C.S.; Lu, L.Y. Electrochemical Impedance and Modelling Studies of the Corrosion of Three Commercial Stainless Steels in Molten Carbonate. Int. J. Corros. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Ma, H.; Cheng, X.; Li, G.; Chen, S.; Quan, Z.; Zhao, S.; Niu, L. The influence of hydrogen sulfide on corrosion of iron under different conditions. Corros. Sci. 2000, 42, 1669–1683. [Google Scholar] [CrossRef]
- Popova, A.; Vasilev, A.; Deligeorgiev, T. Evaluation of the Electrochemical Impedance Measurement of Mild Steel Corrosion in an Acidic Medium, in the Presence of Quaternary Ammonium Bromides. Port. Electrochim. Acta 2018, 36, 423–435. [Google Scholar] [CrossRef]
- Martinez, S.; Metikoš-Huković, M. A nonlinear kinetic model introduced for the corrosion inhibitive properties of some organic inhibitors. J. Appl. Electrochem. 2003, 33, 1137–1142. [Google Scholar] [CrossRef]
- Man, Y.C.; Ammawath, W.; Mirghani, M.E.S. Determining α-tocopherol in refined bleached and deodorized palm olein by Fourier transform infrared spectroscopy. Food Chem. 2005, 90, 323–327. [Google Scholar] [CrossRef]
- Silva, S.D.; Feliciano, R.; Boas, L.V.; Bronze, M. Application of FTIR-ATR to Moscatel dessert wines for prediction of total phenolic and flavonoid contents and antioxidant capacity. Food Chem. 2014, 150, 489–493. [Google Scholar] [CrossRef]
- Krilov, D.; Kosovic, M.; Serec, K. Spectroscopic studies of alpha tocopherol interaction with a model liposome and its influence on oxidation dynamics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 129, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, A.C.S.; De, G.C.R. Traceability of Active Compounds of Essential Oils in Antimicrobial Food Packaging Using a Chemometric Method by ATR-FTIR. Am. J. Anal. Chem. 2017, 8, 726–741. [Google Scholar] [CrossRef] [Green Version]

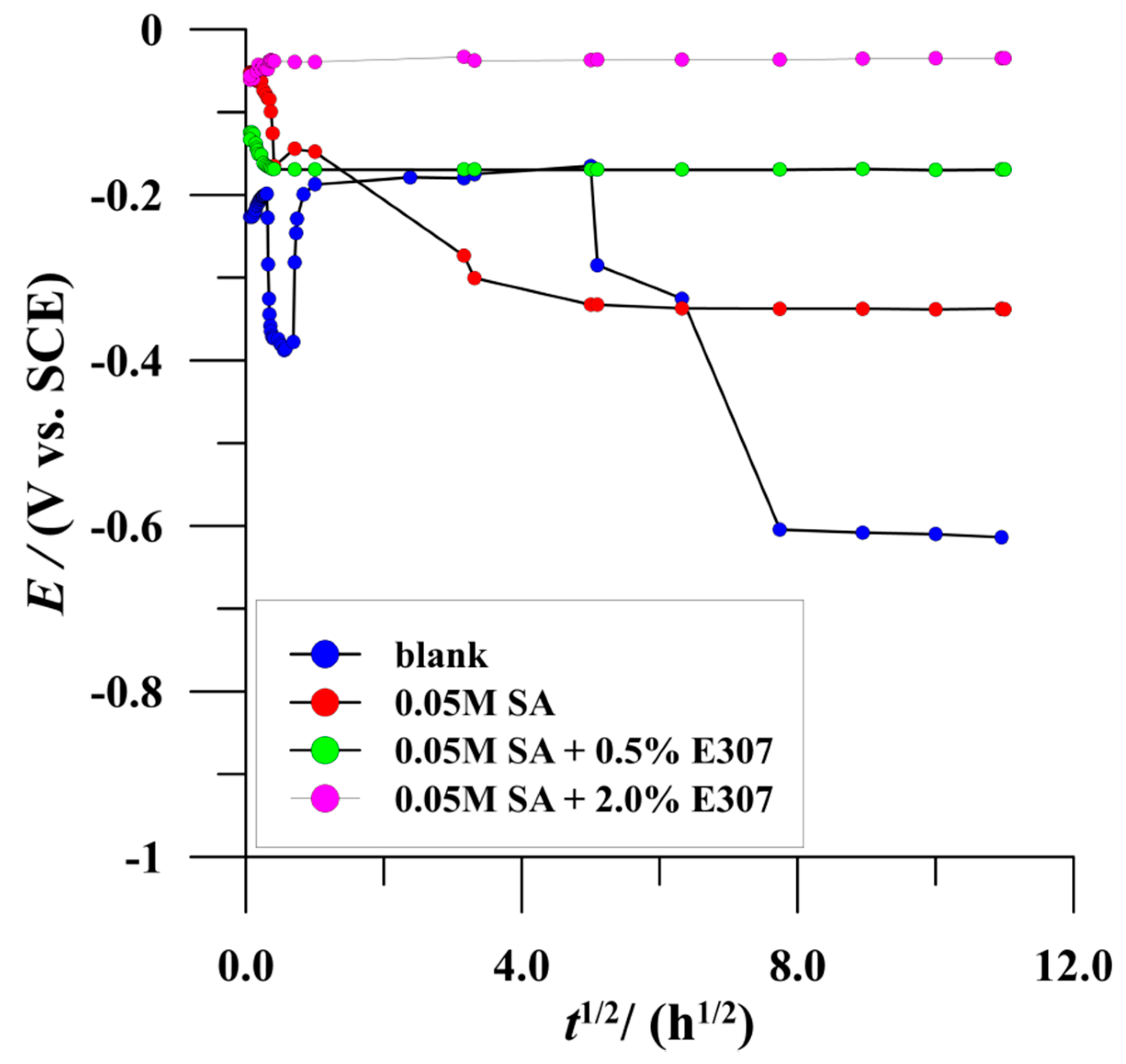





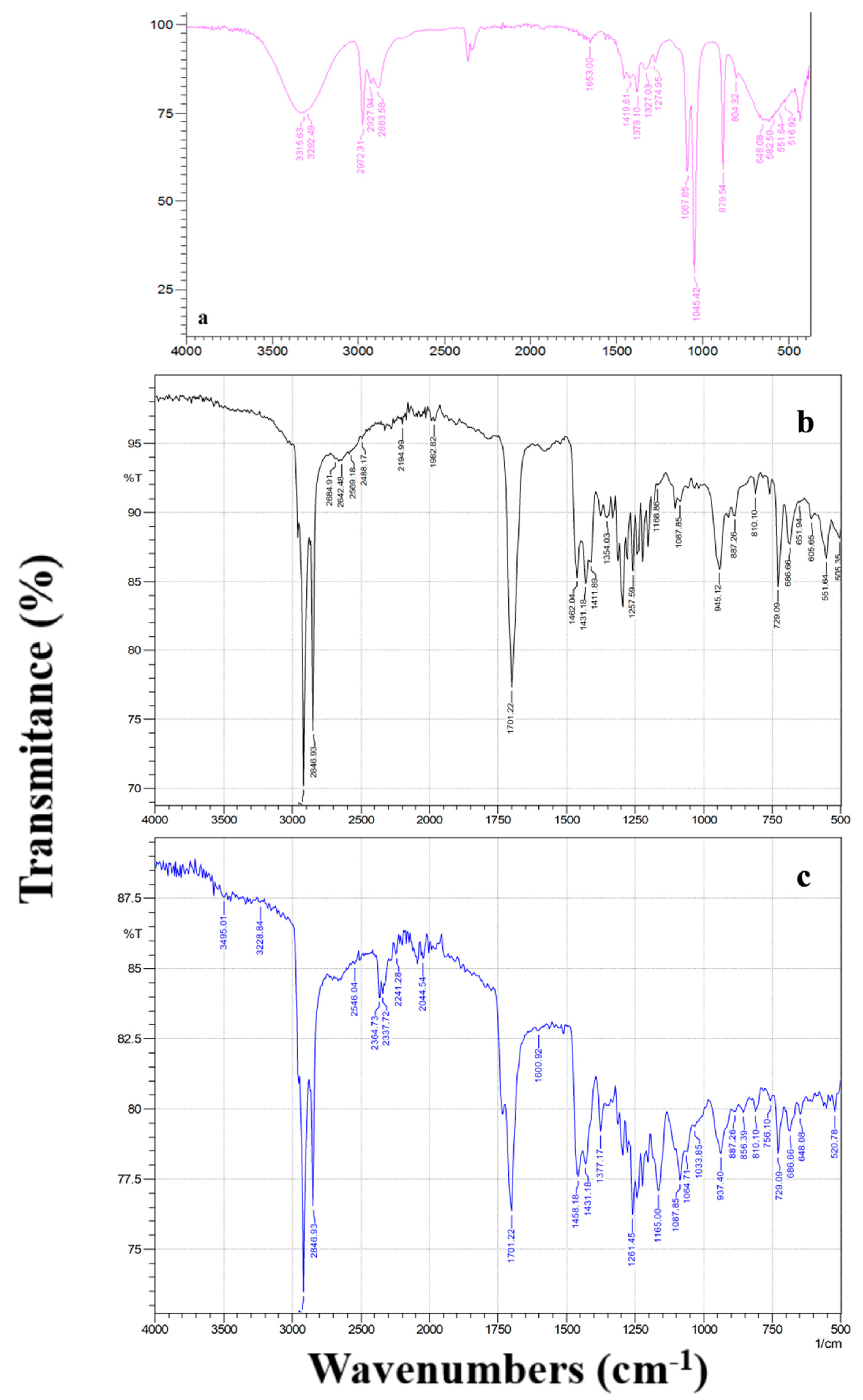
| Corrosive Media 3.0 wt% NaCl | icorr (nA cm−2) | Ecorr (V/SCE) | Rp (MΩ cm2) | bc (mV/dec) | ba (mV/dec) | % ηicorr | %ηRp |
|---|---|---|---|---|---|---|---|
| after 1 h bare surface AISI 410S | 593.50 | −0.270 | 0.021 | −109.1 | 104.4 | ||
| modified surface | |||||||
| wt% E307 | |||||||
| 0* | 244.40 | −0.233 | 0.065 | −156.6 | 137.8 | 58.82 | 66.36 |
| 0.5 | 7.321 | −0.238 | 1.136 | −158.8 | 262.5 | 98.77 | 98.07 |
| 2.0 | 2.214 | −0.272 | 6.573 | −167.3 | 428.8 | 99.63 | 99.67 |
| after 5 days bare surface AISI 410S | 476.00 | −0.403 | 0.0538 | −153.9 | 213.3 | ||
| modified surface * | |||||||
| wt% E307 | |||||||
| 0* | 155.20 | −0.350 | 0.170 | −185.1 | 232.7 | 67.39 | 68.44 |
| 0.5 | 12.270 | −0.213 | 1.394 | −141.2 | 246.8 | 97.42 | 96.14 |
| 2.0 | 0.121 | −0.085 | 22.205 | −117.9 | 251.3 | 99.97 | 99.75 |
| Corrosive Media | Rs | R1 | n1 | C1 | R2 | n2 | C2 | R3 | n3 | C3 | Rp-EIS | ηRp-EIS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3.0 wt% NaCl | (W cm2) | (kW cm2) | (μF cm−2) | (MW cm2) | (μF cm−2) | (MW cm2) | (μF cm−2) | (MW cm2) | (%) | |||
| Bare surface AISI 410S | ||||||||||||
| 1 h | 6.53 | 0.441 | 0.738 | 1.105 | / | / | / | 0.501 | 0.766 | 1.105 | 0.501 | |
| 10 h | 6.55 | 0.263 | 0.776 | 1.122 | / | / | / | 0.514 | 0.79 | 17.981 | 0.514 | |
| 25 h | 6.42 | 0.629 | 0.728 | 1.169 | / | / | / | 0.555 | 0.771 | 12.536 | 0.555 | |
| 5 days | 5.13 | 0.196 | 0.746 | 4.73 | / | / | / | 0.092 | 0.827 | 18.902 | 0.093 | |
| modified surface AISI 410S | SA | |||||||||||
| 1 h | 13.72 | 1.724 | 0.865 | 0.07 | 0.644 | 0.517 | 0.55 | 1.736 | 0.615 | 1.689 | 2.381 | 78.94 |
| 10 h | 13.73 | 2.271 | 0.881 | 0.218 | 0.579 | 0.575 | 0.716 | 1.947 | 0.81 | 1.026 | 2.528 | 79.66 |
| 25 h | 14.21 | 6.195 | 0.87 | 0.453 | 1.28 | 0.89 | 0.868 | 1.488 | 0.636 | 2.749 | 2.774 | 79.99 |
| 5 days | 13.84 | 16.683 | 0.854 | 1.017 | 0.959 | 0.893 | 1.378 | 1.787 | 0.675 | 3.308 | 2.762 | 96.65 |
| modified surface AISI 410S | SA + 0.5%E307 | |||||||||||
| 1 h | 15.13 | 69.991 | 0.918 | 0.04 | 0.11 | 0.588 | 0.119 | 13.998 | 0.627 | 9.215 | 14.178 | 96.46 |
| 10 h | 15.82 | 36.001 | 0.9 | 0.033 | 0.268 | 0.604 | 0.368 | 18.072 | 0.667 | 9.152 | 18.376 | 97.2 |
| 25 h | 16.29 | 20.499 | 0.829 | 0.025 | 0.803 | 0.583 | 1.078 | 21.576 | 0.754 | 9.306 | 22.399 | 97.52 |
| 5 days | 15.81 | 15.47 | 0.88 | 0.043 | 0.242 | 0.542 | 1.246 | 24.079 | 0.669 | 10.111 | 24.337 | 99.62 |
| modified surface AISI 410S | SA + 2.0%E307 | |||||||||||
| 1 h | 17.96 | 76.339 | 0.916 | 0.045 | 0.565 | 0.809 | 0.103 | 13.255 | 0.656 | 2.017 | 13.896 | 96.39 |
| 10 h | 17.37 | 91.5 | 0.923 | 0.045 | 8.02 | 0.897 | 0.49 | 14.19 | 0.745 | 3.74 | 22.302 | 97.69 |
| 25 h | 17.87 | 85.81 | 0.937 | 0.042 | 13.636 | 0.781 | 0.637 | 14.929 | 0.786 | 4.224 | 28.651 | 98.06 |
| 5 days | 17.37 | 128.06 | 0.939 | 0.036 | / | / | / | 28.16 | 0.773 | 4.187 | 28.288 | 99.67 |
| Bare | surface | (SA ) | (SA + 2.0 | wt% E307) | |
|---|---|---|---|---|---|
| X4Cr13 | X4Cr13 | X4Cr13 | |||
| Element | wt% | Element | wt% | Element | wt% |
| C K | 0.52 | C K | 43.04 | C K | 51.02 |
| Si K | 0.48 | Si K | 0.37 | Si K | 0.17 |
| Cr K | 16.31 | Cr K | 7.52 | Cr K | 6.71 |
| Fe K O K S | 80.50 1.64 0.55 | Fe K | 49.08 | Fe K | 42.11 |
| Totals | 100.00 | Totals | 100.00 | Totals | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuchs-Godec, R. A Synergistic Effect between Stearic Acid and (+)-α-Tocopherol as a Green Inhibitor on Ferritic Stainless Steel Corrosion Inhibition in 3.0% NaCl Solution. Coatings 2021, 11, 971. https://doi.org/10.3390/coatings11080971
Fuchs-Godec R. A Synergistic Effect between Stearic Acid and (+)-α-Tocopherol as a Green Inhibitor on Ferritic Stainless Steel Corrosion Inhibition in 3.0% NaCl Solution. Coatings. 2021; 11(8):971. https://doi.org/10.3390/coatings11080971
Chicago/Turabian StyleFuchs-Godec, Regina. 2021. "A Synergistic Effect between Stearic Acid and (+)-α-Tocopherol as a Green Inhibitor on Ferritic Stainless Steel Corrosion Inhibition in 3.0% NaCl Solution" Coatings 11, no. 8: 971. https://doi.org/10.3390/coatings11080971






