Corrosion Behavior of J55 and N80 Carbon Steels in Simulated Formation Water under Different CO2 Partial Pressures
Abstract
:1. Introduction
2. Experimental Section
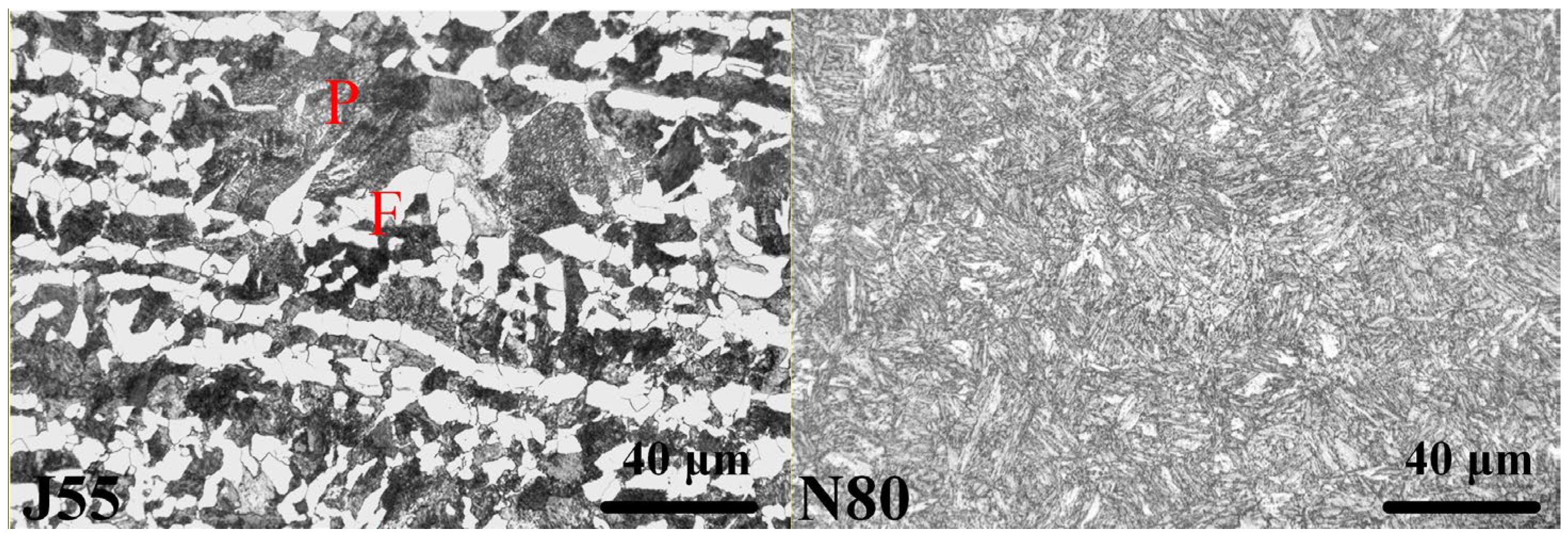
2.1. Materials
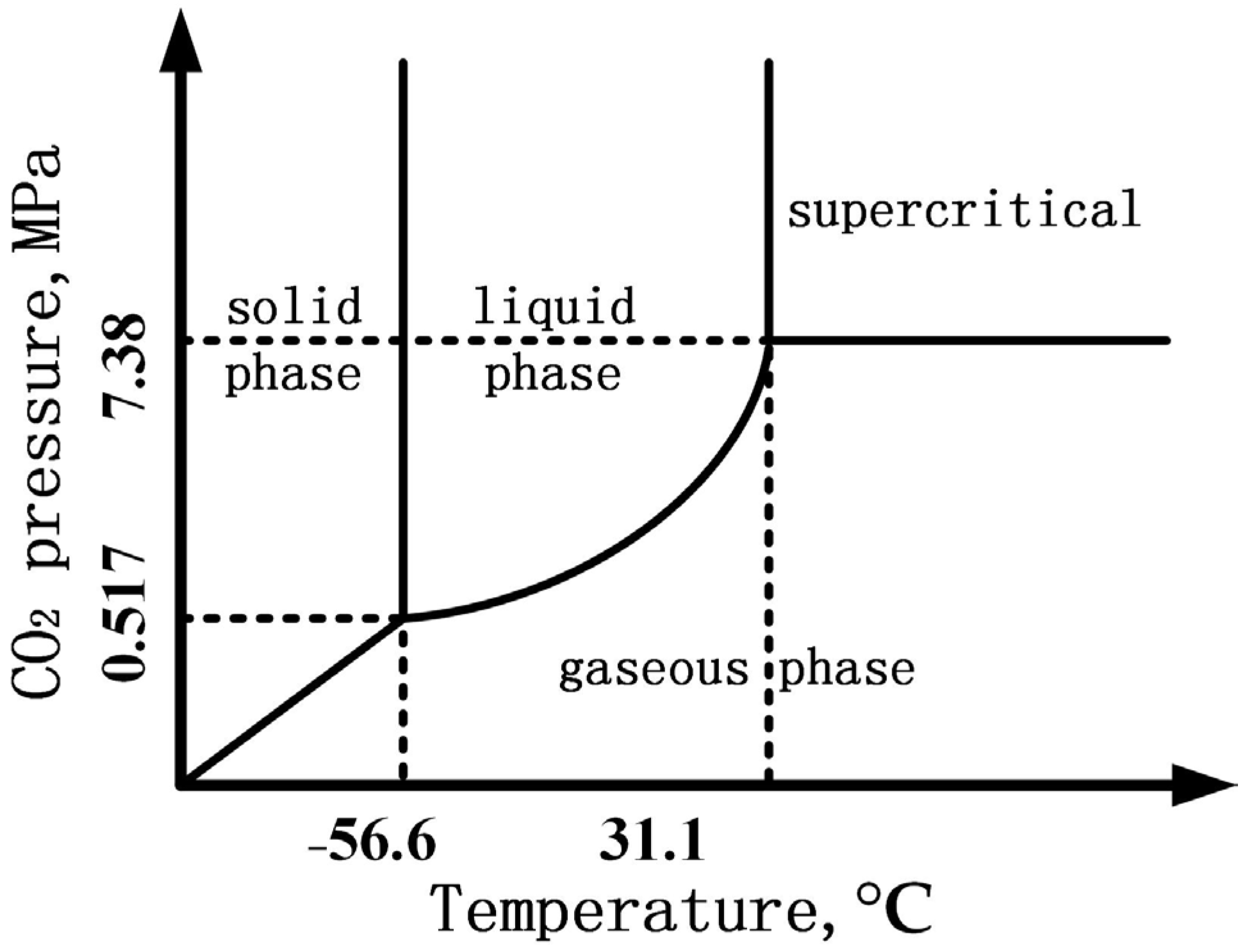
2.2. Immersion Test
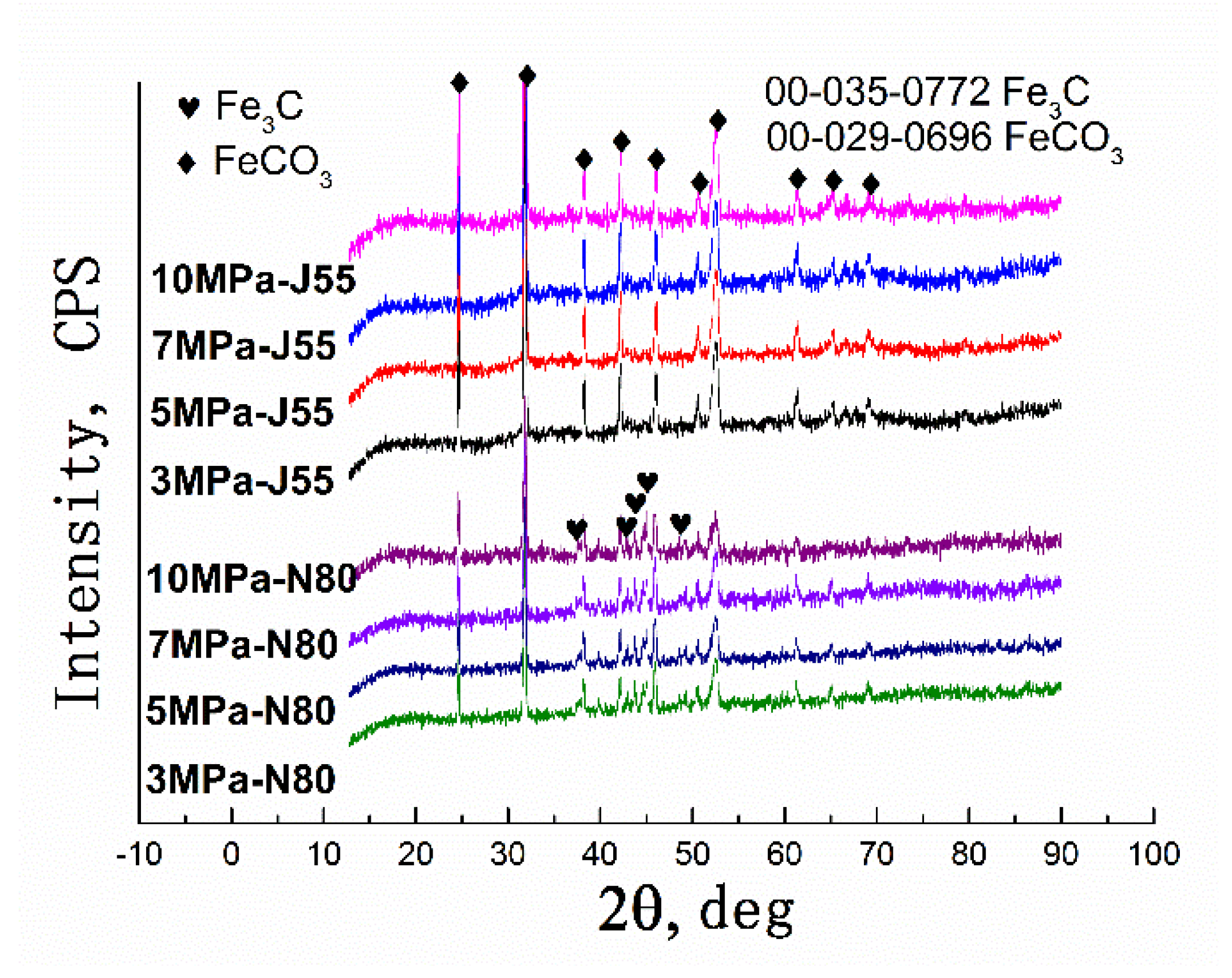
2.3. Characterization of the Corrosion Product
3. Results
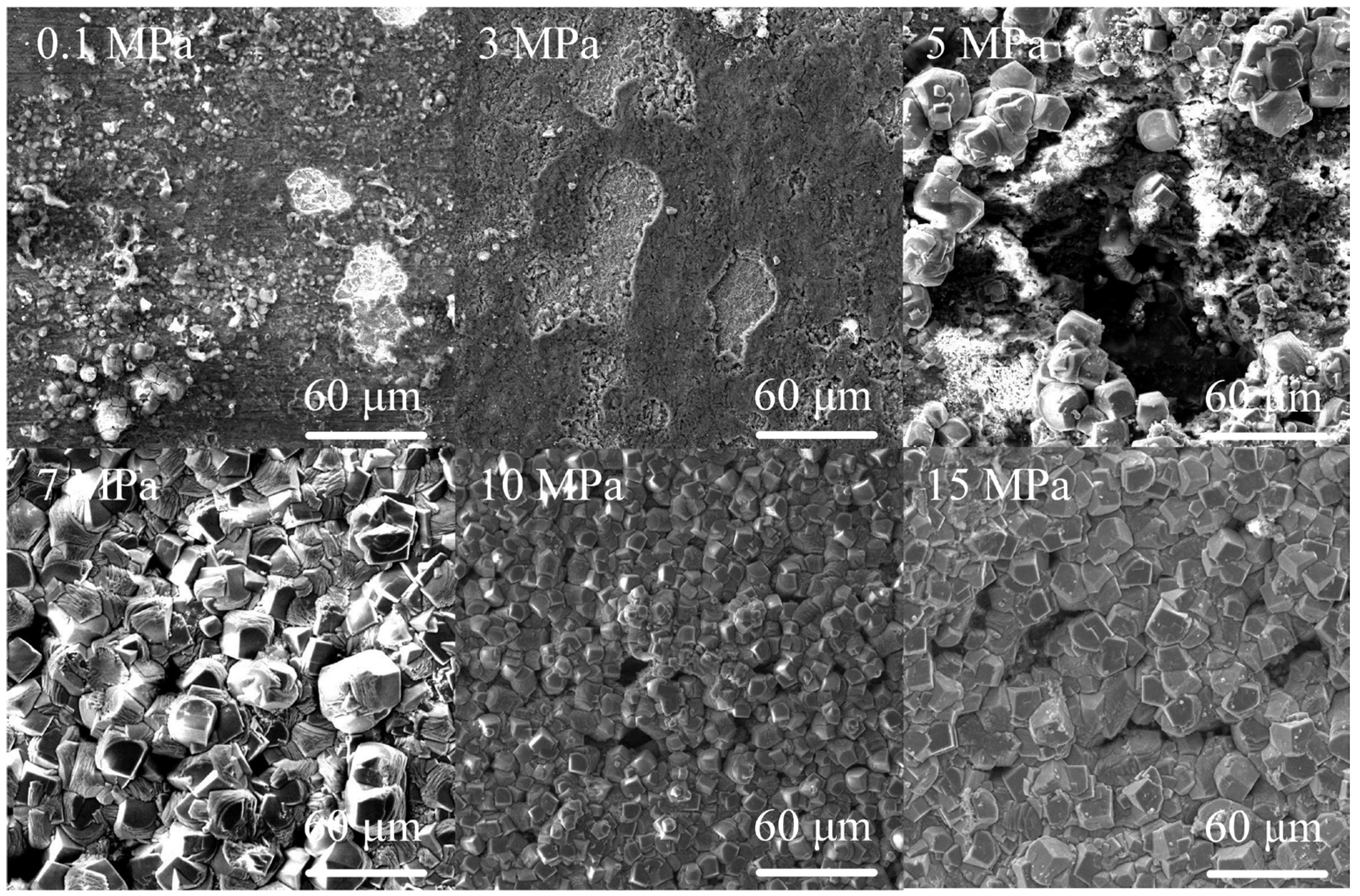
3.1. Corrosion Behavior of J55 and N80 under Different CO2 Partial Pressures
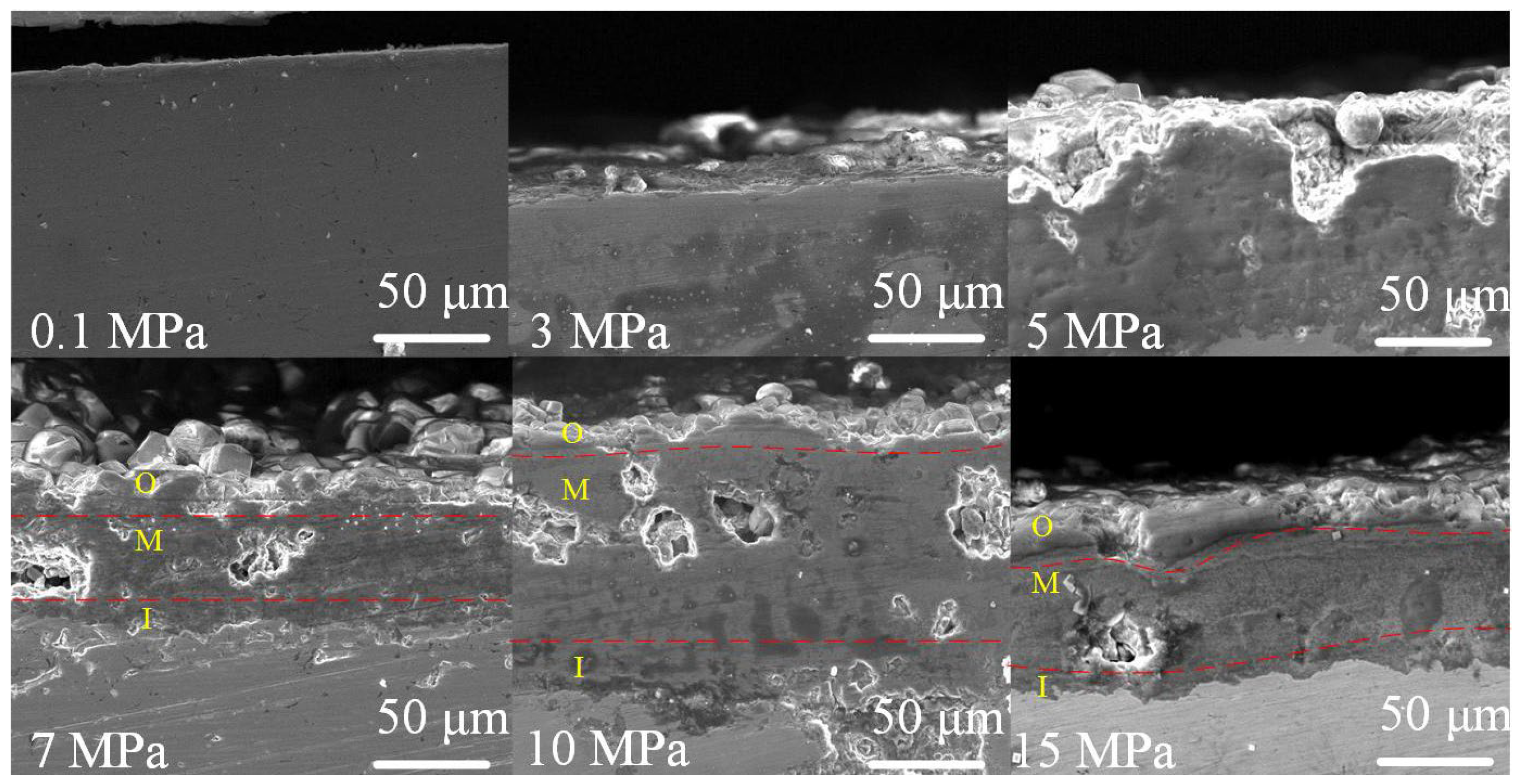
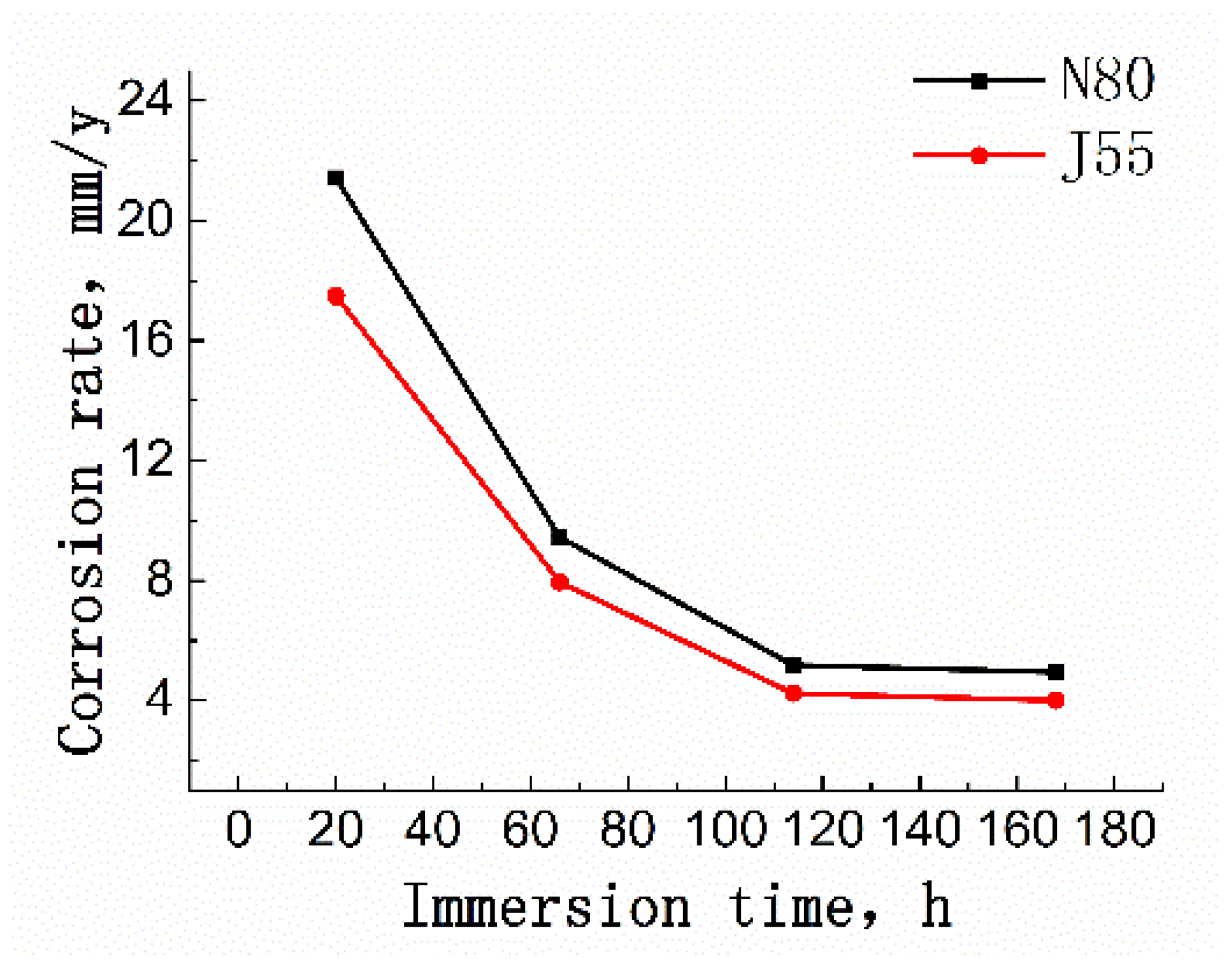
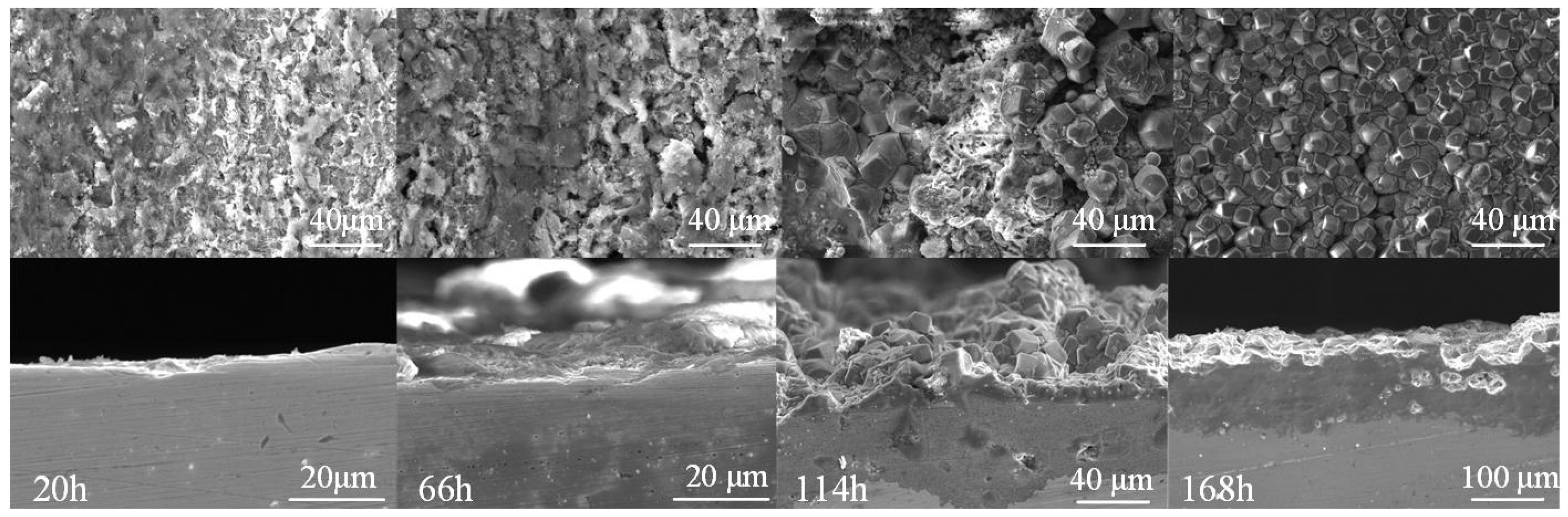
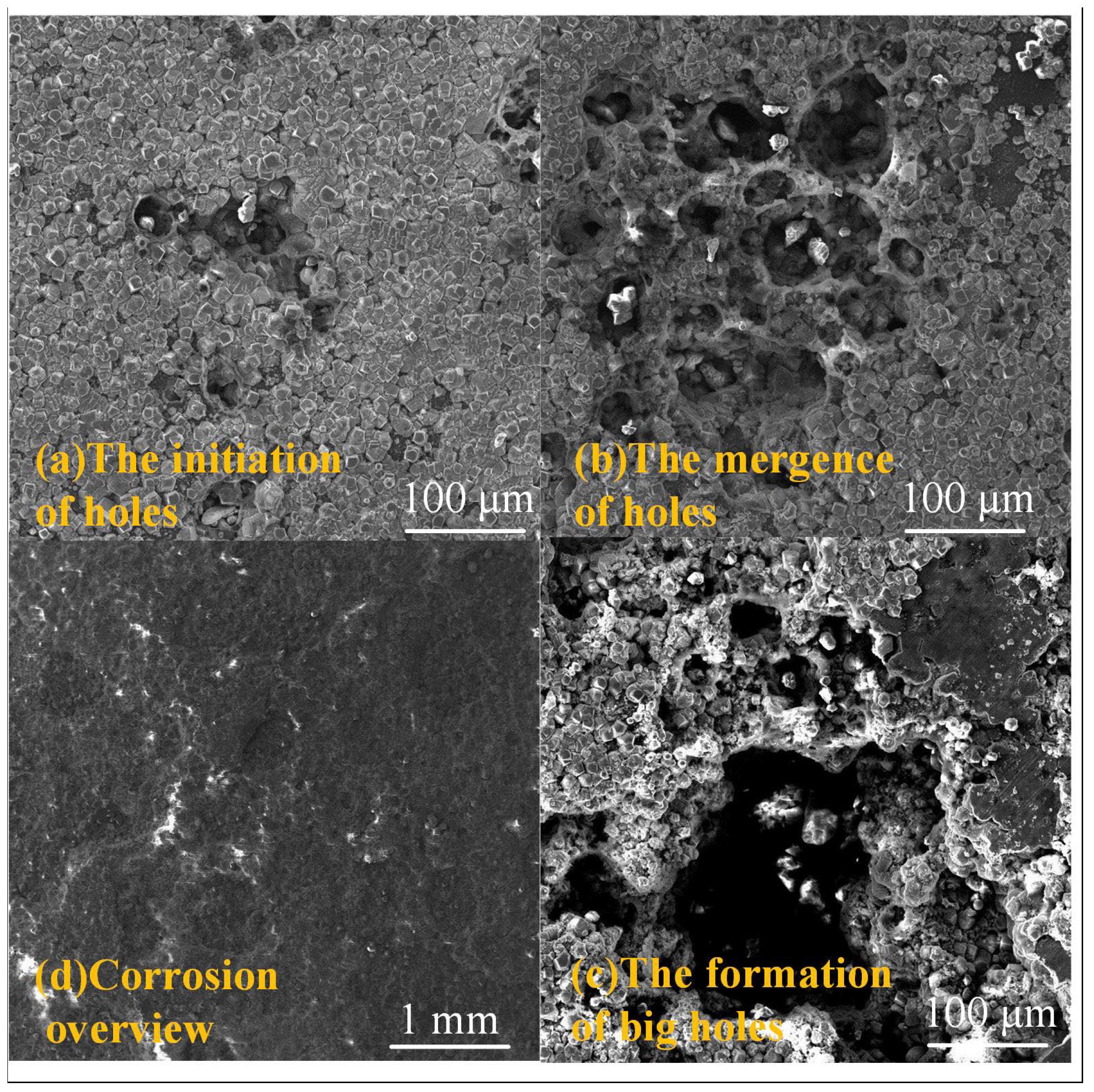
3.2. Corrosion Behavior of J55 and N80 Steels at Different Immersion Times under Supercritical CO2 Conditions
4. Discussion
4.1. Influence of CO2 Partial Pressure on the Corrosion Behavior of J55 and N80 Steels
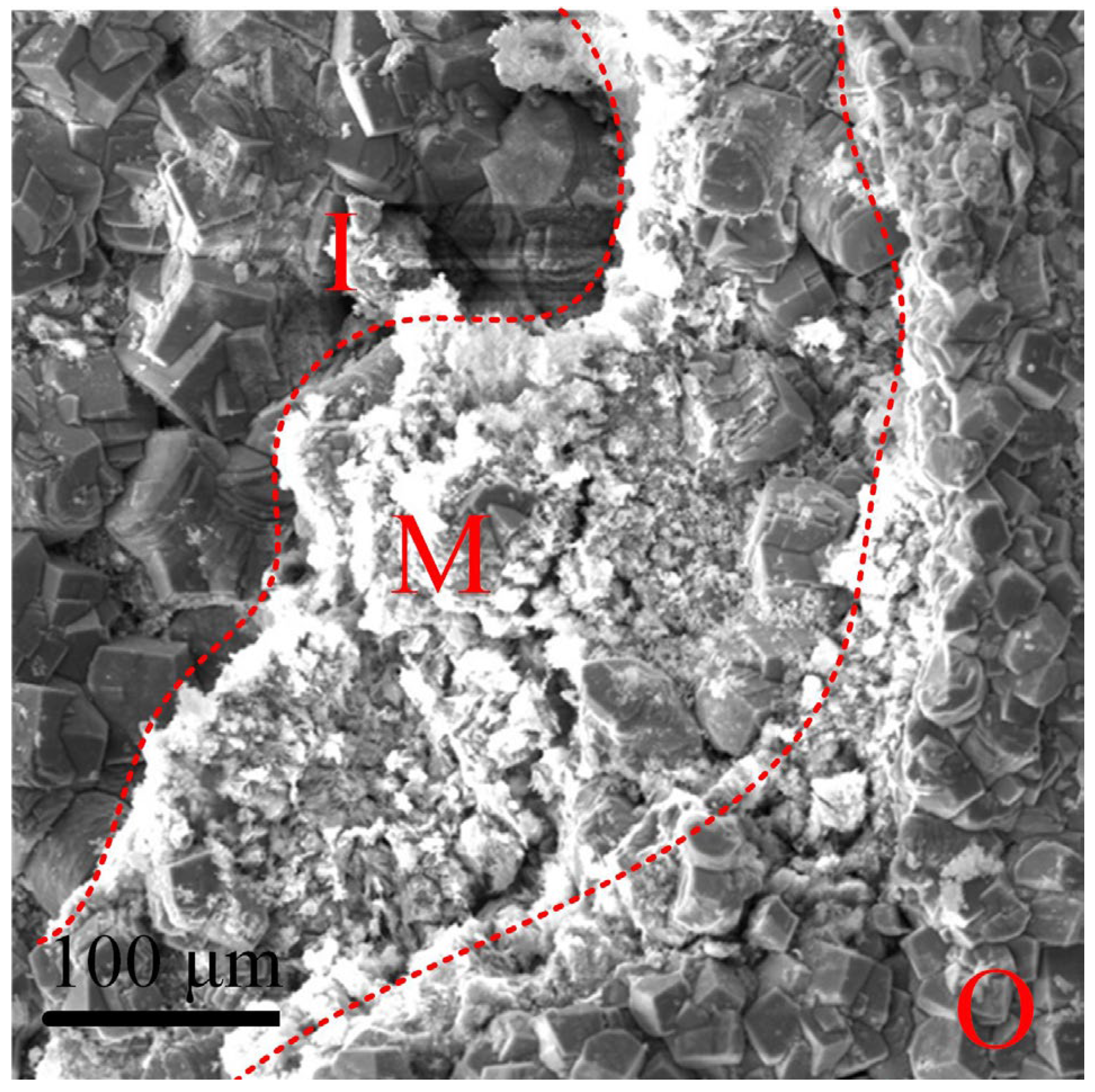
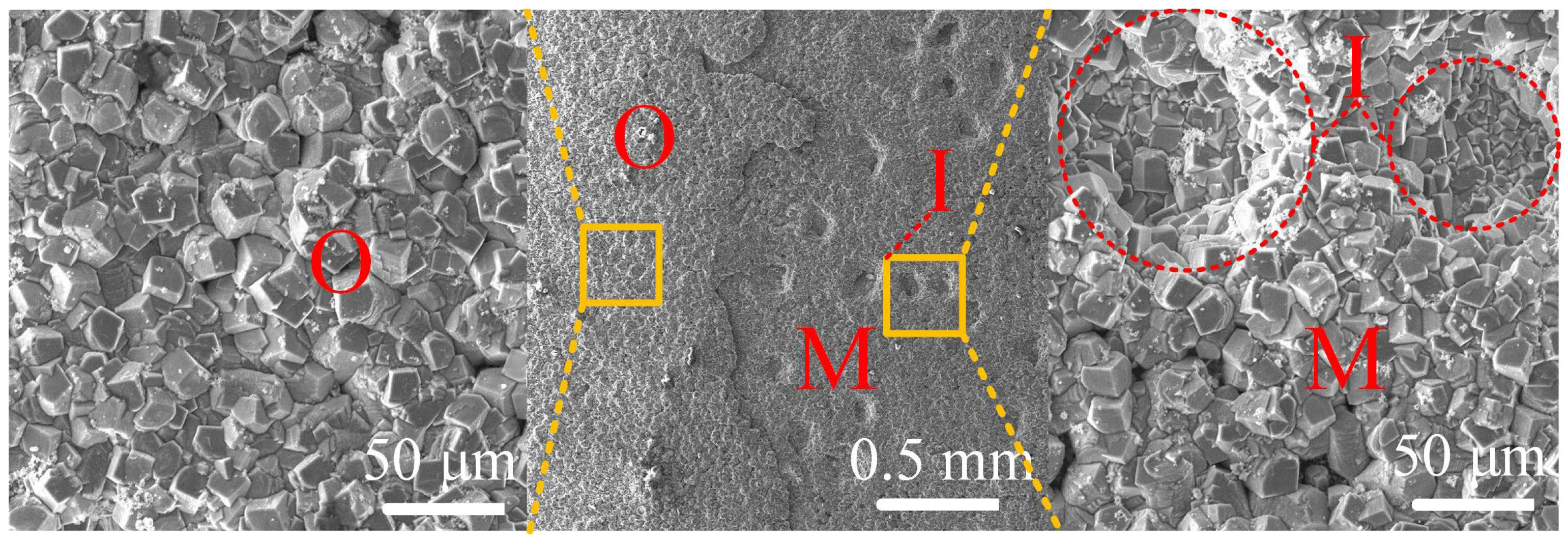
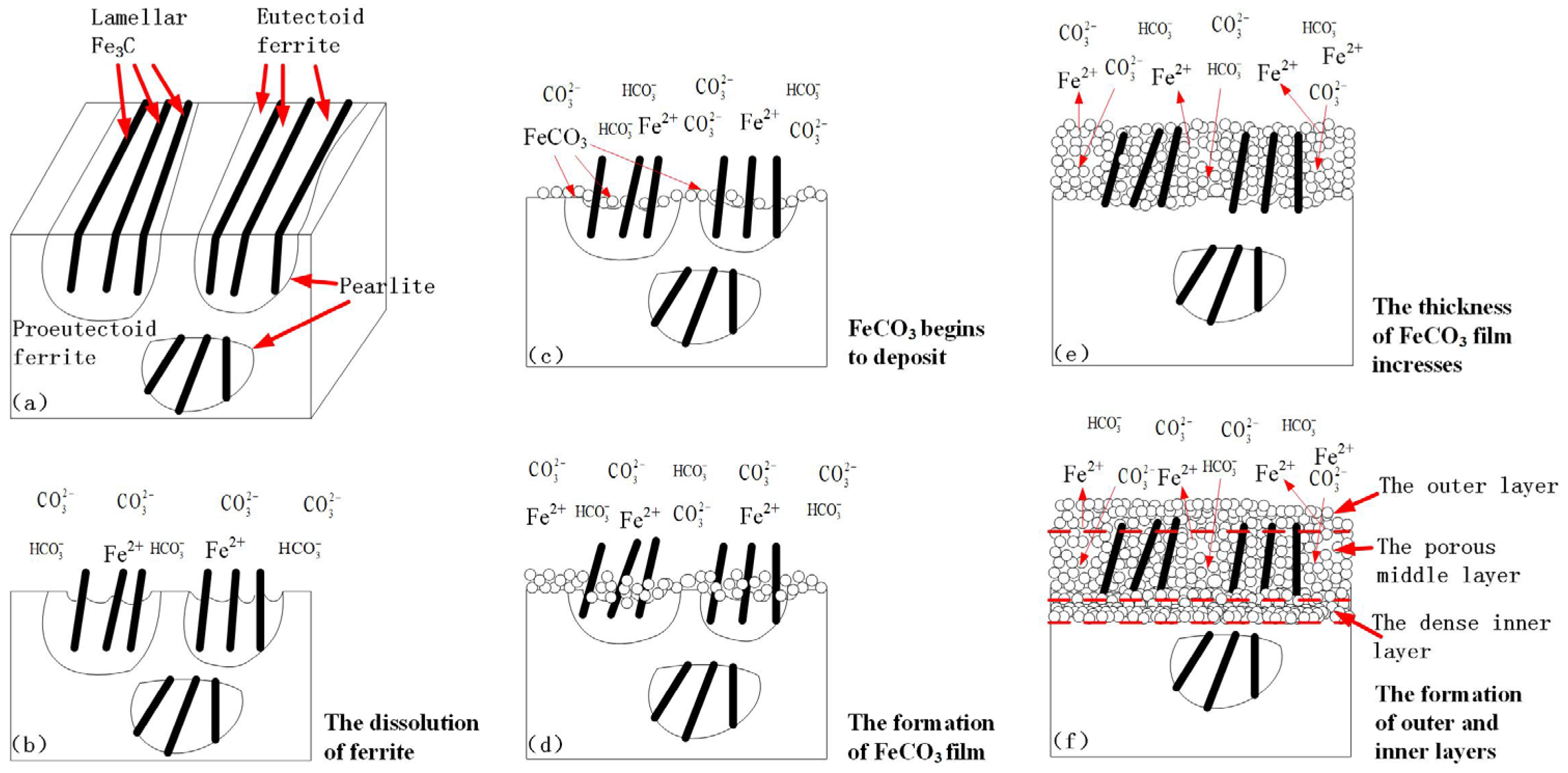
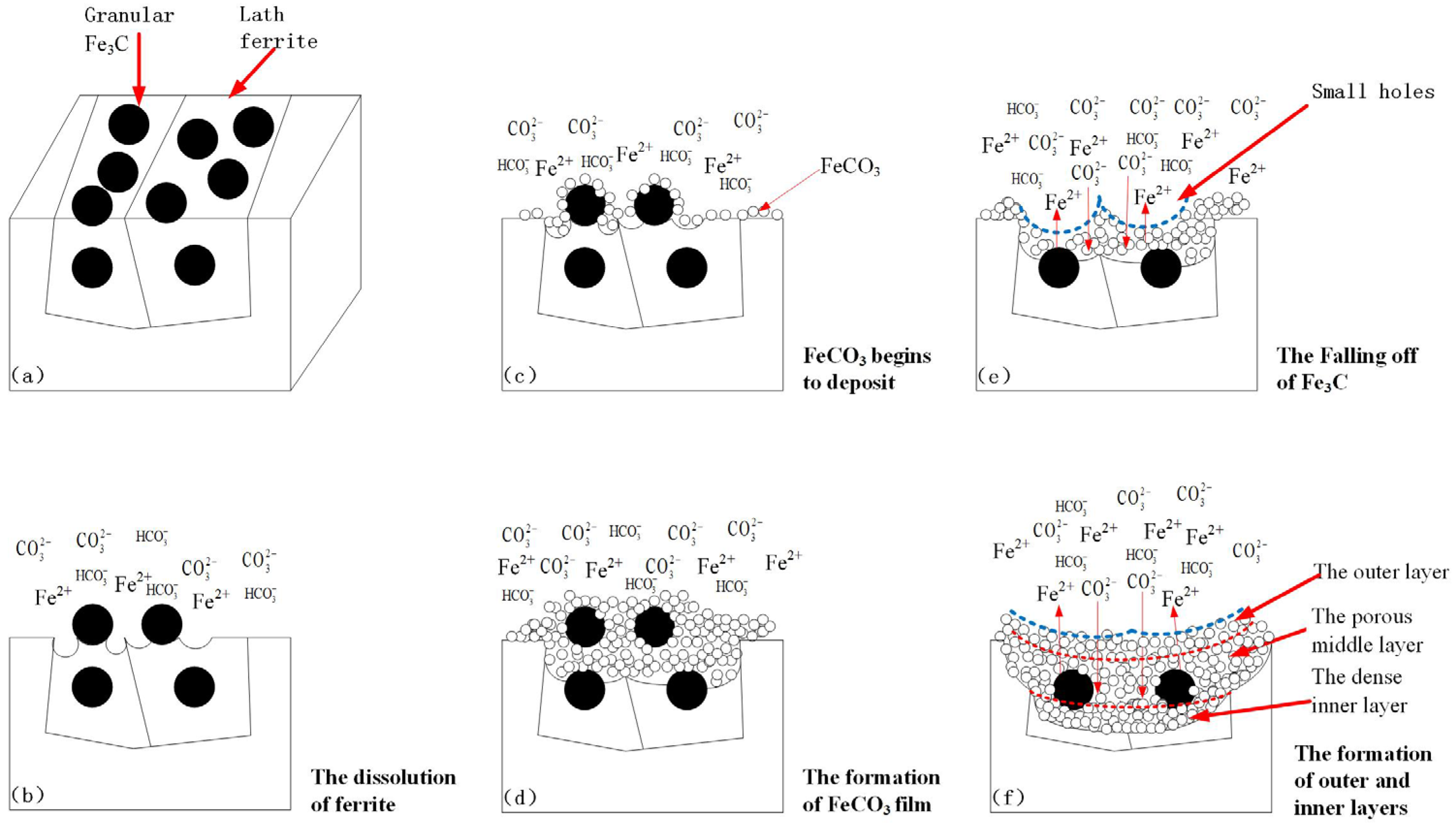
4.2. Formation Process of the Corrosion Product Film under Supercritical CO2 Conditions
4.3. Influence of Microstructure on the Corrosion Behavior of Steels under Supercritical CO2 Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Q.; Zhou, H.; Bartocci, P.; Fantozzi, F.; Mašek, O.; Agblevor, F.A.; Wei, Z.; Yang, H.; Chen, H.; Lu, X.; et al. Prospective contributions of biomass pyrolysis to China’s 2050 carbon reduction and renewable energy goals. Nat. Commun. 2021, 12, 1698. [Google Scholar] [CrossRef] [PubMed]
- Wilberforce, T.; Olabi, A.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Progress in carbon capture technologies. Sci. Total Environ. 2021, 761, 143203. [Google Scholar] [CrossRef] [PubMed]
- Friedlingstein, P.; O’sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Olsen, A.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; et al. Global Carbon Budget 2020. Earth Syst. Sci. Data 2020, 12, 3269–3340. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, W.; Jiang, H. Study on mathematical models for multi-phase porous flow in CO2 drive of extra-low permeability reservoir and field application. Acta Pet. Sin. 2008, 29, 246–251. [Google Scholar]
- Wu, X. Discussion on the influence of CO2 on oil well production. Pet. Geol. Eng. 2008, 22, 97–100. [Google Scholar]
- Wang, Y.; Zhao, Z. Experimental research on the effect of pressure on CO2 oil displacement efficiency. Spec. Oil Gas Reserv. 2017, 24, 132–135. [Google Scholar]
- Li, X. Experimental study on the effect of temperature and injection pressure on CO2 flooding. Pet. Geol. Recovery Effic. 2015, 22, 84–87. [Google Scholar]
- Zhang, G.; Lu, M.; Wu, Y. Formation mechanism of corrosion scales of carbon steel by CO2 corrosion under high temperature and high pressure. J. Univ. Sci. Technol. 2005, 23, 537–548. [Google Scholar]
- Wei, L.; Pang, X.L.; Gao, K.W. Corrosion mechanism discussion of X65 steel in NaCl solution saturated with supercritical CO2. Acta Metall. Sin. 2015, 51, 701–712. [Google Scholar]
- Chen, C.; Zhao, G.; Lu, M. Study of CO2 corrosion scales on N80 steel. J. Chin. Soc. Corros. Prot. 2002, 22, 143–146. [Google Scholar]
- Li, T.; Gao, K.; Lu, M. Formation mechanism of CO2 corrosion product scale on X65 steel. J. Chin. Soc. Corros. Prot. 2007, 38, 411–416. [Google Scholar]
- Zhang, Y.; Pang, X.; Qu, S.; Li, X.; Gao, K. Discussion of the CO2 corrosion mechanism between low partial pressure and supercritical condition. Corros. Sci. 2012, 59, 186–197. [Google Scholar] [CrossRef]
- Wang, S.H.; George, K.; Nesic, S. High Pressure CO2 Corrosion Electrochemistry and the Effect of Acetic Acid, Corrosion/2004; Paper No. 375; NACE International: Houston, TX, USA, 2004. [Google Scholar]
- Zhang, G.; Liu, D.; Li, Y.; Guo, X. Corrosion behaviour of N80 carbon steel in formation water under dynamic supercritical CO2 condition. Corros. Sci. 2017, 120, 107–120. [Google Scholar] [CrossRef]
- Wei, L.; Pang, X.; Liu, C.; Gao, K. Formation mechanism and protective property of corrosion product scale on X70 steel under supercritical CO2 environment. Corros. Sci. 2015, 100, 404–420. [Google Scholar] [CrossRef]
- Fuseini, M.; Zaghloul, M.M.; Elkady, M.F.; El-Shazly, A.H. Evaluation of synthesized polyaniline nanofibresas corrosion protection film coating on coppersubstrate by electrophoretic deposition. J. Mater. Sci. 2022, 57, 6085–6101. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Pang, X.L.; Qu, S.P.; Li, X.; Gao, K.W. The relationship between fracture toughness of CO2 corrosion scale and corrosion rate of X65 pipelinesteel under supercritical CO2 condition. Int. J. Greenh. Gas Control 2011, 5, 1643–1650. [Google Scholar] [CrossRef]
- Gao, K.; Yu, F.; Pang, X.; Zhang, G.; Qiao, L.; Chu, W.; Lu, M. Mechanical properties of CO2 corrosion product scales and their relationship to corrosionrates. Corros. Sci. 2008, 50, 2796–2803. [Google Scholar] [CrossRef]
- Yu, F.; Gao, K.; Su, Y.; Li, J.; Qiao, L.; Chu, W.; Lu, M. The fracture toughness of CO2 corrosion scale in pipeline steel. Mater. Lett. 2005, 59, 1709–1713. [Google Scholar] [CrossRef]
- Lopez, D.A.; Simison, S.N.; De Sanchez, S.R. The influence of steel microstructure on CO2 corrosion. EIS studies on the inhibition efficiency of benzimidazole. Electrochim. Acta 2003, 48, 845–854. [Google Scholar] [CrossRef]
- Ko, M.; Ingham, B.; Laycock, N.; Williams, D. In situ synchrotron X-ray diffraction study of the effect of microstructure and boundary layer conditions on CO2 corrosion of pipeline steels. Corros. Sci. 2015, 90, 192–201. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Pang, X.; Gao, K. Localized CO2 corrosion of carbon steel with different microstructures in brine solutions with an imidazoline-based inhibitor. Appl. Surf. Sci. 2018, 442, 446–460. [Google Scholar] [CrossRef]
- Cui, Z.; Wu, S.; Zhu, S.; Yang, X. Study on corrosion properties of pipelines in simulated produced water saturated with supercritical CO2. Appl. Surf. Sci. 2006, 252, 2368–2374. [Google Scholar] [CrossRef]
- Cui, Z.; Wu, S.; Li, C.; Zhu, S.; Yang, X. Corrosion behavior of oil tube steels under conditions of multiphase flow saturated with super-critical carbon dioxide. Mater. Lett. 2004, 58, 1035–1040. [Google Scholar] [CrossRef]
- Hua, Y.; Barker, R.; Neville, A. Effect of temperature on the critical water content for general and localized corrosion of X65 carbon steel in the transport of supercritical CO2. Int. J. Greenh. Gas Control 2014, 31, 48–60. [Google Scholar] [CrossRef]
- Choi, Y.; Nesic, S. Effect of Water Content on the Corrosion Behavior of Carbon Steel in Supercritical CO2 Phase with Impurities, Corrosion/2011; Paper No. 377; NACE International: Houston, TX, USA, 2011. [Google Scholar]
- Lin, Y.; Deng, K.; Ning, H. CO2 solubility test in formation water and prediction model. J. China Univ. Pet. Ed. Nat. Sci. 2021, 45, 117–126. [Google Scholar]
- Zhang, Y.; Gao, K.; Schmitt, G. Effect of water on steel corrosion under supercritical CO2 conditions. Mater. Perform. 2011, 50, 62–68. [Google Scholar]
- Wei, L.; Gao, K.; Li, Q. Corrosion of low alloy steel containing 0.5% chromium in supercritical CO2-saturated brine and water-saturated supercritical CO2 environments. Appl. Surf. Sci. 2018, 440, 524–534. [Google Scholar] [CrossRef]
- Dugstad, A. Fundamental Aspects of CO2 Metal Loss Corrosion-Part 1: Mechanism, Corrosion/2006; Paper No. 06111; NACE International: Houston, TX, USA, 2006. [Google Scholar]
- Dugstad, A. Mechanism of Protective Film Formation during CO2 Corrosion of Carbon Steel, Corrosion/98; Paper No. 31; NACE International: Houston, TX, USA, 1998. [Google Scholar]
- Chen, C.F.; Lu, M.X.; Zhao, G.X.; Bai, Z.Q. Mechanical properties of CO2 corrosion scale on N80 well tube steel. Acta Metall. Sin. 2003, 39, 175–181. [Google Scholar]
- Sun, W.; Nesic, S. Basics Revisited: Kinetics of Iron Carbonate Scale Precipitation in CO2 Corrosion, Corrosion/2006; Paper No. 365; NACE International: Houston, TX, USA, 2006. [Google Scholar]
- Crolet, J.L.; Thevenot, N.; Nesic, S. Role of Conductive Corrosion Products in the Protectiveness of Corrosion Layers. Corrosion 1998, 54, 194–203. [Google Scholar] [CrossRef]
- Dugstad, A.; Hemmer, H.; Seiersten, M. Effect of Steel Microstructure on Corrosion Rate and Protective Iron Carbonate Film Formation. Corrosion 2001, 57, 369–378. [Google Scholar] [CrossRef]








| Materials | C | Si | Mn | P | S | Mo | Cr | Ni | Cu |
|---|---|---|---|---|---|---|---|---|---|
| J55 | 0.40 | 0.26 | 1.53 | 0.012 | 0.012 | 0.0024 | 0.015 | 0.008 | 0.012 |
| N80 | 0.31 | 0.29 | 1.64 | 0.010 | 0.0034 | 0.013 | 0.044 | 0.021 | 0.037 |
| No. | CO2 Partial Pressure, MPa | Corrosion Rate of J55, mm/y | Corrosion Rate of N80, mm/y |
|---|---|---|---|
| 1 | 0.1 | 0.096 | 0.081 |
| 2 | 3 | 1.524 | 1.095 |
| 3 | 5 | 1.788 | 2.184 |
| 4 | 7 | 2.482 | 2.679 |
| 5 | 10 | 4.570 | 6.549 |
| 6 | 15 | 5.599 | 5.279 |
| 15,610.05 | 113.59 | 114.67 | 305.41 | 1109.32 | 9479.99 |
| No. | Immersion Times, h | Corrosion Rate of J55, mm/y | Corrosion Rate of N80, mm/y |
|---|---|---|---|
| 7 | 20 | 17.485 | 21.425 |
| 8 | 66 | 7.949 | 9.451 |
| 9 | 114 | 4.239 | 5.178 |
| 10 | 168 | 4.002 | 4.946 |
| Reference | Steel | Temperature, °C | CO2 Pressure, MPa | Solution | Structure | Mechanism |
|---|---|---|---|---|---|---|
| [8] | N80 | 90 | 1 | simulated formation water | inner and outer layers | outer layer was formed first |
| [9] | X65 | 80 | 1, 9.5 | 3.5% NaCl solution | inner and outer layers | inner layer was formed first |
| [10] | N80 | 78 | 1 | simulated formation water | three-layer structure | formed at the same time |
| [11] | X65 | 60 | 1 | simulated formation water | three-layer structure | middle layer was formed first |
| [12] | X65 | 50, 80 | 1, 9.5 | simulated formation water | three-layer structure | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.; Zhao, X.; Fu, A.; Li, D.; Yin, C.; Feng, Y. Corrosion Behavior of J55 and N80 Carbon Steels in Simulated Formation Water under Different CO2 Partial Pressures. Coatings 2022, 12, 1402. https://doi.org/10.3390/coatings12101402
Cheng S, Zhao X, Fu A, Li D, Yin C, Feng Y. Corrosion Behavior of J55 and N80 Carbon Steels in Simulated Formation Water under Different CO2 Partial Pressures. Coatings. 2022; 12(10):1402. https://doi.org/10.3390/coatings12101402
Chicago/Turabian StyleCheng, Shixia, Xuehui Zhao, Anqing Fu, Dejun Li, Chengxian Yin, and Yaorong Feng. 2022. "Corrosion Behavior of J55 and N80 Carbon Steels in Simulated Formation Water under Different CO2 Partial Pressures" Coatings 12, no. 10: 1402. https://doi.org/10.3390/coatings12101402






