Dielectric and Antiferroelectric Properties of AgNbO3 Films Deposited on Different Electrodes
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, L.; Gao, J.; Liu, Q.; Zhang, S.; Li, J.-F. Silver Niobate Lead-Free Antiferroelectric Ceramics: Enhancing Energy Storage Density by B-Site Doping. ACS Appl. Mater. Interfaces 2018, 10, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Ren, D.; Sun, L.; Yan, F.; Yang, S.; Zhao, G. Grain size tailoring and enhanced energy storage properties of two-step sintered Nd3+-doped AgNbO3. J. Eur. Ceram. Soc. 2020, 40, 4495–4502. [Google Scholar] [CrossRef]
- Han, K.; Luo, N.; Mao, S.; Zhuo, F.; Liu, L.; Peng, B.; Chen, X.; Hu, C.; Zhou, H.; Wei, Y. Ultrahigh energy-storage density in A-/B-site co-doped AgNbO3 lead-free antiferroelectric ceramics: Insight into the origin of antiferroelectricity. J. Mater. Chem. A 2019, 7, 26293–26301. [Google Scholar] [CrossRef]
- Lu, Z.; Bao, W.; Wang, G.; Sun, S.-K.; Li, L.; Li, J.; Yang, H.; Ji, H.; Feteira, A.; Li, D.; et al. Mechanism of enhanced energy storage density in AgNbO3-based lead-free antiferroelectrics. Nano Energy 2021, 79, 105423. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Song, J.; Zhang, S.; Wang, J.; Dai, X.; Liu, B.; Dong, G.; Zhao, L. AgNbO3 antiferroelectric film with high energy storage performance. J. Mater. 2021, 7, 1294–1300. [Google Scholar] [CrossRef]
- Ahn, Y.; Seo, J.; Lee, K.J.; Son, J.Y. Ferroelectric domain of epitaxial AgNbO3 thin film. J. Cryst. Growth 2016, 437, 10–13. [Google Scholar] [CrossRef]
- Kania, A. An additional phase transition in silver niobate AgNbO3. Ferroelectrics 1998, 205, 19–28. [Google Scholar] [CrossRef]
- Luo, N.; Han, K.; Liu, L.; Peng, B.; Wang, X.; Hu, C.; Zhou, H.; Feng, Q.; Chen, X.; Wei, Y. Lead-free Ag1-3xLaxNbO3 Antiferroelectric Ceramics with High Energy Storage Density and Efficiency. J. Am. Ceram. Soc. 2019, 102, 4640–4647. [Google Scholar] [CrossRef]
- Luo, N.; Han, K.; Zhuo, F.; Xu, C.; Zhang, G.; Liu, L.; Chen, X.; Hu, C.; Zhou, H.; Wei, Y. Aliovalent A-site engineered AgNbO3 lead-free antiferroelectric ceramics toward superior energy storage density. J. Mater. Chem. A 2019, 7, 14118–14128. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Q.; Gao, J.; Zhang, S.; Li, J. Lead-Free Antiferroelectric Silver Niobate Tantalate with High Energy Storage Performance. Adv. Mater. 2017, 29, 1701824. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Endo, M.; Taniguchi, H.; Taniyama, T.; Itoh, M.; Koshihara, S.-Y. Ferroelectricity of Li-doped silver niobate (Ag, Li)NbO3. J. Phys. Condens. Mat. 2011, 23, 075901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, D.; Itoh, M.; Koshihara, S.Y. Dielectric, Ferroelectric, and Piezoelectric Behaviors of AgNbO-KNbO3 Solid Solution. J. Appl. Phys. 2009, 106, 104104. [Google Scholar] [CrossRef]
- Ma, J.; Yan, S.; Xu, C.; Cheng, G.; Mao, C.; Bian, J.; Wang, G. Enhanced energy storage properties of silver niobate ceramics under hydrostatic pressure. Mater. Lett. 2019, 247, 40–43. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, T.; Xue, F.; Nie, H.; Withers, R.; Studer, A.; Kremer, F.; Narayanan, N.; Dong, X.; Yu, D.; et al. Lead-free (Ag, K)NbO3 materials for high-performance explosive energy conversion. Sci. Adv. 2020, 6, eaba0367. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Yamazoe, S.; Wada, T. Ferroelectric and antiferroelectric properties of AgNbO3 films fabricated on (001), (110), and (111) SrTiO3 substrates by pulsed laser deposition. Appl. Phys. Lett. 2010, 97, 042901. [Google Scholar] [CrossRef]
- Shu, L.; Zhang, X.; Li, W.; Gao, J.; Wang, H.; Huang, Y.; Li, Q.; Liu, L.; Li, J.F. Phase-pure antiferroelectric AgNbO3 films on Si substrates: Chemical solution deposition and phase transitions. J. Mater. Chem. A 2022, 10, 12632–12642. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, X.; Lou, X. Effects of epitaxial strain on antiferrodistortion of AgNbO3 from first-principle calculations. Phys. Status Solidi-R. 2018, 12, 1800007. [Google Scholar] [CrossRef]
- Liang, Y.C.; Liang, Y.C. Fabrication and electrical properties of strain-modulated epitaxial Ba0.5Sr0.5TiO3 thin-film capacitors. J. Electrochem. Soc. 2007, 154, G193–G197. [Google Scholar] [CrossRef]
- Hao, J.H.; Luo, Z.; Gao, J. Effects of substrate on the dielectric and tunable properties of epitaxial SrTiO3 thin films. J. Appl. Phys. 2006, 100, 114107. [Google Scholar] [CrossRef]

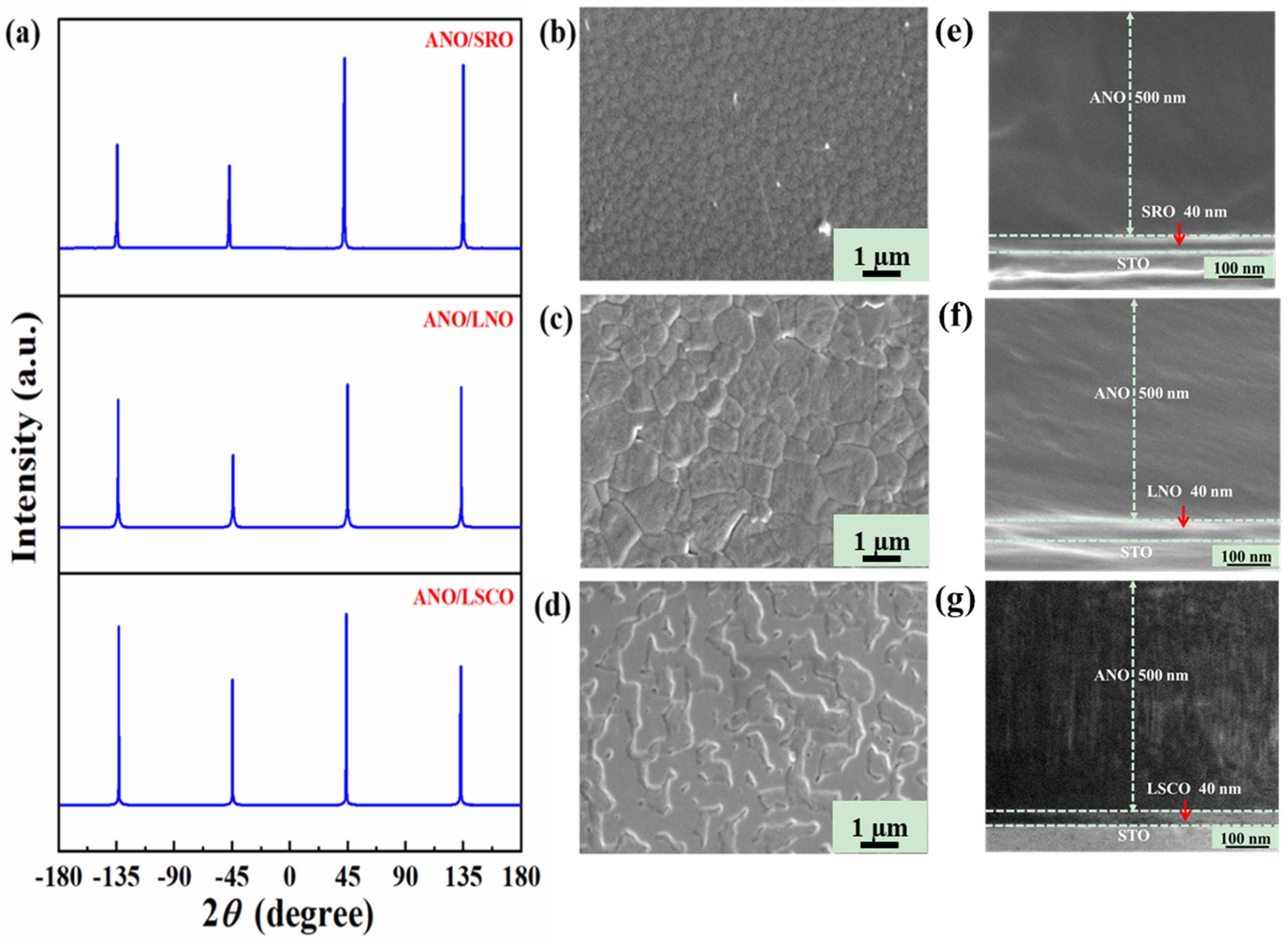
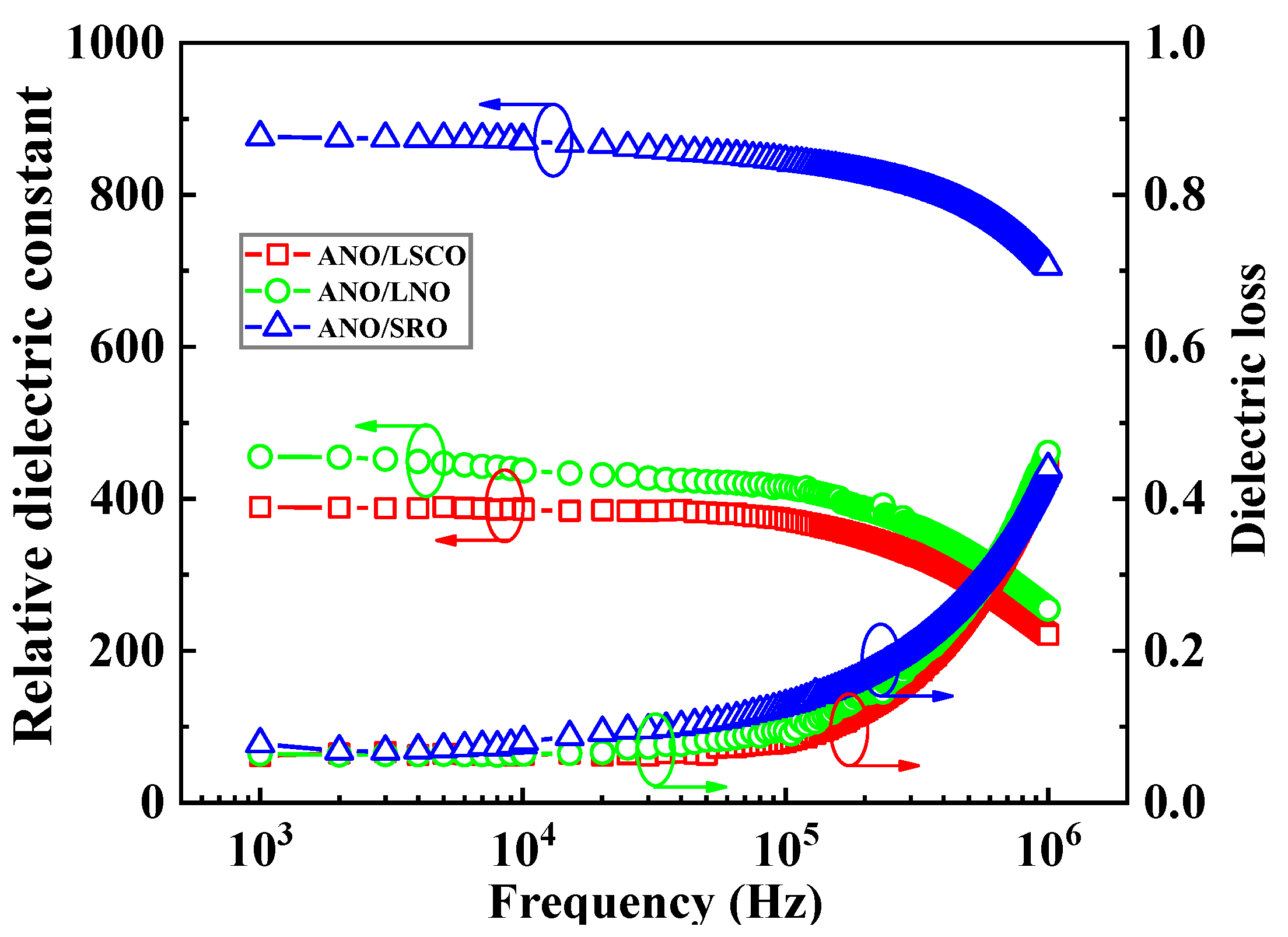
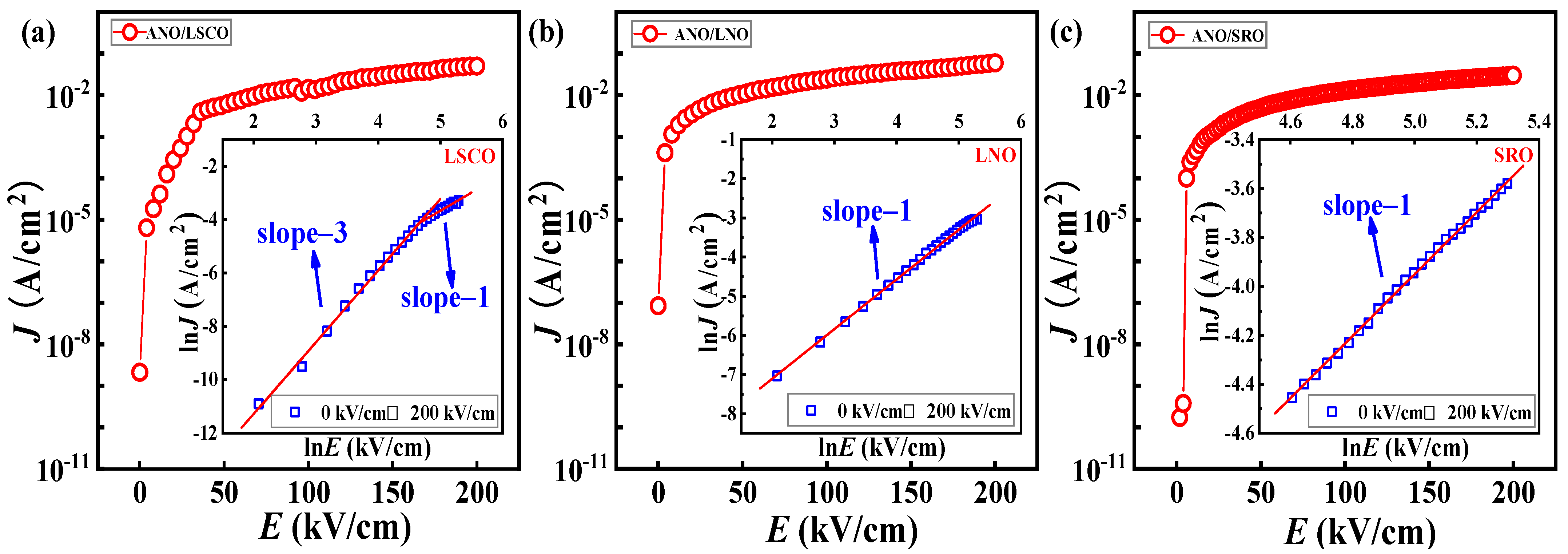
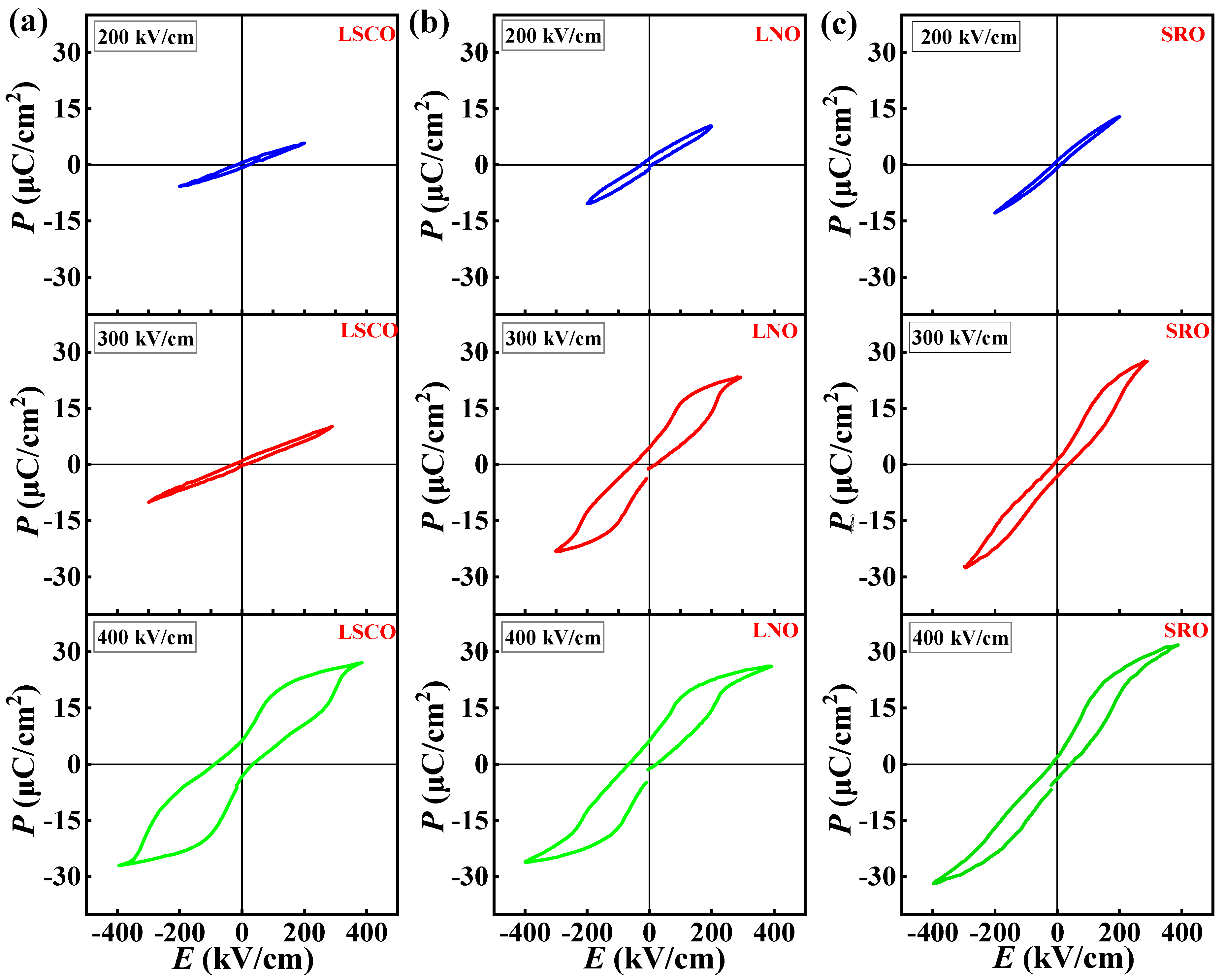
| Bottom Electrodes | Lattice Constant c | |
|---|---|---|
| Bottom Electrodes | ANO Films | |
| LSCO | 3.835 Å | 3.983 Å |
| LNO | 3.849 Å | 3.964 Å |
| SRO | 3.920 Å [15] | 3.949 Å |
| Bottom Electrodes | EF (kV/cm) | EA (kV/cm) | Pmax (μC/cm2) | Pr (μC/cm2) | J (A/cm2) |
|---|---|---|---|---|---|
| LSCO | 272 | 20 | 27.1 | 6.2 | <0.050 |
| LNO | 217 | 25 | 26.1 | 6.0 | <0.059 |
| SRO | 190 | 41 | 24.0 | 2.9 | <0.030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Li, X.; Zhang, Y.; Duo, Z.; Zhang, S.; Zhao, L. Dielectric and Antiferroelectric Properties of AgNbO3 Films Deposited on Different Electrodes. Coatings 2022, 12, 1826. https://doi.org/10.3390/coatings12121826
Ma Q, Li X, Zhang Y, Duo Z, Zhang S, Zhao L. Dielectric and Antiferroelectric Properties of AgNbO3 Films Deposited on Different Electrodes. Coatings. 2022; 12(12):1826. https://doi.org/10.3390/coatings12121826
Chicago/Turabian StyleMa, Qingzhu, Xiang Li, Yanle Zhang, Zhijin Duo, Suwei Zhang, and Lei Zhao. 2022. "Dielectric and Antiferroelectric Properties of AgNbO3 Films Deposited on Different Electrodes" Coatings 12, no. 12: 1826. https://doi.org/10.3390/coatings12121826






