Preparations and Applications of MXene–Metal Composites: A Review
Abstract
:1. Introduction
2. Preparation of MXene–Metal Composites
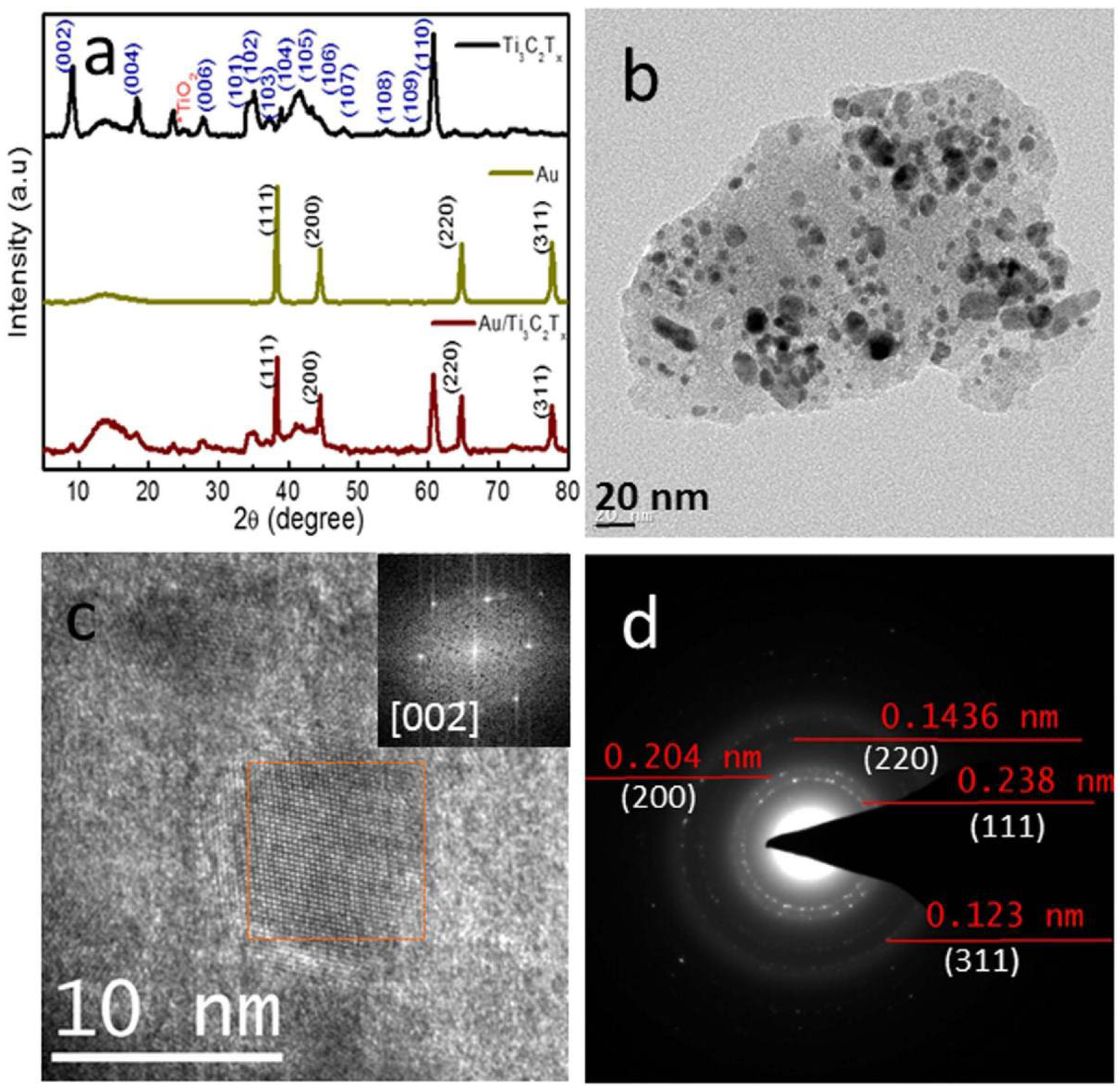
2.1. Au/Ti3C2Tx Nanocomposite
2.2. RhNi/MXene Nanocatalyst
2.3. Ti3C2/DNA/Pd/Pt Nanocomposite
2.4. Ti3C2Tx /Ni MXene Composite
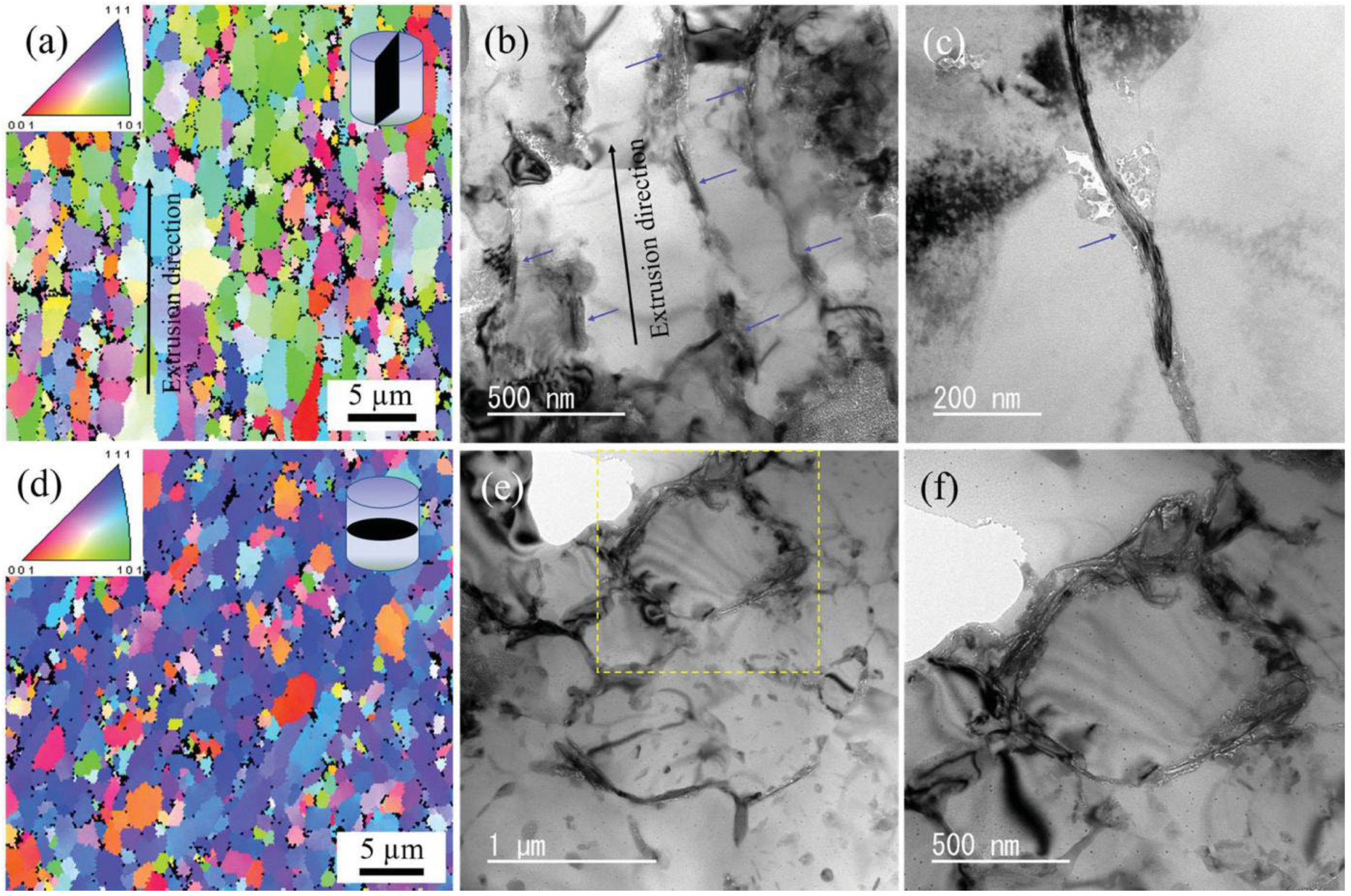
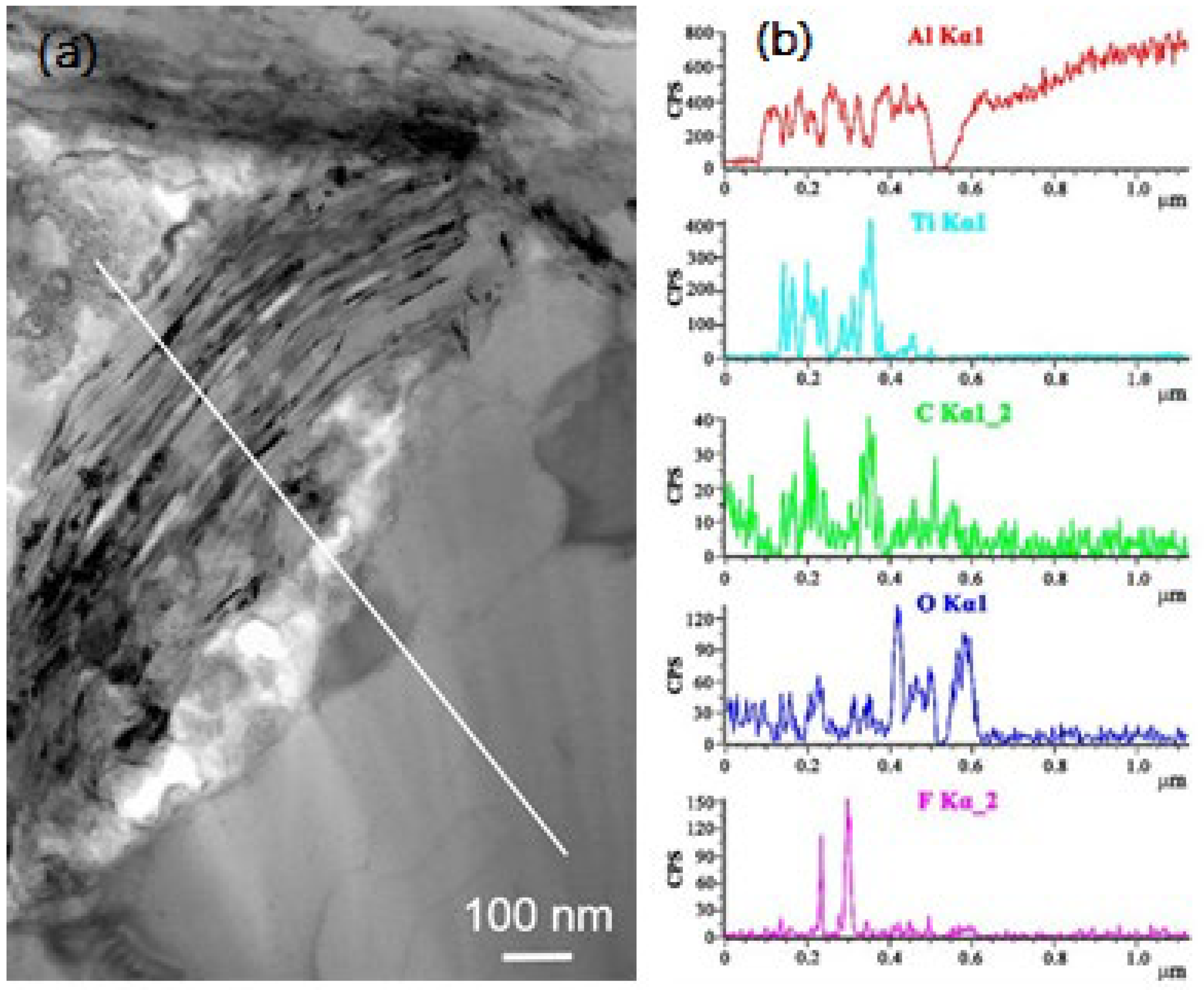
2.5. Ti3C2Tx/Al MXene Composite
2.6. Ti3C2 MXene@Au@CdS Composite
2.7. FLM/Al Composite
2.8. Ag-Ti3C2Tx and Ag-Nb2CTx Composites
3. Properties of MXene and Metal Composites
3.1. Microsructure
3.2. Mechanical Properties
3.3. Electromagnetic Adsorption
3.4. Wettability
4. Application of MXene/Metal Composite
4.1. Electrochemical Performance
4.2. Catalytic Performance
4.3. Corrosion Resistance
5. Critical Overview
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baig, Z.; Mamat, O.; Mustapha, M. Recent progress on the dispersion and the strengthening effect of carbon nanotubes and graphene-reinforced metal nanocomposites: A review. Crit. Rev. Solid State Mater. Sci. 2018, 43, 1–46. [Google Scholar] [CrossRef]
- Li, X.; Sun, M.; Shan, C.; Chen, Q.; Wei, X. Mechanical properties of 2D materials studied by in situ microscopy techniques. Adv. Mater. Interfaces 2018, 5, 1701246. [Google Scholar] [CrossRef]
- Ji, Z.; Zhang, L.; Xie, G.; Xu, W.; Guo, D.; Luo, J.; Prakash, B. Mechanical and tribological properties of nanocomposites incorporated with two-dimensional materials. Friction 2020, 8, 813–846. [Google Scholar] [CrossRef]
- Fan, G.; Li, X.; Ma, Y.; Zhang, Y.; Wu, J.; Xu, B.; Sun, T.; Gao, D.; Bi, J. Magnetic, recyclable Pt y Co 1 − y/Ti 3 C 2 X 2 (X = O, F) catalyst: A facile synthesis and enhanced catalytic activity for hydrogen generation from the hydrolysis of ammonia borane. New J. Chem. 2017, 41, 2793–2799. [Google Scholar] [CrossRef]
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-based composites: Synthesis, properties and environment-related applications. Nanoscale Horiz. 2020, 5, 235–258. [Google Scholar] [CrossRef]
- Guo, J.; Peng, Q.; Fu, H.; Zou, G.; Zhang, Q. Heavy-metal adsorption behavior of two-dimensional alkalization-intercalated MXene by firs-principles calculation. J. Phys. Chem. C 2015, 119, 20923. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, X.; Xiong, Y.; Tang, Y.; Ma, X.; Tao, Q.; Sun, C.; Xu, W. A Review of Etching Methods of MXene and Applications of MXene Conductive Hydrogels. Eur. Polym. J. 2022, 167, 111063. [Google Scholar] [CrossRef]
- Soomro, R.A.; Jawaid, S.; Zhu, Q.; Abbas, Z.; Xu, B. A mini-review on MXenes as versatile substrate for advanced sensors. Chin. Chem. Lett. 2020, 31, 922–930. [Google Scholar] [CrossRef]
- Ding, M.; Chen, W.; Xu, H.; Shen, Z.; Lin, T.; Hu, K.; Kong, Q.; Yang, G.; Xie, Z. Heterogeneous Fe2CoTi3O10-MXene composite catalysts: Synergistic effect of the ternary transition metals in the degradation of 2,4-dichlorophenoxyacetic acid based on peroxymonosulfate activation. Chem. Eng. J. 2019, 378, 122177. [Google Scholar] [CrossRef]
- Seredych, M.; Maleski, K.; Mathis, T.S.; Gogotsi, Y. Delamination of MXenes using bovine serum albumin. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128580. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Gu, Q.; Zhao, C.; Zhang, J.; Xu, S.; Lu, M.; Zhang, B. Multi-ion intercalated Ti3C2Tx MXene and the mutual modulation within interlayer. Particuology 2022. [Google Scholar] [CrossRef]
- Othman, Z.; Mackey, H.R.; Mahmoud, K.A. A critical overview of MXenes adsorption behavior toward heavy metals. Chemosphere 2022, 295, 133849. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-Y.; Gao, M.-H.; Hu, W.-W.; Luo, D.; Hu, S.-Z.; Huang, T.; Zhang, N.; Wang, Y. Largely enhanced adsorption performance and stability of MXene through in-situ depositing polypyrrole nanoparticles. Sep. Purif. Technol. 2022, 287, 120596. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Zhang, J.; Li, G.; Huang, H.; Zhang, X.; Jiang, Q. Enhancement of the electrical properties of MXene Ti3C2 nanosheets by post-treatments of alkalization and calcination. Mater. Lett. 2015, 160, 537–540. [Google Scholar] [CrossRef]
- Yi, H.; Pang, S.; Yang, G.; Yao, X.; Li, C.; Jiang, J.; Li, Y. MXene modified carbon fiber composites with improved mechanical properties based on electrophoretic deposition. Mater. Res. Bull. 2022, 150, 111761. [Google Scholar]
- Zha, X.-H.; Yin, J.; Zhou, Y.; Huang, Q.; Luo, K.; Lang, J.; Du, S. Intrinsic structural, electrical, thermal, and mechanical properties of the promising conductor Mo2C MXene. J. Phys. Chem. C 2016, 120, 15082–15088. [Google Scholar] [CrossRef]
- Yorulmaz, U.; Özden, A.; Perkgöz, N.K.; Ay, F.; Sevik, C. Vibrational and mechanical properties of single layer MXene structures: A first-principles investigation. Nanotechnology 2016, 27, 335702. [Google Scholar] [CrossRef]
- Seyedin, S.; Yanza, E.R.S.; Razal, J.M. Knittable energy storing fiber with high volumetric performance made from predominantly MXene nanosheets. J. Mater. Chem. A 2017, 5, 24076–24082. [Google Scholar] [CrossRef]
- Lipatov, A.; Lu, H.; Alhabeb, M.; Anasori, B.; Gruverman, A.; Gogotsi, Y.; Sinitskii, A. Elastic properties of 2D Ti3C2Tx MXene monolayers and bilayers. Sci. Adv. 2018, 4, eaat0491. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Kong, N.; Uzun, S.; Levitt, A.; Seyedin, S.; Lynch, P.A.; Qin, S.; Han, M.; Yang, W.; Liu, J.; et al. Scalable Manufacturing of Free-Standing, Strong Ti3C2Tx MXene Films with Outstanding Conductivity. Adv. Mater. 2020, 32, 2001093. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Li, X.; Wang, Y.; Chen, Y.; Xie, X.; Yang, R.; Tomsia, A.P.; Jiang, L.; Cheng, Q. Strong sequentially bridged MXene sheets. Proc. Natl. Acad. Sci. USA 2020, 117, 27154–27161. [Google Scholar] [CrossRef] [PubMed]
- Lipatov, A.; Alhabeb, M.; Lu, H.; Zhao, S.; Loes, M.J.; Vorobeva, N.S.; Dall’Agnese, Y.; Gao, Y.; Gruverman, A.; Gogotsi, Y.; et al. Electrical and Elastic Properties of Individual Single-Layer Nb4C3Tx MXene Flakes. Adv. Electron. Mater. 2020, 6, 1901382. [Google Scholar] [CrossRef]
- Zhang, Y.; Zha, X.-H.; Luo, K.; Qin, Y.; Bai, X.; Xu, J.; Lin, C.-T.; Huang, Q.; Du, S. Theoretical study on the electrical and mechanical properties of MXene multilayer structures through strain regulation. Chem. Phys. Lett. 2020, 760, 137997. [Google Scholar] [CrossRef]
- Su, X.; Zhang, J.; Mu, H.; Zhao, J.; Wang, Z.; Zhao, Z.; Han, C.; Ye, Z. Effects of etching temperature and ball milling on the preparation and capacitance of Ti3C2 MXene. J. Alloy. Compd. 2018, 752, 32–39. [Google Scholar] [CrossRef]
- Zukiene, K.; Monastyreckis, G.; Kilikevicius, S.; Procházka, M.; Micusik, M.; Omastová, M.; Aniskevich, A.; Zeleniakiene, D. Wettability of MXene and its interfacial adhesion with epoxy resin. Mater. Chem. Phys. 2021, 257, 123820. [Google Scholar] [CrossRef]
- Choi, E.; Lee, J.; Kim, Y.-J.; Kim, H.; Kim, M.; Hong, J.; Kang, Y.C.; Koo, C.M.; Kim, D.W.; Kim, S.J. Enhanced stability of Ti3C2Tx MXene enabled by continuous ZIF-8 coating. Carbon 2022, 191, 593–599. [Google Scholar] [CrossRef]
- Liu, J.; Jia, X.; Liu, Y.; Wuliu, Y.; Dai, J.; Zhu, X.; Liu, X. A synergetic strategy of well dispersing hydrophilic Ti3C2Tx MXene into hydrophobic polybenzoxazine composites for improved comprehensive performances. Compos. Sci. Technol. 2022, 219, 109248. [Google Scholar] [CrossRef]
- Aakyiir, M.; Oh, J.-A.; Araby, S.; Zheng, Q.; Naeem, M.; Ma, J.; Adu, P.; Zhang, L.; Mai, Y.-W. Combining hydrophilic MXene nanosheets and hydrophobic carbon nanotubes for mechanically resilient and electrically conductive elastomer nanocomposites. Compos. Sci. Technol. 2021, 214, 108997. [Google Scholar] [CrossRef]
- Sliozberg, Y.; Andzelm, J.; Hatter, C.B.; Anasori, B.; Gogotsi, Y.; Hall, A. Interface binding and mechanical properties of MXene-epoxy nanocomposites. Compos. Sci. Technol. 2020, 192, 108124. [Google Scholar] [CrossRef]
- Kvashina, T.; Uvarov, N.; Korchagin, M.; Krutskiy, Y.L.; Ukhina, A. Synthesis of MXene Ti3C2 by selective etching of MAX-phase Ti3AlC2. Mater. Today Proc. 2020, 31, 592–594. [Google Scholar] [CrossRef]
- Benchakar, M.; Loupias, L.; Garnero, C.; Bilyk, T.; Morais, C.; Canaff, C.; Guignard, N.; Morisset, S.; Pazniak, H.; Hurand, S.; et al. One MAX phase, different MXenes: A guideline to understand the crucial role of etching conditions on Ti3C2Tx surface chemistry. Appl. Surf. Sci. 2020, 530, 147209. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.-T.; Shiu, B.-C.; Zhang, X.; Peng, H.-K.; Lou, C.-W.; Lin, J.-H. MXene-decorated nanofiber film based on layer-by-layer assembly strategy for high-performance electromagnetic interference shielding. Appl. Surf. Sci. 2022, 574, 151552. [Google Scholar] [CrossRef]
- Peng, C.; Wei, P.; Chen, X.; Zhang, Y.; Zhu, F.; Cao, Y.; Wang, H.; Yu, H.; Peng, F. A hydrothermal etching route to synthesis of 2D MXene (Ti3C2, Nb2C): Enhanced exfoliation and improved adsorption performance. Ceram. Int. 2018, 44, 18886–18893. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Elanchezhiyan, S.S.; Preethi, J.; Talukdar, K.; Meenakshi, S.; Park, C.M. Two-dimensional (2D) Ti3C2Tx MXene nanosheets with superior adsorption behavior for phosphate and nitrate ions from the aqueous environment. Ceram. Int. 2021, 47, 732–739. [Google Scholar] [CrossRef]
- Tran, N.M.; Ta, Q.T.H.; Sreedhar, A.; Noh, J.-S. Ti3C2Tx MXene playing as a strong methylene blue adsorbent in wastewater. Appl. Surf. Sci. 2021, 537, 148006. [Google Scholar] [CrossRef]
- Lim, G.P.; Soon, C.F.; Morsin, M.; Ahmad, M.K.; Nayan, N.; Tee, K.S. Synthesis, characterization and antifungal property of Ti3C2Tx MXene nanosheets. Ceram. Int. 2020, 46, 20306–20312. [Google Scholar] [CrossRef]
- Cui, C.; Cheng, R.; Zhang, C.; Wang, X. Pt immobilized spontaneously on porous MXene/MAX hybrid monolith for hydrogen evolution reaction. Chin. Chem. Lett. 2020, 31, 988–991. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Mei, C.-T. Optoelectronic properties of Ti3C2Tx MXene transparent conductive electrodes: Microwave synthesis of parent MAX phase. Ceram. Int. 2020, 46, 28114–28119. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Dong, S.; Yang, J.; Liu, X. Flexible electrode based on multi-scaled MXene (Ti3C2Tx) for supercapacitors. J. Alloy. Compd. 2019, 790, 517–523. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Zalnezhad, E.; Hui, K.; Hui, K.; Ko, M.J. 3D hierarchical transition-metal sulfides deposited on MXene as binder-free electrode for high-performance supercapacitors. J. Ind. Eng. Chem. 2020, 82, 309–316. [Google Scholar] [CrossRef]
- Xiao, J.; Wen, J.; Zhao, J.; Ma, X.; Gao, H.; Zhang, X. A safe etching route to synthesize highly crystalline Nb2CTx MXene for high performance asymmetric supercapacitor applications. Electrochim. Acta 2020, 337, 135803. [Google Scholar] [CrossRef]
- Weng, L.; Qi, F.; Min, Y. The Ti3C2Tx MXene coated metal mesh electrodes for stretchable supercapacitors. Mater. Lett. 2020, 278, 128235. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, Z.; Shi, L.; Wang, R.; Sun, J. Enhancing the conductivity, stability and flexibility of Ti3C2Tx MXenes by regulating etching conditions. Appl. Surf. Sci. 2020, 533, 147475. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, D.; Zhao, P.; Yue, G.; Yang, L.; Dan, W. Highly Efficient Methylene Blue Removal by TMAOH Delaminated Ti3C2Tx MXene Suspension and the Mechanistic Aspect. Sep. Purif. Technol. 2022, 288, 120718. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, X.; Huang, C.; Fan, J. Revealing the effects of terminal groups of MXene on the water desalination performance. J. Membr. Sci. 2022, 647, 120334. [Google Scholar] [CrossRef]
- Hu, W.; Huang, Z.; Wang, Y.; Li, X.; Zhai, H.; Zhou, Y.; Chen, L. Layered ternary MAX phases and their MX particulate derivative reinforced metal matrix composite: A review. J. Alloy. Compd. 2020, 856, 157313. [Google Scholar] [CrossRef]
- Yang, J.; Bao, W.; Jaumaux, P.; Zhang, S.; Wang, C.; Wang, G. MXene-based composites: Synthesis and applications in rechargeable batteries and supercapacitors. Adv. Mater. Interfaces 2019, 6, 1802004. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Huang, R.; He, J.; Sun, Y.; Qin, Y.; Shen, L. Stabilizing MXene-based nanofiltration membrane by forming analogous semi-interpenetrating network architecture using flexible poly (acrylic acid) for effective wastewater treatment. J. Membr. Sci. 2022, 648, 120360. [Google Scholar] [CrossRef]
- Zhang, B.; Boretti, A.; Castelletto, S. MXene pseudocapacitive electrode material for capacitive deionization. Chem. Eng. J. 2022, 435, 134959. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, Y.; Geng, W.; Dai, G.; Sheng, X.; Xie, D.; Wu, H.; Mei, Y. Fabrication of thermoplastic polyurethane with functionalized MXene towards high mechanical strength, flame-retardant, and smoke suppression properties. J. Colloid Interface Sci. 2022, 606, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Gong, K.; Gao, F.; Yin, L. Facile strategy to synthesize MXene@ LDH nanohybrids for boosting the flame retardancy and smoke suppression properties of epoxy. Compos. Part A Appl. Sci. Manuf. 2022, 157, 106912. [Google Scholar] [CrossRef]
- Shen, B.; Liao, X.; Zhang, X.; Ren, H.-T.; Lin, J.-H.; Lou, C.-W.; Li, T.-T. Synthesis of Nb2C MXene-based 2D layered structure electrode material for high-performance battery-type supercapacitors. Electrochim. Acta 2022, 413, 140144. [Google Scholar] [CrossRef]
- Liao, S.-Y.; Cui, S.-F.; Li, Y.-Z.; Cheng, W.-X.; Huang, X.-W.; Zhang, J.; Cui, T.-T.; Shu, X.-G.; Min, Y.-G. Wrinkled and flexible N-doped MXene additive for improving the mechanical and electrochemical properties of the nickel-rich LiNi0.8Co0.1Mn0.1O2 cathode. Electrochim. Acta 2022, 410, 139989. [Google Scholar] [CrossRef]
- George, S.M.; Kandasubramanian, B. Advancements in MXene-Polymer composites for various biomedical applications. Ceram. Int. 2020, 46, 8522–8535. [Google Scholar] [CrossRef]
- Aslfattahi, N.; Saidur, R.; Arifutzzaman, A.; Sadri, R.; Bimbo, N.; Sabri, M.F.M.; Maughan, P.; Bouscarrat, L.; Dawson, R.; Said, S.M.; et al. Experimental investigation of energy storage properties and thermal conductivity of a novel organic phase change material/MXene as A new class of nanocomposites. J. Energy Storage 2020, 27, 101115. [Google Scholar] [CrossRef]
- Rubbi, F.; Habib, K.; Saidur, R.; Aslfattahi, N.; Yahya, S.M.; Das, L. Performance optimization of a hybrid PV/T solar system using Soybean oil/MXene nanofluids as A new class of heat transfer fluids. Sol. Energy 2020, 208, 124–138. [Google Scholar] [CrossRef]
- Samylingam, L.; Aslfattahi, N.; Saidur, R.; Yahya, S.M.; Afzal, A.; Arifutzzaman, A.; Tan, K.; Kadirgama, K. Thermal and energy performance improvement of hybrid PV/T system by using olein palm oil with MXene as a new class of heat transfer fluid. Sol. Energy Mater. Sol. Cells 2020, 218, 110754. [Google Scholar] [CrossRef]
- Tu, S.; Jiang, Q.; Zhang, X.; Alshareef, H.N. Large dielectric constant enhancement in MXene percolative polymer composites. ACS Nano 2018, 12, 3369–3377. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Feng, J. Flexible and freestanding silicon/MXene composite papers for high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 10004–10011. [Google Scholar] [CrossRef]
- Li, C.; Xu, J.; Xu, Q.; Xue, G.; Yu, H.; Wang, X.; Lu, J.; Cui, G.; Gu, G. Synthesis of Ti3C2 MXene@ PANI composites for excellent anticorrosion performance of waterborne epoxy coating. Prog. Org. Coat. 2022, 165, 106673. [Google Scholar] [CrossRef]
- Rakhi, R.; Nayak, P.; Xia, C.; Alshareef, H.N. Novel amperometric glucose biosensor based on MXene nanocomposite. Sci. Rep. 2016, 6, 36422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Wang, Q.; Yuan, J.; Zhao, X.; Gao, G. Highly dispersed bimetallic nanoparticles supported on titanium carbides for remarkable hydrogen release from hydrous hydrazine. ChemCatChem 2018, 10, 2200–2204. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, B.; Ding, A.; Weng, B.; Chen, J. Synthesis of MXene/DNA/Pd/Pt nanocomposite for sensitive detection of dopamine. J. Electroanal. Chem. 2018, 816, 189–194. [Google Scholar] [CrossRef]
- Li, N.; Xie, X.; Lu, H.; Fan, B.; Wang, X.; Zhao, B.; Zhang, R.; Yang, R. Novel two-dimensional Ti3C2Tx/Ni-spheres hybrids with enhanced microwave absorption properties. Ceram. Int. 2019, 45, 22880–22888. [Google Scholar] [CrossRef]
- Hu, J.; Li, S.; Zhang, J.; Chang, Q.; Yu, W.; Zhou, Y. Mechanical properties and frictional resistance of Al composites reinforced with Ti3C2Tx MXene. Chin. Chem. Lett. 2020, 31, 996–999. [Google Scholar] [CrossRef]
- Yin, J.; Zhan, F.; Jiao, T.; Wang, W.; Zhang, G.; Jiao, J.; Jiang, G.; Zhang, Q.; Gu, J.; Peng, Q. Facile preparation of self-assembled MXene@ Au@ CdS nanocomposite with enhanced photocatalytic hydrogen production activity. Sci. China Mater. 2020, 63, 2228–2238. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, Z.; Fan, Y.; Nomura, N. Significant strengthening effect in few-layered MXene-reinforced Al matrix composites. Mater. Res. Lett. 2021, 9, 148–154. [Google Scholar] [CrossRef]
- Rajavel, K.; Hu, Y.; Zhu, P.; Sun, R.; Wong, C. MXene/metal oxides-Ag ternary nanostructures for electromagnetic interference shielding. Chem. Eng. J. 2020, 399, 125791. [Google Scholar] [CrossRef]
- Satheeshkumar, E.; Makaryan, T.; Melikyan, A.; Minassian, H.; Gogotsi, Y.; Yoshimura, M. One-step solution processing of Ag, Au and Pd@ MXene hybrids for SERS. Sci. Rep. 2016, 6, 32049. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, S.; Hu, S.; Zhou, Y. Chemical stability of Ti3C2 MXene with Al in the temperature range 500–700 °C. Materials 2018, 11, 1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; An, Y.; Wei, C.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Flexible and Free-Standing Ti3C2Tx MXene@ Zn Paper for Dendrite-Free Aqueous Zinc Metal Batteries and Nonaqueous Lithium Metal Batteries. ACS Nano 2019, 13, 11676–11685. [Google Scholar] [CrossRef] [PubMed]
- Kamysbayev, V.; James, N.M.; Filatov, A.S.; Srivastava, V.; Anasori, B.; Jaeger, H.M.; Gogotsi, Y.; Talapin, D.V. Colloidal gelation in liquid metals enables functional nanocomposites of 2D metal carbides (MXenes) and lightweight metals. ACS Nano 2019, 13, 12415–12424. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, S.; Wang, Q.; Ren, F.; Wang, Y. Preparation, microstructure and tensile properties of two dimensional MXene reinforced copper matrix composites. Mater. Sci. Eng. A 2021, 803, 140699. [Google Scholar] [CrossRef]
- Li, M.; Wang, S.; Wang, Q.; Ren, F.; Wang, Y. Microstructure and tensile properties of Ni nano particles modified MXene reinforced copper matrix composites. Mater. Sci. Eng. A 2021, 808, 140932. [Google Scholar] [CrossRef]
- He, J.; Liu, X.; Deng, Y.; Peng, Y.; Deng, L.; Luo, H.; Cheng, C.; Yan, S. Improved magnetic loss and impedance matching of the FeNi-decorated Ti3C2Tx MXene composite toward the broadband microwave absorption performance. J. Alloy. Compd. 2021, 862, 158684. [Google Scholar] [CrossRef]
- Liu, P.; Yao, Z.; Ng, V.M.H.; Zhou, J.; Kong, L.B. Novel multilayer-like structure of Ti3C2Tx/CNZF composites for low-frequency electromagnetic absorption. Mater. Lett. 2019, 248, 214–217. [Google Scholar] [CrossRef]
- Zou, G.; Zhang, Z.; Guo, J.; Liu, B.; Zhang, Q.; Fernandez, C.; Peng, Q. Synthesis of MXene/Ag composites for extraordinary long cycle lifetime lithium storage at high rates. ACS Appl. Mater. Interfaces 2016, 8, 22280–22286. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, P.; Xue, T.; Ge, Y.; Ai, S.; Sheng, Y.; Wu, R.; Xu, L.; Tang, K.; Wen, Y. A novel graphene-like titanium carbide MXene/Au–Ag nanoshuttles bifunctional nanosensor for electrochemical and SERS intelligent analysis of ultra-trace carbendazim coupled with machine learning. Ceram. Int. 2021, 47, 173–184. [Google Scholar] [CrossRef]
- Song, D.; Jiang, X.; Li, Y.; Lu, X.; Luan, S.; Wang, Y.; Li, Y.; Gao, F. Metal−organic frameworks-derived MnO2/Mn3O4 microcuboids with hierarchically ordered nanosheets and Ti3C2 MXene/Au NPs composites for electrochemical pesticide detection. J. Hazard. Mater. 2019, 373, 367–376. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Xiong, S.; Feng, J.; Qian, Y. A general method for constructing robust, flexible and freestanding MXene@ metal anodes for high-performance potassium-ion batteries. J. Mater. Chem. A 2019, 7, 9716–9725. [Google Scholar] [CrossRef]
- Chandran, M.; Aswathy, E.; Shamna, I.; Vinoba, M.; Kottappara, R.; Bhagiyalakshmi, M. Laccase immobilized on Au confined MXene based electrode for electrochemical detection of catechol. Mater. Today Proc. 2021, 46, 3136–3143. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Zou, G.; Fernandez, C.; Liu, B.; Zhang, Q.; Hu, J.; Peng, Q. Self-reduction synthesis of new MXene/Ag composites with unexpected electrocatalytic activity. ACS Sustain. Chem. Eng. 2016, 4, 6763–6771. [Google Scholar] [CrossRef]
- Li, K.; Jiao, T.; Xing, R.; Zou, G.; Zhou, J.; Zhang, L.; Peng, Q. Fabrication of tunable hierarchical MXene@ AuNPs nanocomposites constructed by self-reduction reactions with enhanced catalytic performances. Sci. China Mater. 2018, 61, 728–736. [Google Scholar] [CrossRef] [Green Version]
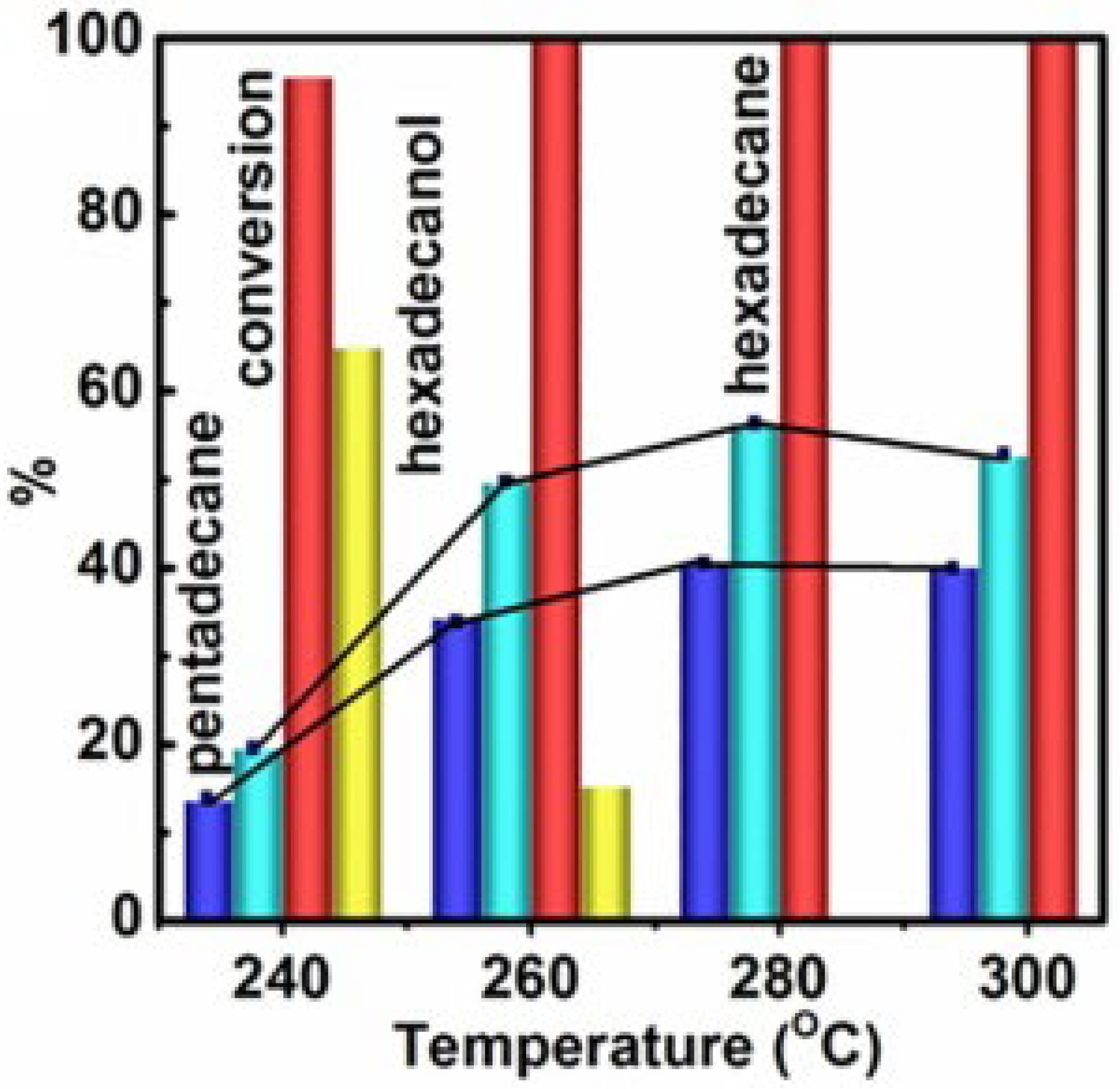
- Liang, J.; Chen, T.; Liu, J.; Zhang, Q.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Chemoselective hydrodeoxygenation of palmitic acid to diesel-like hydrocarbons over Ni/MoO2@ Mo2CTx catalyst with extraordinary synergic effect. Chem. Eng. J. 2020, 391, 123472. [Google Scholar] [CrossRef]
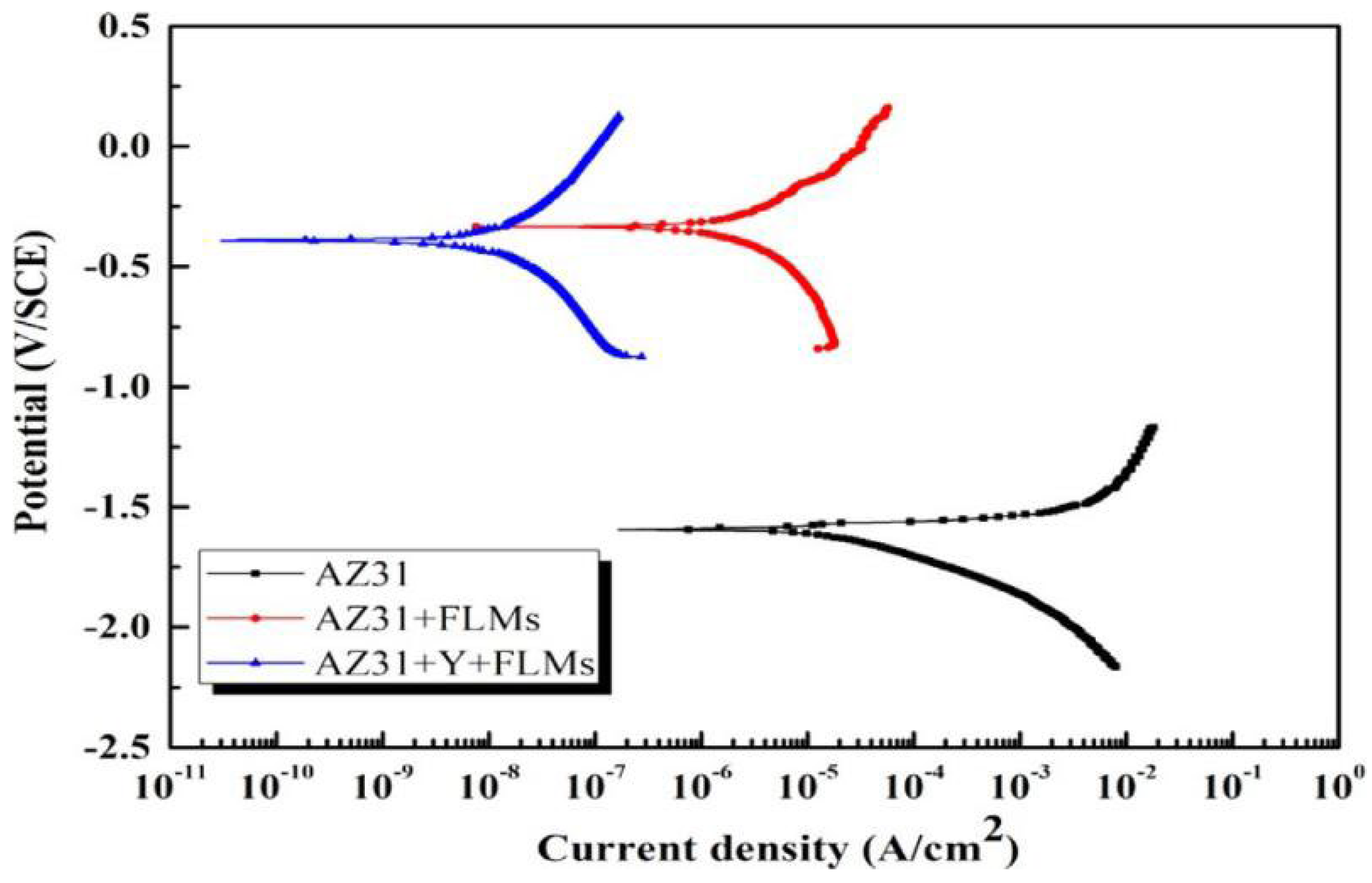
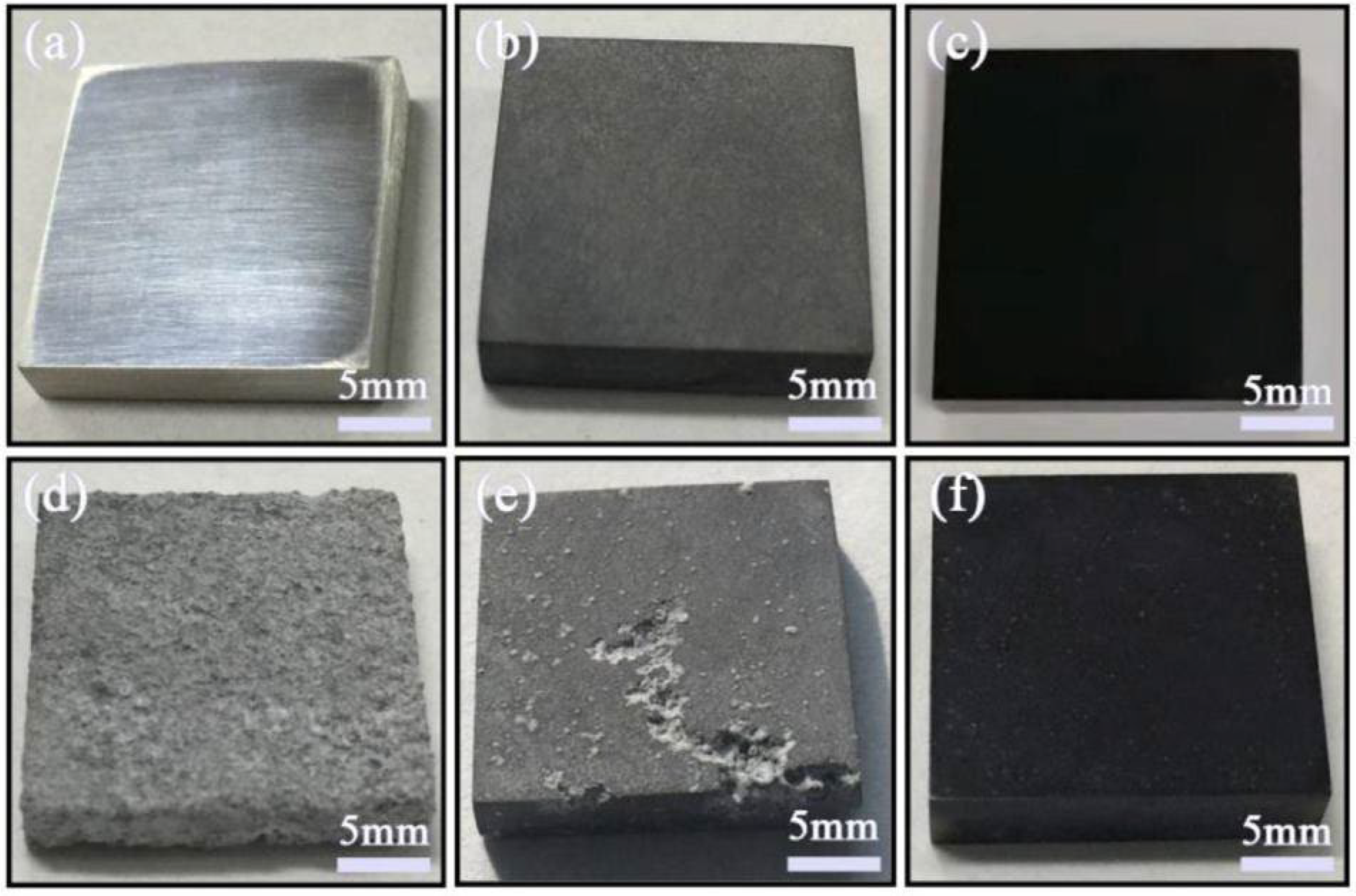
- Wu, Y.; Wu, L.; Yao, W.; Jiang, B.; Wu, J.; Chen, Y.; Chen, X.-B.; Zhan, Q.; Zhang, G.; Pan, F. Improved corrosion resistance of AZ31 Mg alloy coated with MXenes/MgAl-LDHs composite layer modified with yttrium. Electrochim. Acta 2021, 374, 137913. [Google Scholar] [CrossRef]







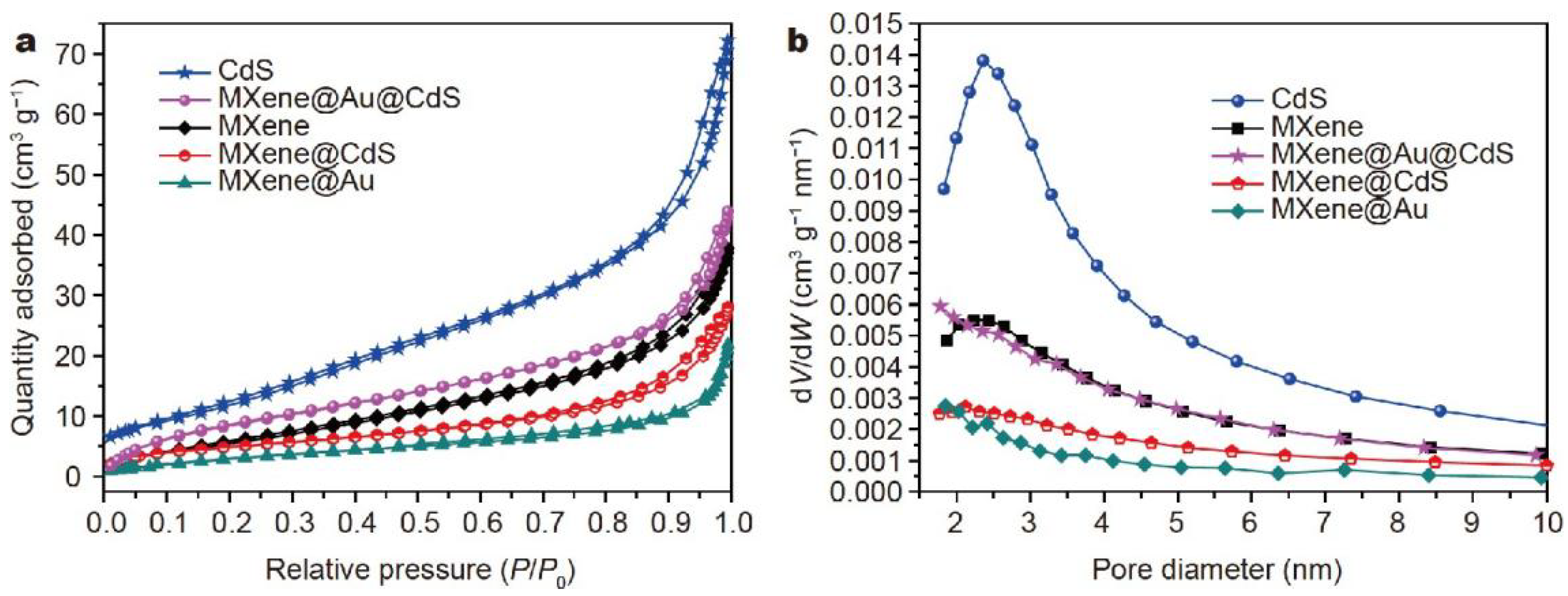




































| Catalysts | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Pore Size (nm) |
|---|---|---|---|
| MXene | 22.2672 | 0.0563 | 9.7948 |
| MXene@Au | 12.2502 | 0.0316 | 9.4052 |
| MXene@CdS | 17.8133 | 0.0421 | 9.0111 |
| MXene@Au@CdS | 34.5170 | 0.0622 | 7.3110 |
| CdS | 45.4100 | 0.1096 | 9.1249 |
| Samples | EORR, V | E1/2, V | J mA·cm−2 | n | Ep (V) | Refs. | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 100 | 500 | 1000 | ||||||
| MXene/N-Ag | 0.880 | 0.571 | 3.31 | 2.06 | 0.514 | 0.506 | 0.501 | 0.495 | [49] |
| MXene/NT-Ag | 0.901 | 0.631 | 3.34 | 2.19 | 0.526 | 0.523 | 0.512 | 0.503 | - |
| MXene/NW-Ag0.9Ti0.1 | 0.921 | 0.782 | 3.64 | 3.95 | 0.565 | 0.558 | 0.553 | 0.549 | - |
| MXene/SS-Ag0.9Ti0.1 | 0.881 | 0.554 | 2.78 | 3.15 | 0.508 | 0.504 | 0.504 | 0.499 | - |
| 20 wt.%/Ag/C | 0.88 | 0.57 | 3.29 | 3.71 | - | ||||
| Supportless Ag nanowire | 0.92 | 0.78 | 3.51 | 3.85 | - | ||||
| 20 wt.%/Ag/C | 0.85 | 0.56 | 3.29 | 3.70 | - | ||||
| Ag nanorods | 0.57 | 3.80 | - | ||||||
| Ag-GNR | 0.618 | 3.51 | - | ||||||
| Ag/B-MWCNTs | 0.69 | 3.80 | - | ||||||
| Ag-MnO2/graphene | 0.67 | 3.70 | - | ||||||
| Ag/GNP | 0.72 | 4 | - | ||||||
| Ag/TiO2 | 0.69 | 4 | - | ||||||
| MXene–Metal Composites | Methods | Properties | Applications | References |
|---|---|---|---|---|
| Au/Ti3C2Tx | chemical reduction | microstructure | electrochemical and catalytic performance | [62] |
| RhNi/MXene | one-step wet chemical | microstructure | catalytic performance | [63] |
| Ti3C2/DNA/Pd/Pt | In-situ process | sensor and catalytic performance | [64] | |
| Ti3C2Tx /Ni | In-situ hydrothermal | EMA | electromagnetic wave absorption | [65] |
| Ti3C2Tx/Al | pressureless sintering followed by hot extrusion | microstructure and mechanical properties | solid lubricant | [66] |
| Ti3C2@Au@CdS | self reduction | microstructure | photocatalytic hydrogen production activity | [67] |
| FLM/Al composite | self assembly protocol and powder metallurgy | microstructure, mechanical properties | automotive, aerospace, packaging industries | [68] |
| Ag-Ti3C2Tx and Ag-Nb2CTx | self chemical reduction | electromagnetic Interference | EM wave shielding | [69] |
| Pd@MXene | one-step soft solution processing | microstructure, surface-enhanced Raman spectroscopy | Sensors, catalysis, biomedical | [70] |
| Ti3C2TxMXene@Zn | facile in situ electroplating | flexibility, wettability, electronic conductivity | energy storage system | [72] |
| Ti3C2Tx /Mg-Li | liquid metal gelation | mechanical properties | alloys, batteries and supercapacitor | [73] |
| MXene/Cu | high energy ball milling | microstructure, mechanical | automotive and aerospace industries | [74] |
| Ni-MXene/Cu composites | high energy ball milling | microstructure, mechanical and wettability | automotive and aerospace industries | [75] |
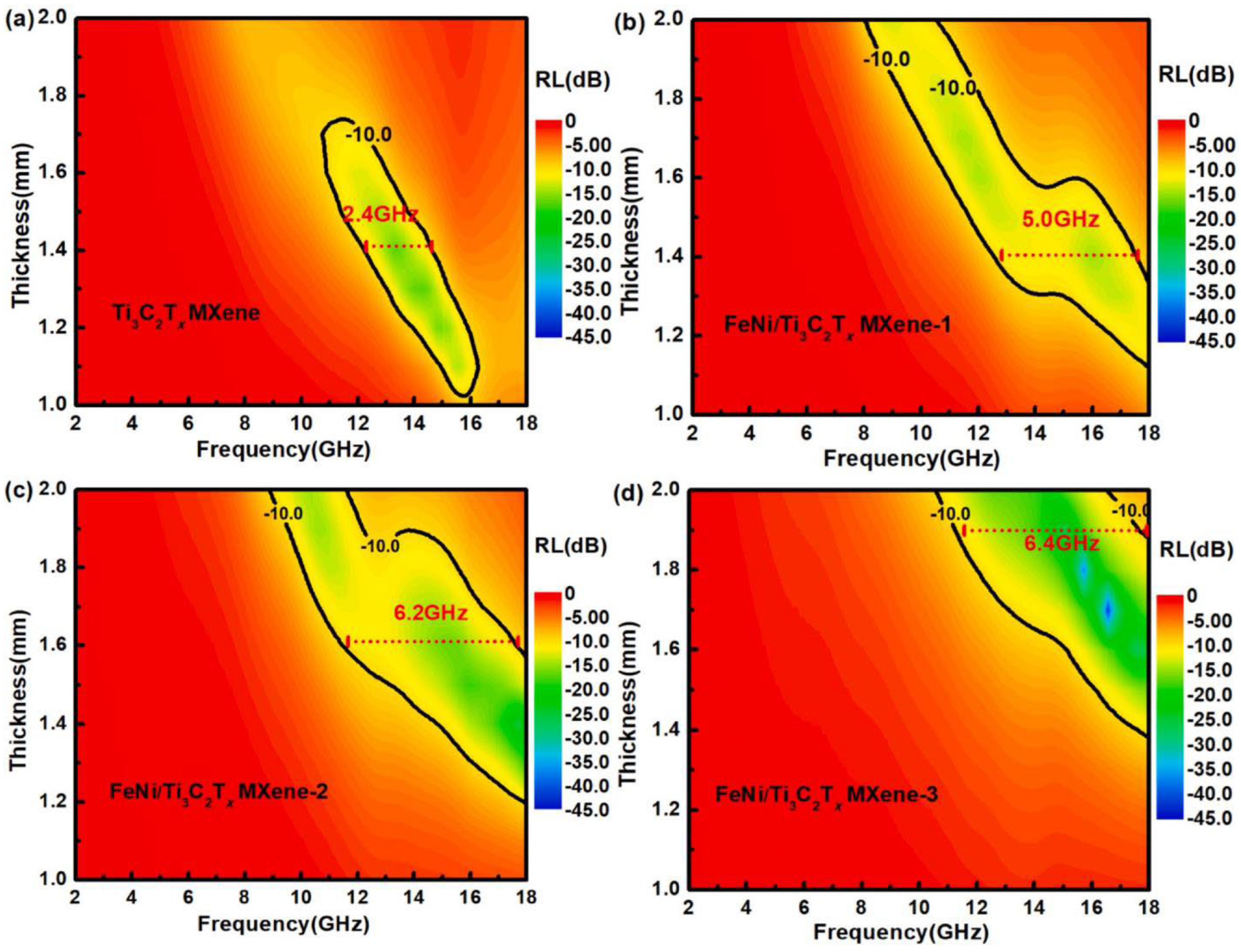
| FeNi/Ti3C2Tx | facile in situ hydrothermal | microstructure, magnetic and microwave absorption | Radar detection technology | [76] |
| Ag-Ti3C2Tx and Ag-Nb2CTx Composites | simultaneous self-reduction and oxidation | EMI shielding | wireless technologies and radar systems | [77] |
| MXene/Ag | direct reduction method | lithium-ion batteries | [78] | |
| Ti2C/Au-Ag | machine learning | electrochemical and SERS intelligent analysis | [79] | |
| MOF-derived MnO2/Mn3O4 and Ti3C2 MXene/Au | enzymatic inhibition | electrochemical pesticides detection | [80] | |
| MXene@Sb | one-step electrodeposition approach | flexible | catalyst, batteries, sensors | [81] |
| Lac/Au/MXene/GCE | reduction process | Electrochemical detection of catechol | [82] | |
| MXene-Ag0.9Ti0.1 | self reduction | electrocatalytic activity | [83] | |
| MXene@AuNPs | self reduction | catalytic performance | [84] | |
| Ni/MoO2@Mo2CTx | wet impregnation method | catalytic performance | [85] | |
| MXene/MgAl-LDHs | in situ synthesis | anticorrosion | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.U.; Du, L.; Fu, S.; Wan, D.; Bao, Y.; Feng, Q.; Grasso, S.; Hu, C. Preparations and Applications of MXene–Metal Composites: A Review. Coatings 2022, 12, 516. https://doi.org/10.3390/coatings12040516
Khan MU, Du L, Fu S, Wan D, Bao Y, Feng Q, Grasso S, Hu C. Preparations and Applications of MXene–Metal Composites: A Review. Coatings. 2022; 12(4):516. https://doi.org/10.3390/coatings12040516
Chicago/Turabian StyleKhan, Maaz Ullah, LiJing Du, Shuai Fu, Detian Wan, Yiwang Bao, Qingguo Feng, Salvatore Grasso, and Chunfeng Hu. 2022. "Preparations and Applications of MXene–Metal Composites: A Review" Coatings 12, no. 4: 516. https://doi.org/10.3390/coatings12040516









