Hydrothermal Synthesis of NiCo2O4 @NiCo2O4 Core-Shell Nanostructures Anchored on Ni Foam for Efficient Oxygen Evolution Reactions Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of NiCo2O4 Nanocones/NF
2.3. Synthesis of Core-Shell NiCo2O4@NiCo2O4/NF
2.4. Synthesis of IrO2/NF
2.5. Characterization
2.6. Electrochemical Measurements
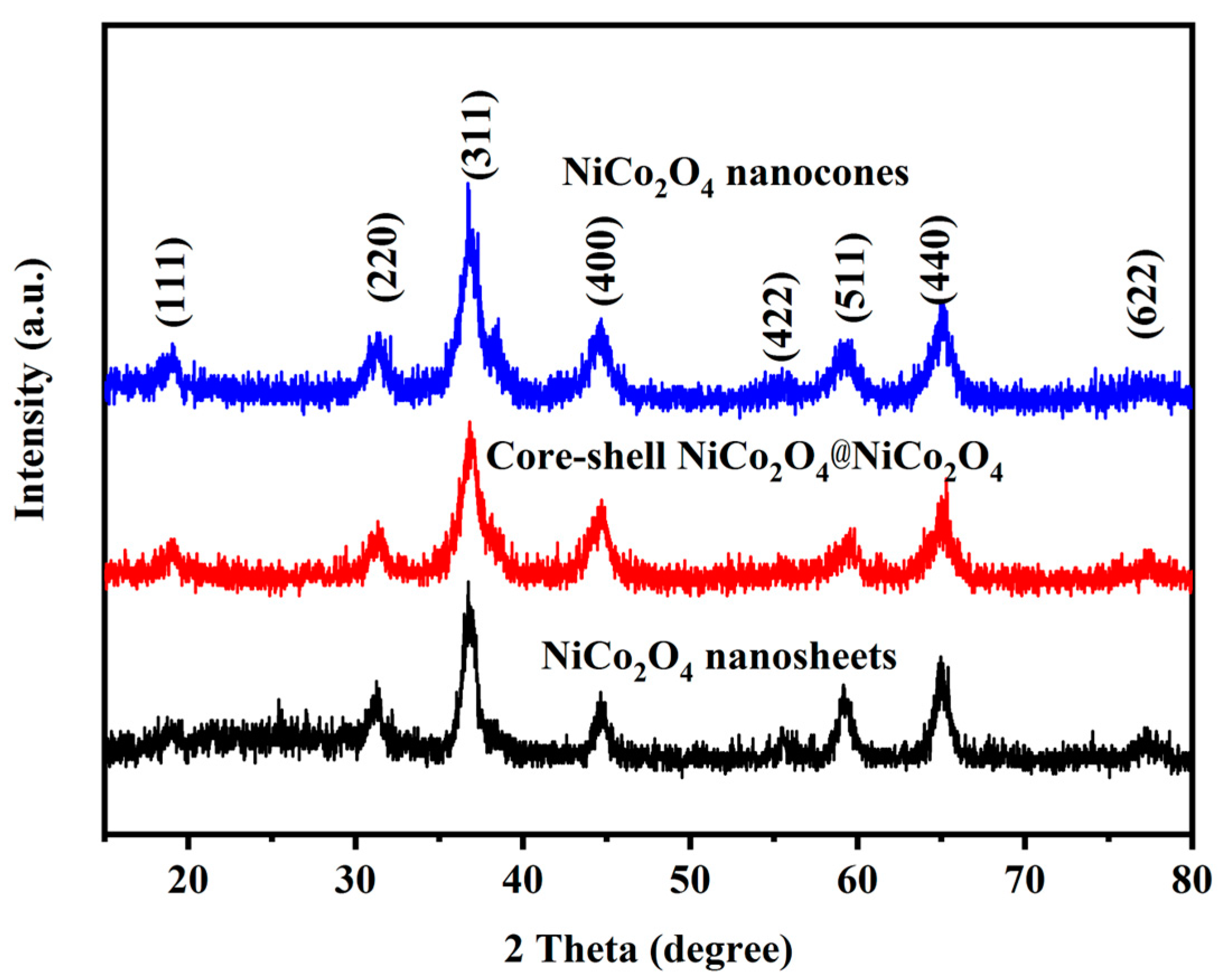
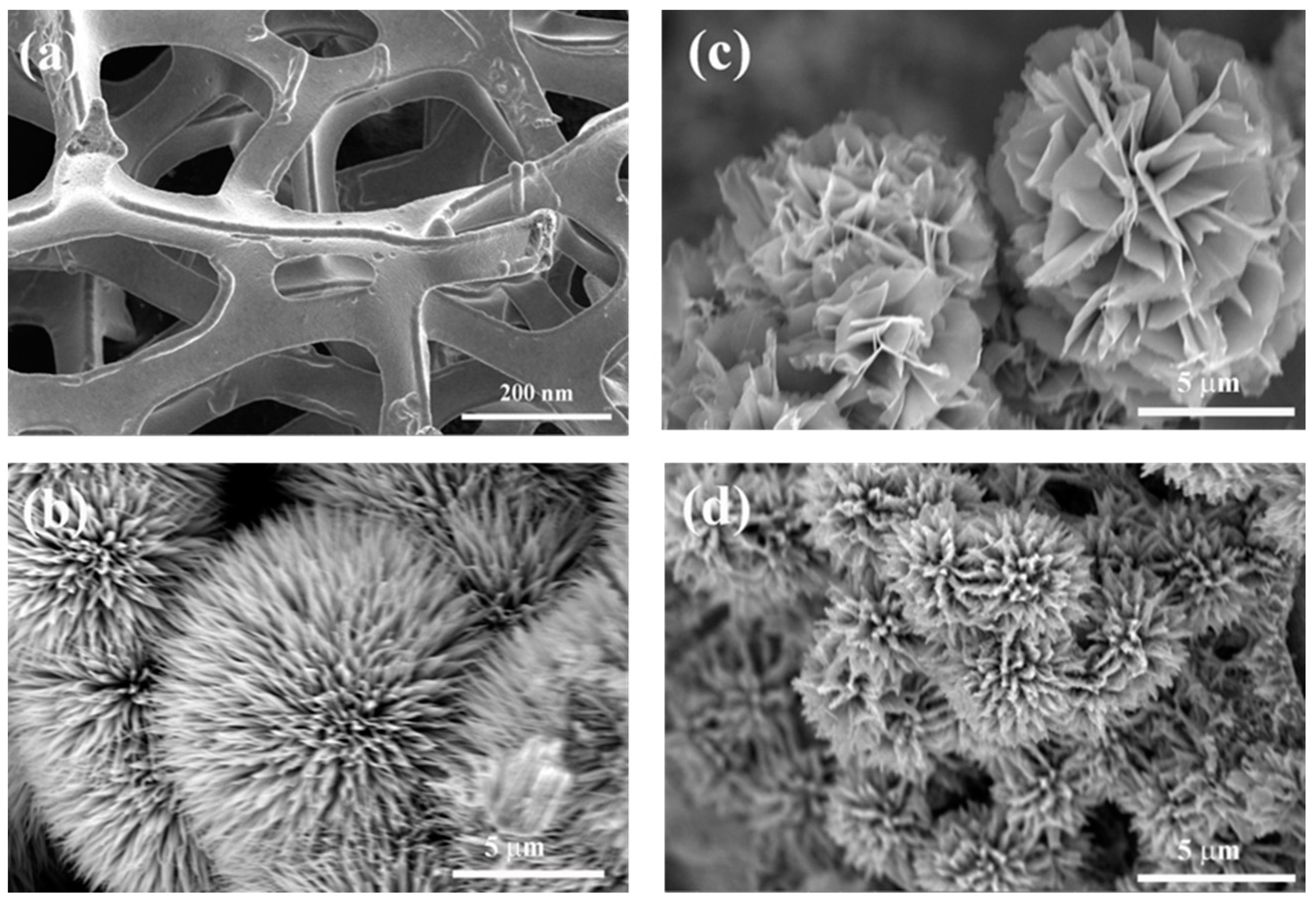
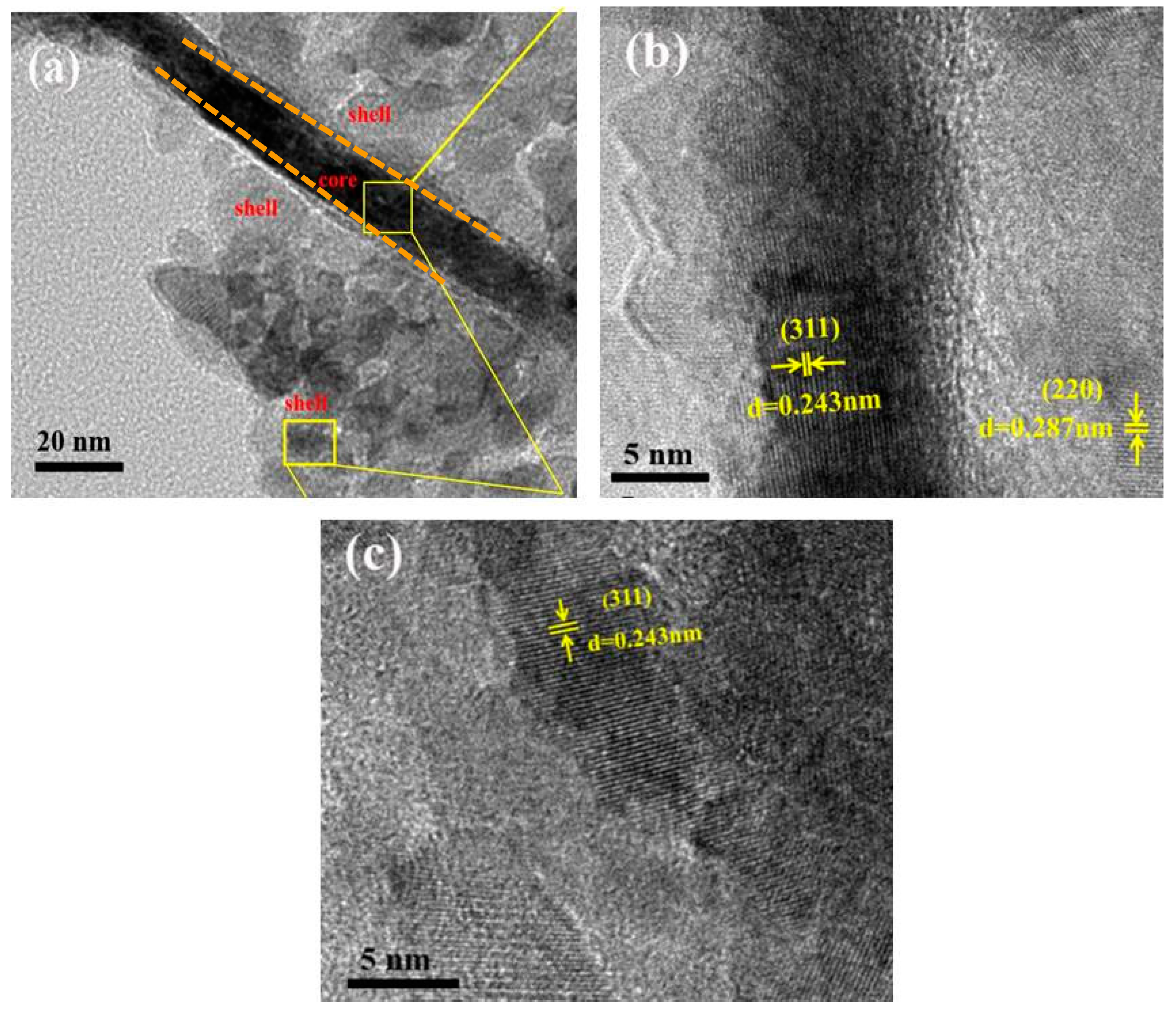
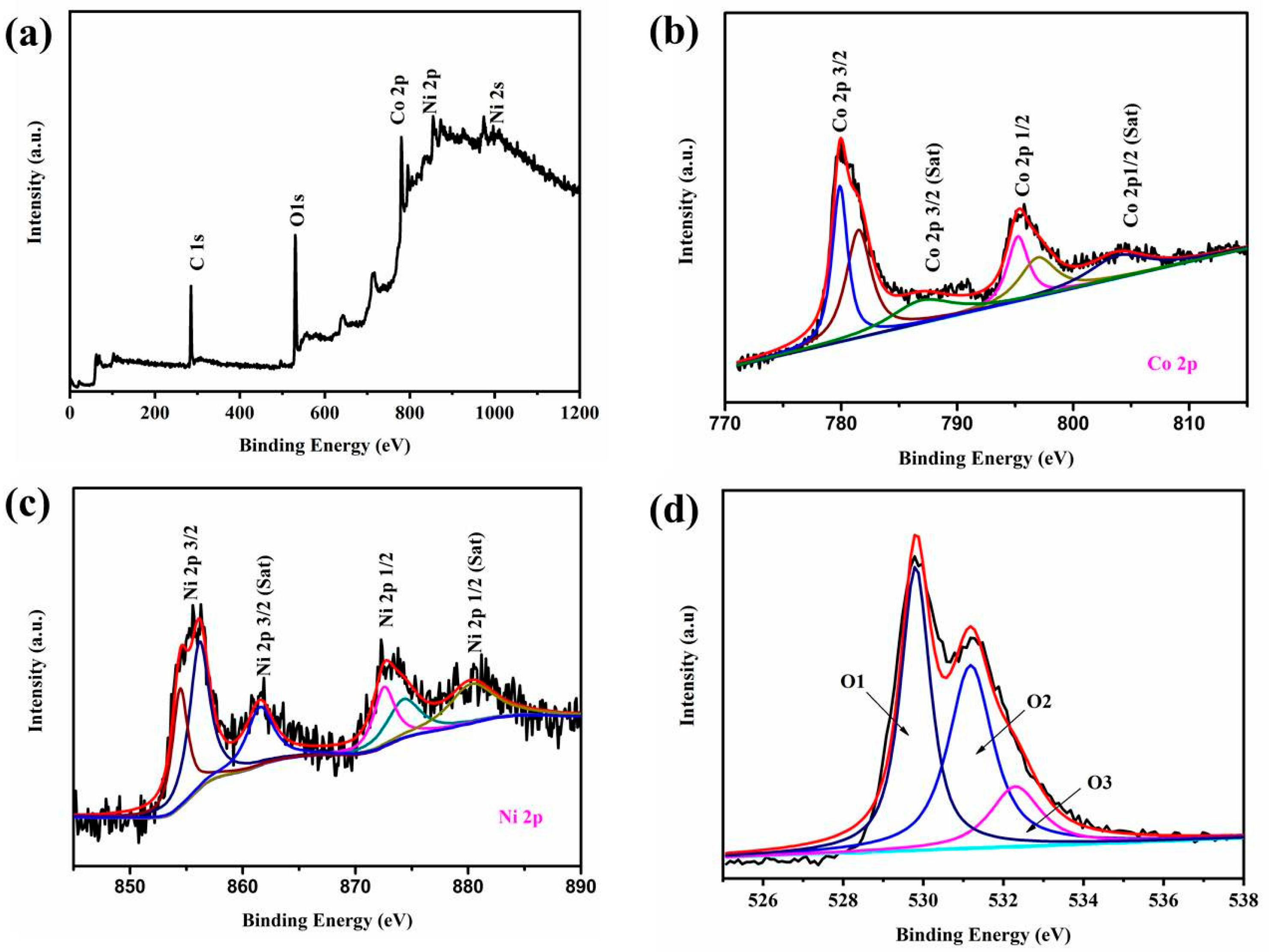
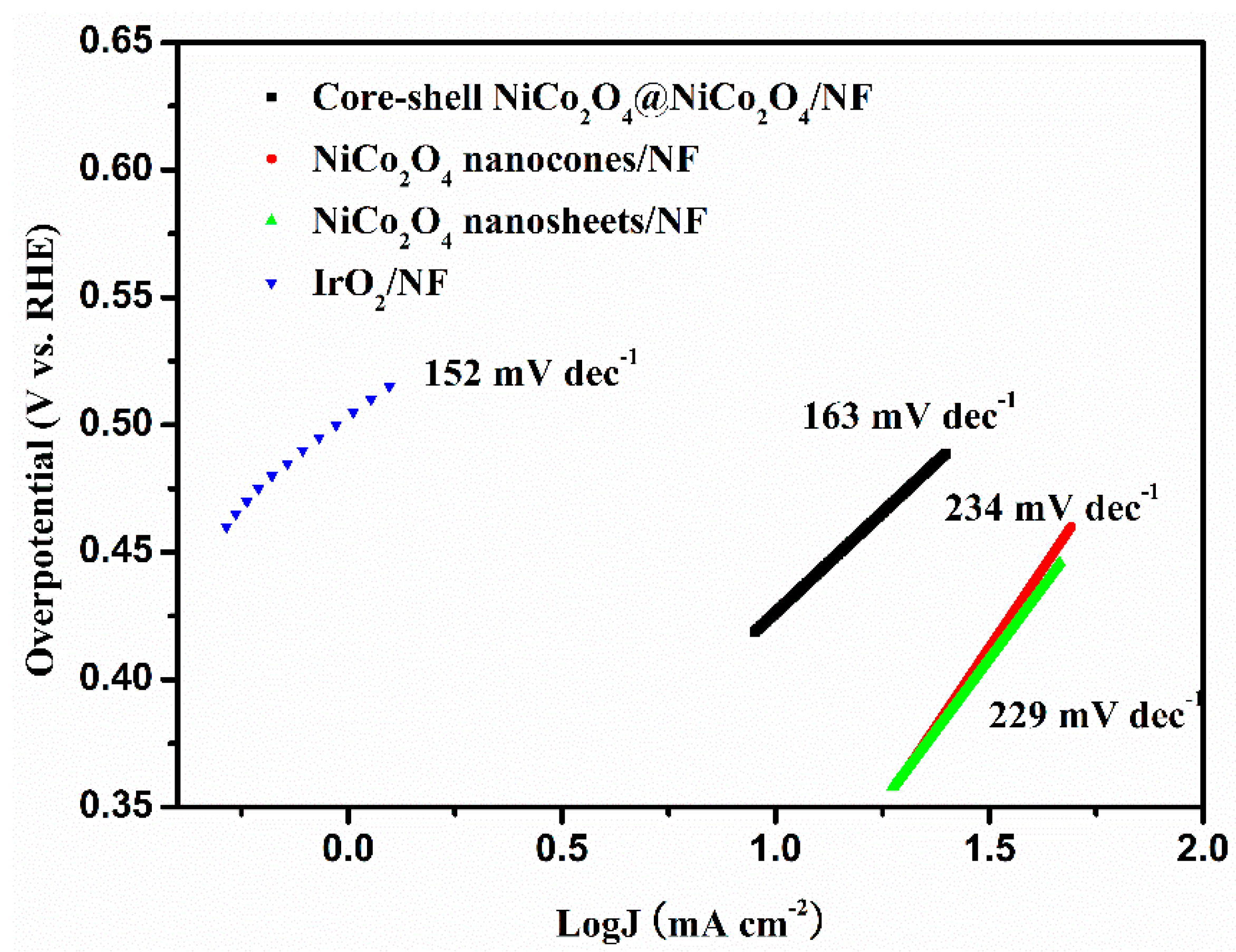
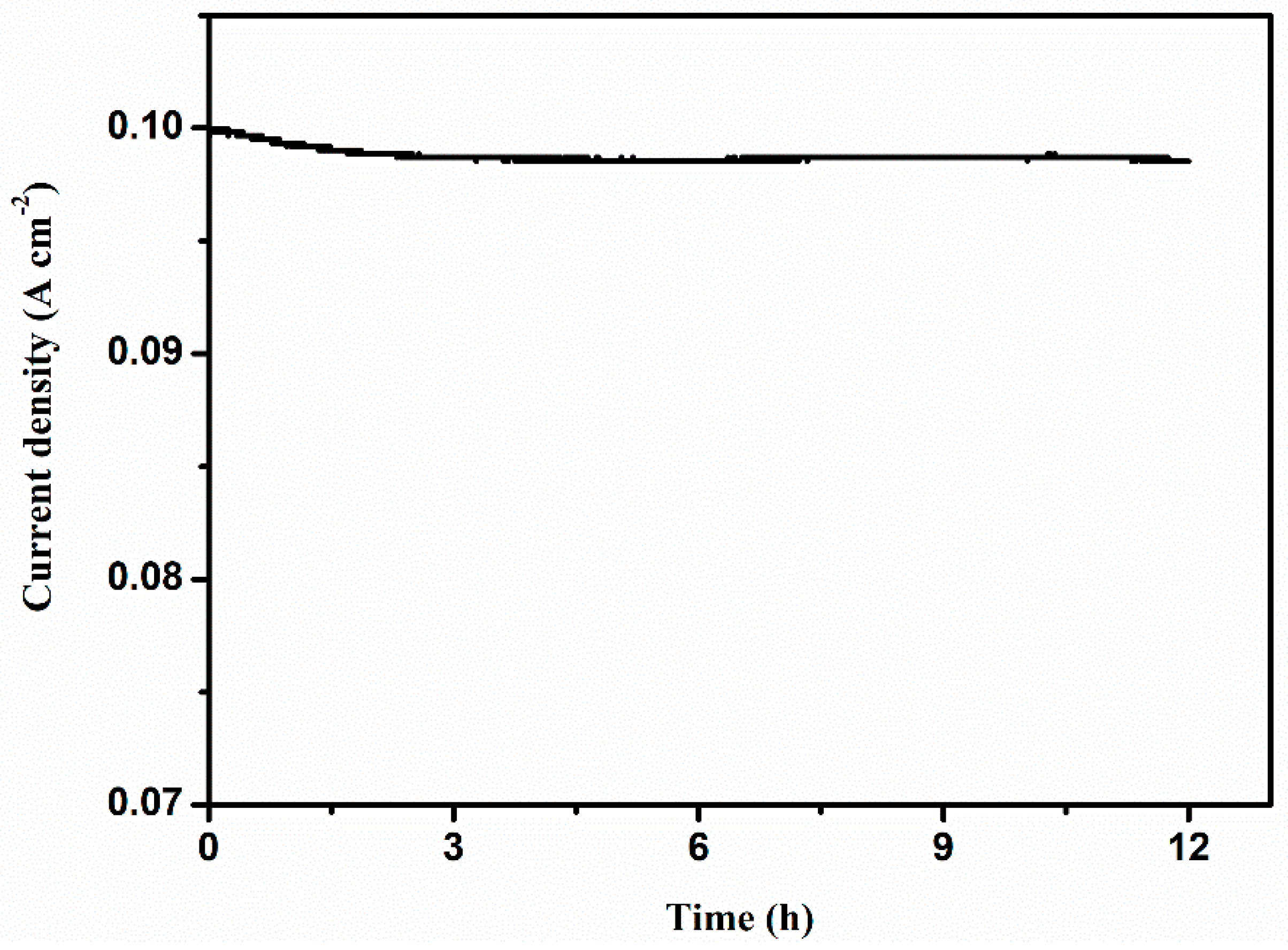
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, J.T.; Ji, S.; Wang, H.; Linkov, V.; Gai, H.J.; Liu, F.S.; Liu, Q.B.; Wang, R.F. N-doped 3D porous Ni/C bifunctional electrocatalysts for alkaline water electrolysis. ACS Sustain. Chem. Eng. 2019, 7, 3974–3981. [Google Scholar] [CrossRef]
- Xiang, W.K.; Yang, N.T.; Li, X.P.; Linnemann, J.L.; Hagemann, U.; Ruediger, O.; Heidelmann, M.; Falk, T.; Aramini, M.; DeBeer, S. 3D atomic-scale imaging of mixed Co-Fe spinel oxide nanoparticles during oxygen evolution reaction. Nat.Commun. 2022, 13, 179. [Google Scholar] [CrossRef]
- Grimaud, A.; Diaz-Morales, O.; Han, B.H.; Hong, W.T.; Lee, Y.L.; Giordano, L.; Stoerzinger, K.A.; Koper, M.T.M.; Horn, Y.S. Activating lattice oxygen redox reactions in metal oxides to catalyst oxygen evolution. Nat. Chem. 2017, 9, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.D.; Dai, X.P.; Liu, M.Z.; Yong, J.X.; Qiao, H.Y.; Jin, A.X.; Li, Z.Z.; Huang, X.L.; Wang, H.; Zhang, X. Strongly Coupled FeNi alloys/NiFe2O4@Carbonitride layers-assembled microboxes for enhanced oxygen evolution raction. ACS Appl. Mater. Interfaces 2016, 8, 34396–34404. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Shi, L.X.; Yan, X.X.; Chen, Z.L.; Li, Y.P.; Xu, W.; Wu, R.B. Multifunctional electrocatalysis on a porous N-doped NiCo2O4@C nanonetwork. ACS Appl. Mater. Interfaces 2019, 11, 45546–45553. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.S.; Shah, S.S.A.; Wei, Z.D. Recent developments in metal phosphide and sulfide electrocatalysts for oxygen evolution reaction. Chin. J. Catal. 2018, 39, 1575–1593. [Google Scholar] [CrossRef]
- Burke, M.; Kast, M.G.; Trotochaud, L.; Smith, A.M.; Boettcher, S.W. Cobalt-Iron (oxy)hydroxide oxygen evolution electrocatalysts: The role of structure and composition on activity, stability, and mechanism. J. Am. Chem. Soc. 2015, 137, 3638–3648. [Google Scholar] [CrossRef]
- Sumboj, A.; Chen, J.W.; Zong, Y.; Lee, P.S.; Liu, Z.L. NiMn Layered Double Hydroxide as efficient electrocatalyst for oxygen evolution reaction and its application in rechargeable Zn-Air batteries. Nanoscle 2017, 9, 774–780. [Google Scholar] [CrossRef]
- Prabhu, C.P.K.; Aralekallu, S.; Sajjan, V.A.; Palanna, M.; Kumarb, S.; Sannegowda, L.K. Non-precious cobalt phthalocyanine-embedded iron ore electrocatalysts for hydrogen evolution reactions. Sustain. Energy Fuels 2021, 5, 1448–1457. [Google Scholar]
- Amirzhanova, A.; Akmanşen, N.; Karakaya, I.; Dag, Ö. Mesoporous MnCo2O4, NiCo2O4, and ZnCo2O4 thin-film electrodes as electrocatalysts for the oxygen evolution reaction in alkaline solutions. ACS Appl. Mater. Interfaces 2021, 4, 2769–2785. [Google Scholar] [CrossRef]
- Han, L.; Dong, S.J.; Wang, E.K. Transition-metal (Co, Ni, and Fe)-based electrocatalysts for the water oxidation reaction. Adv. Mater. 2016, 28, 9266–9291. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sui, G.Z.; Guo, D.X.; Li, J.L.; Ma, X.Y.; Zhuang, Y.; Chai, D.F. Oxygen vacancies-rich NiCo2O4-4x nanowires assembled on porous carbon derived from cigarette ash: A competitive candidate for hydrogen evolution reaction and supercapacitor. J. Energy Storage 2022, 50, 104280. [Google Scholar] [CrossRef]
- Zhang, L.X.; Zhang, S.L.; Zhang, K.J.; Xu, G.J.; He, X.; Dong, S.M.; Liu, Z.H.; Huang, C.S.; Gu, L.; Cui, G.L. Mesoporous NiCo2O4 nanoflakes as electrocatalysts for rechargeable Li-O2 batteries. Chem. Commun. 2013, 49, 3540–3542. [Google Scholar] [CrossRef]
- Liu, W.J.; Bao, J.; Xu, L.; Guan, M.L.; Wang, Z.L.; Qiu, J.X.; Huang, Y.P.; Xia, J.X.; Lei, Y.C.; Li, H.M. NiCo2O4 ultrathin nanosheets with oxygen vacancies as bifunctional electrocatalysts for Zn-air battery. Appl. Surf. Sci. 2019, 478, 552–559. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, F.; Yao, S.Y.; Sun, Z.P.; Wu, X. Hierarchical NiCo2O4 nanowalls composed of ultrathin nanosheets as electrode materials for supercapacitor and Li ion battery applications. Mater. Res. Bul. 2017, 93, 303–309. [Google Scholar] [CrossRef]
- Han, G.Q.; Liu, Y.R.; Hu, W.H.; Dong, B.; Li, X.; Shang, X.; Chai, Y.M.; Liu, Y.Q.; Liu, C.G. Three dimensional Nickel Oxides/Nickel structure by in situ electro-oxidation of nickel foam as robust electrocatalyst for oxygen evolution reaction. Appl. Surf. Sci. 2015, 359, 172–176. [Google Scholar] [CrossRef]
- Yu, Y.J.; Chen, C.; Liu, Y.H.; Yu, H.L.; Li, S.J.; Xue, Y.N.; Cai, N.; Wang, J.Z.; Yu, F.Q. Hierarchical Ni/Ni(OH)2-NiCo2O4 supported on Ni foam as efficient bifunctional electrocatalysts for water splitting. J. Phys. Chem. C 2022, 126, 5493–5501. [Google Scholar] [CrossRef]
- Kamblea, G.P.; Kashalea, A.A.; Rasalab, A.S.; Manea, S.A.; Chavana, R.A.; Chang, J.Y.; Ling, Y.C.; Kolekard, S.S.; Ghule, A.V. Marigold micro-flower like NiCo2O4 grown on flexible stainless-steel mesh as an electrode for supercapacitors. RSC Adv. 2021, 11, 3666–3672. [Google Scholar] [CrossRef]
- Zhang, C.; Geng, X.P.; Tang, S.L.; Deng, M.; Du, Y. NiCo2O4@rGO hybrid nanostructures on Ni foam as high-performance supercapacitor electrodes. J. Mater. Chem. A 2017, 5, 5912–5919. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, X.L.; Wang, T.; Xiao, F.X. A facile, green and time-saving method to prepare partially crystalline NiFe layered double hydroxide nanosheets on nickel foam for superior OER catalysis. J. Alloys Compd. 2020, 844, 156224. [Google Scholar] [CrossRef]
- Zhu, J.W.; Liu, Z.H.; Zhai, M.H.; Ni, Y.H. Ni1-xCoxSe nanostructures deposited on nickel foam by a facile potentiostatic route for enhanced OER performance. J. Phys. Chem. Solids 2021, 148, 109658. [Google Scholar] [CrossRef]
- Ren, Q.Q.; Wang, Z.B.; Ke, K.; Zhang, S.W.; Yin, B.S. NiCo2O4 nanosheets and nanocones as additive-free anodes for high-performance Li-ion batteries. Ceram. Int. 2017, 43, 13710–13716. [Google Scholar] [CrossRef]
- Ni, L.; Zhou, J.H.; Chen, N.N.; Li, X.G.; Xu, S.C.; Zhang, L.; Lu, C.L.; Chen, J.; Xu, L.; Hou, W.H. In situ direct growth of flower-like hierarchical architecture of CoNi-layered double hydroxide on Ni foam as an efficient self-supported oxygen evolution electrocatalyst. Int. J. Hydrogen Energ. 2020, 45, 22788–22796. [Google Scholar] [CrossRef]
- Zhao, W.; Li, X.M.; Yin, R.; Qian, L.; Huang, X.S.; Liu, H.; Zhang, J.X.; Wang, J.; Ding, T.; Guo, Z.H. Urchin-like NiO-NiCo2O4 heterostructure microsphere catalysts for enhanced rechargeable non-aqueous Li-O2 batteries. Nanoscale 2019, 11, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Li, B.L.; Chen, J.M.; Pang, Q.; Shen, P.K. Spinel NiCo2O4 3-D nanoflowers supported on graphene nanosheets as efficient electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energ. 2019, 44, 16120–16131. [Google Scholar] [CrossRef]
- Wang, X.H.; Fang, Y.; Shi, B.; Huang, F.F.; Rong, F.; Que, R.H. Three-dimensional NiCo2O4@NiCo2O4 core-shell nanocones arrays for high-performance supercapacitors. Langmuir 2017, 33, 10446–10454. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Zhang, J.M.; Peng, X.M.; Liu, Y.Y.; Cen, T.L.; Ye, Z.F.; Yuan, D.S. Co3O4-NiCo2O4 hybrid nanoparticles anchored on N-doped reduced graphene oxide nanosheets as an efficient catalyst for Zn-Air batteries. Energy Fuels 2021, 35, 4550–4558. [Google Scholar] [CrossRef]
- Pu, J.; Wang, J.; Jin, X.; Cui, F.; Sheng, E.; Wang, Z. Porous hexagonal NiCo2O4 nanoplates as electrode materials for supercapacitors. Electrochim. Acta 2013, 106, 226–234. [Google Scholar] [CrossRef]
- Ma, F.X.; Yu, L.; Xu, C.Y.; Lou, X.W. Self-Supported formation of hierarchical NiCo2O4 tetragonal microtubes with enhanced electrochemical properties. Energy Environ. Sci. 2016, 9, 862–866. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.P.; Mao, Y.Y.; Wei, M.; Chu, W.; Huang, K. Rapid synthesis of ultrafine NiCo2O4 nanoparticles loaded carbon nanotubes for lithium ion battery anode materials. Chem. Phys. Lett. 2019, 715, 278–283. [Google Scholar] [CrossRef]
- He, G.G.; Tian, L.L.; Cai, Y.H.; Wu, S.P.; Su, Y.Y.; Yan, H.Q.; Pu, W.R.; Zhang, J.K.; Li, L. Sensitive nonenzymatic electrochemical glucose detection based on hollow porous NiO. Nanoscale Res. Lett. 2018, 3, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Z.X.; Du, X.Q.; Zhang, X.S. Controlled synthesis of NiCo2O4@Ni-MOF on Ni foam as efficient electrocatalyst for urea oxidation reaction and oxygen evolution reaction. Int. J. Hydrogen Energy 2022, 4, 17252–17262. [Google Scholar] [CrossRef]
- Xiang, K.; Wu, D.; Fan, Y.; You, W.; Zhang, D.D.; Luo, J.L.; Fu, X.Z. Enhancing bifunctional electrodes of oxygen vacancy abundant ZnCo2O4 nanosheets for supercapacitor and oxygen evolution. Chem. Eng. J. 2021, 425, 130583. [Google Scholar] [CrossRef]
- Gong, Y.Q.; Yang, Z.; Lin, Y.; Wang, J.L.; Pan, H.; Xu, Z. Hierarchical heterostructure NiCo2O4@CoMoO4/NF as an efficient bifunctional electrocatalyst for overall water splitting. J. Mater. Chem. A 2018, 6, 16950–16958. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Zhang, M.; Gong, Y. Vertically stacked bilayer heterostructure CoFe2O4@Ni3S2 on a 3D nickel foam as a high-performance electrocatalyst for the oxygen evolution reaction. New J. Chem. 2020, 44, 1455–1462. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Xia, J.; Pan, Z.; Wang, X.; Zhu, G.; Zheng, B.; Liu, G.; Lang, L. Reduced CoFe2O4/graphene composite with rich oxygen vacancies as a high efficient electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 2020, 45, 11052–11061. [Google Scholar] [CrossRef]
- Tao, B.; Yang, L.; Miao, F.; Zang, Y.; Chu, P.K. An MoS2/NiCo2O4 composite supported on Ni foam as a bifunctional electrocatalyst for efficient overall water splitting. J. Phys. Chem. Solids 2021, 150, 109842. [Google Scholar] [CrossRef]
- Long, X.; Li, J.; Xiao, S.; Yan, K.Y.; Wang, Z.L.; Chen, H.N.; Yang, S.H. A Strongly coupled graphene and FeNi double hydroxide hybrid as an excellent electrocatalyst for the oxygen evolution reaction. Angew. Chem. Int. Ed. 2014, 53, 7584–7588. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, L.W.; Liu, X.L.; Tan, T.; Liu, H.; Wang, J.J. Precisely engineering the electronic structure of active sites boosts the activity of iron-nickel selenide on nickel foam for highly efficient and stable overall water splitting. Appl. Catal. B Environ. 2021, 299, 120678. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Choi, B.; Kim, Y.B. Development of highly active bifunctional electrocatalyst using Co3O4 on carbon nanotubes for oxygen reduction and oxygen evolution. Sci. Rep. 2018, 8, 2543. [Google Scholar] [CrossRef]
- Su, Y.Z.; Xu, Q.Z.; Chen, G.F.; Cheng, H.; Li, N.; Liu, Z.Q. One dimensionally spinel NiCo2O4 nanowire arrays: Facile synthesis, water oxidation, and magnetic properties. Electrochim. Acta 2015, 174, 1216–1224. [Google Scholar] [CrossRef]
- Feng, H.F.; Gao, S.Y.; Shi, J.; Zhang, L.; Peng, Z.M.; Cao, S.K. Construction of 3D hierarchical porous NiCo2O4/graphene hydrogel/Ni foam electrode for high-performance supercapacitor. Electrochim. Acta 2019, 299, 116–124. [Google Scholar] [CrossRef]
- Zhang, L.J.; Li, H.J.; Li, K.Z.; Li, L.; Wei, J.F.; Feng, L.; Fu, Q.G. Morphology-controlled fabrication of Co3O4 nanostructures and their comparative catalytic activity for oxygen evolution reaction. J. Alloys Compd. 2016, 680, 146–154. [Google Scholar] [CrossRef]
- Jiang, S.; Ithisuphalap, K.; Zeng, X.R.; Wu, G.; Yang, H.P. 3D porous celluar NiCoO2/graphene network as durable bifunctional electrocatalyst for oxygen evolution and reduction reactions. J. Power Sources 2018, 399, 66–75. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Yuan, H.; Li, X.; Wang, Y. Hydrothermal Synthesis of NiCo2O4 @NiCo2O4 Core-Shell Nanostructures Anchored on Ni Foam for Efficient Oxygen Evolution Reactions Catalysts. Coatings 2022, 12, 1240. https://doi.org/10.3390/coatings12091240
Zhang L, Yuan H, Li X, Wang Y. Hydrothermal Synthesis of NiCo2O4 @NiCo2O4 Core-Shell Nanostructures Anchored on Ni Foam for Efficient Oxygen Evolution Reactions Catalysts. Coatings. 2022; 12(9):1240. https://doi.org/10.3390/coatings12091240
Chicago/Turabian StyleZhang, Lijuan, Haichen Yuan, Xiang Li, and Yan Wang. 2022. "Hydrothermal Synthesis of NiCo2O4 @NiCo2O4 Core-Shell Nanostructures Anchored on Ni Foam for Efficient Oxygen Evolution Reactions Catalysts" Coatings 12, no. 9: 1240. https://doi.org/10.3390/coatings12091240






