Suppression of Secondary Electron Emissions on the Graphene-Coated Polyimide Materials Prepared by Chemical Vapor Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
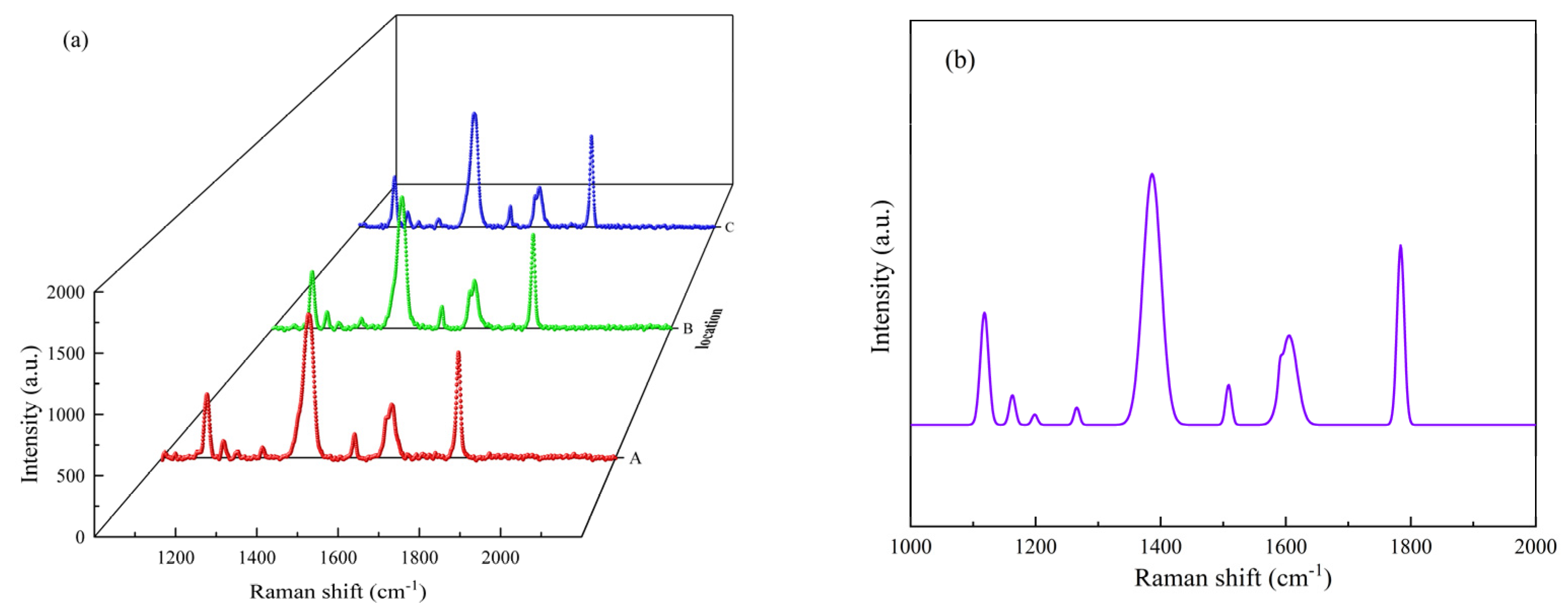
2.2. Material Characterization
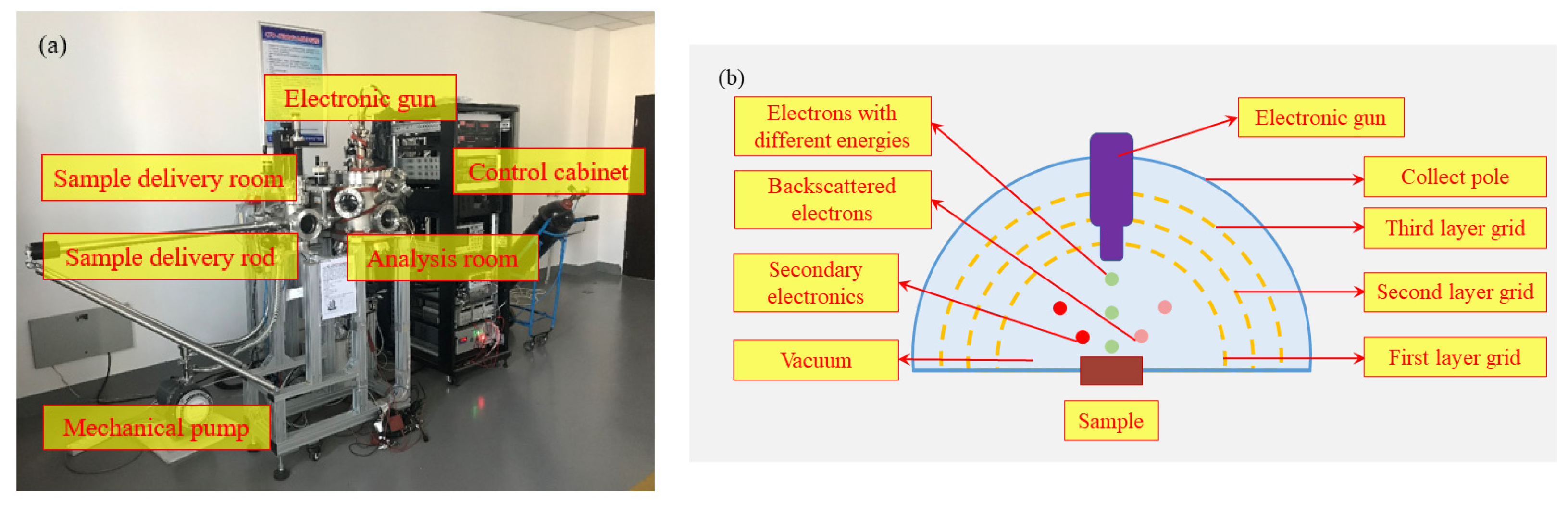
2.3. Secondary Electron Yield Measurement
3. Results
3.1. SEY and Theoretical Analysis
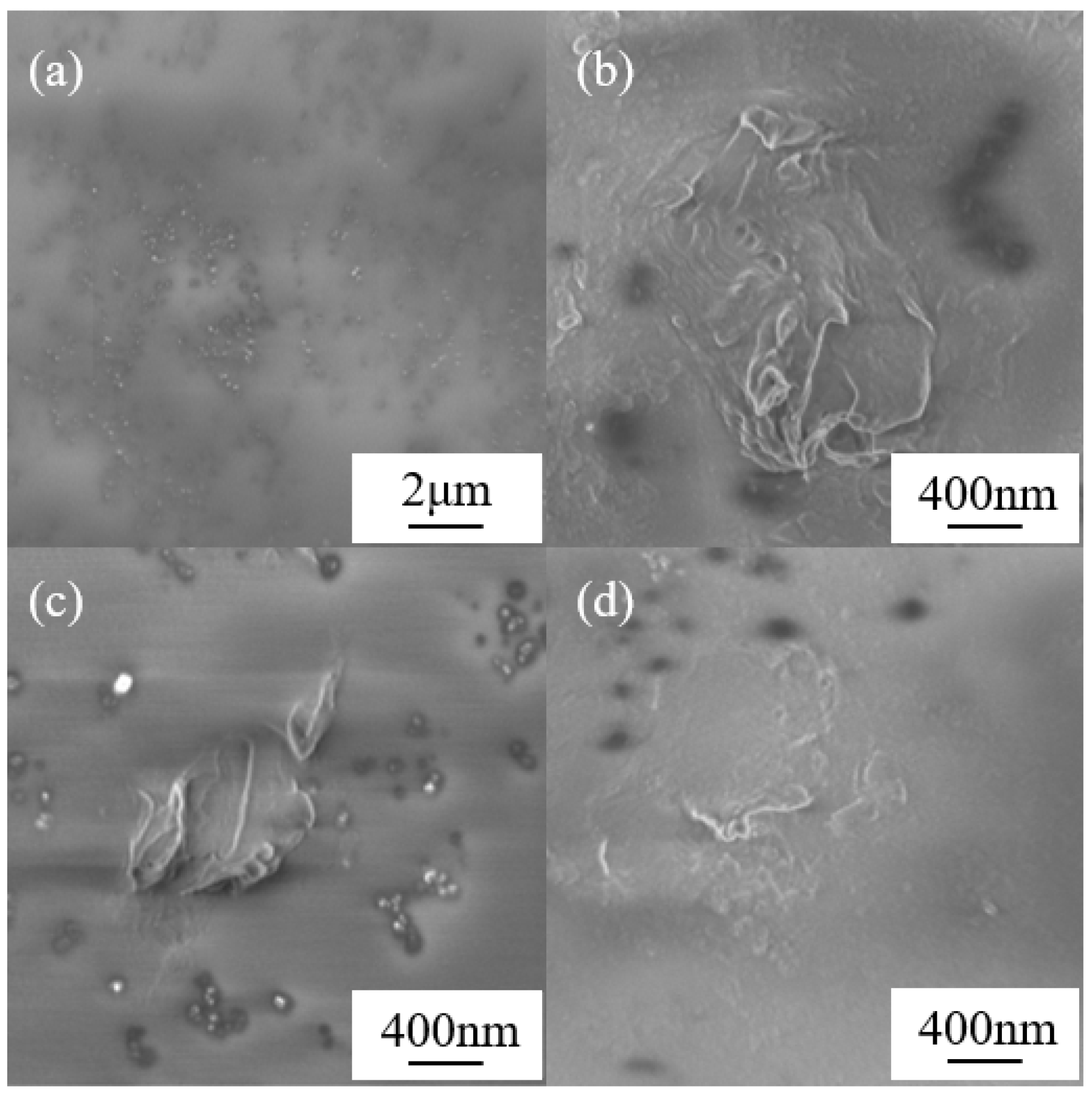
3.2. Surface Morphology Study of the Graphene-Coated Polyimide Materials
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Kong, F.; Zhang, P.H.; Yu, W.X.; Zhang, C.; Liu, J.B.; Ren, C.Y.; Shao, T. Enhanced surface insulating performance for polystyrene by atmospheric pressure plasma jet deposition. Appl. Surf. Sci. 2020, 527, 146826. [Google Scholar] [CrossRef]
- Wang, D.; He, Y.N.; Cui, W.Z. Secondary electron emission characteristics of TiN coatings produced by RF magnetron sputtering. J. Appl. Phys. 2018, 124, 053301. [Google Scholar] [CrossRef]
- Santos, A.; Bundaleski, N.; Shaw, B.J.; Silva, A.G.; Teodoro, O. Increase of secondary electron yield of amorphous carbon coatings under high vacuum conditions. Vacuum 2013, 98, 37–40. [Google Scholar] [CrossRef]
- Lapington, J.S.; Thompson, D.P.; May, P.W.; Fox, N.A.; Howorth, J.; Milnes, J.; Taillandier, V. Investigation of the secondary emission characteristics of CVD diamond films for electron amplification. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2009, 610, 253–257. [Google Scholar] [CrossRef]
- Costin, C. Secondary electron emission under magnetic constraint: From Monte Carlo simulations to analytical solution. Sci. Rep. 2021, 11, 1874. [Google Scholar] [CrossRef]
- Liu, X.W.; Mang, G.J.; Li, J.; Shi, G.L.; Zhou, M.Y.; Huang, B.Q.; Tang, Y.J.; Song, X.H.; Yang, W.F. Deep Learning for Feynman’s Path Integral in Strong-Field Time-Dependent Dynamics. Phys. Rev. Lett. 2020, 124, 113202. [Google Scholar] [CrossRef]
- Raitses, Y.; Kaganovich, I.D.; Khrabrov, A.; Sydorenko, D.; Fisch, N.J.; Smolyakov, A. Effect of Secondary Electron Emission on Electron Cross-Field Current in E x B Discharges. IEEE Trans. Plasma Sci. 2011, 39, 995–1006. [Google Scholar] [CrossRef]
- Liu, L.; Meng, X.N.; Miao, Z.; Zhou, S. Design of a novel thermoelectric module based on application stability and power generation. Case Stud. Therm. Eng. 2022, 31, 101836. [Google Scholar] [CrossRef]
- Choi, H.J.; Jung, S.M.; Seo, J.M.; Chang, D.W.; Dai, L.M.; Baek, J.B. Graphene for energy conversion and storage in fuel cells and supercapacitors. Nano Energy 2012, 1, 534–551. [Google Scholar] [CrossRef]
- Ma, J.Z.; Wei, L.F.; Bai, Y.R.; Bao, Y.; Kang, Q.L.; Zhang, W.B.; Cui, W.Z.; Hu, T.C.; Wu, D.D. Controllable preparation of silver nano-bowl coatings for suppressing secondary electron emission. Thin Solid Film. 2021, 725, 138633. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; You, Z.M.; Fan, J.K.; Zhang, J.; Wang, S.; Xu, Z.L. Laser Induced Nano and Micro Structures of Molybdenum Surface Applied in Multistage Depressed Collector for Secondary Electron Suppression. Appl. Sci. 2019, 9, 4374. [Google Scholar] [CrossRef]
- Swanson, C.; Kaganovich, I.D. Modeling of reduced secondary electron emission yield from a foam or fuzz surface. J. Appl. Phys. 2018, 123, 023302. [Google Scholar] [CrossRef]
- Cimino, R.; Commisso, M.; Grosso, D.R.; Demma, T.; Baglin, V.; Flammini, R.; Larciprete, R. Nature of the Decrease of the Secondary-Electron Yield by Electron Bombardment and its Energy Dependence. Phys. Rev. Lett. 2012, 109, 064801. [Google Scholar] [CrossRef] [PubMed]
- Pivi, M.; King, F.K.; Kirby, R.E.; Raubenheimer, T.O.; Stupakov, G.; Le Pimpec, F. Sharp reduction of the secondary electron emission yield from grooved surfaces. J. Appl. Phys. 2008, 104, 104904. [Google Scholar] [CrossRef]
- Liu, L.; Feng, G.B.; Chen, B.D.; Wang, N.; Cui, W.Z. Characteristics of secondary electron emission and multipactor from a nested microtrap structure surface. AIP Adv. 2021, 11, 025332. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, Y.G.; Wang, S.H.; Wei, W.; Ge, X.Q.; Zhu, B.L.; Shao, J.Q.; Wang, Y. Comparison of Carbon Thin Films with Low Secondary Electron Yield Deposited in Neon and Argon. Coatings 2020, 10, 884. [Google Scholar] [CrossRef]
- Wang, G.D.; Liu, D.M.; Fan, S.Q.; Li, Z.Y.; Su, J. High-k erbium oxide film prepared by sol-gel method for low-voltage thin-film transistor. Nanotechnology 2021, 32, 215202. [Google Scholar] [CrossRef]
- Nivas, J.J.J.; Valadan, M.; Salvatore, M.; Fittipaldi, R.; Himmerlich, M.; Rimoldi, M.; Passarelli, A.; Allahyari, E.; Oscurato, S.L.; Vecchione, A.; et al. Secondary electron yield reduction by femtosecond pulse laser-induced periodic surface structuring. Surf. Interfaces 2021, 25, 101179. [Google Scholar] [CrossRef]
- Jo, G.; Choe, M.; Lee, S.; Park, W.; Kahng, Y.H.; Lee, T. The application of graphene as electrodes in electrical and optical devices. Nanotechnology 2012, 23, 112001. [Google Scholar] [CrossRef]
- Park, J.H.; Park, J.M. Electrophoretic deposition of graphene oxide on mild carbon steel for anti-corrosion application. Surf. Coat. Technol. 2014, 254, 167–174. [Google Scholar] [CrossRef]
- Tang, H.C.; Yang, C.; Lin, Z.Y.; Yang, Q.H.; Kang, F.Y.; Wong, C.P. Electrospray-deposition of graphene electrodes: A simple technique to build high-performance supercapacitors. Nanoscale 2015, 7, 9133–9139. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, C.L.P.; Sarada, B.V.; Rajulapati, K.V.; Rao, T.N.; Sundararajan, G. A New Electrochemical Approach for the Synthesis of Copper-Graphene Nanocomposite Foils with High Hardness. Sci. Rep. 2014, 4, 4049. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.S.; Yang, S.L.; Zhang, Y.; Yang, J.L.; Liu, J.; Li, R.Y.; Sham, T.K.; Sun, X.L.; Ye, S.Y.; Knights, S. Nitrogen doping effects on the structure of graphene. Appl. Surf. Sci. 2011, 257, 9193–9198. [Google Scholar] [CrossRef]
- Mai, Y.J.; Zhou, M.P.; Ling, H.J.; Chen, F.X.; Lian, W.Q.; Jie, X.H. Surfactant-free electrodeposition of reduced graphene oxide/copper composite coatings with enhanced wear resistance. Appl. Surf. Sci. 2018, 433, 232–239. [Google Scholar] [CrossRef]
- Larciprete, R.; Grosso, D.R.; Di Trolio, A.; Cimino, R. Evolution of the secondary electron emission during the graphitization of thin C films. Appl. Surf. Sci. 2015, 328, 356–360. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, X.S.; Liu, W.H.; Wang, H.G.; Li, Y.D. Secondary electron emission of graphene-coated copper. Diam. Relat. Mater. 2017, 73, 199–203. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Xu, Y.H.; Zhang, Y.X.; Zhang, B.; Wei, W. Secondary electron emission characteristics of graphene films with copper substrate. Chin. Phys. C 2016, 40, 117003. [Google Scholar] [CrossRef]
- Sian, T.; Lin, Y.; Valizadeh, R.; Malyshev, O.B.; Xia, G.; Valles, C.; Yu, G.; Kinloch, I. Graphene coating for the reduction of the secondary electron yield. Proc. IPAC 2016, 2, 3688–3690. [Google Scholar]
- Aguincha, R.; Bundaleski, N.; Bundaleska, N.; Novakovi, M.; Teodoro, O. Low total electron yield graphene coatings produced by electrophoretic deposition. Appl. Surf. Sci. 2019, 504, 143870. [Google Scholar] [CrossRef]
- Luo, J.; Tian, P.; Pan, C.T.; Robertson, A.W.; Warner, J.H.; Hill, E.W.; Briggs, G. Ultralow Secondary Electron Emission of Graphene. ACS Nano 2011, 5, 1047. [Google Scholar] [CrossRef]
- Bundaleski, N.; Belhaj, M.; Gineste, T.; Teodoro, O. Calculation of the angular dependence of the total electron yield. Vacuum 2015, 122, 255–259. [Google Scholar] [CrossRef]
- Nguyen, H.K.A.; Mankowski, J.; Dickens, J.C.; Neuber, A.A.; Joshi, R.P. Calculations of secondary electron yield of graphene coated copper for vacuum electronic applications. AIP Adv. 2018, 8, 015325. [Google Scholar] [CrossRef]
- Fernandez, H.M.; Himmerlich, M.; Pinto, P.C.; Coroa, J.; Sousa, D.; Baris, A.; Taborelli, M. The impact of H-2 and N-2 on the material properties and secondary electron yield of sputtered amorphous carbon films for anti-multipacting applications. Appl. Surf. Sci. 2021, 542, 148552. [Google Scholar] [CrossRef]
- Fitting, H.J.; Touzin, M. Time-dependent start-up and decay of secondary electron emission in dielectrics. J. Appl. Phys. 2010, 108, 033711. [Google Scholar] [CrossRef]
- Tatarova, E.; Dias, A.; Henriques, J.; Abrashev, M.; Bundaleska, N.; Kovacevic, E.; Bundaleski, N.; Cvelbar, U.; Valcheva, E.; Arnaudov, B.; et al. Towards large-scale in free-standing graphene and N-graphene sheets. Sci. Rep. 2017, 7, 10175. [Google Scholar] [CrossRef]
- Montero, I.; Aguilera, L.; Davila, M.E.; Nistor, V.C.; Gonzalez, L.A.; Galan, L.; Raboso, D.; Ferritto, R. Secondary electron emission under electron bombardment from graphene nanoplatelets. Appl. Surf. Sci. 2014, 291, 74–77. [Google Scholar] [CrossRef]
- Zhang, W.H.; Nefedov, A.; Naboka, M.; Cao, L.; Woll, C. Molecular orientation of terephthalic acid assembly on epitaxial graphene: NEXAFS and XPS study. Phys. Chem. Chem. Phys. 2012, 14, 10125–10131. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Kadari, A.; Schemme, T.; Kadri, D.; Wollschlager, J. XPS and morphological properties of Cr2O3 thin films grown by thermal evaporation method. Results Phys. 2017, 7, 3124–3129. [Google Scholar] [CrossRef]
- Jung, R.H.; Tsuchiya, H.; Fujimoto, S. XPS characterization of passive films formed on Type 304 stainless steel in humid atmosphere. Corros. Sci. 2012, 58, 62–68. [Google Scholar] [CrossRef]
- Yang, X.; Sun, G.Y.; Zhou, R.D.; Huang, K.; Li, W.D.; Wang, C.; Dong, J.F.; Song, B.P.; Zhang, G.J. Ultralow secondary electron emission and improved vacuum surface insulation of polyimide with scalable nanocomposite coating. Appl. Surf. Sci. 2022, 592, 153221. [Google Scholar] [CrossRef]
- Zhang, H.F.; Ge, Y.; Pan, P.; Du, Y.H.; Fu, H.; Yan, M.J.; Li, P.; Long, H.M.; Zhang, C.Z.; Cai, J.; et al. Suppression of secondary electron emission on oxygen-free copper surface of reduced graphene oxide coatings prepared by electrophoretic deposition. Appl. Surf. Sci. 2022, 603, 1544900. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, B.; Hu, Z.Q.; He, Y.; Hu, T.C.; Zhao, Y.N.; Wang, Z.G.; Zhou, Z.Y.; Cui, W.; Liu, M. Surface roughness evolution induced low secondary electron yield in carbon coated Ag/Al substrates for space microwave devices. Appl. Surf. Sci. 2020, 501, 144236. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, X.; Ma, Y.; Liu, S.; Nie, X.; Zhang, T.; Wu, Y.; Peng, W.; Hu, G. Suppression of Secondary Electron Emissions on the Graphene-Coated Polyimide Materials Prepared by Chemical Vapor Deposition. Coatings 2023, 13, 1805. https://doi.org/10.3390/coatings13101805
Qi X, Ma Y, Liu S, Nie X, Zhang T, Wu Y, Peng W, Hu G. Suppression of Secondary Electron Emissions on the Graphene-Coated Polyimide Materials Prepared by Chemical Vapor Deposition. Coatings. 2023; 13(10):1805. https://doi.org/10.3390/coatings13101805
Chicago/Turabian StyleQi, Xin, Yanzhao Ma, Sisheng Liu, Xiangyu Nie, Tao Zhang, Yong Wu, Weiping Peng, and Guoming Hu. 2023. "Suppression of Secondary Electron Emissions on the Graphene-Coated Polyimide Materials Prepared by Chemical Vapor Deposition" Coatings 13, no. 10: 1805. https://doi.org/10.3390/coatings13101805



