AgNWs–Silane Coatings for the Functionalization of Aramid Woven Fabrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Silver Nanowire (AgNWs) Colloid
2.2.2. Silane Sol Preparation
2.2.3. Low-Pressure Air RF Plasma Treatment and Polydopamine Modification of Aramid Woven Fabrics
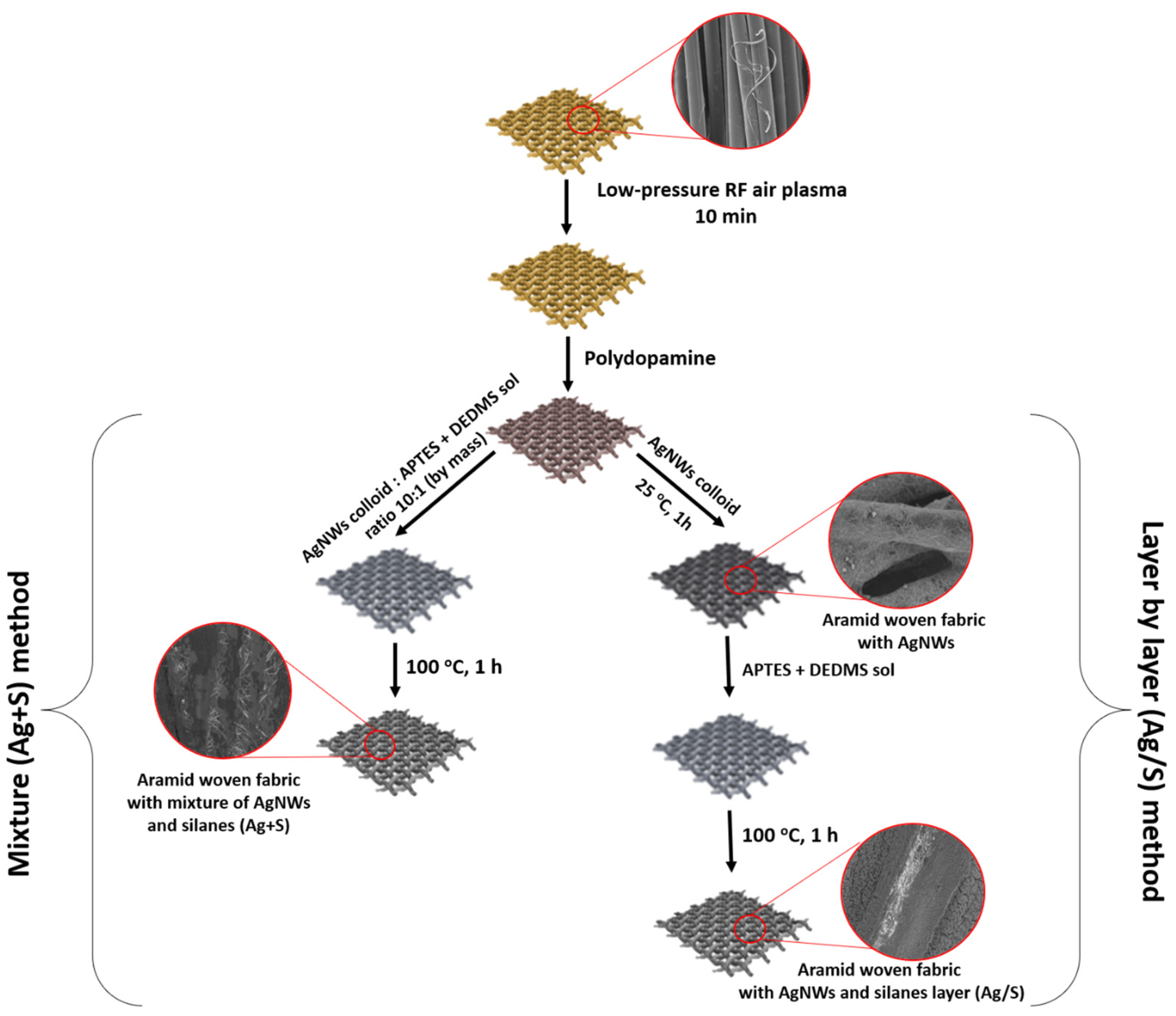
2.2.4. Functionalization of Aramid Woven Fabrics with AgNWs and Silanes
2.2.5. Instrumental Techniques
3. Results and Discussion
3.1. Application Effect of Coatings
3.2. SEM Analysis
3.3. AAS Analysis
3.4. FTIR Analysis
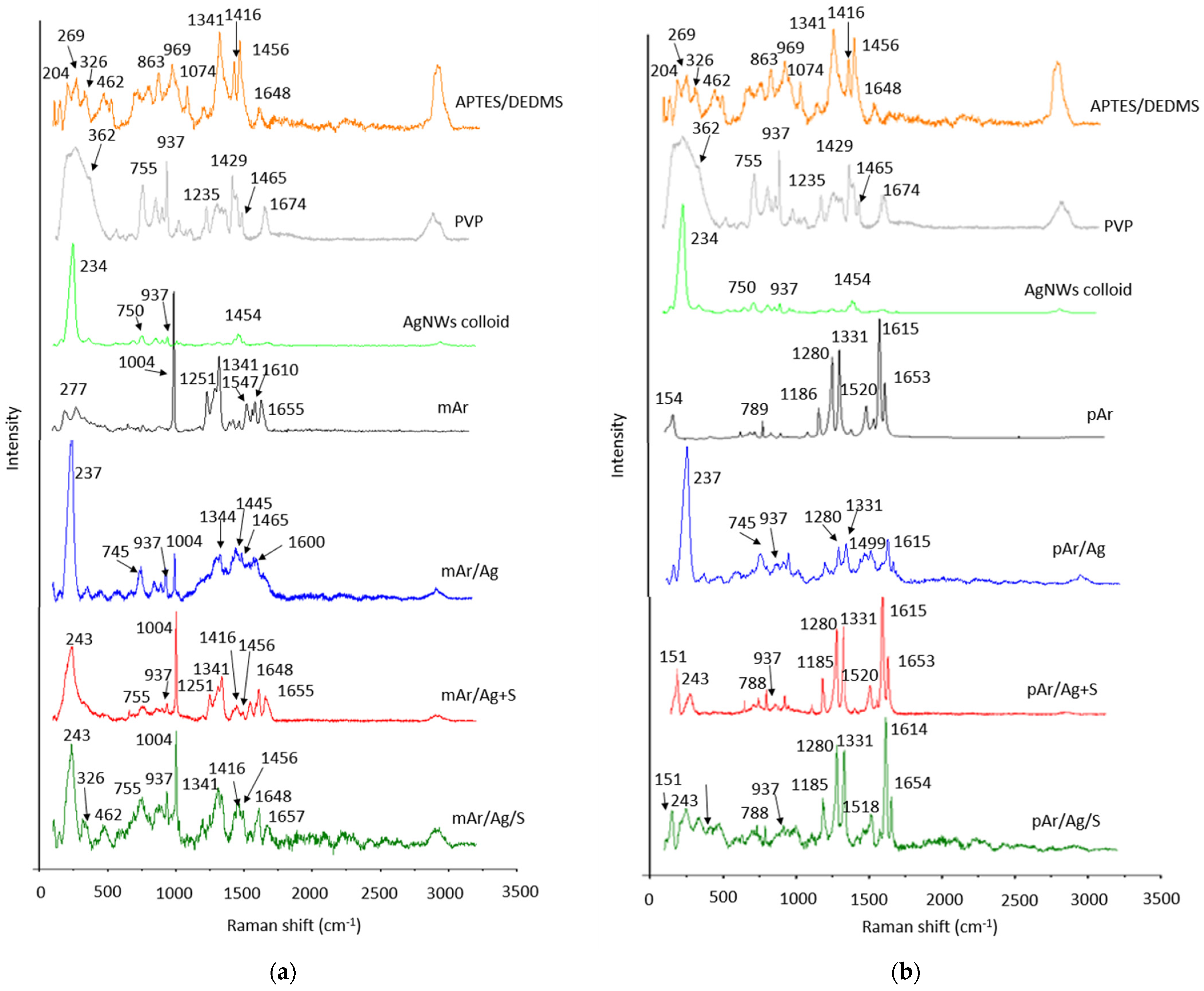
3.5. Raman Analysis
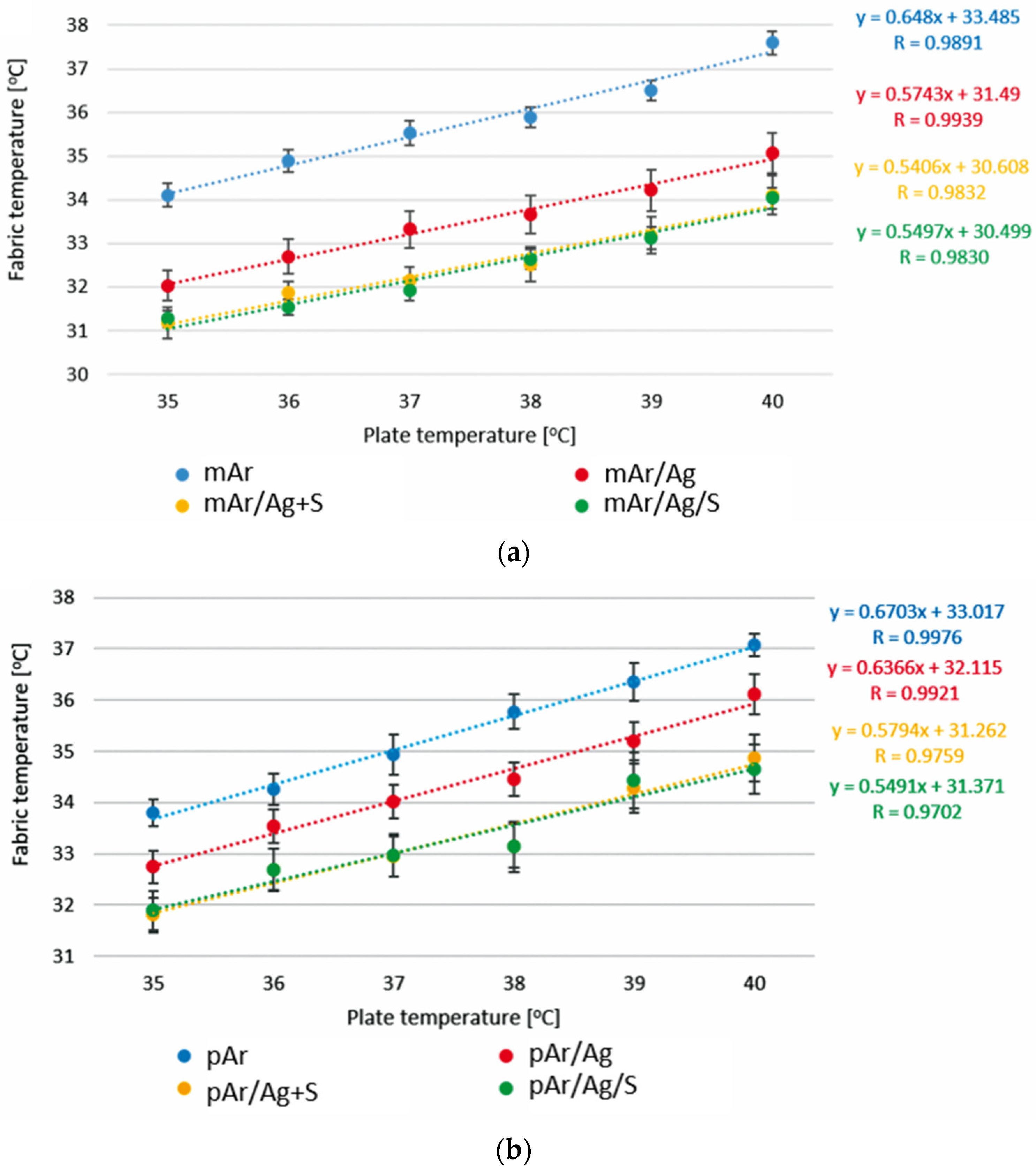
3.6. Active Infrared Thermography
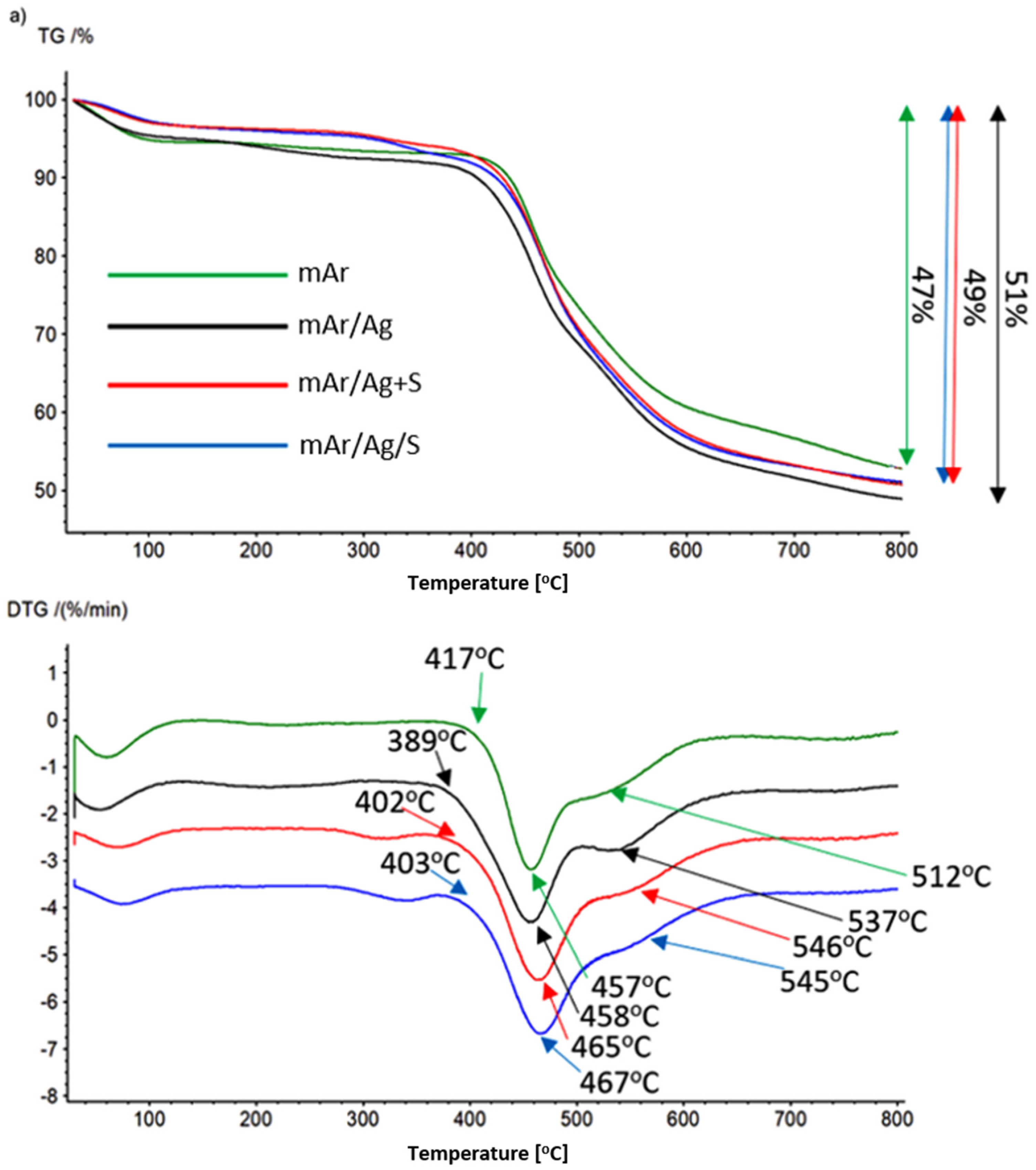
3.7. TG/DTG Analysis
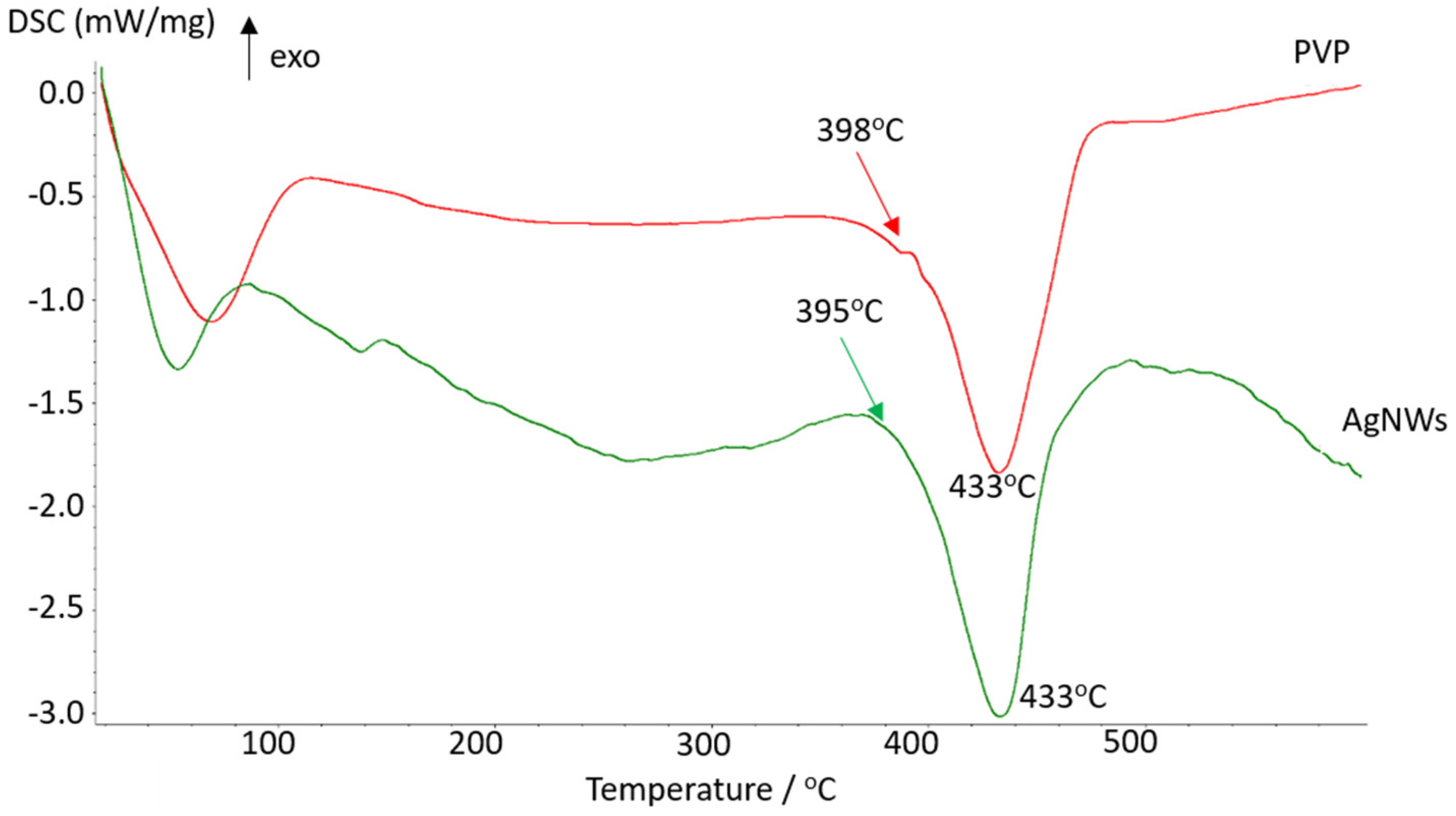
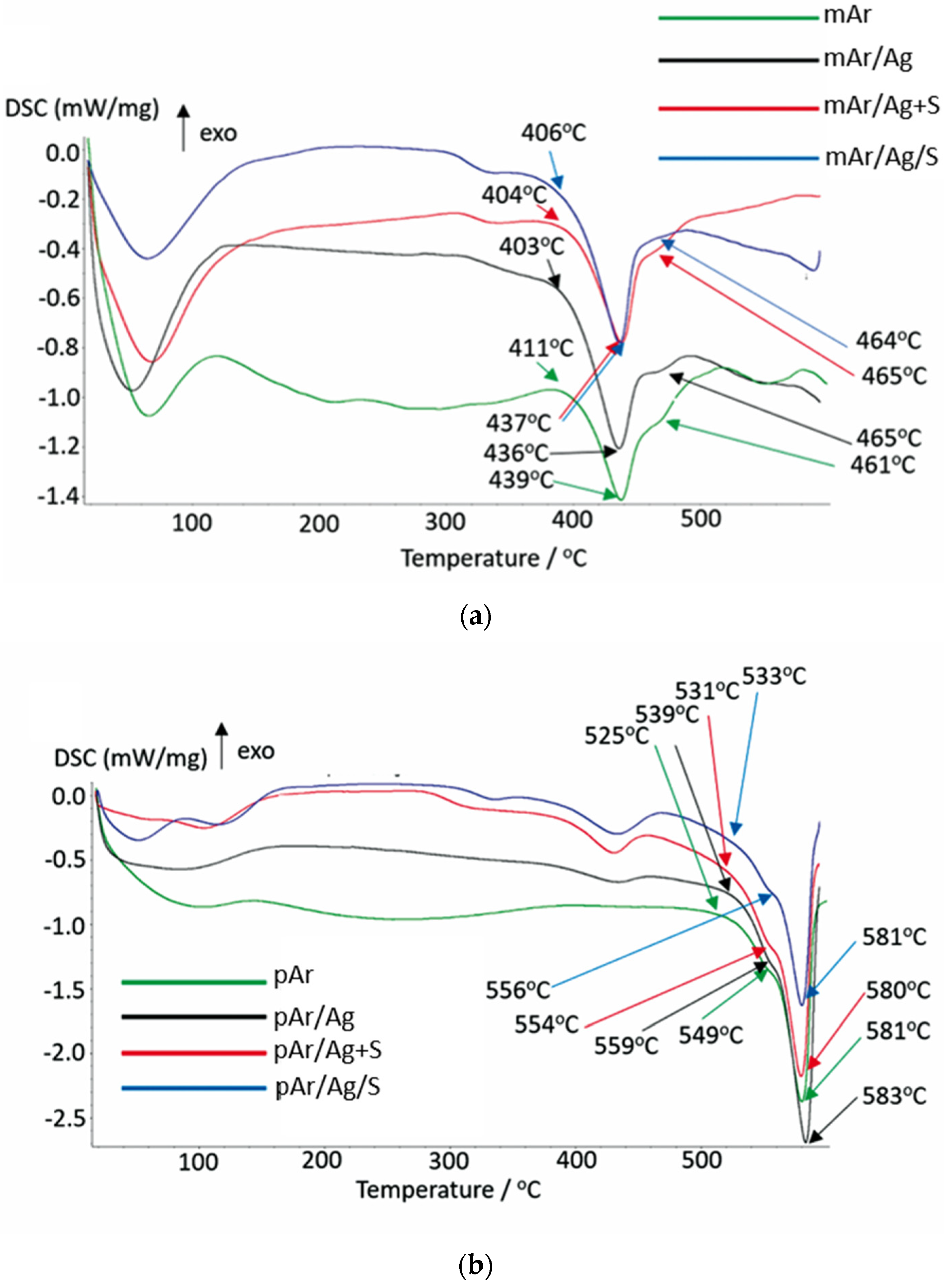
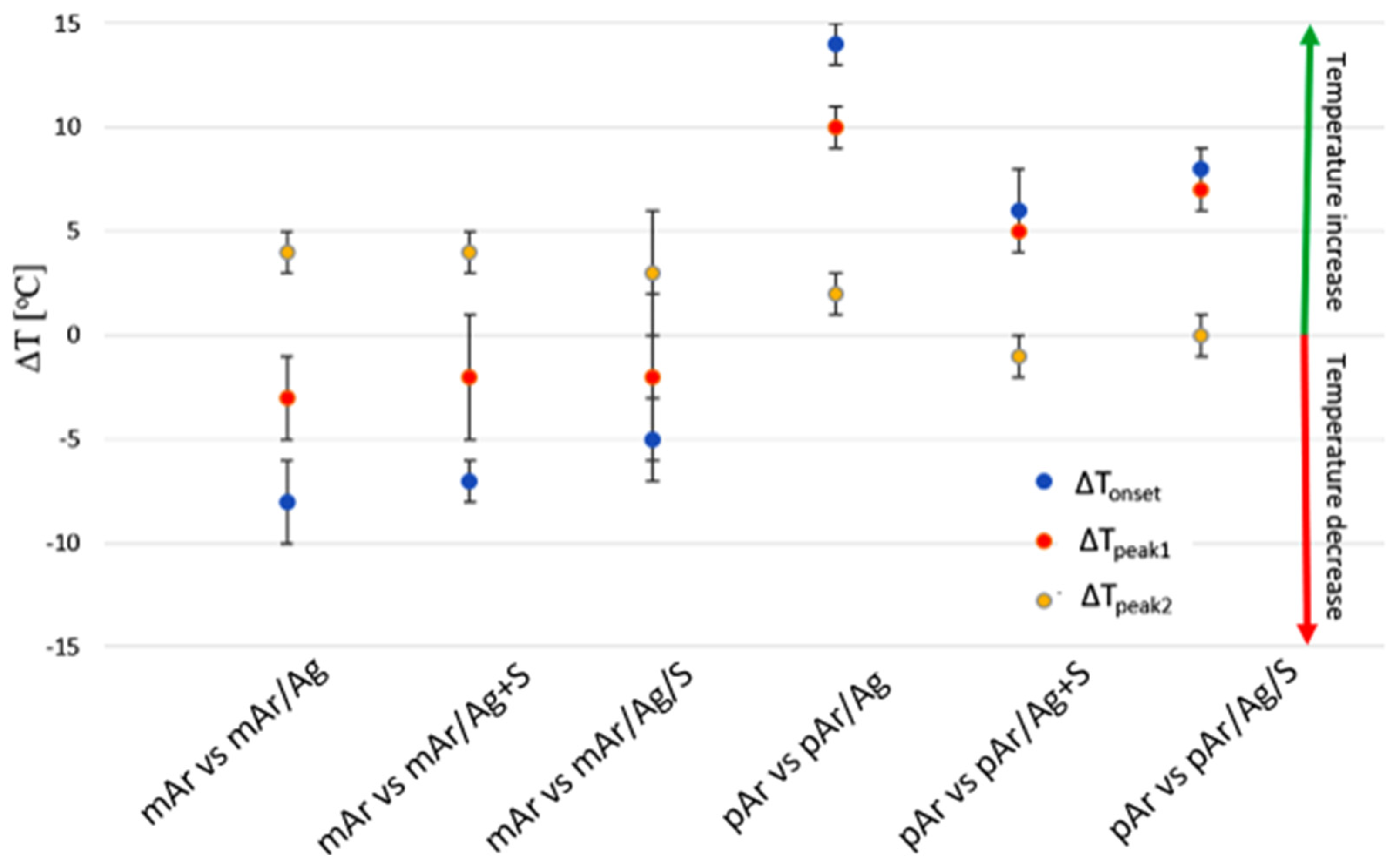
3.8. DSC Analysis
3.9. Antibacterial Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, G.; Ding, D.; Chitrphiromsri, P. Numerical Simulations of Heat and Moisture Transport in Thermal Protective Clothing under Flash Fire Conditions. Int. J. Occup. Saf. Ergon. 2008, 14, 89–106. [Google Scholar] [CrossRef]
- Fu, M.; Weng, W.; Yuan, H. Thermal Insulations of Multilayer Clothing Systems Measured by a Bench Scale Test in Low Level Heat Exposures. Int. J. Cloth. Sci. Technol. 2014, 26, 412–423. [Google Scholar] [CrossRef]
- Naeem, J.; Mazari, A.; Naeem, M.S.; Rasheed, A.; Ahmad, F.; Ahmad, S. Durability of Metallic Coating for Improvement of Thermal Protective Performance of Firefighter Protective Clothing. J. Text. Inst. 2022, 114, 820–833. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Organic Phase Change Materials and Their Textile Applications: An Overview. Thermochim. Acta 2012, 540, 7–60. [Google Scholar] [CrossRef]
- Balamurugan, M.; Saravanan, S.; Soga, T. Coating of Green-Synthesized Silver Nanoparticles on Cotton Fabric. J. Coat. Technol. Res. 2017, 14, 735–745. [Google Scholar] [CrossRef]
- Giesz, P.; Mackiewicz, E.; Nejman, A.; Celichowski, G.; Cieślak, M. Investigation on Functionalization of Cotton and Viscose Fabrics with AgNWs. Cellulose 2017, 24, 409–422. [Google Scholar] [CrossRef]
- Jones, R.S.; Draheim, R.R.; Roldo, M. Silver Nanowires: Synthesis, Antibacterial Activity and Biomedical Applications. Appl. Sci. 2018, 8, 673. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ma, J.; Jo, S.; Lee, S.; Kim, C.S. Enhancement of Antibacterial Properties of a Silver Nanowire Film via Electron Beam Irradiation. ACS Appl. Bio Mater. 2020, 3, 2117–2124. [Google Scholar] [CrossRef]
- Kiran Kumar, A.B.V.; Wan Bae, C.; Piao, L.; Kim, S.H. Silver Nanowire Based Flexible Electrodes with Improved Properties: High Conductivity, Transparency, Adhesion and Low Haze. Mater. Res. Bull. 2013, 48, 2944–2949. [Google Scholar] [CrossRef]
- Kaikanov, M.; Kemelbay, A.; Amanzhulov, B.; Demeuova, G.; Akhtanova, G.; Bozheyev, F.; Tikhonov, A. Electrical Conductivity Enhancement of Transparent Silver Nanowire Films on Temperature-Sensitive Flexible Substrates Using Intense Pulsed Ion Beam. Nanotechnology 2021, 32, 145706–145715. [Google Scholar] [CrossRef]
- Nejman, A.; Baranowska-Korczyc, A.; Ranoszek-Soliwoda, K.; Jasińska, I.; Celichowski, G.; Cieślak, M. Silver Nanowires and Silanes in Hybrid Functionalization of Aramid Fabrics. Molecules 2022, 27, 1952. [Google Scholar] [CrossRef]
- Tomiyama, T.; Mukai, I.; Yamazaki, H.; Takeda, Y. Optical Properties of Silver Nanowire/Polymer Composite Films: Absorption, Scattering, and Color Difference. Opt. Mater. Express 2020, 10, 3202–3214. [Google Scholar] [CrossRef]
- Luu, Q.N.; Doorn, J.M.; Berry, M.T.; Jiang, C.; Lin, C.; May, P.S. Preparation and Optical Properties of Silver Nanowires and Silver-Nanowire Thin Films. J. Colloid Interface Sci. 2011, 356, 151–158. [Google Scholar] [CrossRef]
- Nateghi, M.R.; Shateri-Khalilabad, M. Silver Nanowire-Functionalized Cotton Fabric. Carbohydr Polym. 2015, 117, 160–168. [Google Scholar] [CrossRef]
- Baranowska-Korczyc, A.; Mackiewicz, E.; Ranoszek-Soliwoda, K.; Nejman, A.; Trasobares, S.; Grobelny, J.; Cieślak, M.; Celichowski, G. A SnO2 Shell for High Environmental Stability of Ag Nanowires Applied for Thermal Management. RSC Adv. 2021, 11, 4174–4185. [Google Scholar] [CrossRef]
- Hsu, P.C.; Liu, X.; Liu, C.; Xie, X.; Lee, H.R.; Welch, A.J.; Zhao, T.; Cui, Y. Personal Thermal Management by Metallic Nanowire-Coated Textile. Nano Lett. 2015, 15, 365–371. [Google Scholar] [CrossRef]
- PN-P-04613:1997; Textiles—Knitted and Stitch Bonded Fabrics—Determination of Mass per Unit Length and Mass per Unit Area. Polish Standardization Committee: Warsaw, Poland, 1997.
- PN-EN ISO 5089:1999; Textiles—Determination of Thickness of Textiles and Textile Products. Polish Standardization Committee: Warsaw, Poland, 1999.
- PN-EN ISO 9237:1998; Textiles—Determination of Permeability of Fabrics to Air. Polish Standardization Committee: Warsaw, Poland, 1998.
- AATCC 100-2012; Assessment of Antibacterial Finishes on Textile Materials. American Association of Textile Chemists & Colorists: Research Triangle Park, NC, USA, 2012.
- PN-EN ISO 20743; Determination of the Antibacterial Activity of Finished Products with Antibacterial Finish. Absorption Method. Polish Standardization Committee: Warsaw, Poland, 2021.
- Lee, C.Y.; Van Le, Q.; Kim, C.; Kim, S.Y. Use of Silane-Functionalized Graphene Oxide in Organic Photovoltaic Cells and Organic Light-Emitting Diodes. Phys. Chem. Chem. Phys. 2015, 17, 9369–9374. [Google Scholar] [CrossRef]
- Girardi, F.; Bergamonti, L.; Isca, C.; Predieri, G.; Graiff, C.; Lottici, P.P.; Cappelletto, E.; Ataollahi, N.; Di Maggio, R. Chemical–Physical Characterization of Ancient Paper with Functionalized Polyamidoamines (PAAs). Cellulose 2017, 24, 1057–1068. [Google Scholar] [CrossRef]
- Wang, C.B.; Deo, G.; Wachs, I.E. Interaction of Polycrystalline Silver with Oxygen, Water, Carbon Dioxide, Ethylene, and Methanol: In Situ Raman and Catalytic Studies. J. Phys. Chem. B 1999, 103, 5645–5656. [Google Scholar] [CrossRef]
- Jena, K.K.; Alhassan, S.M.; Tiwari, A.; Hihara, L.H. Functional Nano-Coating Materials by Michael Addition and Ring-Opening Polymerization: Reactivity, Molecular Architecture and Refractive Index. Sci. Rep. 2018, 8, 11912. [Google Scholar] [CrossRef]
- Lian, Y.; Yu, H.; Wang, M.; Yang, X.; Li, Z.; Yang, F.; Wang, Y.; Tai, H.; Liao, Y.; Wu, J.; et al. A Multifunctional Wearable E-Textile via Integrated Nanowire-Coated Fabrics. J. Mater. Chem. C. 2020, 8, 8399–8409. [Google Scholar] [CrossRef]
- Liu, H.; Lee, Y.Y.; Norsten, T.B.; Chong, K. In Situ Formation of Anti-Bacterial Silver Nanoparticles on Cotton Textiles. J. Ind. Text. 2014, 44, 198–210. [Google Scholar] [CrossRef]
- Usmanova, L.S.; Ziganshin, M.A.; Rakipov, I.T.; Lyadov, N.M.; Klimovitskii, A.E.; Mukhametzyanov, T.A.; Gerasimov, A.V. Microspherical Particles of Solid Dispersion of Polyvinylpyrrolidone K29-32 for Inhalation Administration. Biomed Res. Int. 2018, 2018, 2412156. [Google Scholar] [CrossRef] [PubMed]
- Zhenhua, S.; Yanfen, Z.; Wenyue, L.; Shaojuan, C.; Shihua, Y.; Jianwei, M. Preparation of Silver-Plated Para-Aramid Fiber by Employing Low-Temperarure Oxygen Plasm Treatment and Dopamine Functionalization. Coatings 2019, 9, 559. [Google Scholar]
- Morsi, M.A.; Abdelrazek, E.M.; Abdelghany, A.M.; Badr, S.I. Morphological, Thermal and Electrical Properties of (PEO/PVP)/Au Nanocomposite Before and After Gamma-Irradiation. J. Res. Updat. Polym. Sci. 2017, 6, 45–54. [Google Scholar]
- Amato, E.; Diaz-Fernandez, Y.A.; Taglietti, A.; Pallavicini, P.; Pasotti, L.; Cucca, L.; Milanese, C.; Grisoli, P.; Dacarro, C.; Fernandez-Hechavarria, J.M.; et al. Synthesis, Characterization and Antibacterial Activity against Gram Positive and Gram Negative Bacteria of Biomimetically Coated Silver Nanoparticles. Langmuir 2011, 27, 9165–9173. [Google Scholar] [CrossRef]
- Cieślak, M.; Kowalczyk, D.; Krzyżowska, M.; Janicka, M.; Witczak, E.; Kamińska, I. Effect of Cu Modified Textile Structures on Antibacterial and Antiviral Protection. Materials 2022, 15, 6164. [Google Scholar] [CrossRef]








| Woven Fabric Parameter | mAr | pAr | |
|---|---|---|---|
| Linear mass of yarn, tex | 25 × 2 | 20 × 2 | |
| Weave | Plain | Plain | |
| Thread number/10 cm | Warp | 230 | 240 |
| Weft | 160 | 150 | |
| Mass per unit area, g/m2 | 205 ± 2 | 165 ± 3 | |
| Thickness, mm | 0.56 ± 0.01 | 0.42 ± 0.01 | |
| Air permeability, mm/s | 299 ± 18 | 610 ± 18 | |
| Volume porosity, % | 73.27 | 72.72 |
| Mass per Unit Area, g/m2 | ||||
|---|---|---|---|---|
| Fabric Symbol | Modified Fabric | Ag Coating | Silanes (S) Coating | Ag/S and Ag + S Coating |
| mAr/Ag | 248 ± 7 | 43 ± 2 | ||
| mAr/Ag + S | 273 ± 7 | 68 ± 5 | ||
| mAr/Ag/S | 305 ± 9 | 43 ± 2 | 62 ± 1 | 105 ± 3 |
| pAr/Ag | 200 ± 1 | 35 ± 3 | ||
| pAr/Ag + S | 222 ± 5 | 57 ± 2 | ||
| pAr/Ag/S | 263 ± 4 | 35 ± 3 | 63 ± 5 | 98 ± 6 |
| mAr/Ag | mAr/Ag + S | mAr/Ag/S | ||||
|---|---|---|---|---|---|---|
| S. aureus | K. pneumoniae | S. aureus | K. pneumoniae | S. aureus | K. pneumoniae | |
| Concentration of inoculum, CFU/mL | 1.8 × 105 | 1.5 × 105 | 1.8 × 105 | 1.5 × 105 | 1.8 × 105 | 1.5 × 105 |
| Reduction in bacterial growth, R | 90.9% | 99.9% | 90.0% | 90.0% | 90.0% | 90.0% |
| Value of the bacteriostatic coefficient, S * | 7.7 | 7.8 | 7.7 | 7.8 | 8.1 | 8.3 |
| Value of the bactericidal coefficient, L ** | 3.1 | 3.2 | 3.1 | 3.2 | 3.2 | 3.4 |
| pAr/Ag | pAr/Ag + S | pAr/Ag/S | ||||
| S. aureus | K. pneumoniae | S. aureus | K. pneumoniae | S. aureus | K. pneumoniae | |
| Concentration of inoculum, CFU/mL | 1.8 × 105 | 1.5 × 105 | 1.8 × 105 | 1.5 × 105 | 1.8 × 105 | 1.5 × 105 |
| Reduction in bacterial growth, R | 72.0% | 99.9% | 90.0% | 95.7% | 90.0% | 90.0% |
| Value of the bacteriostatic coefficient, S * | 5.5 | 7.9 | 7.7 | 7.8 | 8.1 | 8.3 |
| Value of the bactericidal coefficient, L ** | 0.6 | 3.0 | 3.1 | 3.2 | 3.2 | 3.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nejman, A.; Baranowska-Korczyc, A.; Celichowski, G.; Cieślak, M. AgNWs–Silane Coatings for the Functionalization of Aramid Woven Fabrics. Coatings 2023, 13, 1852. https://doi.org/10.3390/coatings13111852
Nejman A, Baranowska-Korczyc A, Celichowski G, Cieślak M. AgNWs–Silane Coatings for the Functionalization of Aramid Woven Fabrics. Coatings. 2023; 13(11):1852. https://doi.org/10.3390/coatings13111852
Chicago/Turabian StyleNejman, Alicja, Anna Baranowska-Korczyc, Grzegorz Celichowski, and Małgorzata Cieślak. 2023. "AgNWs–Silane Coatings for the Functionalization of Aramid Woven Fabrics" Coatings 13, no. 11: 1852. https://doi.org/10.3390/coatings13111852







