Preparation and Applications of Superhydrophobic Coatings on Aluminum Alloy Surface for Anti-Corrosion and Anti-Fouling: A Mini Review
Abstract
:1. Introduction
2. Coating Types on the Surface of Aluminum Alloy for Anti-Corrosion and Anti-Fouling
2.1. Chemical Conversion Film Coatings
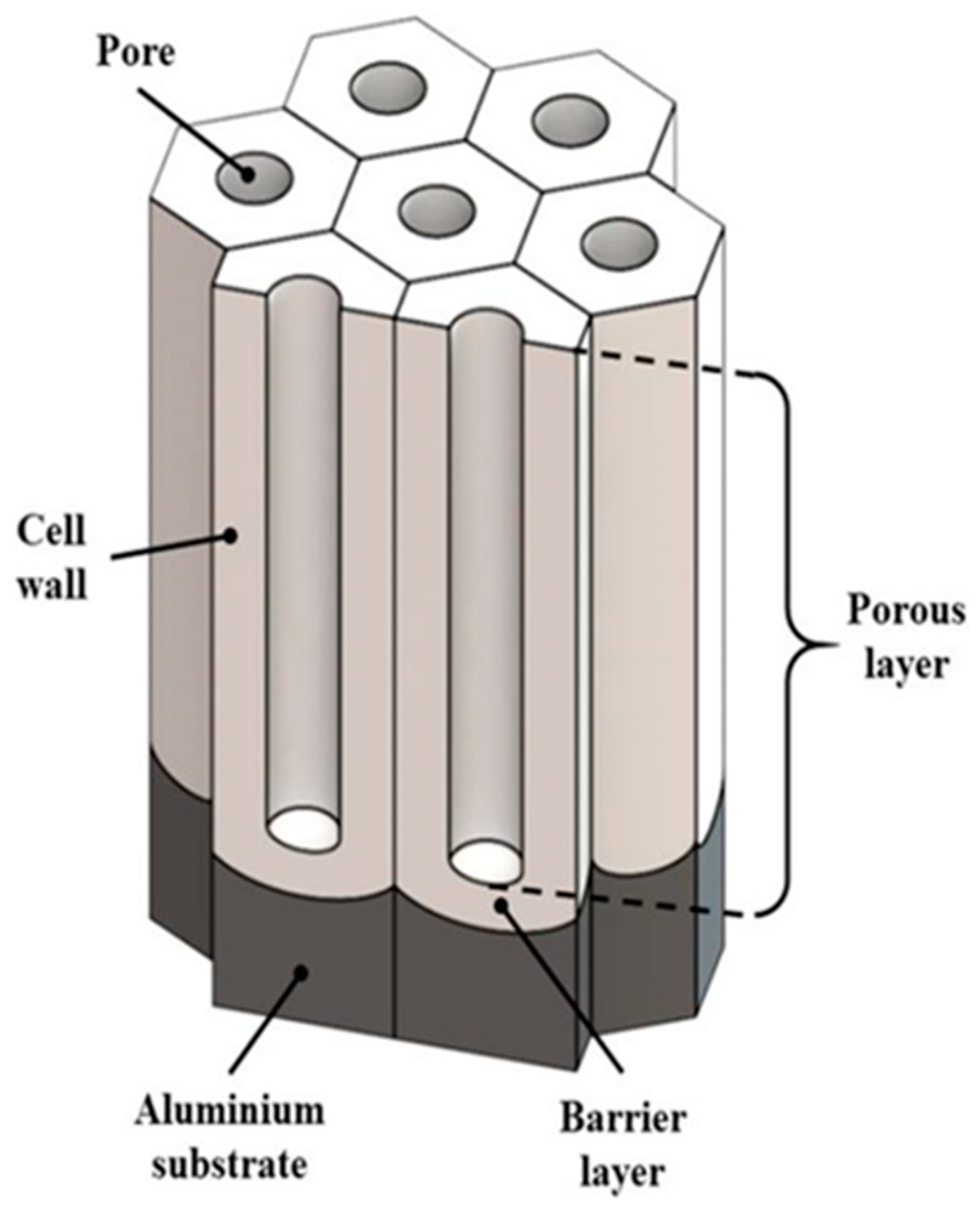
2.2. Anodizing Film Coatings
2.3. Organic Painting Coatings
2.4. Superhydrophobic Coatings
3. Preparation Methods for Superhydrophobic Coating on the Surface of Aluminum Alloy for Anti-Corrosion and Anti-Fouling
3.1. Impregnation Method
3.2. Spraying Method
3.3. Anodization Method
3.4. Plasma Electrolytic Oxidation Method
3.5. Electrodeposition Method
3.6. Other Methods
4. Application of Superhydrophobic Coating on the Surface of Aluminum Alloy for Anti-Corrosion and Anti-Fouling
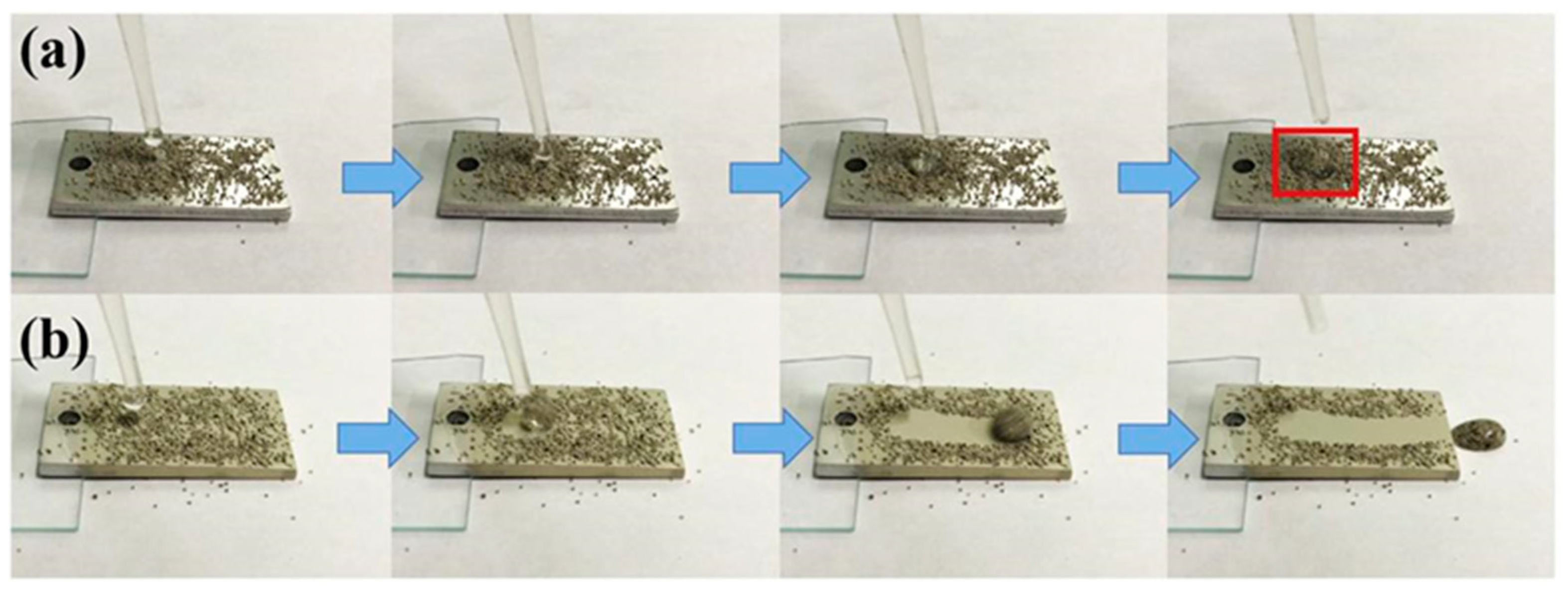
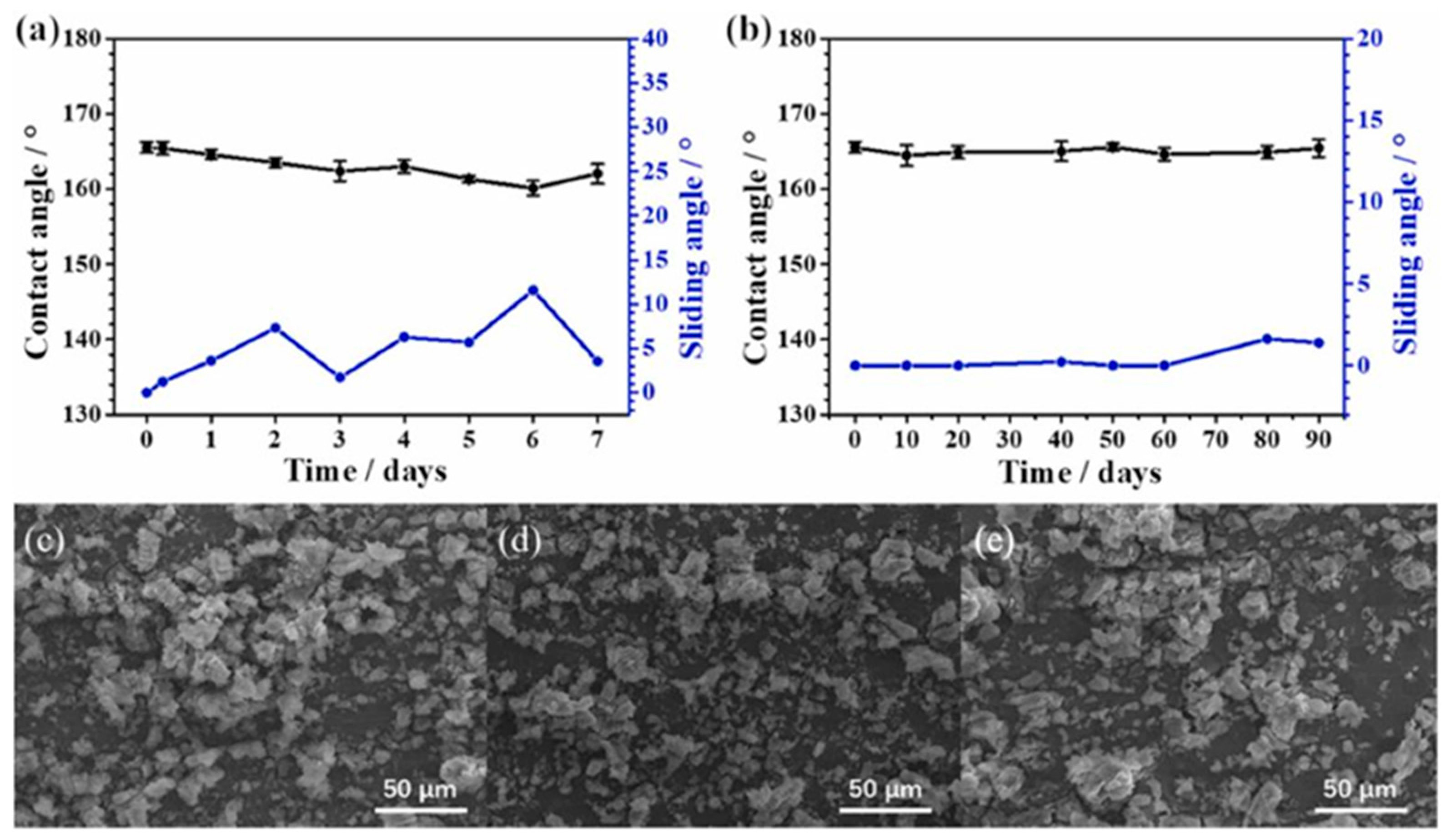
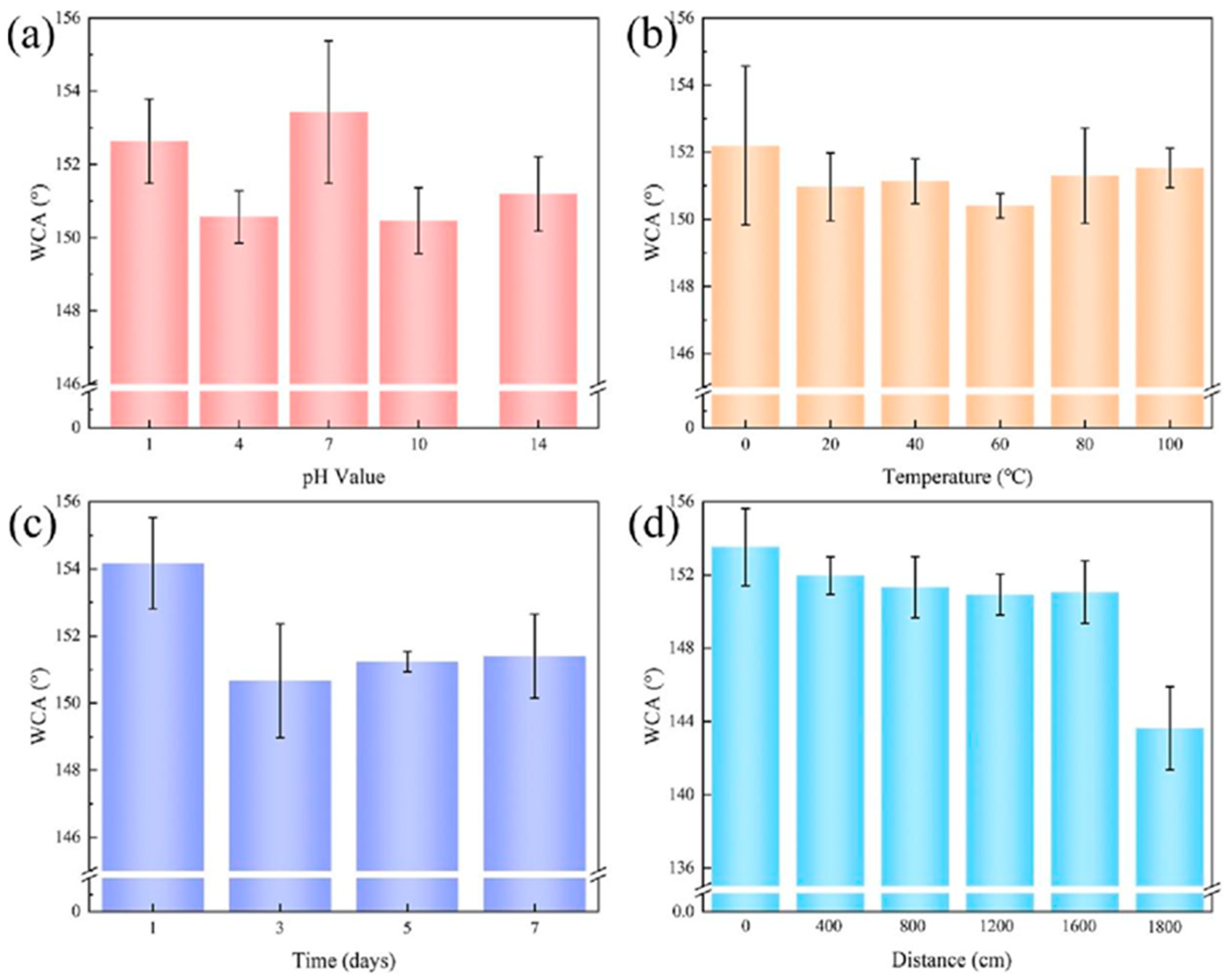
4.1. Application Status and Problems of Superhydrophobic Coating on Aluminum Alloy Surface for Anti-Corrosion and Anti-Fouling
4.2. Methods for Improving the Performance of Superhydrophobic Coating on the Surface of Aluminum Alloy for Anti-Corrosion and Anti-Fouling
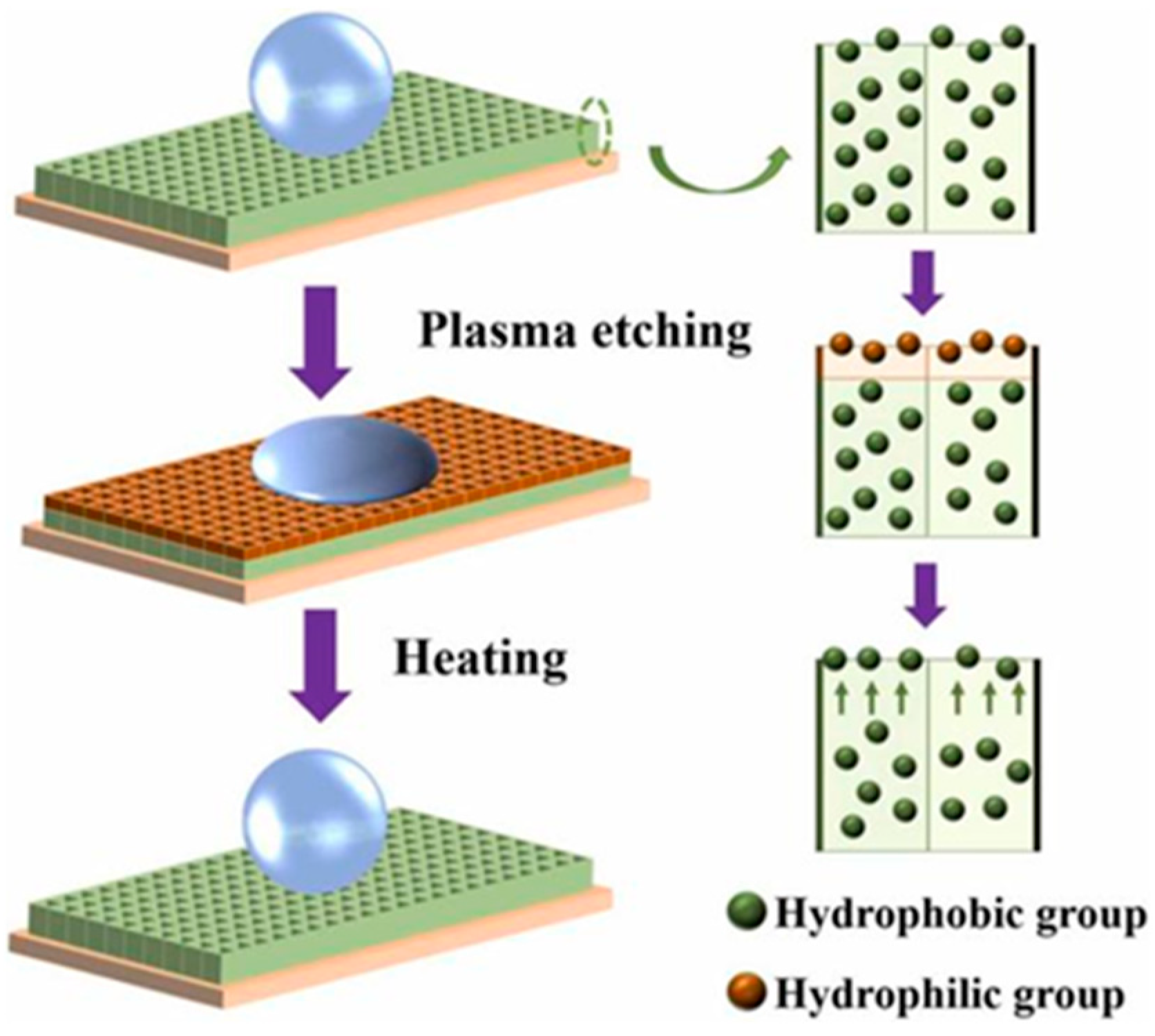
4.2.1. Improve the Preparation Process of Superhydrophobic Coating on the Surface of Aluminum Alloy
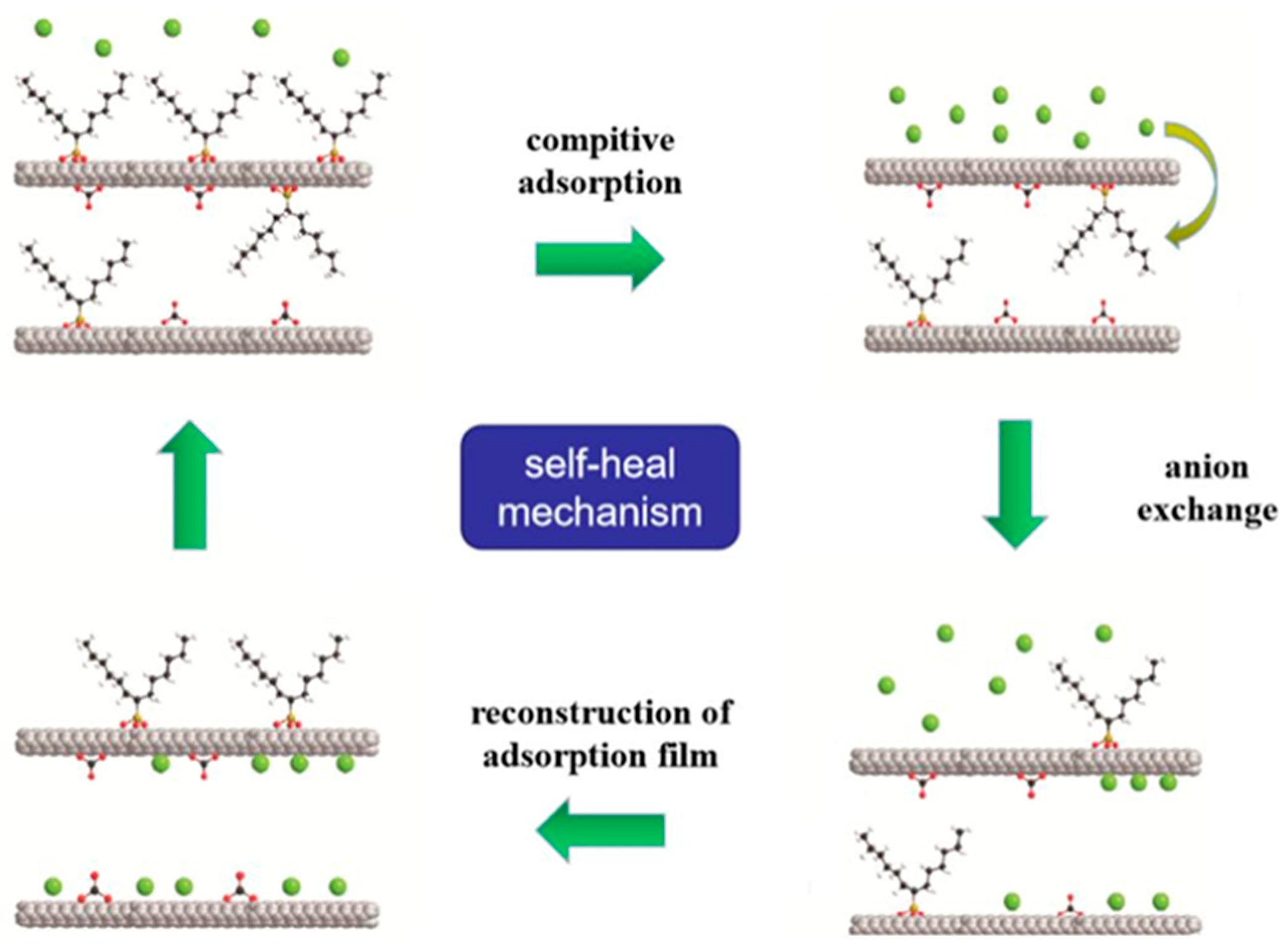
4.2.2. Preparation of Superhydrophobic Coatings with Self-Healing Properties
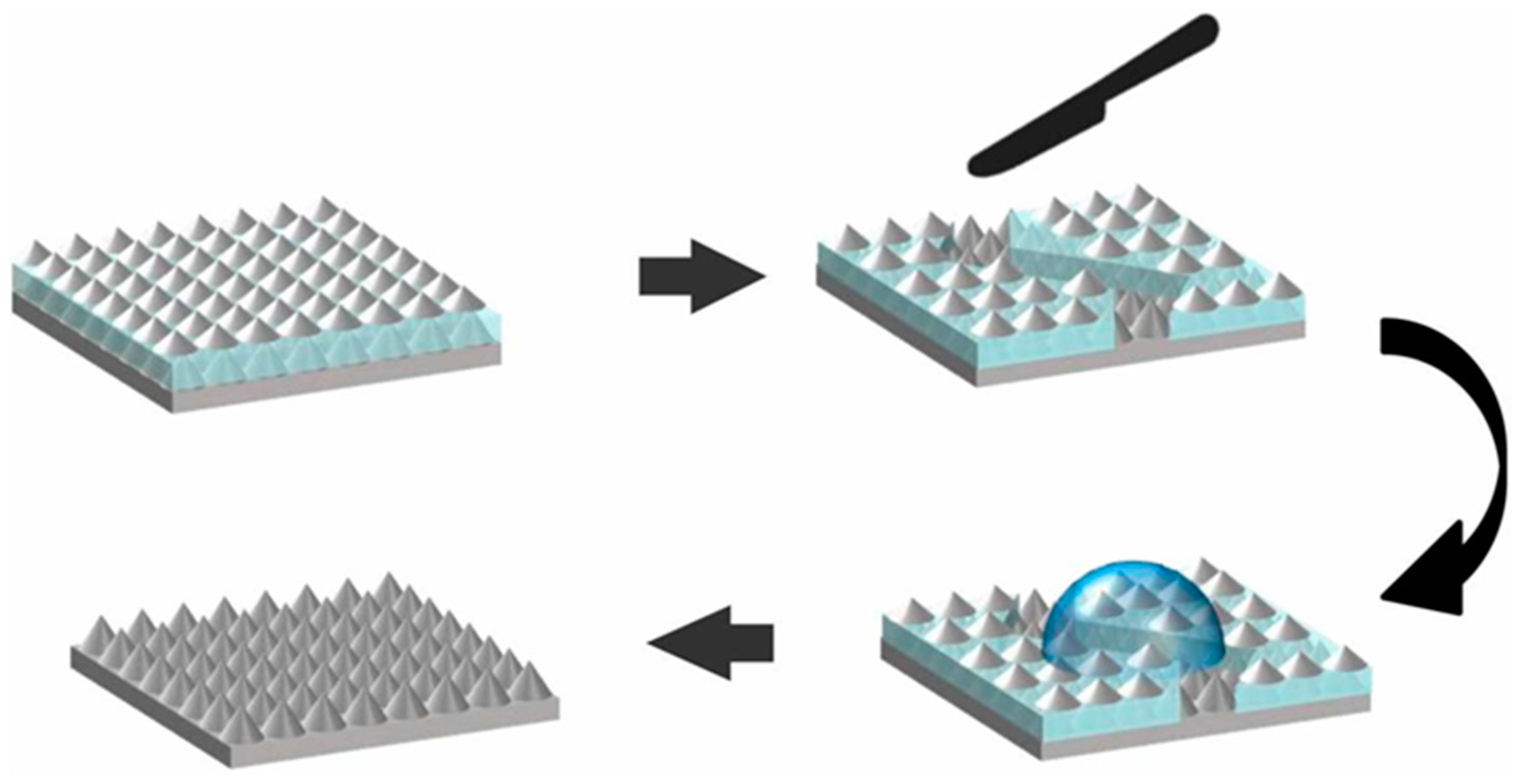
4.2.3. Introduction of Buffer or Sacrificial Layer into a Superhydrophobic Coating
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Cai, Y.; Liu, S.; Su, Z.; Jiang, T. Life cycle assessment of aluminum-silicon alloy production from secondary aluminum in China. J. Clean. Prod. 2023, 392, 136214. [Google Scholar]
- Raabe, D.; Ponge, D.; Uggowitzer, P.J.; Roscher, M.; Paolantonio, M.; Liu, C.; Antrekowitsch, H.; Kozeschnik, E.; Seidmann, D.; Gault, B.; et al. Making sustainable aluminum by recycling scrap: The science of “dirty” alloys. Prog. Mater. Sci. 2022, 128, 100947. [Google Scholar]
- Georgantzia, E.; Gkantou, M.; Kamaris, G.S. Aluminium alloys as structural material: A review of research. Eng. Struct. 2021, 227, 111372. [Google Scholar]
- Chang, L.; Yuan, S.; Huang, X.; Cai, Z. Determination of Johnson-Cook damage model for 7xxx laminated aluminum alloy and simulation application. Mater. Today Commun. 2023, 34, 105224. [Google Scholar]
- Chen, B.; Han, X. Corrosion and Protection of Aluminum Alloys. Constr. Eng. Technol. Des. 2018, 25, 23–27. [Google Scholar]
- Fakhri, M.; Rezaee, B.; Pakzad, H.; Moosavi, A. Facile, scalable, and low-cost superhydrophobic coating for frictional drag reduction with anti-corrosion property. Tribol. Int. 2023, 178, 108091. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Tran, H.N.; Plamondon, C.O.; Tuduri, L.; Vo, D.-V.N.; Nanda, S.; Mishra, A.; Chao, H.-P.; Bajpai, A.K. Recent progress in the preparation, properties and applications of superhydrophobic nano-based coatings and surfaces: A review. Prog. Org. Coat. 2019, 132, 235–256. [Google Scholar]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of superhydrophobic coatings as a corrosion barrier: A review. Surf. Coat. Technol. 2018, 341, 40–56. [Google Scholar]
- Zhang, B.; Xu, W.; Zhu, Q.; Sun, Y.; Li, Y. Mechanically robust superhydrophobic porous anodized AA5083 for marine corrosion protection. Corros. Sci. 2019, 158, 108083. [Google Scholar] [CrossRef]
- Abbasi, S.; Nouri, M.; Sabour Rouhaghdam, A. A novel combined method for fabrication of stable corrosion resistance superhydrophobic surface on Al alloy. Corros. Sci. 2019, 159, 108144. [Google Scholar]
- Ma, J.; Chang, M.L.; He, H.Y.; Wei, H.Y.; Huang, Y.C.; Du, X.Q.; Chen, D.C. Corrosion Resistance of Li-Al LDHs Film Modified by Methionine for 6063 Al Alloy in 3.5 wt.% NaCl Solution. Coatings 2022, 12, 507. [Google Scholar] [CrossRef]
- Becker, M. Chromate-free chemical conversion coatings for aluminum alloys. Corros. Rev. 2019, 37, 321–342. [Google Scholar]
- Liang, G.; Zhu, S.; Wang, W.Y.; Wang, X.M.; Han, G.F.; Ren, Z.Q. Research Status and Development Trend of Aluminum Alloy Anti-corrosion Technology. Mater. Rep. 2020, 34, 429–436. [Google Scholar]
- Wang, W.Q.; Zuo, H.Y.; Yang, H.; Ma, Y.L. Research and Development Status of Trivalent Chromium Conversion Coatings on Aluminum Alloys. J. Chongqing Univ. Technol. 2021, 35, 81–94. [Google Scholar]
- Guo, B.; Li, D.D.; Shu, J.J.; Zhang, Z.; Liu, L.X.; Wang, Y.; Liu, X.S. Research Progressin Preparation Technology of Chemical Conversion Coatingon Aluminum Alloy Surface. Mater. Prot. 2021, 54, 106–113. [Google Scholar]
- Peltier, F.; Thierry, D. Review of Cr-Free Coatings for the Corrosion Protection of Aluminum Aerospace Alloys. Coatings 2022, 12, 518. [Google Scholar] [CrossRef]
- Kim, M.M.; Kapun, B.; Tiringer, U.; Sekularac, G.; Milosev, I. Protection of Aluminum Alloy 3003 in Sodium Chloride and Simulated Acid Rain Solutions by Commercial Conversion Coatings Containing Zr and Cr. Coatings 2019, 9, 563. [Google Scholar] [CrossRef]
- Kharitonov, D.S.; Sommertune, J.; Örnek, C.; Ryl, J.; Kurilo, I.I.; Claesson, P.M.; Pan, J. Corrosion inhibition of aluminium alloy AA6063-T5 by vanadates: Local surface chemical events elucidated by confocal Raman micro-spectroscopy. Corros. Sci. 2019, 148, 237–250. [Google Scholar] [CrossRef]
- Huang, S.; Wang, J.; Wei, X.; Zhou, Y.; Wang, L.; Zhang, J. Microstructural characterization and film-forming mechanism of a phosphate chemical conversion ceramic coating prepared on the surface of 2A12 aluminum alloy. RSC Adv. 2019, 9, 18767–18775. [Google Scholar] [CrossRef] [PubMed]
- Mamizadeh Janqour, L.; Sarabi, A.A. Optimization of coating process parameters and surface characterization for vanadium-based conversion coating on 2024 aluminum alloy. Prog. Org. Coat. 2019, 133, 33–43. [Google Scholar] [CrossRef]
- Niu, Y.S.; Yao, X.Y.; Li, Y.L.; Jiang, Y.Y.; Li, Y.L. Progress of Cerium-based Conversion Coating on Aluminum Alloy Surface. Mater. Rep. 2021, 35, 15169–15174. [Google Scholar]
- Valdez, B.; Kiyota, S.; Stoytcheva, M.; Zlatev, R.; Bastidas, J.M. Cerium-based conversion coatings to improve the corrosion resistance of aluminium alloy 6061-T6. Corros. Sci. 2014, 87, 141–149. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Sun, L.Y.; Barrett, A.T.; Fedel, M. Layered Double Hydroxide Protective Films Developed on Aluminum and Aluminum Alloys: Synthetic Methods and Anti-Corrosion Mechanisms. Coatings 2020, 10, 428. [Google Scholar] [CrossRef]
- Bouali, A.C.; Serdechnova, M.; Blawert, C.; Tedim, J.; Ferreira, M.G.S.; Zheludkevich, M.L. Layered double hydroxides (LDHs) as functional materials for the corrosion protection of aluminum alloys: A review. Appl. Mater. Today 2020, 21, 100857. [Google Scholar] [CrossRef]
- He, Q.-Q.; Zhou, M.-J.; Hu, J.-M. Electrodeposited Zn-Al layered double hydroxide films for corrosion protection of aluminum alloys. Electrochim. Acta 2020, 355, 136796. [Google Scholar] [CrossRef]
- Araujo, J.V.D.; da Silva, R.M.P.; Klumpp, R.E.; Costa, I. The anodizing process of aluminum and its alloys: A historical and electrochemical approach. Quim. Nova 2021, 44, 999–1011. [Google Scholar]
- Scampone, G.; Timelli, G. Anodizing Al–Si Foundry Alloys: A Critical Review. Adv. Eng. Mater. 2022, 24, 2101480. [Google Scholar] [CrossRef]
- Pashchanka, M. Conceptual Progress for Explaining and Predicting Self-Organization on Anodized Aluminum Surfaces. Nanomaterials 2021, 11, 2271. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, D.Z.; Pan, X.Z.; Xie, G.J.; Zhao, T.; Wang, Z.; Mao, Z.G.; Ding, Y.H. Mechanism, application, and research progress of sealing technology for anodic oxide films on aluminum and its alloys. Electroplat. Finish. 2022, 41, 1305–1312. [Google Scholar]
- Khan, M.F.; Kumar, A.M.; Ul-Hamid, A.; Al-Hems, L.M. Achieving non-adsorptive anodized film on Al-2024 alloy: Surface and electrochemical corrosion investigation. Surf. Interfaces 2019, 15, 78–88. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, G.; Zhang, Z.; Wang, Y. Research on corrosion resistance of anodized and sealed 6061 aluminum alloy in 3.5% sodium chloride solution. Int. J. Electrochem. Sci. 2023, 18, 100092. [Google Scholar] [CrossRef]
- Deyab, M.A. Anti-corrosion properties of nanocomposites coatings: A critical review. J. Mol. Liq. 2020, 313, 113533. [Google Scholar] [CrossRef]
- Song, W.; Zhao, X.; Jin, Z.; Fan, L.; Ji, X.; Deng, J.; Duan, J. Poly(vinyl alcohol) for multi-functionalized corrosion protection of metals: A review. J. Clean. Prod. 2023, 394, 136390. [Google Scholar] [CrossRef]
- Olajire, A.A. Recent advances on organic coating system technologies for corrosion protection of offshore metallic structures. J. Mol. Liq. 2018, 269, 572–606. [Google Scholar] [CrossRef]
- Rivero, G.; Fasce, L.A.; Ceré, S.M.; Manfredi, L.B. Furan resins as replacement of phenolic protective coatings: Structural, mechanical and functional characterization. Prog. Org. Coat. 2014, 77, 247–256. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, T.; He, Y.; Wang, Y.; Bi, Y. Corrosion and aging of organic aviation coatings: A review. Chin. J. Aeronaut. 2023, 36, 1–35. [Google Scholar] [CrossRef]
- Hussain, A.K.; Seetharamaiah, N.; Pichumani, M.; Chakra, C.S. Research progress in organic zinc rich primer coatings for cathodic protection of metals—A comprehensive review. Prog. Org. Coat. 2021, 153, 106040. [Google Scholar] [CrossRef]
- Ge, X.; Fan, W.; Tang, H.; Yang, J.; Ding, R.; Zhao, X. Anticorrosion performance of an eco-friendly coating system including an epoxy tie primer with aluminum tripolyphosphates and a polyurethane topcoat for marine aluminum alloy. Prog. Org. Coat. 2023, 174, 107294. [Google Scholar] [CrossRef]
- Gad, S.M.; Zhou, X.; Lyon, S.B.; Emad, S. Inhibition mechanism of anticorrosion pigments leached from organic coatings: Comparison between salt spray and immersion testing. Prog. Org. Coat. 2023, 174, 107266. [Google Scholar] [CrossRef]
- Kotrikla, A. Environmental management aspects for TBT antifouling wastes from the shipyards. J. Environ. Manag. 2009, 90, S77–S85. [Google Scholar] [CrossRef]
- Bagley, F.; Atlar, M.; Charles, A.; Anderson, C. The use of copper-based antifoulings on aluminium ship hulls. Ocean Eng. 2015, 109, 595–602. [Google Scholar] [CrossRef]
- Wang, Z.H.; Cong, W.W.; Zhang, K.; Gui, T.J. Research and Development of Environmental Friendly Strategies of Marine Anti-fouling Coatings. Mater. Rep. 2022, 36, 480–485. [Google Scholar]
- Sha, J.; Yu, J.; Chen, R.; Liu, Q.; Liu, J.; Zhu, J.; Liu, P.; Li, R.; Wang, J. Eco-friendly self-polishing anti-fouling coating via eugenol ester hydrolysis. Prog. Org. Coat. 2022, 172, 107077. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, L.; Mou, J.; Wu, D.; Xu, M.; Zhou, P.; Ren, Y. Research Strategies to Develop Environmentally Friendly Marine Antifouling Coatings. Mar. Drugs 2020, 18, 371. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, J.; Chen, H.; Chen, D. Progress of marine biofouling and anti-fouling technologies. Chin. Sci. Bull. 2010, 56, 598–612. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D.; Jiang, Y.; Gao, C.; Liu, L.; Liu, Y. A silicone coating containing natural borneol fluorinated side chains with excellent static anti-fouling properties. Eur. Polym. J. 2023, 193, 112064. [Google Scholar] [CrossRef]
- Liu, W.; Wang, S.; Wang, G.; Zhang, J.; Zhou, C. Investigation on the differences of surface cleaning properties of series of superhydrophobic aluminum alloys. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129614. [Google Scholar] [CrossRef]
- Cao, H. Low adhesive and superhydrophobic LDH coating for anti-corrosion and self-cleaning. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129893. [Google Scholar] [CrossRef]
- Li, Y.; Si, W.; Gao, R. Facile preparation of superamphiphobic aluminum alloy surfaces and their corrosion resistance. Surf. Coat. Technol. 2022, 430, 127997. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Zhang, J. Bioinspired one step hydrothermal fabricated superhydrophobic aluminum alloy with favorable corrosion resistance. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124469. [Google Scholar] [CrossRef]
- Rasitha, T.P.; Vanithakumari, S.C.; Nanda Gopala Krishna, D.; George, R.P.; Srinivasan, R.; Philip, J. Facile fabrication of robust superhydrophobic aluminum surfaces with enhanced corrosion protection and antifouling properties. Prog. Org. Coat. 2022, 162, 106560. [Google Scholar] [CrossRef]
- Hooda, A.; Goyat, M.S.; Pandey, J.K.; Kumar, A.; Gupta, R. A review on fundamentals, constraints and fabrication techniques of superhydrophobic coatings. Prog. Org. Coat. 2020, 142, 105557. [Google Scholar] [CrossRef]
- Ma, M.; Cao, Y.; Wang, Q.; Shang, W.; Peng, N.; Wen, Y. Study on the forming mechanism and corrosion resistance of doping graphene composite film on the 6061 Al alloy. J. Ind. Eng. Chem. 2022, 107, 483–492. [Google Scholar] [CrossRef]
- Kumar, A.; Gogoi, B. Development of durable self-cleaning superhydrophobic coatings for aluminium surfaces via chemical etching method. Tribol. Int. 2018, 122, 114–118. [Google Scholar] [CrossRef]
- Lomga, J.; Varshney, P.; Nanda, D.; Satapathy, M.; Mohapatra, S.S.; Kumar, A. Fabrication of durable and regenerable superhydrophobic coatings with excellent self-cleaning and anti-fogging properties for aluminium surfaces. J. Alloys Compd. 2017, 702, 161–170. [Google Scholar] [CrossRef]
- Peng, H.; Yang, H.; Shi, T.; Liu, Y.; Li, Z.; Ma, X.; Liu, X. Novel superhydrophobic polystyrene microspheres/polydimethylsiloxane coating on aluminum alloy with excellent anti-freezing and self-cleaning performances. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130660. [Google Scholar] [CrossRef]
- Gong, A.; Zheng, Y.; Yang, Z.; Guo, X.; Gao, Y.; Li, X. Spray fabrication of superhydrophobic coating on aluminum alloy for corrosion mitigation. Mater. Today Commun. 2021, 26, 101828. [Google Scholar] [CrossRef]
- Zang, J.; Yu, S.; Zhu, G.; Zhou, X. Fabrication of superhydrophobic surface on aluminum alloy 6061 by a facile and effective anodic oxidation method. Surf. Coat. Technol. 2019, 380, 125078. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, R.; Long, F.; Li, X.; Zhou, T.; Hu, W.; Liu, L. The long-term degradation behavior of the durable superhydrophobic coating on Al matrix. Surf. Coat. Technol. 2022, 434, 128203. [Google Scholar] [CrossRef]
- Ao, N.; Liu, D.; Zhang, X.; He, G. Microstructural characteristics of PEO coating: Effect of surface nanocrystallization. J. Alloys Compd. 2020, 823, 153823. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Molaei, M.; Nouri, M.; Babaei, K. Review of the role of graphene and its derivatives in enhancing the performance of plasma electrolytic oxidation coatings on titanium and its alloys. Appl. Surf. Sci. Adv. 2021, 6, 100140. [Google Scholar] [CrossRef]
- Fu, J.; Sun, Y.; Ji, Y.; Zhang, J. Fabrication of robust ceramic based superhydrophobic coating on aluminum substrate via plasma electrolytic oxidation and chemical vapor deposition methods. J. Mater. Process. Technol. 2022, 306, 117641. [Google Scholar] [CrossRef]
- Yang, C.; Cui, S.; Weng, Y.; Wu, Z.; Liu, L.; Ma, Z.; Tian, X.; Fu, R.K.Y.; Chu, P.K.; Wu, Z. Scalable superhydrophobic T-shape micro/nano structured inorganic alumina coatings. Chem. Eng. J. 2021, 409, 128142. [Google Scholar] [CrossRef]
- Yang, H.; He, Y.; Wu, Z.; Miao, J.; Yang, F.; Lu, Z. Fabrication of a superhydrophobic and high-glossy copper coating on aluminum substrates. Appl. Surf. Sci. 2018, 433, 1192–1196. [Google Scholar] [CrossRef]
- Zhao, Q.; Tang, T.; Wang, F. Fabrication of Superhydrophobic AA5052 Aluminum Alloy Surface with Improved Corrosion Resistance and Self Cleaning Property. Coatings 2018, 8, 390. [Google Scholar] [CrossRef]
- Xiong, J.; Sarkar, D.K.; Chen, X.G. Superhydrophobic honeycomb-like cobalt stearate thin films on aluminum with excellent anti-corrosion properties. Appl. Surf. Sci. 2017, 407, 361–370. [Google Scholar] [CrossRef]
- Xu, N.; Sarkar, D.K.; Chen, X.G.; Tong, W.P. Corrosion performance of superhydrophobic nickel stearate/nickel hydroxide thin films on aluminum alloy by a simple one-step electrodeposition process. Surf. Coat. Technol. 2016, 302, 173–184. [Google Scholar] [CrossRef]
- Jiang, J.; Shen, Y.; Wang, Z.; Tao, J.; Liu, W.; Chen, H.; Liu, S.; Xie, X.; Zeng, C. Anti/de-icing performance of the one-step electrodeposited superhydrophobic surfaces: Role of surface polarity regulated by hydrocarbon radical length. Chem. Eng. J. 2022, 431, 133276. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Wu, Y.; Zhu, B.; Song, H. One-step method for fabrication of bioinspired hierarchical superhydrophobic surface with robust stability. Appl. Surf. Sci. 2019, 473, 493–499. [Google Scholar] [CrossRef]
- Liu, E.; Zhu, G.; Dai, P.; Liu, L.; Yu, S.; Wang, B.; Xiong, W. Preparation of self-healing Ni-Al layered double hydroxide superhydrophobic coating with nanowall arrays on aluminum alloy. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129916. [Google Scholar] [CrossRef]
- Peng, H.; Yang, H.; Ma, X.; Shi, T.; Li, Z.; Xue, S.; Wang, Q. In situ fabrication of flower-like ZnO on aluminum alloy surface with superhydrophobicity. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128800. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Z. Insitu growth of durable superhydrophobic Mg–Al layered double hydroxides nanoplatelets on aluminum alloys for corrosion resistance. J. Alloys Compd. 2018, 767, 382–391. [Google Scholar] [CrossRef]
- Zeng, Q.; Min, X.; Luo, Z.; Dai, H.; Liao, B. In-situ preparation of superhydrophobic Zn-Al layered double hydroxide coatings for corrosion protection of aluminum alloy. Mater. Lett. 2022, 328, 133077. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Z.; Zhang, Z.; Xin, Y.; Fujita, T.; Wei, Y. In situ one-step fabrication of superhydrophobic layered double hydroxide on Al alloys for anti-corrosion. Appl. Surf. Sci. 2022, 593, 153400. [Google Scholar] [CrossRef]
- Lee, J.-W.; Hwang, W. Exploiting the silicon content of aluminum alloys to create a superhydrophobic surface using the sol–gel process. Mater. Lett. 2016, 168, 83–85. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, G.; Tong, Q.; Yang, W.; Hao, W. Fluorine-free superhydrophobic coatings from polydimethylsiloxane for sustainable chemical engineering: Preparation methods and applications. Chem. Eng. J. 2021, 426, 130829. [Google Scholar] [CrossRef]
- Ivvala, J.; Arora, H.S.; Grewal, H.S. Towards development of sustainable metallic superhydrophobic materials. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131047. [Google Scholar] [CrossRef]
- Shakourian, M.; Rahemi Ardekani, S.; Bayat, A.; Saievar-Iranizad, E.; Deferme, W. Ultrasonic atomization based fabrication of superhydrophobic and corrosion-resistant hydrolyzed MTMS/PVDF coatings. JCIS Open 2022, 7, 100059. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, Y. Preparation of superhydrophobic composite coating on 2024 aluminum alloy and its stability and corrosion resistance. Int. J. Electrochem. Sci. 2023, 18, 100088. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, Y.; Xu, S.; Jin, T.; Wei, D.; Ouyang, J.; Jia, D.; Zhou, Y. Superhydrophobic double-layer coating for efficient heat dissipation and corrosion protection. Chem. Eng. J. 2019, 362, 638–649. [Google Scholar] [CrossRef]
- Fu, J.; Sun, Y.; Wang, J.; Zhang, H.; Zhang, J.; Ji, Y. Fabrication of Fluorine-Free Superhydrophobic Surface on Aluminum Substrate for Corrosion Protection and Drag Reduction. J. Mar. Sci. Eng. 2023, 11, 520. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, H.; Wang, Z.; Liu, Y. Superhydrophobic aluminum alloy surface: Fabrication, structure, and corrosion resistance. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 319–325. [Google Scholar] [CrossRef]
- da Silva, R.G.C.; Malta, M.I.C.; de Carvalho, L.A.P.; da Silva, J.J.; da Silva Filho, W.L.C.; Oliveira, S.H.; de Araújo, E.G.; Urtiga Filho, S.L.; Vieira, M.R.S. Low-cost superhydrophobic coating on aluminum alloy with self-cleaning and repellency to water-based mixed liquids for anti-corrosive applications. Surf. Coat. Technol. 2023, 457, 129293. [Google Scholar] [CrossRef]
- Su, W.; Lu, X.; Shu, Y.; Liu, X.; Gao, W.; Yao, J.; Niu, Z.; Xie, Y. Robust, Superhydrophobic Aluminum Fins with Excellent Mechanical Durability and Self-Cleaning Ability. Micromachines 2023, 14, 704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Li, X.-D.; Wang, N.; Liu, Y.-J.; Tian, F.; Wang, C.-X. Robust superhydrophobic SiO2/epoxy composite coating prepared by one-step spraying method for corrosion protection of aluminum alloy: Experimental and theoretical studies. Mater. Des. 2023, 228, 111833. [Google Scholar] [CrossRef]
- Aparna, A.; Sethulekshmi, A.S.; Saritha, A.; Joseph, K. Recent advances in superhydrophobic epoxy based nanocomposite coatings and their applications. Prog. Org. Coat. 2022, 166, 106819. [Google Scholar] [CrossRef]
- Raj Sharma, A.; Arora, H.S.; Grewal, H.S. Nano sponge like metal-based coating with self-regenerative superhydrophobicity using simple flame spraying. Mater. Lett. 2023, 334, 133709. [Google Scholar] [CrossRef]
- Liu, S.; Wang, C.; Li, K.; Wang, Z.; Luo, H.; Fan, W.; Wang, H. Simple spray method preparation of slow-release porous microcapsule for long-term active anti-corrosion and scale-inhibiting coatings. Prog. Org. Coat. 2022, 162, 106589. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, K.; Xu, G.; Yang, C.; Wang, D. Facile preparation of super-oleophobic TiO2/SiO2 composite coatings by spraying method. Prog. Org. Coat. 2021, 159, 106411. [Google Scholar] [CrossRef]
- Dalawai, S.P.; Saad Aly, M.A.; Latthe, S.S.; Xing, R.; Sutar, R.S.; Nagappan, S.; Ha, C.-S.; Kumar Sadasivuni, K.; Liu, S. Recent Advances in durability of superhydrophobic self-cleaning technology: A critical review. Prog. Org. Coat. 2020, 138, 105381. [Google Scholar] [CrossRef]
- Chen, G.H.; Wang, G.W.; Song, D. Research Progress of Preparation Technology of Superhydrophobic Surface on Magnesium Alloys. Mater. Prot. 2022, 55, 134–140. [Google Scholar]
- Zhao, M.R.; Zhou, H.Y.; Kang, W.Q.; Huang, Y.G.; Zheng, Y.L. Comparison of methods for fabricating superhydrophobic surface. Acta Mater. Compos. Sin. 2021, 38, 361–379. (In Chinese) [Google Scholar]
- Esmaeili, M.; Asgari, M.; Daneshmand, H.; Karimi, M.; Rouhaghdam, A.S. Effect of anode position on the incorporation of nano/microparticles during the PEO coating on AZ31B. Appl. Surf. Sci. Adv. 2021, 6, 100147. [Google Scholar] [CrossRef]
- Saji, V.S. Superhydrophobic surfaces and coatings by electrochemical anodic oxidation and plasma electrolytic oxidation. Adv. Colloid Interface Sci. 2020, 283, 102245. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fang, X.; Zhu, C.; Xing, X.; Cui, G.; Li, Z. Fabrication of superhydrophobic coatings for corrosion protection by electrodeposition: A comprehensive review. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125498. [Google Scholar] [CrossRef]
- Noorbakhsh Nezhad, A.H.; Mohammadi Zahrani, E.; Alfantazi, A.M. Erosion-corrosion of electrodeposited superhydrophobic Ni-Al2O3 nanocomposite coatings under jet saline-sand slurry impingement. Corros. Sci. 2022, 197, 110095. [Google Scholar] [CrossRef]
- Wang, T.; Guo, Q.; Zhang, T.C.; Zhang, Y.-X.; Yuan, S. Large-scale prepared superhydrophobic HDTMS-modified diatomite/epoxy resin composite coatings for high-performance corrosion protection of magnesium alloys. Prog. Org. Coat. 2022, 170, 106999. [Google Scholar] [CrossRef]
- Ge-Zhang, S.; Yang, H.; Ni, H.; Mu, H.; Zhang, M. Biomimetic superhydrophobic metal/nonmetal surface manufactured by etching methods: A mini review. Front. Bioeng. Biotechnol. 2022, 10, 958095. [Google Scholar] [CrossRef]
- Dang-Sheng, X.; Wei, T. Bioinspired Superhydrophobic Progress and Recent Advances of Its Functional Application. J. Inorg. Mater. 2019, 34, 5–1144. [Google Scholar]
- Wan, T.; Wang, B.; Han, Q.; Chen, J.; Li, B.; Wei, S. A review of superhydrophobic shape-memory polymers: Preparation, activation, and applications. Appl. Mater. Today 2022, 29, 101665. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Yu, X.F.; Wang, H.T.; Shi, X.T.; Feng, L.B. Research progress in preparation and properties of superhydrophobic surface on metal substrates. J. Mater. Eng. 2019, 47, 26–33. [Google Scholar]
- Fan, H.-Q.; Lu, P.; Zhu, X.; Behnamian, Y.; Li, Q. Development of superhydrophobic and corrosion resistant coatings on carbon steel by hydrothermal treatment and fluoroalkyl silane self-assembly. Mater. Chem. Phys. 2022, 290, 126569. [Google Scholar] [CrossRef]
- Wang, D.F.; Li, X.L.; Xie, W.L. Research progress on superhydrophobic coatings on magnesium alloys. Light Alloy Fabr. Technol. 2021, 49, 4–12. [Google Scholar]
- Zou, R.Q.; Tang, J.B.; Zhang, X.; Wang, J. Superhydrophobicity and Rapid Rebounding Induced via a Unique Nonfluorinated Aluminum-Based Multiscale Multilayer Nickel "Trampoline" Structure. ACS Appl. Mater. Interfaces 2020, 12, 58412–58427. [Google Scholar] [CrossRef]
- Huang, Y.; Sarkar, D.K.; Chen, X.G. Fabrication of Superhydrophobic Surfaces on Aluminum Alloy Via Electrodeposition of Copper Followed by Electrochemical Modification. Nano-Micro Lett. 2011, 3, 160–165. [Google Scholar] [CrossRef]
- Ye, Y.; Kang, Z.; Wang, F.; Long, Y.; Guo, T.; Chen, D.; Kong, J.; Xu, L. Achieving hierarchical structure with superhydrophobicity and enhanced anti-corrosion via electrochemical etching and chemical vapor deposition. Appl. Surf. Sci. 2023, 610, 155362. [Google Scholar] [CrossRef]
- Gupta, R.; Verma, R.; Kango, S.; Constantin, A.; Kharia, P.; Saini, R.; Kudapa, V.K.; Mittal, A.; Prakash, J.; Chamoli, P. A critical review on recent progress, open challenges, and applications of corrosion-resistant superhydrophobic coating. Mater. Today Commun. 2023, 34, 105201. [Google Scholar] [CrossRef]
- George, J.S.; Vijayan, P.P.; Hoang, A.T.; Kalarikkal, N.; Nguyen-Tri, P.; Thomas, S. Recent advances in bio-inspired multifunctional coatings for corrosion protection. Prog. Org. Coat. 2022, 168, 106858. [Google Scholar] [CrossRef]
- Lv, Z.; Yu, S.; Song, K.; Zhou, X.; Yin, X. Fabrication of a leaf-like superhydrophobic CuO coating on 6061Al with good self-cleaning, mechanical and chemical stability. Ceram. Int. 2020, 46, 14872–14883. [Google Scholar] [CrossRef]
- Zhang, B.; Guan, F.; Zhao, X.; Zhang, Y.; Li, Y.; Duan, J.; Hou, B. Micro-nano textured superhydrophobic 5083 aluminum alloy as a barrier against marine corrosion and sulfate-reducing bacteria adhesion. J. Taiwan Inst. Chem. Eng. 2019, 97, 433–440. [Google Scholar] [CrossRef]
- Rodič, P.; Milošev, I. One-step ultrasound fabrication of corrosion resistant, self-cleaning and anti-icing coatings on aluminium. Surf. Coat. Technol. 2019, 369, 175–185. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, R.; Long, F.; Zhou, T.; Hu, W.; Liu, L. Durable superhydrophobic coating derived from hard-soft technology with enhanced anticorrosion performance. Corros. Sci. 2021, 193, 109889. [Google Scholar] [CrossRef]
- da Silva, R.G.C.; Vieira, M.R.S.; Malta, M.I.C.; da Silva, C.H.; de Oliveira, S.H.; Filho, S.L.U. Effect of initial surface treatment on obtaining a superhydrophobic surface on 5052 aluminum alloy with enhanced anticorrosion properties. Surf. Coat. Technol. 2019, 369, 311–322. [Google Scholar] [CrossRef]
- Sun, R.; Zhao, J.; Li, Z.; Qin, N.; Mo, J.; Pan, Y.; Luo, D. Robust superhydrophobic aluminum alloy surfaces with anti-icing ability, thermostability, and mechanical durability. Prog. Org. Coat. 2020, 147, 105745. [Google Scholar] [CrossRef]
- Khajavian, E.; Attar, M.R.; Mohammadi Zahrani, E.; Liu, W.; Davoodi, A.; Hosseinpour, S. Tuning surface wettability of aluminum surface and its correlation with short and long term corrosion resistance in saline solutions. Surf. Coat. Technol. 2022, 429, 127950. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Q.; Jiang, Y.; Li, H.; Zhang, B. One-step spraying achieved superhydrophobic fluoroSiO2@epoxy coating with corrosion-wear resistance and anti-wetting stability. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130702. [Google Scholar] [CrossRef]
- Nazeer, A.A.; Madkour, M. Potential use of smart coatings for corrosion protection of metals and alloys: A review. J. Mol. Liq. 2018, 253, 11–22. [Google Scholar] [CrossRef]
- Li, F.; Zhao, M.; Zhan, Y.; Wu, C.; Zhang, Y.; Jiang, X.; Sun, Z. Facile fabrication of novel superhydrophobic Al2O3/polysiloxane hybrids coatings for aluminum alloy corrosion protection. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128444. [Google Scholar] [CrossRef]
- Fan, X.; Song, S.; Shi, Y.; Cai, M.; Huang, Y.; Zhang, B.; Zhu, M. Mechanochemical stable superhydrophobic coating toward lasting corrosion protection. Prog. Org. Coat. 2023, 178, 107478. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, H.; Liu, X. Anti-wetting surfaces with self-healing property: Fabrication strategy and application. J. Ind. Eng. Chem. 2023, 117, 54–69. [Google Scholar] [CrossRef]
- Thakur, A.; Kaya, S.; Kumar, A. Recent Trends in the Characterization and Application Progress of Nano-Modified Coatings in Corrosion Mitigation of Metals and Alloys. Appl. Sci 2023, 13, 730. [Google Scholar] [CrossRef]
- Yin, B.; Wu, C.; Hou, D.; Li, S.; Jin, Z.; Wang, M.; Wang, X. Research and application progress of nano-modified coating in improving the durability of cement-based materials. Prog. Org. Coat. 2021, 161, 106529. [Google Scholar] [CrossRef]
- Liu, G.Y.; Yuan, Y.; Liao, R.J.; Xiang, H.Y.; Wang, L.; Yu, Q.; Zhang, C. Robust and self-healing superhydrophobic aluminum surface with excellent anti-icing performance. Surf. Interfaces 2022, 28, 101588. [Google Scholar] [CrossRef]
- Pan, X.; Luo, X.; Li, J.; Zhang, X.; Yu, X.; Zhou, C.; Chen, B.; Liu, Y. Superhydrophobicity and high corrosion resistance of secondary alkane sulphonate (SAS) modified Li-Al LDH film in-situ grown on aluminum alloy. Corros. Commun. 2023, 9, 27–35. [Google Scholar] [CrossRef]
- Wu, S.; Wu, R.; Liu, R.; Jiang, H.; Chen, Z.; Liu, X. Superhydrophobic surface on Al alloy with self-healing performance via snakeskin-like shedding. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128555. [Google Scholar] [CrossRef]
- Lv, C.; Wang, H.; Liu, Z.; Zhang, W.; Wang, C.; Tao, R.; Li, M.; Zhu, Y. A sturdy self-cleaning and anti-corrosion superhydrophobic coating assembled by amino silicon oil modifying potassium titanate whisker-silica particles. Appl. Surf. Sci. 2018, 435, 903–913. [Google Scholar] [CrossRef]




| The types of Coatings | Characteristics | Advantages | Drawbacks | References |
|---|---|---|---|---|
| Chemical Conversion Film Coatings | A few micrometers in thickness As a connecting layer between the topcoat and the aluminum alloy substrate, it increases the adhesion of the coating. | Low cost Simple production process Wear resistant Good corrosion resistance Suitable for large-scale industrialization | Cr (VI) is poisonous and carcinogenic. Phosphorus-containing wastewater could cause eutrophication of water bodies. | [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25] |
| Anodizing Film Coatings | Thickness in micrometers Composed of alumina in the shape of nano-channels Post-treatment for sealing holes or use with coatings. | Good adhesion on the substrate Suitable for large-scale industrialization | Once the coating is damaged, the substrate is corroded | [26,27,28,29,30,31] |
| Organic Painting Coatings | Consists of three layers, with the layer in contact with the aluminum alloy being a priming coat, the top layer being a topcoat, and the intermediate coat connecting the priming coat and topcoat. Suitable for substrates of any size and shape. | Good weather resistance Anti-fouling and preventing adhesion of marine organisms Suitable for large-scale industrialization | It may degrade in aqueous solution. Environmentally unfriendly Poor adhesion on the substrate | [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] |
| Superhydrophobic coatings | Non-infiltration. Synergistic effect of micro-nano graded rough structure and low surface energy material modification. | Good anti-corrosion and anti-fouling Self-healing | Poor chemical stability Poor durability | [7,8,47,48,49,50,51] |
| Al alloy Type | Preparation Method | Coating Composition | The Performance of the Coating | References | |||
|---|---|---|---|---|---|---|---|
| Water Contact Angle (WCA) | Sliding Angle (SA) | Ecorr (V) | Icorr (A·cm−2) | ||||
| 6061 | Hydrothermal method + impregnation modification | Ni-Al LDHs, Stearic acid (SA) | 162.1 ± 0.4° | 1.9 ± 0.3° | −0.28 | 2.88 × 10−8 | [70] |
| 4037 | Hydrothermal method + impregnation modification | ZnO, Poly-dimethyl-siloxane (PDMS) | 161.1° | 3.3° | −0.59 | 8.12 × 10−8 | [71] |
| 6061 | Hydrothermal method + impregnation modification | Mg-Al LDHs, Triethoxy-1H,1H,2H,2H-trideca-fluoro-n-octylsilane (FAS-13) | 160° | −0.33 | 7.94 × 10−6 | [72] | |
| 3003 | Hydrothermal method+ impregnation modification | Zn-Al LDHs, Cetyl trimethoxy-silane, hexadecyl-trimethoxy-silane | 156.3° | −0.66 | 3.87 × 10−8 | [73] | |
| 6061 | Hydrothermal method | Zn-Al LDHs, Sodium dodecyl sulfate (SDS) | 161° | −0.26 | 2.01 × 10−8 | [74] | |
| 6020 | Shot peening + chemical etching + impregnation modification | Methyl trichlorosilane (MTCS) | 153 ± 2° | 8 ± 2° | [10] | ||
| 5083 | Thermo-mechanical and microwave-assisted hydrothermal processing+ Chemical vapor deposition | 1H,1H,2H,2H-Perfluorooctyltrieth-oxysilane (FOTES) | 162 ± 1° | 1 ± 2° | [77] | ||
| 6061 | Coating method+ Ultrasonic spray hydrolysis deposition | Polyvinylidene fluoride (PVDF), hydrolyzed methyl-trimethoxy-silane (HMTMS) | 167° | 7 ± 1° | −0.61 | 2.87 × 10−8 | [78] |
| 6061 | Anodization + sealing treatment+ impregnation modification | TiO2 nanoparticles, Octadecyl trimethoxy-silane (OTS) | 154.2 ± 1.7° | 8° | −0.348 | 4.78 × 10−11 | [59] |
| 2024 | Anodization + spraying | Triethoxy-silane containing ZnO nanoparticles | 151.2° | 7° | −0.503 | 3.79 × 10−6 | [79] |
| Pure Al | Plasma electrolytic oxidation+ electrodeposition | Al2O3, Cerium hexa-decylate | 165.5° | 5.2° | −0.73 | 1.8 × 10−7 | [80] |
| 6061 | Electrodeposition | Cobalt stearate | 161 ± 1° | 2° | −0.706 | 0.8 × 10−8 | [66] |
| Pure Al | Two-step electrodeposition | SiC particles, Cetyl-trimethyl-cerium, Palmitic acid | 162.3° | 1.5° | −0.755 | 5.224 × 10−9 | [81] |
| 6063 | water treatment + impregnation modification | Stearic acid | 154.1° | −0.96 | 5.01 × 10−5 | [82] | |
| 5052 | chemical etching + Deposition + impregnation modification | Zn-Al LDH, Stearic acid | 164 ± 3° | 1 ± 0.5° | 0.49 | 3.5 × 10−7 | [83] |
| Pure Al | laser etching+ impregnation modification | 1H,1H,2H,2H- Perfluoro-octane triethoxy-silane (PFOTES) | 152.8° | 0.6° | [84] | ||
| Al-Si alloy | Sol-gel process | Heptadecafluoro-1,1,2,2-tetrahydrodecyl trichlorosilane (HDFS) | 166.04° | 8.86° | [75] | ||
| 2024-T3 | Spraying | SiO2 nanoparticles, Dodecyl-trimethoxy-silane, Epoxy resin | 165° | ≈0° | −0.63 | 2.66 × 10−9 | [85] |
| Preparation Method | Advantages | Disadvantages | References |
|---|---|---|---|
| Spraying | Simple one-step preparation; Low cost; Large-scale preparation. | The adhesion of the coating is low, and it is easy to fail after being damaged by external forces, resulting in unsatisfactory durability. Organic coatings are potentially harmful to humans and the environment. | [86,87,88,89] |
| Impregnation | Simple and economical; Suitable for large-scale preparation; Suitable for surfaces of all shapes or sizes; Able to realize one-step preparation. | The uniformity of the coating is uncontrollable; The adhesion of the coating to the substrate is poor, and it is easy to fall off. The use of fluorine-containing organic reagents contradicts environmental protection. | [7,76,90,91] |
| Anodization | Low cost and simple and fast manufacturing; Precise control of surface roughness and nanostructure; Better adhesion to substrates; Not prone to aging and wear. | The prepared film layer has pores; subsequent pore-sealing treatment or modification of low surface energy substances is required. Obtaining a relatively uniform coating is challenging. | [92,93,94] |
| Electrodeposition | Easy control of coating topography and thickness; The process is relatively simple; Suitable for industrial-scale production; Environmentally friendly; Able to realize one-step preparation. | Limited cathode metal area leads to low preparation efficiency; low surface energy substances in the electrolyte are not used efficiently; The coating adhesion is poor, and the surface is easy to wear. | [95,96,97] |
| Chemical etching | The preparation process is simple; Low cost; Able to precisely select the processing area. | The micro-nanostructure of the coating is not easy to control; The solutions or by-products used are not environmentally friendly; Subsequent modification of low surface energy substances is required. | [52,98,99] |
| Laser etching | It is possible to obtain regular and controllable micro-nano structures; The preparation process is environmentally friendly. | Special equipment is required; it is Costly. Subsequent modification with low surface energy substances is needed. | [84,92,100] |
| Hydrothermal method | The microscopic size of the coating is relatively uniform; Able to realize one-step preparation. | High temperature and high-pressure conditions are required; High requirements for preparation equipment. | [8,101,102] |
| Sol-gel process | High temperature and pressure conditions are not required; Suitable for surfaces of different shapes or sizes. | There is thermal cracking behavior, Inaccurate coating thickness, and Expensive and environmentally unfriendly. | [7,75,76,103] |
| The Primary Preparation Process of the Coating | Chemical Stability | Mechanical Durability | References |
|---|---|---|---|
| Sodium hydroxide etching; Lauric acid impregnation modification | WCA dropped to 125.3° after immersion in 5% acetic acid solution for 6 days. | The peel test was carried out with 100 N/m insulation tape, and the coating lost its superhydrophobicity after 15 viscous peels. | [55] |
| Hydrochloric acid etching; Deposition of Zn-Al LDH film; Stearic acid impregnation modification. | Lost superhydrophobicity after 7 days of immersion in 0.6 mol/L NaCl solution; Lost superhydrophobicity after 14 days of immersion in distilled water. | Using 1500 mesh SiC abrasive paper, the coating lost its superhydrophobicity after applying 2 N pressure friction for 250 cm. | [83] |
| Anodizing; electrodeposited TiO2 nanoparticles; Octadecyl trimethoxy-silane impregnation modification | WCA dropped to 141.8° and lost superhydrophobicity after 7 days of immersion in seawater. | [112] | |
| Anodizing; Lauric acid impregnation modification. | Using 2000 mesh SiC sandpaper load 100 g for uniform friction, lost superhydrophobicity when the wear distance is 300 cm. | [58] | |
| Scrub treatment; Hydrochloric acid etching; Stearic acid impregnation modification. | Lost superhydrophobicity after 14 days of immersion in NaCl solution (0.6 mol/L). | [113] | |
| High-speed wire discharge machining; Hydrochloric acid etching; Perfluorooctanoic acid impregnation modification | Lost its superhydrophobicity after rubbing the sample for 260 cm at 20 kPa using 1000 sand-grained sandpaper. Using a 200 g weight drop from a height of 12 cm and hitting the coatings directly at approximately 15.50 cm/s, it lost superhydrophobicity after 35 times. | [114] | |
| Hydrochloric acid etching; Stearic acid impregnation modification. | After 1 day of immersion in 3.5 wt% NaCl solution, WCA < 150°. Lost superhydrophobicity after a 14-h salt spray test. | [115] | |
| Laser etching; 1H, 1H, 2H, 2H- Perfluoro-octyl-triethoxy-silane impregnation modification. | The coating lost superhydrophobicity after rubbing 150 cm with a 1000 mesh sandpaper load of 200 g. | [84] | |
| Spray nano-SiO2 particles and 1H, 1H, 2H, 2H-perfluorodialkyltriethoxysilane modified epoxy resins. | A peel test was performed using 3 M VHB tape, and the coating lost superhydrophobicity at the 40 th peel. The coating lost its superhydrophobicity after wearing 900 cm with a 2000 mesh sandpaper load of 100 g. | [116] | |
| Spray polystyrene microspheres and polydimethylsiloxane. | Heated at 260 °C for 1 h, the coating lost its superhydrophobicity. | [56] | |
| Spray F-SiO2 nanoparticles, epoxy resin, fluoro-silicone paint, and fluorinated polyurethane. | 50 μL of water is used for dripping onto the surface of the coating at a rate of 160 drops per minute from a height of 40 cm, and the coating lost its superhydrophobicity after 2 h. | [57] | |
| Spray SiO2 nanoparticles and epoxy resins modified with dodecyl-trimethoxy-silane. | After 40 h of irradiation under ultraviolet light, the WCA of the coating drops to 149.3°, while after 12 h of irradiation, the SA of the coating increases to 15.7°. | [85] | |
| Plasma oxidation; Chemical vapor deposition of fluorinated SiO2 nanoparticles. | After 9 days of immersion in 3.5 wt% NaCl solution, the coating lost its superhydrophobicity. | After peeling off 20 cycles with 3 M tape, the coating lost its superhydrophobicity. | [62] |
| One-step electrodeposition of Iron (III) chloride hexahydrate and myristic acid. | After rubbing at a constant speed of 60 cm with a load of 40 g using 240 mesh sandpaper, the coating lost its superhydrophobicity. | [69] | |
| Thermomechanical and microwave-assisted hydrothermal treatment; Chemical vapor deposition perfluoro-octyl-triethoxy-silane. | After 6 days of immersion in 3.5 wt% NaCl solution, the coating lost its superhydrophobicity. | [77] | |
| Electrodeposition of SiC particles, trimethyl cerium palmitate, and palmitic acid first; then electrodeposition of palmitic acid. | After 9 days of immersion in 3.5 wt% NaCl solution, the WCA of the coating decreases to 147.5°, while on day 8, the SA of the coating increases to 10.5°. | After rubbing 300 cm with a 50 g weight load of 1000 mesh sandpaper, the WCA of the coating is reduced to 135.4°. A 2 kg steel rod is rolled over the specimen surface, and the coating loses its superhydrophobicity after 18 cycles. | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Du, X.; Liu, Y.; Fang, X.; Chen, D.; Zhang, Z. Preparation and Applications of Superhydrophobic Coatings on Aluminum Alloy Surface for Anti-Corrosion and Anti-Fouling: A Mini Review. Coatings 2023, 13, 1881. https://doi.org/10.3390/coatings13111881
Zhu Q, Du X, Liu Y, Fang X, Chen D, Zhang Z. Preparation and Applications of Superhydrophobic Coatings on Aluminum Alloy Surface for Anti-Corrosion and Anti-Fouling: A Mini Review. Coatings. 2023; 13(11):1881. https://doi.org/10.3390/coatings13111881
Chicago/Turabian StyleZhu, Qianyi, Xiaoqing Du, Yudie Liu, Xuming Fang, Dongchu Chen, and Zhao Zhang. 2023. "Preparation and Applications of Superhydrophobic Coatings on Aluminum Alloy Surface for Anti-Corrosion and Anti-Fouling: A Mini Review" Coatings 13, no. 11: 1881. https://doi.org/10.3390/coatings13111881
APA StyleZhu, Q., Du, X., Liu, Y., Fang, X., Chen, D., & Zhang, Z. (2023). Preparation and Applications of Superhydrophobic Coatings on Aluminum Alloy Surface for Anti-Corrosion and Anti-Fouling: A Mini Review. Coatings, 13(11), 1881. https://doi.org/10.3390/coatings13111881






