A Comprehensive Review of Surface Modification Techniques for Enhancing the Biocompatibility of 3D-Printed Titanium Implants
Abstract
:1. Introduction
| Surface Modification Technology | Advantages | Disadvantages | References |
|---|---|---|---|
| Shot peening/sandblasting | Improves the fatigue and wear resistance of implants. Improves surface hydrophilicity and surface roughness. | Surface has impurities that may cause damage to the surface of the material. | Żebrowski et al., 2019 [23], Bernhardt et al., 2021 [24] |
| LSE | Improved corrosion resistance and mechanical properties, increased surface roughness, and improved biocompatibility and osseointegration. | May lead to surface microcracking and the need to optimize parameters. | Arthur et al., 2023 [25], Simões et al., 2023 [26], Kang et al., 2016 [27] |
| Acid etching | Increasing the surface roughness and improving the surface activity favor the adhesion and growth of osteoblasts and can be used as a pre-treatment. | Time and conditions need to be controlled and over-treatment leads to unstable or damaged surfaces. | Yan et al., 2022 [28], Yu et al., 2020 [29], Ren et al., 2021 [30] |
| Anodization | The formation of an oxide layer to improve osteogenic properties and drug loading to enhance implant biocompatibility. | The high cost of preparation may also affect the mechanical properties of the implant. | Gulati et al. [31], 2017, Maher et al., 2016 [32], Liang et al., 2021 [33], Hunate et al., 2021 [34] |
| EPD | Preparation of the coating on the implant surface results in good surface coverage, more surface material particles, and better coating properties. | Complex coating preparation equipment and processes; the thickness of the coating may not be easily controlled. | Zhao et al., 2022 [35], Teng et al., 2019 [36], Jahanmard et al., 2020 [37], Dian Juliadmi et al., 2020 [38] |
| CVD | It promotes osteoblast adhesion and growth by precisely controlling the composition and structure of the coating, providing strong customization, coating uniformity, and durability. | High cost; gas selection and condition control require precision. | Rifai et al., 2018 [39], Youn et al., 2019 [40] |
| MAO | The formation of a dense oxide film and the loading of drugs to improve surface hardness and abrasion resistance, which are conducive to cell adhesion and the growth of bone tissue towards the implant surface and growth. | The bonding strength between the coating and the substrate material may be insufficient, which will weaken its loading capacity. Treatment parameters are difficult to control accurately and the thickness and nature of the oxide layer may be uneven in different areas. | Kozelskaya et al., 2021 [41], Xiu et al., 2016 [42], Sun et al., 2021 [43], Huang et al., 2021 [44], Hu et al., 2020 [45], Tang et al., 2022 [46] |


2. Physical–Mechanical Methods
2.1. Sandblasting and Shot Peen
2.2. Physical Mechanical Surface Coating Technology
2.3. Laser Surface Engineering
3. Chemical Modification Technology
3.1. Acid Etching
3.2. Anodization
3.3. Microarc Oxidation
3.4. Electrophoretic Deposition
3.5. Chemical Vapor Deposition
4. Bioconvergence Modification Technology
4.1. Antimicrobial Coating
4.2. Other Antibacterial Coatings
4.3. Biologically Active Organic Coatings
4.4. Dopamine Coating
5. Functional Composite Coatings
6. Clinical Significance
7. Future Directions and Challenges
8. Summary
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti Based Biomaterials, the Ultimate Choice for Orthopaedic Implants—A Review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.; Ding, C. Surface Modification of Titanium, Titanium Alloys, and Related Materials for Biomedical Applications. Mater. Sci. Eng. R 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Pałka, K.; Pokrowiecki, R. Porous titanium implants: A review. Adv. Eng. Mater. 2018, 20, 1700648. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological Design and Additive Manufacturing of Porous Metals for Bone Scaffolds and Orthopaedic Implants: A Review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Bahl, S.; Suwas, S.; Chatterjee, K. Comprehensive Review on Alloy Design, Processing, and Performance of β Titanium Alloys as Biomedical Materials. Int. Mater. Rev. 2021, 66, 114–139. [Google Scholar] [CrossRef]
- Avila, J.D.; Stenberg, K.; Bose, S.; Bandyopadhyay, A. Hydroxyapatite Reinforced Ti6Al4V Composites for Load-Bearing Implants. Acta Biomater. 2021, 123, 379–392. [Google Scholar] [CrossRef]
- Markopoulos, A.; Galanis, N.; Karkalos, N.; Manolakos, D. Precision CNC Machining of Femoral Component of Knee Implant: A Case Study. Machines 2018, 6, 10. [Google Scholar] [CrossRef]
- Sidambe, A. Biocompatibility of Advanced Manufactured Titanium Implants—A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef]
- Minto, J.; Zhou, X.; Osborn, J.; Zhang, L.G.; Sarkar, K.R.; Rao, R.D. Three-Dimensional Printing: A Catalyst for a Changing Orthopaedic Landscape. JBJS Rev. 2020, 8, e0076. [Google Scholar] [CrossRef]
- Bak, D. Rapid Prototyping or Rapid Production? 3D Printing Processes Move Industry towards the Latter. Assem. Autom. 2003, 23, 340–345. [Google Scholar] [CrossRef]
- Liaw, C.Y.; Guvendiren, M. Current and Emerging Applications of 3D Printing in Medicine. Biofabrication 2017, 9, 024102. [Google Scholar] [CrossRef] [PubMed]
- Rengier, F.; Mehndiratta, A.; von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.-U.; Giesel, F.L. 3D Printing Based on Imaging Data: Review of Medical Applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, C.; Zhou, Q.; Gong, Y.; Li, R.; Li, C.; Klämpfl, F.; Freund, S.; Wu, X.; Sun, Y.; et al. Laser Beam Melting 3D Printing of Ti6Al4V Based Porous Structured Dental Implants: Fabrication, Biocompatibility Analysis and Photoelastic Study. Sci. Rep. 2017, 7, 45360. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Snis, A.; Matic, A.; Thomsen, P.; Palmquist, A. 3D Printed Ti6Al4V Implant Surface Promotes Bone Maturation and Retains a Higher Density of Less Aged Osteocytes at the Bone-Implant Interface. Acta Biomater. 2016, 30, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of Titanium Surface Modification Techniques and Coatings for Antibacterial Applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Szesz, E.M.; De Souza, G.B.; De Lima, G.G.; Da Silva, B.A.; Kuromoto, N.K.; Lepienski, C.M. Improved Tribo-Mechanical Behavior of CaP-Containing TiO2 Layers Produced on Titanium by Shot Blasting and Micro-Arc Oxidation. J. Mater. Sci. Mater. Med. 2014, 25, 10. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on Titanium and Titanium Based Alloys as Biomaterials for Orthopaedic Applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Faria, D.; Abreu, C.S.; Buciumeanu, M.; Dourado, N.; Carvalho, O.; Silva, F.S.; Miranda, G. Ti6Al4V Laser Surface Preparation and Functionalization Using Hydroxyapatite for Biomedical Applications. J. Biomed. Mater. Res. Part A 2018, 106, 1534–1545. [Google Scholar] [CrossRef]
- Costa Valente, M.L.; Oliveira, T.T.; Kreve, S.; Batalha, R.L.; Oliveira, D.P.; Pauly, S.; Bolfarini, C.; Bachmann, L.; Reis, A.C. Analysis of the Mechanical and Physicochemical Properties of Ti-6Al-4V Discs Obtained by Selective Laser Melting and Subtractive Manufacturing Method. J. Biomed. Mater. Res. Part A 2021, 109, 420–427. [Google Scholar] [CrossRef]
- Abar, B.; Kelly, C.; Pham, A.; Allen, N.; Barber, H.; Kelly, A.; Mirando, A.J.; Hilton, M.J.; Gall, K.; Adams, S.B. Effect of Surface Topography on In Vitro Osteoblast Function and Mechanical Performance of 3D Printed Titanium. J. Biomed. Mater. Res. Part A 2021, 109, 1792–1802. [Google Scholar] [CrossRef]
- Piszczek, P.; Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Sznarkowska, A.; Sadowska, B.; Bartmański, M.; Erdoğan, Y.K.; Ercan, B.; Jędrzejczyk, W. Comprehensive Evaluation of the Biological Properties of Surface-Modified Titanium Alloy Implants. J. Clin. Med. 2020, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Wan, Y.; Zhao, Z.; Wang, T.; Yu, M.; Wang, H.; Fan, S.; Liu, Z.; Liu, C. Polydopamine and Magnesium Ions Loaded 3D-Printed Ti-6Al-4V Implants Coating with Enhanced Osteogenesis and Antibacterial Abilities. Adv. Mater. Technol. 2022, 7, 2200598. [Google Scholar] [CrossRef]
- Żebrowski, R.; Walczak, M.; Korga, A.; Iwan, M.; Szala, M. Effect of Shot Peening on the Mechanical Properties and Cytotoxicity Behaviour of Titanium Implants Produced by 3D Printing Technology. J. Healthc. Eng. 2019, 2019, 8169538. [Google Scholar] [CrossRef]
- Bernhardt, A.; Schneider, J.; Schroeder, A.; Papadopoulous, K.; Lopez, E.; Brückner, F.; Botzenhart, U. Surface Conditioning of Additively Manufactured Titanium Implants and Its Influence on Materials Properties and In Vitro Biocompatibility. Mater. Sci. Eng. C 2021, 119, 111631. [Google Scholar] [CrossRef] [PubMed]
- Arthur, N.K.; Kubjane, S.M.; Popoola, A.P.I.; Masina, B.N.; Pityana, S.L. The Effect of Laser Shock Processing on the Anti-Corrosion Performance of LENS-Fabricated Ti-6Al-4V Alloy. J. Compos. Sci. 2023, 7, 218. [Google Scholar] [CrossRef]
- Simões, I.G.; Dos Reis, A.C.; Da Costa Valente, M.L. Analysis of the Influence of Surface Treatment by High-Power Laser Irradiation on the Surface Properties of Titanium Dental Implants: A Systematic Review. J. Prosthet. Dent. 2023, 129, 863–870. [Google Scholar] [CrossRef]
- Kang, H.K.; Chu, T.M.; Dechow, P.; Stewart, K.; Kyung, H.M.; Liu, S.S. Laser-Treated Stainless Steel Mini-Screw Implants: 3D Surface Roughness, Bone-Implant Contact, and Fracture Resistance Analysis. Eur. J. Orthod. 2016, 38, 154–162. [Google Scholar] [CrossRef]
- Yan, J.; Huang, W.; Kuang, H.; Wang, Q.; Li, B. The Effect of Etched 3D Printed Cu-Bearing Titanium Alloy on the Polarization of Macrophage. Front. Mater. 2022, 9, 941311. [Google Scholar] [CrossRef]
- Yu, M.; Wan, Y.; Ren, B.; Wang, H.; Zhang, X.; Qiu, C.; Liu, A.; Liu, Z. 3D Printed Ti-6Al-4V Implant with a Micro/Nanostructured Surface and Its Cellular Responses. ACS Omega 2020, 5, 31738–31743. [Google Scholar] [CrossRef]
- Ren, B.; Wan, Y.; Liu, C.; Wang, H.; Yu, M.; Zhang, X.; Huang, Y. Improved Osseointegration of 3D Printed Ti-6Al-4V Implant with a Hierarchical Micro/Nano Surface Topography: An In Vitro and In Vivo Study. Mater. Sci. Eng. C 2021, 118, 111505. [Google Scholar] [CrossRef]
- Gulati, K.; Prideaux, M.; Kogawa, M.; Lima-Marques, L.; Atkins, G.J.; Findlay, D.M.; Losic, D. Anodized 3D-Printed Titanium Implants with Dual Micro- and Nano-Scale Topography Promote Interaction with Human Osteoblasts and Osteocyte-Like Cells: 3D Printed Titanium Implants with Dual Micro- and Nano-Scale Topography. J. Tissue Eng. Regen. Med. 2017, 11, 3313–3325. [Google Scholar] [CrossRef]
- Maher, S.; Qin, J.; Gulati, K.; Elmekawy, A.; Losic, D. 3D Printed Titanium Implants with Nano-Engineered Surface Titania Nanotubes for Localized Drug Delivery. In Proceedings of the Chemeca 2016, Adelaide, SA, USA, 25–28 September 2016. [Google Scholar]
- Liang, C.-Y.; Jiang, X.-J.; Ji, R.-L.; Li, B.-E.; Zou, X.-R.; Wang, H.-S.; Hao, J.-Z.; Yang, T. Preparation and Surface Modification of 3D Printed Ti–6Al–4V Porous Implant. Rare Met. 2021, 40, 1164–1172. [Google Scholar] [CrossRef]
- Chunate, H.; Khamwannah, J.; Aliyu, A.A.A.; Tantavisut, S.; Puncreobutr, C.; Khamkongkaeo, A.; Tongyam, C.; Tumkhanon, K.; Phetrattanarangsi, T.; Chanamuangkon, T.; et al. Titania Nanotube Architectures Synthesized on 3D-Printed Ti-6Al-4V Implant and Assessing Vancomycin Release Protocols. Materials 2021, 14, 6576. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ma, S.; Wu, C.; Li, X.; Ma, X.; Hu, H.; Wu, J.; Wang, Y.; Liu, Z. Chimeric Peptides Quickly Modify the Surface of Personalized 3D Printing Titanium Implants to Promote Osseointegration. ACS Appl. Mater. Interfaces 2021, 13, 33981–33994. [Google Scholar] [CrossRef]
- Teng, F.-Y.; Tai, I.-C.; Ho, M.-L.; Wang, J.-W.; Weng, L.W.; Wang, Y.J.; Wang, M.-W.; Tseng, C.-C. Controlled Release of BMP-2 from Titanium with Electrodeposition Modification Enhancing Critical Size Bone Formation. Mater. Sci. Eng. C 2019, 105, 109879. [Google Scholar] [CrossRef]
- Jahanmard, F.; Dijkmans, F.M.; Majed, A.; Vogely, H.C.; van der Wal, B.C.H.; Stapels, D.A.C.; Ahmadi, S.M.; Vermonden, T.; Amin Yavari, S. Toward Antibacterial Coatings for Personalized Implants. ACS Biomater. Sci. Eng. 2020, 6, 5486–5492. [Google Scholar] [CrossRef]
- Juliadmi, D.; Nuswantoro, N.F.; Fajri, H.; Indriyani, I.Y.; Affi, J.; Manjas, M.; Suharti, N.; Tjong, D.H.; Niinomi, M.; Gunawarman. The Coating of Bovine-Source Hydroxyapatite on Titanium Alloy (Ti-6Al-4V ELI) Using Electrophoretic Deposition for Biomedical Application. Mater. Sci. Forum 2020, 1000, 97–106. [Google Scholar] [CrossRef]
- Rifai, A.; Tran, N.; Lau, D.W.; Elbourne, A.; Zhan, H.; Stacey, A.D.; Mayes, E.L.H.; Sarker, A.; Ivanova, E.P.; Crawford, R.J.; et al. Polycrystalline Diamond Coating of Additively Manufactured Titanium for Biomedical Applications. ACS Appl. Mater. Interfaces 2018, 10, 8474–8484. [Google Scholar] [CrossRef]
- Youn, Y.H.; Lee, S.J.; Choi, G.R.; Lee, H.R.; Lee, D.; Heo, D.N.; Kim, B.-S.; Bang, J.B.; Hwang, Y.-S.; Correlo, V.M.; et al. Simple and Facile Preparation of Recombinant Human Bone Morphogenetic Protein-2 Immobilized Titanium Implant via Initiated Chemical Vapor Deposition Technique to Promote Osteogenesis for Bone Tissue Engineering Application. Mater. Sci. Eng. C 2019, 100, 949–958. [Google Scholar] [CrossRef]
- Kozelskaya, A.I.; Rutkowski, S.; Gogolev, A.S.; Chistyakov, S.G.; Krasovsky, I.B.; Zheravin, A.A.; Tverdokhlebov, S.I. Bioactive Coatings on 3D Printed Titanium Implants with a Complex Internal Structure for Bone Replacement. J. Phys. Conf. Ser. 2021, 2144, 012015. [Google Scholar] [CrossRef]
- Xiu, P.; Jia, Z.; Lv, J.; Yin, C.; Cheng, Y.; Zhang, K.; Song, C.; Leng, H.; Zheng, Y.; Cai, H.; et al. Tailored Surface Treatment of 3D Printed Porous Ti6Al4V by Microarc Oxidation for Enhanced Osseointegration via Optimized Bone In-Growth Patterns and Interlocked Bone/Implant Interface. ACS Appl. Mater. Interfaces 2016, 8, 17964–17975. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Tong, S.; Yang, S. The Effects of Graphene on the Biocompatibility of a 3D-Printed Porous Titanium Alloy. Coatings 2021, 11, 1509. [Google Scholar] [CrossRef]
- Huang, L.; Cai, B.; Huang, Y.; Wang, J.; Zhu, C.; Shi, K.; Song, Y.; Feng, G.; Liu, L.; Zhang, L. Comparative Study on 3D Printed Ti6Al4V Scaffolds with Surface Modifications Using Hydrothermal Treatment and Microarc Oxidation to Enhance Osteogenic Activity. ACS Omega 2021, 6, 1465–1476. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, H.; Wang, X.; Yang, L.; Chen, M.; Wang, R.; Qin, G.; Chen, D.-F.; Zhang, E. Effect of Ultrasonic Micro-Arc Oxidation on the Antibacterial Properties and Cell Biocompatibility of Ti-Cu Alloy for Biomedical Application. Mater. Sci. Eng. C 2020, 115, 110921. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wu, Z.; Yao, X.; Zhou, Y.; Xiong, Y.; Li, Y.; Xu, J.; Dargusch, M.S.; Yan, M. From Bio-Inertness to Osseointegration and Antibacterial Activity: A One-Step Micro-Arc Oxidation Approach for Multifunctional Ti Implants Fabricated by Additive Manufacturing. Mater. Des. 2022, 221, 110962. [Google Scholar] [CrossRef]
- Maver, T.; Mastnak, T.; Mihelič, M.; Maver, U.; Finšgar, M. Clindamycin-Based 3D-Printed and Electrospun Coatings for Treatment of Implant-Related Infections. Materials 2021, 14, 1464. [Google Scholar] [CrossRef]
- Qin, J.; Yang, D.; Maher, S.; Lima-Marques, L.; Zhou, Y.; Chen, Y.; Atkins, G.J.; Losic, D. Micro- and Nano-Structured 3D Printed Titanium Implants with a Hydroxyapatite Coating for Improved Osseointegration. J. Mater. Chem. B 2018, 6, 3136–3144. [Google Scholar] [CrossRef]
- Arifvianto, B.; Suyitno. Surface Modification of Titanium Using Steel Slag Ball and Shot Blasting Treatment for Biomedical Implant Applications. Int. J. Miner. Metall. Mater. 2013, 20, 788–795. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Iannello, G.; Santonocito, D.; Risitano, G.; Cicciù, M. Sandblasted and Acid Etched Titanium Dental Implant Surfaces Systematic Review and Confocal Microscopy Evaluation. Materials 2019, 12, 1763. [Google Scholar] [CrossRef]
- Darimont, G.L.; Cloots, R.; Heinen, E.; Seidel, L.; Legrand, R. In Vivo Behaviour of Hydroxyapatite Coatings on Titanium Implants: A Quantitative Study in the Rabbit. Biomaterials 2002, 23, 2569–2575. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P. Surface Treatments of Titanium Dental Implants for Rapid Osseointegration. Dent Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Suzuki, K.; Aoki, K.; Ohya, K. Effects of Surface Roughness of Titanium Implants on Bone Remodeling Activity of Femur in Rabbits. Bone 1997, 21, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Von Maltzahn, N.F.; Holstermann, J.; Kohorst, P. Retention Forces between Titanium and Zirconia Components of Two-Part Implant Abutments with Different Techniques of Surface Modification: Retention Forces of Two-Part Implant Abutments. Clin. Implant Dent. Relat. Res. 2016, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Feller, L.; Chandran, R.; Khammissa, R.A. Osseointegration: Biological Events in Relation to Characteristics of the Implant Surface. S. Afr. Dent. J. 2014, 69, 112, 114–117. [Google Scholar]
- Tung, J.-H.; Chen, C.-S.; Zhao, W.-Y. Optimization of Sandblasting Process of Complex 3D Surface Polishing Using Variable Viscoelastic Diamond Particles Abrasive. Mach. Sci. Technol. 2019, 23, 118–130. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of Titanium Surface Topography on Bone Integration: A Systematic Review. Clin. Oral Implants Res. 2009, 20, 172–184. [Google Scholar] [CrossRef]
- Yang, G.; He, F.; Yang, X.; Wang, X.; Zhao, S. Bone Responses to Titanium Implants Surface-Roughened by Sandblasted and Double Etched Treatments in a Rabbit Model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, 516–524. [Google Scholar] [CrossRef]
- Chang, Y.R.; Xu, F.F.; Li, J.; You, Y.H.; Liu, C.; Yin, L.H. Surface Morphology and Surface Properties of Ti and TiZr Implant Materials. Zhonghua Kou Qiang Yi Xue Za Zhi 2019, 54, 118–123. [Google Scholar]
- John, M.; Kalvala, P.R.; Misra, M.; Menezes, P.L. Peening Techniques for Surface Modification: Processes, Properties, and Applications. Materials 2021, 14, 3841. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, X.; Zheng, X.; Ding, C.; Chu, P.K. Improved Stability of Plasma-Sprayed Dicalcium Silicate/Zirconia Composite Coating. Thin Solid Films 2006, 515, 1214–1218. [Google Scholar] [CrossRef]
- Fouda, M.A.; Nemat, A.; Gawish, A. Does the Coating of Titanium Implants by Hydroxyapatite Affect the Elaboration of Free Radicals. Exp. Study 2009, 3, 1122–1129. [Google Scholar]
- Dong, S.; Zeng, J.; Li, L.; Sun, J.; Yang, X. Significance of In-Situ Dry-Ice Blasting on the Microstructure, Crystallinity and Bonding Strength of Plasma-Sprayed Hydroxyapatite Coatings. J. Mech. Behav. Biomed. Mater. 2017, 71, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Wu, X.; Yuan, L.; Zhang, Z.; Zhang, Y.; Zhou, Y. Fabrication and In Vitro Bioactivity of Robust Hydroxyapatite Coating on Porous Titanium Implant. Chem. Res. Chin. Univ. 2019, 35, 686–692. [Google Scholar] [CrossRef]
- Bonfante, E.A.; Witek, L.; Tovar, N.; Suzuki, M.; Marin, C.; Granato, R. Physicochemical Characterization and In Vivo Evaluation of Amorphous and Partially Crystalline Calcium Phosphate Coatings Fabricated on Ti-6Al-4V Implants by the Plasma Spray Method. Int. J. Biomater. 2012, 2012, 603826. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Luo, M.; Hu, W.; Zheng, L.; Huang, R.; Greven, J.; Hildebrand, F.; Yuan, F. Plasma Spray vs. Electrochemical Deposition: Toward a Better Osteogenic Effect of Hydroxyapatite Coatings on 3D-Printed Titanium Scaffolds. Front. Bioeng. Biotechnol. 2021, 9, 705774. [Google Scholar] [CrossRef]
- Yang, C.; Yu, Z.; Long, Y.; Chen, L. Preparation of Porous Titanium Dental Implant by 3D Printing/Composite Coating and Its Biomechanical Properties and Flexural Strength. Sci. Technol. Adv. Mater. 2020, 12, 1492–1501. [Google Scholar] [CrossRef]
- Bose, S.; Banerjee, D.; Shivaram, A.; Tarafder, S. Calcium Phosphate Coated 3D Printed Porous Titanium with Nanoscale Surface Modification for Orthopedic and Dental Applications. Mater. Des. 2018, 151, 102–112. [Google Scholar] [CrossRef]
- Su, S.; Chen, W.; Zheng, M.; Lu, G.; Tang, W.; Huang, H.; Qu, D. Facile Fabrication of 3D-Printed Porous Ti6Al4V Scaffolds with a Sr-CaP Coating for Bone Regeneration. ACS Omega 2022, 7, 8391–8402. [Google Scholar]
- Zhang, Y.C.; Li, J.J.; Hou, W.T.; Zhang, H.F.; Liu, J.H. A Preliminary Study of Three-Dimensional Printed Porous Titanium Plate Integrated Implant for the Repair of Comminuted Acetabular Posterior Wall Fracture with Bone Defect. Zhongguo Gu Shang 2019, 32, 469–474. [Google Scholar]
- Kurella, A.; Dahotre, N.B. Review Paper: Surface Modification for Bioimplants: The Role of Laser Surface Engineering. J. Biomater. Appl. 2005, 20, 5–50. [Google Scholar] [CrossRef]
- Fathi-Hafshejani, P.; Johnson, H.; Ahmadi, Z.; Roach, M.; Shamsaei, N.; Mahjouri-Samani, M. Phase-Selective and Localized TiO2 Coating on Additive and Wrought Titanium by a Direct Laser Surface Modification Approach. ACS Omega 2020, 5, 16744–16751. [Google Scholar] [CrossRef]
- Shivakoti, I.; Kibria, G.; Cep, R.; Pradhan, B.B.; Sharma, A. Laser Surface Texturing for Biomedical Applications: A Review. Coatings 2021, 11, 124. [Google Scholar] [CrossRef]
- Jaritngam, P.; Tangwarodomnukun, V.; Qi, H.; Dumkum, C. Surface and Sub-Surface Characteristics of Laser Polished Ti6Al4V Titanium Alloy. Opt. Laser Technol. 2020, 126, 106102. [Google Scholar] [CrossRef]
- Lamolle, S.F.; Monjo, M.; Rubert, M.; Haugen, H.J.; Lyngstadaas, S.P.; Ellingsen, J.E. The Effect of Hydrofluoric Acid Treatment of Titanium Surface on Nanostructural and Chemical Changes and the Growth of MC3T3-E1 Cells. Biomaterials 2009, 30, 736–742. [Google Scholar] [CrossRef]
- Attar, H.; Bönisch, M.; Calin, M.; Zhang, L.C.; Zhuravleva, K.; Funk, A.; Scudino, S.; Yang, C.; Eckert, J. Comparative Study of Microstructures and Mechanical Properties of In Situ Ti–TiB Composites Produced by Selective Laser Melting, Powder Metallurgy, and Casting Technologies. J. Mater. Res. 2014, 29, 1941–1950. [Google Scholar] [CrossRef]
- Ban, S.; Iwaya, Y.; Kono, H.; Sato, H. Surface Modification of Titanium by Etching in Concentrated Sulfuric Acid. Dent. Mater. 2006, 22, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Akeuchi, M.; Abe, Y.; Yoshida, Y.; Nakayama, Y.; Okazaki, M.; Akagawa, Y. Acid Pretreatment of Titanium Implants. Biomaterials 2003, 24, 1821–1827. [Google Scholar] [CrossRef]
- Rosales-Leal, J.I.; Rodríguez-Valverde, M.A.; Mazzaglia, G.; Ramón-Torregrosa, P.J.; Díaz-Rodríguez, L.; García-Martínez, O.; Vallecillo-Capilla, M.; Ruiz, C.; Cabrerizo-Vílchez, M.A. Effect of Roughness, Wettability and Morphology of Engineered Titanium Surfaces on Osteoblast-Like Cell Adhesion. Colloids Surf. A 2010, 365, 222–229. [Google Scholar] [CrossRef]
- Liu, X.; Poon, R.W.Y.; Kwok, S.C.H.; Chu, P.K.; Ding, C. Plasma Surface Modification of Titanium for Hard Tissue Replacements. Surf. Coat. Technol. 2004, 186, 227–233. [Google Scholar] [CrossRef]
- Chang, E.; Chang, W.J.; Wang, B.C.; Yang, C.Y. Plasma Spraying of Zirconia-Reinforced Hydroxyapatite Composite Coatings on Titanium: Part I: Phase, Microstructure and Bonding Strength. J. Mater. Sci. Mater. Med. 1997, 8, 193–200. [Google Scholar] [CrossRef]
- Cho, S. The Removal Torque of Titanium Screw Inserted in Rabbit Tibia Treated by Dual Acid Etching. Biomaterials 2003, 24, 3611–3617. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.Y.; She, P.L.; Shih, H.C. Microstructure Effect on Microtopography of Chemically Etched α + β Ti Alloys. Appl. Surf. Sci. 2006, 253, 449–458. [Google Scholar] [CrossRef]
- Tang, H.; Li, Y.; Ma, J.; Zhang, X.; Li, B.; Liu, S.; Zhang, K. Improvement of Biological and Mechanical Properties of Titanium Surface by Anodic Oxidation. BME 2016, 27, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Lee, M.H.; Bae, T.S. Effects of Anodic Oxidation Parameters on a Modified Titanium Surface. J. Biomed. Mater. Res. Part B 2008, 84, 422–429. [Google Scholar] [CrossRef]
- Peng, Z.; Ni, J.; Zheng, K.; Shen, Y.; Wang, X.; He, G.; Jin, S.; Tang, T. Dual effects and mechanism of TiO2 nanotube arrays in reducing bacterial colonization and enhancing C3H10T1/2 cell adhesion. Int. J. Nanomed. 2013, 8, 3093–3105. [Google Scholar]
- Sarraf, M.; Nasiri-Tabrizi, B.; Yeong, C.H.; Madaah Hosseini, H.R.; Saber-Samandari, S.; Basirun, W.J.; Tsuzuki, T. Mixed Oxide Nanotubes in Nanomedicine: A Dead-End or a Bridge to the Future? Ceram. Int. 2021, 47, 2917–2948. [Google Scholar] [CrossRef]
- Micheletti, C.; Suriano, R.; Grandfield, K.; Turri, S. Drug Release from Polymer-Coated TiO2 Nanotubes on Additively Manufactured Ti-6Al-4V Bone Implants: A Feasibility Study. Nano Express 2021, 2, 010018. [Google Scholar] [CrossRef]
- Maher, S.; Kaur, G.; Lima-Marques, L.; Evdokiou, A.; Losic, D. Engineering of Micro- to Nanostructured 3D-Printed Drug-Releasing Titanium Implants for Enhanced Osseointegration and Localized Delivery of Anticancer Drugs. ACS Appl. Mater. Interfaces 2017, 9, 29562–29570. [Google Scholar] [CrossRef]
- Yao, Q.; Jiang, Y.; Tan, S.; Fu, X.; Li, B.; Liu, L. Composition and Bioactivity of Calcium Phosphate Coatings on Anodic Oxide Nanotubes Formed on Pure Ti and Ti-6Al-4V Alloy Substrates. Mater. Sci. Eng. C 2020, 110, 110687. [Google Scholar] [CrossRef]
- Neuhoff, D.; Thompson, R.E.; Frauchiger, V.M.; Ganser, A.; Steiner, A. Anodic Plasma Chemical Treatment of Titanium Schanz Screws Reduces Pin Loosening. J. Orthop. Trauma 2005, 19, 543–550. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma Electrolytic Oxidation (PEO) Process—Processing, Properties, and Applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wei, D.; Yang, H.; Feng, W.; Cheng, S.; Li, B.; Wang, Y.; Jia, D.; Zhou, Y. MC3T3-E1 Cell Response of Amorphous Phase/TiO2 Nanocrystal Composite Coating Prepared by Microarc Oxidation on Titanium. Mater. Sci. Eng. C 2014, 39, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Song, W.H.; Jun, Y.K.; Han, Y. Biomimetic Apatite Coatings on Micro-Arc Oxidized Titania. Biomaterials 2004, 25, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wu, C.; Lei, X.; Zhang, K.; Liu, C.; Ding, J.; Shi, X. Effect of Oxidation Time on Cytocompatibility of Ultrafine-Grained Pure Ti in Micro-Arc Oxidation Treatment. Surf. Coat. Technol. 2018, 342, 12–22. [Google Scholar] [CrossRef]
- Li, X.-J.; Zhang, M.; Wen, S.; Mao, X.; Huo, W.-G.; Guo, Y.-Y.; Wang, Y.-X. Microstructure and Wear Resistance of Micro-Arc Oxidation Ceramic Coatings Prepared on 2A50 Aluminum Alloys. Surf. Coat. Technol. 2020, 394, 125853. [Google Scholar] [CrossRef]
- Babaei, M.; Dehghanian, C.; Taheri, P. Effect of Duty Cycle and Electrolyte Additive on Photocatalytic Performance of TiO2-ZrO2 Composite Layers Prepared on CP Ti by Micro Arc Oxidation Method. Surf. Coat. Technol. 2016, 307, 554–564. [Google Scholar] [CrossRef]
- Yang, W.; Xu, D.P.; Guo, Q.Q. Influence of Electrolyte Composition on Microstructure and Properties of Coatings Formed on Pure Ti Substrate by Micro Arc Oxidation. Surf. Coat. Technol. 2018, 349, 522–528. [Google Scholar] [CrossRef]
- Ni, R.; Jing, Z.; Xiong, C.; Meng, D.; Wei, C.; Cai, H. Effect of Micro-Arc Oxidation Surface Modification of 3D-Printed Porous Titanium Alloys on Biological Properties. Ann. Transl. Med. 2022, 10, 710. [Google Scholar] [CrossRef]
- Yao, Z.Q.; Ivanisenko, Y.; Diemant, T.; Caron, A.; Chuvilin, A.; Jiang, J.Z.; Valiev, R.Z.; Qi, M.; Fecht, H.-J. Synthesis and Properties of Hydroxyapatite-Containing Porous Titania Coating on Ultrafine-Grained Titanium by Micro-Arc Oxidation. Acta Biomater. 2010, 6, 2816–2825. [Google Scholar] [CrossRef]
- Shen, X.; Fang, K.; Ru Yie, K.H.; Zhou, Z.; Shen, Y.; Wu, S.; Zhu, Y.; Deng, Z.; Ma, P.; Ma, J.; et al. High Proportion Strontium-Doped Micro-Arc Oxidation Coatings Enhance Early Osseointegration of Titanium in Osteoporosis by Anti-Oxidative Stress Pathway. Bioact. Mater. 2022, 10, 405–419. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, Q.; Zhou, H.; Zhou, W.; Fan, D.; Lin, X.; Jing, Z.; Cai, H.; Cheng, Y.; Liu, X.; et al. Sustainable Release of Vancomycin from Micro-Arc Oxidised 3D-Printed Porous Ti6Al4V for Treating Methicillin-Resistant Staphylococcus Aureus Bone Infection and Enhancing Osteogenesis in a Rabbit Tibia Osteomyelitis Model. Biomater. Sci. 2020, 8, 3106–3115. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.; Kossenko, A.; Zinigrad, M.; Danchuk, V.; Sobolev, A. Cleaning Strategies of Synthesized Bioactive Coatings by PEO on Ti-6Al-4V Alloys of Organic Contaminations. Materials 2023, 16, 4624. [Google Scholar] [CrossRef] [PubMed]
- Maciąg, F.; Moskalewicz, T.; Kowalski, K.; Łukaszczyk, A.; Hadzhieva, Z.; Boccaccini, A.R. The Effect of Electrophoretic Deposition Parameters on the Microstructure and Adhesion of Zein Coatings to Titanium Substrates. Materials 2021, 14, 312. [Google Scholar] [CrossRef] [PubMed]
- Seuss, S.; Boccaccini, A. Alternating Current Electrophoretic Deposition of Antibacterial Bioactive Glass-Chitosan Composite Coatings. Int. J. Mol. Sci. 2014, 15, 12231–12242. [Google Scholar] [CrossRef]
- Nuswantoro, N.F.; Manjas, M.; Suharti, N.; Juliadmi, D.; Fajri, H.; Tjong, D.H.; Affi, J.; Niinomi, M.; Gunawarman. Hydroxyapatite Coating on Titanium Alloy TNTZ for Increasing Osseointegration and Reducing Inflammatory Response In Vivo on Rattus Norvegicus Wistar Rats. Ceram. Int. 2021, 47, 16094–16100. [Google Scholar] [CrossRef]
- Gaafar, M.S.; Yakout, S.M.; Barakat, Y.F.; Sharmoukh, W. Electrophoretic Deposition of Hydroxyapatite/Chitosan Nanocomposites: The Effect of Dispersing Agents on the Coating Properties. RSC Adv. 2022, 12, 27564–27581. [Google Scholar] [CrossRef]
- Fiołek, A.; Zimowski, S.; Kopia, A.; Łukaszczyk, A.; Moskalewicz, T. Electrophoretic Co-Deposition of Polyetheretherketone and Graphite Particles: Microstructure, Electrochemical Corrosion Resistance, and Coating Adhesion to a Titanium Alloy. Materials 2020, 13, 3251. [Google Scholar] [CrossRef]
- Pipattanachat, S.; Qin, J.; Rokaya, D.; Thanyasrisung, P.; Srimaneepong, V. Biofilm Inhibition and Bactericidal Activity of NiTi Alloy Coated with Graphene Oxide/Silver Nanoparticles via Electrophoretic Deposition. Sci. Rep. 2021, 11, 14008. [Google Scholar] [CrossRef]
- Srimaneepong, V.; Rokaya, D.; Thunyakitpisal, P.; Qin, J.; Saengkiettiyut, K. Corrosion Resistance of Graphene Oxide/Silver Coatings on Ni–Ti Alloy and Expression of IL-6 and IL-8 in Human Oral Fibroblasts. Sci. Rep. 2020, 10, 3247. [Google Scholar] [CrossRef]
- Danlei, Z.; Haoran, D.; Yuting, N.; Wenjie, F.; Muqi, J.; Ke, L.; Qingsong, W.; William, M.P.; Zhen, Z. Electrophoretic Deposition of Novel Semi-Permeable Coatings on 3D-Printed Ti-Nb Alloy Meshes for Guided Alveolar Bone Regeneration. Dent. Mater. 2022, 38, 431–443. [Google Scholar]
- Grandfield, K.; Zhitomirsky, I. Electrophoretic Deposition of Composite Hydroxyapatite–Silica–Chitosan Coatings. Mater. Charact. 2008, 59, 61–67. [Google Scholar] [CrossRef]
- Han, C.; Yao, Y.; Cheng, X.; Luo, J.; Luo, P.; Wang, Q.; Yang, F.; Wei, Q.; Zhang, Z. Electrophoretic Deposition of Gentamicin-Loaded Silk Fibroin Coatings on 3D-Printed Porous Cobalt-Chromium-Molybdenum Bone Substitutes to Prevent Orthopedic Implant Infections. Biomacromolecules 2017, 18, 3776–3787. [Google Scholar] [CrossRef] [PubMed]
- Choy, K. Chemical Vapour Deposition of Coatings. Prog. Mater Sci. 2003, 48, 57–170. [Google Scholar] [CrossRef]
- Rupprecht, S.; Bloch, A.; Rosiwal, S.; Neukam, F.W.; Wiltfang, J. Examination of the Bone-Metal Interface of Titanium Implants Coated by the Microwave Plasma Chemical Vapor Deposition Method. Int. J. Oral Maxillofac. Implants 2002, 17, 778–785. [Google Scholar] [PubMed]
- Montali, A. Antibacterial Coating Systems. Injury 2006, 37, S81–S86. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthcare Mater. 2018, 7, e1701503. [Google Scholar] [CrossRef]
- Amin Yavari, S.; Loozen, L.; Paganelli, F.L.; Bakhshandeh, S.; Lietaert, K.; Groot, J.A.; Fluit, A.C.; Boel, C.H.; Alblas, J.; Vogely, H.C.; et al. Antibacterial Behavior of Additively Manufactured Porous Titanium with Nanotubular Surfaces Releasing Silver Ions. ACS Appl Mater. Interfaces 2016, 27, 17080–17089. [Google Scholar] [CrossRef]
- Wu, Z.; Luo, J.; Zhang, J.; Huang, H.; Xie, Z.; Xie, X. Silver-Releasing Micro-/Nanoporous Coating on Additively Manufactured Macroporous Ti-Ta-Nb-Zr Scaffolds with High Osseointegration and Antibacterial Properties. Coatings 2021, 11, 716. [Google Scholar] [CrossRef]
- Xue, C.; Song, X.; Liu, M.; Ai, F.; Liu, M.; Shang, Q.; Shi, X.; Li, F.; He, X.; Xie, L.; et al. A Highly Efficient, Low-Toxic, Wide-Spectrum Antibacterial Coating Designed for 3D Printed Implants with Tailorable Release Properties. J. Mater. Chem. B 2017, 5, 4128–4136. [Google Scholar] [CrossRef]
- Surmeneva, M.; Lapanje, A.; Chudinova, E.; Ivanova, A.; Koptyug, A.; Loza, K.; Prymak, O.; Epple, M.; Ennen-Roth, F.; Ulbricht, M.; et al. Decreased Bacterial Colonization of Additively Manufactured Ti6Al4V Metallic Scaffolds with Immobilized Silver and Calcium Phosphate Nanoparticles. Appl. Surf. Sci. 2019, 480, 822–829. [Google Scholar] [CrossRef]
- Suchý, T.; Vištejnová, L.; Šupová, M.; Klein, P.; Bartoš, M.; Kolinko, Y.; Blassová, T.; Tonar, Z.; Pokorný, M.; Sucharda, Z.; et al. Vancomycin-Loaded Collagen/Hydroxyapatite Layers Electrospun on 3D Printed Titanium Implants Prevent Bone Destruction Associated with S. Epidermidis Infection and Enhance Osseointegration. Biomedicines 2021, 9, 531. [Google Scholar] [CrossRef] [PubMed]
- Guarch-Pérez, C.; Shaqour, B.; Riool, M.; Verleije, B.; Beyers, K.; Vervaet, C.; Cos, P.; Zaat, S.A.J. 3D-Printed Gentamicin-Releasing Poly-ε-Caprolactone Composite Prevents Fracture-Related Staphylococcus Aureus Infection in Mice. Pharmaceutics 2022, 14, 1363. [Google Scholar] [CrossRef] [PubMed]
- Rifai, A.; Tran, N.; Reineck, P.; Elbourne, A.; Mayes, E.; Sarker, A.; Dekiwadia, C.; Ivanova, E.P.; Crawford, R.J.; Ohshima, T.; et al. Engineering the Interface: Nanodiamond Coating on 3D-Printed Titanium Promotes Mammalian Cell Growth and Inhibits Staphylococcus Aureus Colonization. ACS Appl. Mater. Interfaces 2019, 11, 24588–24597. [Google Scholar] [CrossRef]
- Rodríguez-Contreras, A.; Torres, D.; Guillem-Marti, J.; Sereno, P.; Ginebra, M.P.; Calero, J.A.; Manero, J.M.; Rupérez, E. Development of Novel Dual-Action Coatings with Osteoinductive and Antibacterial Properties for 3D-Printed Titanium Implants. Surf. Coat. Technol. 2020, 403, 126381. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Wang, X.; Zhang, Y.; Wu, Y.; Jiang, D.; Jia, R. Construction and Osteogenic Effects of 3D-Printed Porous Titanium Alloy Loaded with VEGF/BMP-2 Shell-Core Microspheres in a Sustained-Release System. Front. Bioeng. Biotechnol. 2022, 10, 1028278. [Google Scholar] [CrossRef] [PubMed]
- Guillem-Marti, J.; Vidal, E.; Girotti, A.; Heras-Parets, A.; Torres, D.; Arias, F.J.; Ginebra, M.-P.; Rodriguez-Cabello, J.C.; Manero, J.M. Functionalization of 3D-Printed Titanium Scaffolds with Elastin-Like Recombinamers to Improve Cell Colonization and Osteoinduction. Pharmaceutics 2023, 15, 872. [Google Scholar] [CrossRef]
- You, Y.; Wang, W.; Li, Y.; Song, Y.; Jiao, J.; Wang, Y.; Chen, B.; Liu, J.; Qi, H.; Liang, Y. Aspirin/PLGA Coated 3D-Printed Ti-6Al-4V Alloy Modulate Macrophage Polarization to Enhance Osteoblast Differentiation and Osseointegration. J. Mater. Sci. Mater. Med. 2023, 33, 73. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Q.; He, Y.; Geng, X.; Wang, C.; Li, Y.; Li, Z.; Wang, C.; Qiu, D.; Tian, H. A pH-Neutral Bioactive Glass Coated 3D-Printed Porous Ti6Al4V Scaffold with Enhanced Osseointegration. J. Mater. Chem. B 2023, 11, 1203–1212. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, C.; Lin, K.; Zhu, R.; Zhang, S. Modifying a 3D-Printed Ti6Al4V Implant with Polydopamine Coating to Improve BMSCs Growth, Osteogenic Differentiation, and In Situ Osseointegration In Vivo. Front. Bioeng. Biotechnol. 2021, 309, 761911. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Yang, L.; Yu, F.; Zhang, K.; Jin, J.; Shi, J.; Zhu, L.; Liang, H.; Wang, X.; et al. Polydopamine Coating Promotes Early Osteogenesis in 3D Printing Porous Ti6Al4V Scaffolds. Ann. Transl. Med. 2019, 7, 240. [Google Scholar] [CrossRef]
- Ma, X.Y.; Ma, T.C.; Feng, Y.F.; Xiang, G.; Lei, W.; Zhou, D.P.; Yu, H.L.; Xiang, L.B.; Wang, L. Promotion of Osteointegration under Diabetic Conditions by a Silk Fibroin Coating on 3D-Printed Porous Titanium Implants via a ROS-Mediated NF-κB Pathway. Biomed. Mater. 2021, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Sidane, D.; Rammal, H.; Beljebbar, A.; Gangloff, S.C.; Chicot, D.; Velard, F.; Khireddine, H.; Montagne, A.; Kerdjoudj, H. Biocompatibility of Sol-Gel Hydroxyapatite-Titania Composite and Bilayer Coatings. Mater. Sci. Eng. C 2017, 72, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, R.B.; Baldin, E.K.; Steffens, D.; Pavulack, D.; Pranke, P.; Brandalise, R.N.; de Fraga Malfatti, C. HA-Hybrid Matrix Composite Coating on Ti-Cp for Biomedical Application. J. Mater. Sci. Mater. Med. 2020, 31, 82. [Google Scholar] [CrossRef] [PubMed]
- Fahad, N.D.; Radhi, N.S.; Al-Khafaji, Z.S.; Diwan, A.A. Surface Modification of Hybrid Composite Multilayers Spin Cold Spraying for Biomedical Duplex Stainless Steel. Heliyon 2023, 9, e14103. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Siva, T.; Ramadoss, A. Surface Functionalized Bioceramics Coated on Metallic Implants for Biomedical and Anticorrosion Performance—A Review. J. Mater. Chem. B 2021, 9, 9433–9460. [Google Scholar] [CrossRef]
- Huang, T.; Wang, H.; Zhang, Z.; Feng, K.; Xiang, L. Incorporation of Inorganic Elements onto Titanium-Based Implant Surfaces by One-Step Plasma Electrolytic Oxidation: An Efficient Method to Enhance Osteogenesis. Biomater. Sci. 2022, 10, 6656–6674. [Google Scholar] [CrossRef]
- Liu, C.-F.; Chang, K.-C.; Sun, Y.-S.; Nguyen, D.T.; Huang, H.-H. Combining Sandblasting, Alkaline Etching, and Collagen Immobilization to Promote Cell Growth on Biomedical Titanium Implants. Polymers 2021, 13, 2550. [Google Scholar] [CrossRef]
- Xie, Y.; Zhai, W.; Chen, L.; Chang, J.; Zheng, X.; Ding, C. Preparation and In Vitro Evaluation of Plasma-Sprayed Mg2SiO4 Coating on Titanium Alloy. Acta Biomater. 2009, 5, 2331–2337. [Google Scholar] [CrossRef]
- Song, P.; Hu, C.; Pei, X.; Sun, J.; Sun, H.; Wu, L.; Jiang, Q.; Fan, H.; Yang, B.; Zhou, C.; et al. Dual Modulation of Crystallinity and Macro-/Microstructures of 3D Printed Porous Titanium Implants to Enhance Stability and Osseointegration. J. Mater. Chem. B 2019, 7, 2865–2877. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, S.; Qiu, J.; Qiao, Y.; Qian, S.; Tan, J.; Yeung, K.W.K.; Liu, X. Atomic Layer Deposition of Tantalum Oxide Films on 3D-Printed Ti6Al4V Scaffolds with Enhanced Osteogenic Property for Orthopedic Implants. ACS Biomater. Sci. Eng. 2023, 9, 4197–4207. [Google Scholar]
- Berger, M.B.; Cohen, D.J.; Snyder, K.; Sions, J.; Boyan, B.D.; Schwartz, Z. Bone Marrow Stromal Cells Are Sensitive to Discrete Surface Alterations in Build and Post-Build Modifications of Bioinspired Ti6Al4V 3D-Printed In Vitro Testing Constructs. J. Biomed. Mater. Res. Part A 2023, 111, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, C.; Sheng, R.; Lin, K.; Wang, X.; Zhang, S. Construction of a Hierarchical Micro-/Submicro-/Nanostructured 3D-Printed Ti6Al4V Surface Feature to Promote Osteogenesis: Involvement of Sema7A through the ITGB1/FAK/ERK Signaling Pathway. ACS Appl. Mater. Interfaces 2022, 14, 30571–30581. [Google Scholar] [CrossRef]
- Rieger, E.; Dupret-Bories, A.; Salou, L.; Metz-Boutigue, M.-H.; Layrolle, P.; Debry, C.; Lavalle, P.; Engin Vrana, N. Controlled Implant/Soft Tissue Interaction by Nanoscale Surface Modifications of 3D Porous Titanium Implants. Nanoscale 2015, 7, 9908–9918. [Google Scholar] [CrossRef] [PubMed]
- Körmöczi, K.; Komlós, G.; Papócsi, P.; Horváth, F.; Joób-Fancsaly, Á. The early loading of different surface-modified implants: A randomized clinical trial. BMC Oral Health 2021, 21, 207. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Shao, H.; Li, H.; Zhou, Y. Is surface modification effective to prevent periprosthetic joint infection? A systematic review of preclinical and clinical studies. Orthop. Traumatol. Surg. Res. 2019, 105, 967–974. [Google Scholar] [CrossRef]



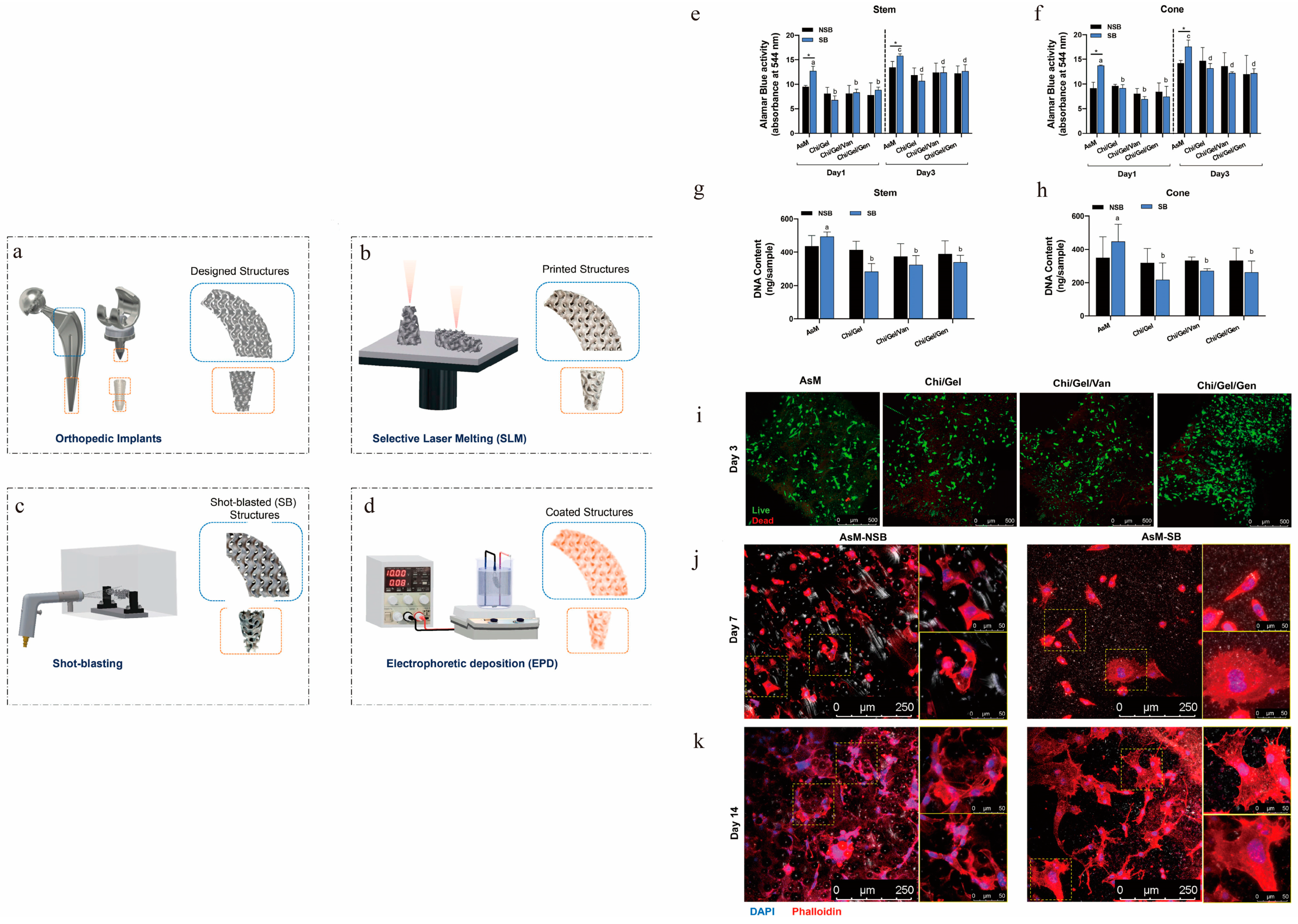

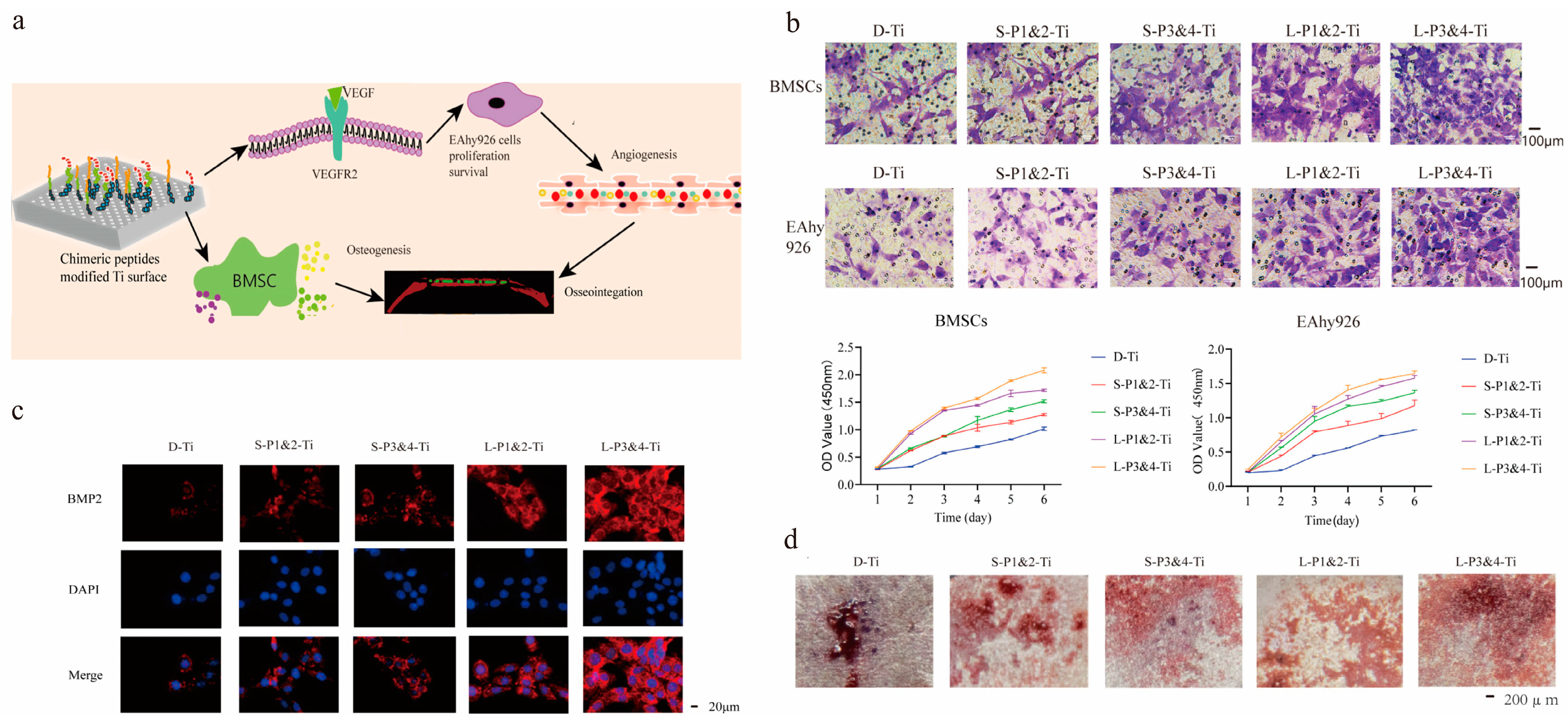

| Implant Material | 3D-Printed Method | Coating Materials | Function | References |
|---|---|---|---|---|
| Ti6Al4V | SLM | Ag + coating | Provides strong antibacterial behavior and promotes osteogenesis. | Wu et al., 2021 [119], Amin et al., 2016 [118], Surmeneva et al., 2021 [121] |
| Ti6Al4V | SLM | Antibiotic coating | Inhibits the growth and reproduction of bacteria, reducing the risk of infection. | Maver et al., 2021 [47], Guarch et al., 2022 [123] |
| Ti6Al4V | SLM | HA coating | Improves bone integration ability and osteoinduction; potential for better promotion of bone mesenchymal stem cell adhesion, proliferation, and osteogenic differentiation. | Fouda et al., 2019 [62], Sun et al., 2021 [66], Suchý et al., 2021 [122] |
| Ti6Al4V | SLM | Nano-diamond coating | Inhibits bacterial proliferation and increases the density of bone and fiber cells. | Rifai et al., 2019 [124] |
| Ti6Al4V | SLM | Organic active coating | Effectively beneficial for bone differentiation and osteosynthesis; improves clinical treatment effectiveness for patients with underlying diseases during Ti alloy implantation. | Liu et al., 2022 [126], Guillem et al., 2023 [127], Ma et al., 2021 [132] |
| Ti6Al4V | LENS™ | Cap coating | Improves interface bonding between the bone host tissue and implant surface; reduces healing time by enhancing early bone integration in the body. | Bose et al., 2018 [68] |
| Ti6Al4V | SLM | Polydopamine coating | Forms a uniform and sturdy coating; improves proliferation and osteogenic differentiation; and helps reduce stress shielding and increases bone growth. | Wang et al., 2021 [130], Li et al., 2019 [131] |
| Type | Advantage | Disadvantage |
|---|---|---|
| Physical–mechanical methods | Physical–mechanical methods are simpler and more cost-effective modifications that can improve the surface roughness and, thus, the osseointegration of the implant, improving the mechanical properties of the surface more significantly. | Physical–mechanical methods may induce poor bioadaptation and interfacial adhesion, have a low capacity to enhance bioactivity, and have limited bioactivity promotion ability. |
| Chemical modification technology | Chemical surface modification methods can achieve better bioactivity results, improve the osseointegration of implants, improve bioadaptability by changing the chemical components of the surface, and be less damaging to the substrate. | Chemical surface modification methods to improve the mechanical properties are limited, coating adhesion and stability are poor, some modification methods are complicated to operate, the cost of raw materials and equipment is high, and the control of the technicians in this field still needs to be improved. |
| Bioconvergence Modification Technology | Promotes cell adhesion, inhibits bacterial colonization, enhances bone tissue growth and integration, and improves the biocompatibility and bioactivity of the implant, making changes to the implant surface at the microscopic and macroscopic levels in order to promote a strong bond between the implant and the surrounding bone tissue. | Biofusion modification technology is less stable and mechanically robust than physical–mechanical and chemical modification methods, and the technology is more cumbersome to operate. |
| Functional Coating Lamination | Composite methods for the different purposes of surface modification have their respective advantages, and the advantages of a variety of methods make up for the shortcomings of a single method. A multi-layer structure can be designed to give full play to the functions of each layer to improve antibacterial properties, mechanical properties, and corrosion resistance, and a coating can improve biological activity. | Composite functional coatings and Ti alloy substrates are poor and easy to peel off; the coating performance is not uniform; the processing technology is complex; and the long-term compatibility of composite coatings with the human physiological environment and other issues remain to be confirmed by further research. |
| Coating | XRD | XPS | SEM | Corrosion Resistance | Bioactivity | Disadvantage |
|---|---|---|---|---|---|---|
| Ag+ coating | Diffraction peaks from silver crystals in coatings. | Appearance of silver elemental peaks. | Usually distributed as tiny particles on the surface; white or gray in color. | Achieves some improvement. | Powerful antibacterial activity. | Some cytotoxicity. |
| Antibiotic coating | May show a flat background rather than sharp diffraction peaks. | Characteristic peaks of the antibiotic elements involved, such as sulphur, oxygen, and nitrogen, can be detected. | May be unevenly distributed with areas of aggregation; color may be close to untreated implant surface. | No significant change. | Prevents infections and inhibits the growth of a wide range of bacteria. | May develop bacterial resistance. |
| HA coating | Characteristic diffraction peaks of HA can be detected. | The characteristic peaks of the elements phosphorus and calcium can be seen. | Forms a homogeneous film, which may appear grayish white in color. | Poor corrosion resistance. | Ability to promote bone cell adhesion and growth. | Poor mechanical properties; easily falls off. |
| ND coating | Diffraction peaks of visible diamonds. | Characteristic peaks of visible carbon. | May be highly dispersed or may form agglomerates; bright, grayish, or blackish in color. | Typically high corrosion resistance. | The promotion of osteoblast growth and osseointegration. | Complex process with high cost. |
| Organic active coating | Organic coatings usually do not have a crystal structure and have no visible crystal diffraction peaks. | Characteristic peaks of elements in proteins such as carbon, oxygen, nitrogen, and sulphur can be detected. | Uniform distribution of organic protein coatings; color close to untreated implant surface. | Poor corrosion resistance. | Positive effects on cell adhesion, biomolecular interactions, etc. | Low corrosion resistance. |
| Cap coating | Clear characteristic peaks. | The characteristic peaks of elemental Ca and P can be seen. | Microstructure showing the surface morphology and particle distribution of Cap coatings is usually varying shades of gray. | Better corrosion resistance. | Potential promotion of bone tissue growth and osseointegration. | Susceptible to mechanical abrasion or flaking. |
| Dopamine coating | Amorphous; no obvious diffraction peaks. | A characteristic peak of a high concentration of nitrogen can be seen. | Highly uniform coverage; color may be close to the implant base: slightly darker or shiny. | Poor corrosion resistance. | Promotes improved osseointegration, proliferation, and osteogenic differentiation. | Relatively poor corrosion resistance. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, S.; Zhu, J.; Jing, Y.; He, S.; Cheng, L.; Shi, Z. A Comprehensive Review of Surface Modification Techniques for Enhancing the Biocompatibility of 3D-Printed Titanium Implants. Coatings 2023, 13, 1917. https://doi.org/10.3390/coatings13111917
Long S, Zhu J, Jing Y, He S, Cheng L, Shi Z. A Comprehensive Review of Surface Modification Techniques for Enhancing the Biocompatibility of 3D-Printed Titanium Implants. Coatings. 2023; 13(11):1917. https://doi.org/10.3390/coatings13111917
Chicago/Turabian StyleLong, Shuai, Jiang Zhu, Yiwan Jing, Si He, Lijia Cheng, and Zheng Shi. 2023. "A Comprehensive Review of Surface Modification Techniques for Enhancing the Biocompatibility of 3D-Printed Titanium Implants" Coatings 13, no. 11: 1917. https://doi.org/10.3390/coatings13111917








