Effect of Amorphous Boron on the Microstructure and Corrosion Properties of Ni-W Coatings
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gurrappa, I.; Binder, L. Electrodeposition of nanostructured coatings and their characterization—A review. Sci. Technol. Adv. Mater. 2008, 9, 043001. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, S.; Kalaignan, G.P. Tribological and electrochemical corrosion behavior of Ni–W/BN (hexagonal) nano-composite coatings. Ceram. Int. 2015, 41, 10415–10424. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; Fan, Y.; Xu, W.; Yang, Q. Pulse electrodeposition and corrosion behavior of Ni–W/MWCNT nanocomposite coatings. RSC Adv. 2015, 5, 68890–68899. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, X.; Umporntheep, K.; Auejitthavorn, V.; Li, R.; Wangyao, P.; Boonyongmaneerat, Y.; Limpanart, S.; Ma, M.; Liu, R. Electrodeposition and Mechanical Properties of Ni-W Matrix Composite Coatings with Embedded Amorphous Boron Particles. Int. J. Electrochem. Sci. 2016, 11, 9529–9541. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, J.; Perasinjaroen, T.; Aeksen, W.; Das, M.K.; Hao, R.; Zhang, B.; Wangyao, P.; Boonyongmaneerat, Y.; Limpanart, S.; et al. Preparation and hardness of pulse electrodeposited Ni–W–diamond composite coatings. Surf. Coat. Technol. 2015, 276, 228–232. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, J.; Das, M.K.; Hao, R.; Zhong, H.; Thueploy, A.; Limpanart, S.; Boonyongmaneerat, Y.; Ma, M.; Liu, R. Co-electrodeposition of hard Ni-W/diamond nanocomposite coatings. Sci. Rep. 2016, 6, 22285. [Google Scholar] [CrossRef]
- Das, M.K.; Li, R.; Qin, J.; Zhang, X.; Das, K.; Thueploy, A.; Limpanart, S.; Boonyongmaneerat, Y.; Ma, M.; Liu, R. Effect of electrodeposition conditions on structure and mechanical properties of Ni-W/diamond composite coatings. Surf. Coat. Technol. 2017, 309, 337–343. [Google Scholar] [CrossRef]
- Schuh, C.; Nieh, T.; Iwasaki, H. The effect of solid solution W additions on the mechanical properties of nanocrystalline Ni. Acta Mater. 2003, 51, 431–443. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.H.; Aliofkhazraei, M.; Rezvanian, A.R.; Torabinejad, V.; Rouhaghdam, A.S. Ni-W electrodeposited coatings: Characterization, properties and applications. Surf. Coat. Technol. 2016, 307, 978–1010. [Google Scholar] [CrossRef]
- Sriraman, K.; Raman, S.G.S.; Seshadri, S. Corrosion behaviour of electrodeposited nanocrystalline Ni–W and Ni–Fe–W alloys. Mater. Sci. Eng. A 2007, 460, 39–45. [Google Scholar] [CrossRef]
- Alimadadi, H.; Ahmadi, M.; Aliofkhazraei, M.; Younesi, S. Corrosion properties of electrodeposited nanocrystalline and amorphous patterned Ni–W alloy. Mater. Des. 2009, 30, 1356–1361. [Google Scholar] [CrossRef]
- Chianpairot, A.; Lothongkum, G.; Schuh, C.A.; Boonyongmaneerat, Y. Corrosion of nanocrystalline Ni–W alloys in alkaline and acidic 3.5wt.% NaCl solutions. Corros. Sci. 2011, 53, 1066–1071. [Google Scholar] [CrossRef]
- Beltowska-Lehman, E.; Indyka, P.; Bigos, A.; Kot, M.; Tarkowski, L. Electrodeposition of nanocrystalline Ni–W coatings strengthened by ultrafine alumina particles. Surf. Coat. Technol. 2012, 211, 62–66. [Google Scholar] [CrossRef]
- Kumar, K.A.; Kalaignan, G.P.; Muralidharan, V. Direct and pulse current electrodeposition of Ni–W–TiO2 nanocomposite coatings. Ceram. Int. 2013, 39, 2827–2834. [Google Scholar] [CrossRef]
- Hou, K.-H.; Sheu, H.-H.; Ger, M.-D. Preparation and wear resistance of electrodeposited Ni–W/diamond composite coatings. Appl. Surf. Sci. 2014, 308, 372–379. [Google Scholar] [CrossRef]
- Yao, Y.W.; Yao, S.W.; Zhang, L. Preparation, mechanical properties and wear resistance of Ni–W/SiC nanocomposite coatings. Mater. Sci. Technol. 2008, 24, 237–240. [Google Scholar] [CrossRef]
- Kumar, K.A.; Kalaignan, G.P.; Muralidharan, V.S. Enhanced corrosion resistance of Ni–W alloy with inclusion of TiN nanoparticles by electrodeposition method. Trans. IMF 2013, 91, 202–206. [Google Scholar] [CrossRef]
- Han, B.; Lu, X. Tribological and anti-corrosion properties of Ni–W–CeO2 coatings against molten glass. Surf. Coat. Technol. 2008, 202, 3251–3256. [Google Scholar] [CrossRef]
- Xing, S.; Wang, L.; Jiang, C.; Liu, H.; Zhu, W.; Ji, V. Influence of Y2O3 nanoparticles on microstructures and properties of electrodeposited Ni–W–Y2O3 nanocrystalline coatings. Vacuum 2020, 181, 109665. [Google Scholar] [CrossRef]
- Bin Humam, S.; Gyawali, G.; Joshi, B.; Kim, T.H.; Lee, S.W. Influence of WC and TaC particles on the microstructure and scratch resistance of electrodeposited nickel-tungsten alloy. J. Alloys Compd. 2021, 893, 162371. [Google Scholar] [CrossRef]
- Klepper, C.C.; Hazelton, R.C.; Yadlowsky, E.J.; Carlson, E.P.; Keitz, M.D.; Williams, J.M.; Zuhr, R.A.; Poker, D.B. Amorphous boron coatings produced with vacuum arc deposition technology. J. Vac. Sci. Technol. A 2002, 20, 725–732. [Google Scholar] [CrossRef]
- Wang, J.; Gu, Y.; Li, Z.; Wang, W.; Fu, Z. Synthesis of nano-sized amorphous boron powders through active dilution self-propagating high-temperature synthesis method. Mater. Res. Bull. 2013, 48, 2018–2022. [Google Scholar] [CrossRef]
- Harachai, K.; Qin, J.Q.; Boonyongmaneerat, Y.; Jaroenapibal, P. Influences of Boron Concentration on Mechanical Properties of Ni-W-B Composite Coatings. Key Eng. Mater. 2019, 801, 166–171. [Google Scholar] [CrossRef]
- Das, M.K.; Pinitpuwadol, W.; Wonlopsiri, K.; Wangyao, P.; Qin, J. Influence of Annealing on the Microstructure and Mechanical Properties of Ni-W/Boron Composite Coatings. Coatings 2022, 12, 1992. [Google Scholar] [CrossRef]
- Steffani, C.P.; Dint, J.W.; Groza, J.R.; Palazoğlu, A. Electrodeposition and corrosion resistance of Ni-W-B coatings. J. Mater. Eng. Perform. 1997, 6, 413–416. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, Q.; Liu, J. Study of corrosion resistance of Ni-WB amorphous alloy deposits. Plat. Surf. Finish. 2000, 87, 74–77. [Google Scholar]
- Yildiz, R.A.; Genel, K.; Gulmez, T. Effect of electroless Ni-B and Ni-W-B coatings on the corrosion-fatigue behaviour of 7075 Al alloy. Int. J. Fatigue 2021, 144, 106040. [Google Scholar] [CrossRef]
- Islam, T.; Rashed, H.M.M.A. Classification and Application of Plain Carbon Steels. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Li, H.; He, Y.; He, T.; Qing, D.; Luo, F.; Fan, Y.; Chen, X. Ni-W/BN(h) electrodeposited nanocomposite coating with functionally graded microstructure. J. Alloys Compd. 2017, 704, 32–43. [Google Scholar] [CrossRef]
- Shaik, S.; Basu, A. Effect of multiple dissimilar nanoparticles in Ni–W alloy matrix composite coating and evaluation of surface-mechanical, corrosion, and hydrophobic properties. Mater. Chem. Phys. 2022, 278, 125585. [Google Scholar] [CrossRef]
- Robin, A.; de Santana, J.C.P.; Sartori, A.F. Co-electrodeposition and characterization of Cu–Si3N4 composite coatings. Surf. Coat. Technol. 2011, 205, 4596–4601. [Google Scholar] [CrossRef]
- Balaraju, J.; Selvi, V.E.; Grips, V.W.; Rajam, K. Electrochemical studies on electroless ternary and quaternary Ni–P based alloys. Electrochim. Acta 2006, 52, 1064–1074. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, H.; Li, M.-K.; Shi, Y.-L.; Huang, Y.; Li, H.-L. Preparation and properties of Ni/P/single-walled carbon nanotubes composite coatings by means of electroless plating. Thin Solid Films 2004, 466, 86–91. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.; Xiao, H.; Cheng, F.; Zhang, G.; Yi, G. Corrosion behavior of carbon nanotubes–Ni composite coating. Surf. Coatings Technol. 2005, 191, 351–356. [Google Scholar] [CrossRef]
- Younes, O.; Gileadi, E. Electroplating of Ni /W Alloys. J. Electrochem. Soc. 2002, 149, C100–C111. [Google Scholar] [CrossRef]
- Dehgahi, S.; Amini, R.; Alizadeh, M. Microstructure and corrosion resistance of Ni-Al2O3-SiC nanocomposite coatings produced by electrodeposition technique. J. Alloys Compd. 2017, 692, 622–628. [Google Scholar] [CrossRef]
- Ahmadiyeh, S.; Rasooli, A.; Hosseini, M.G.; Farhood, A. Superior corrosion and wear resistance of pulse plated Ni–W–B/SiC composite coatings. Mater. Chem. Phys. 2021, 270, 124761. [Google Scholar] [CrossRef]
- Ranganatha, S.; Venkatesha, T.V.; Vathsala, K. Electroless Ni–W–P Coating and Its Nano-WS2 Composite: Preparation and Properties. Ind. Eng. Chem. Res. 2012, 51, 7932–7940. [Google Scholar] [CrossRef]
- Ranganatha, S.; Venkatesha, T.V.; Vathsala, K. Development of high performance electroless Ni–P–HNT composite coatings. Appl. Surf. Sci. 2012, 263, 149–156. [Google Scholar] [CrossRef]

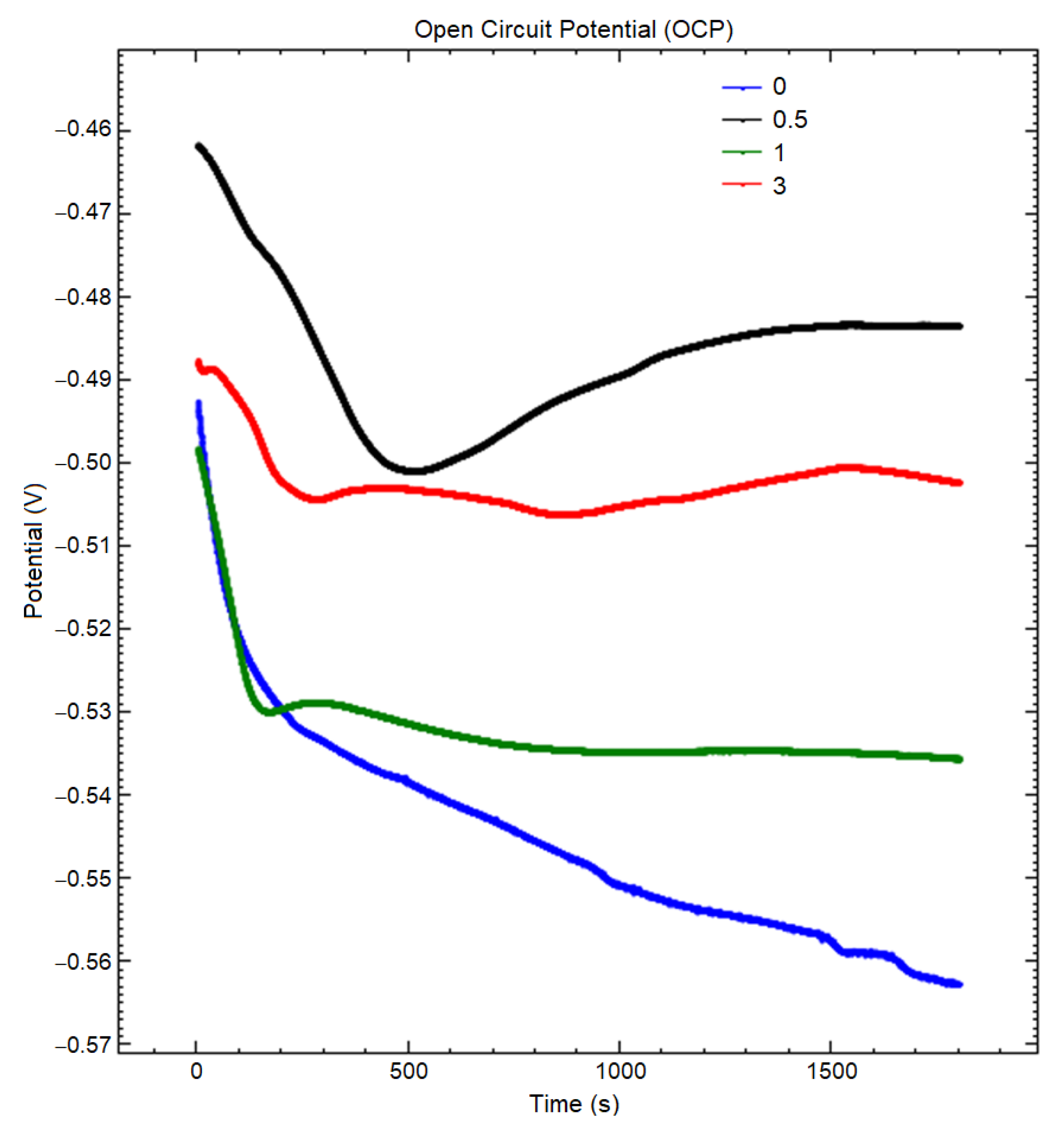

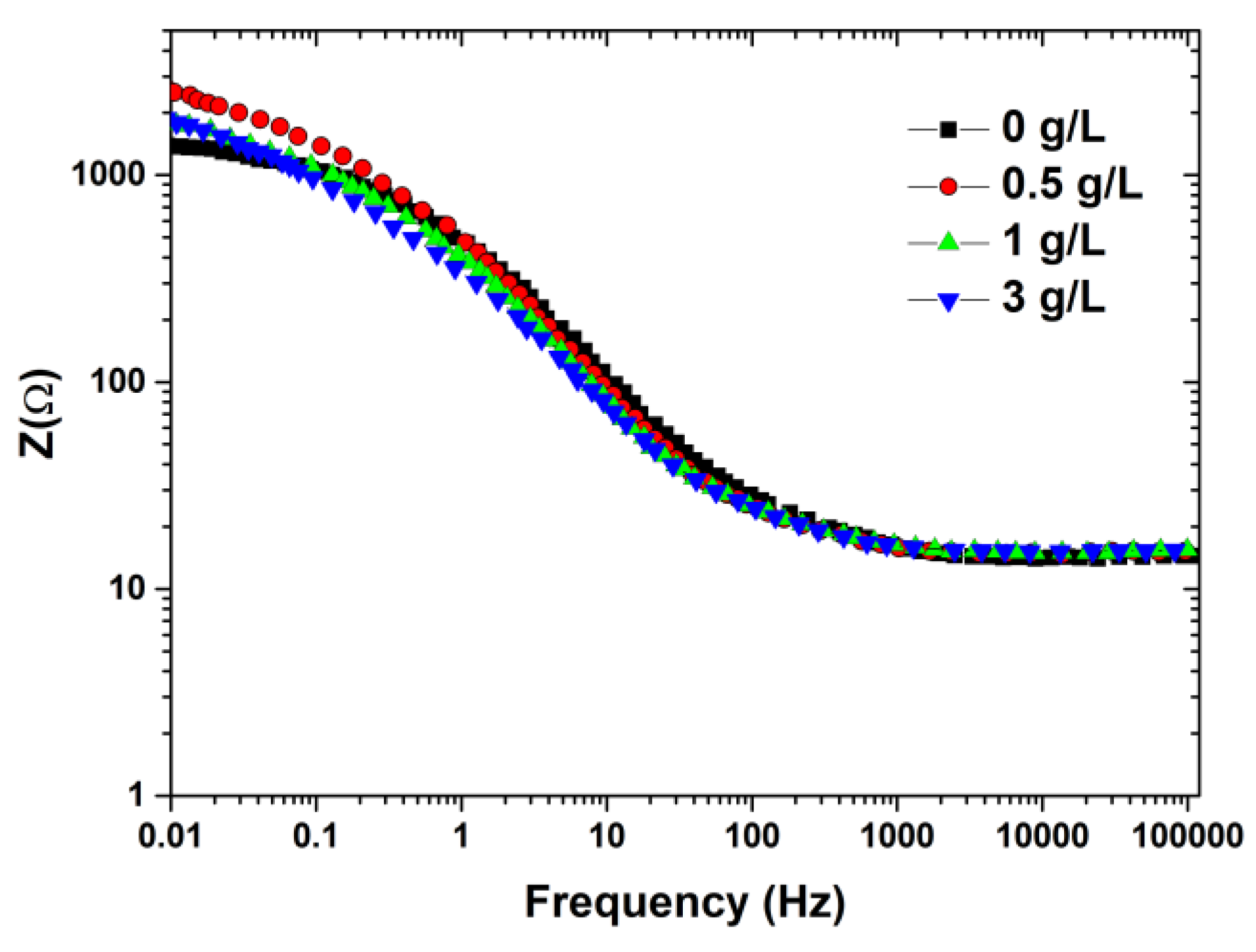
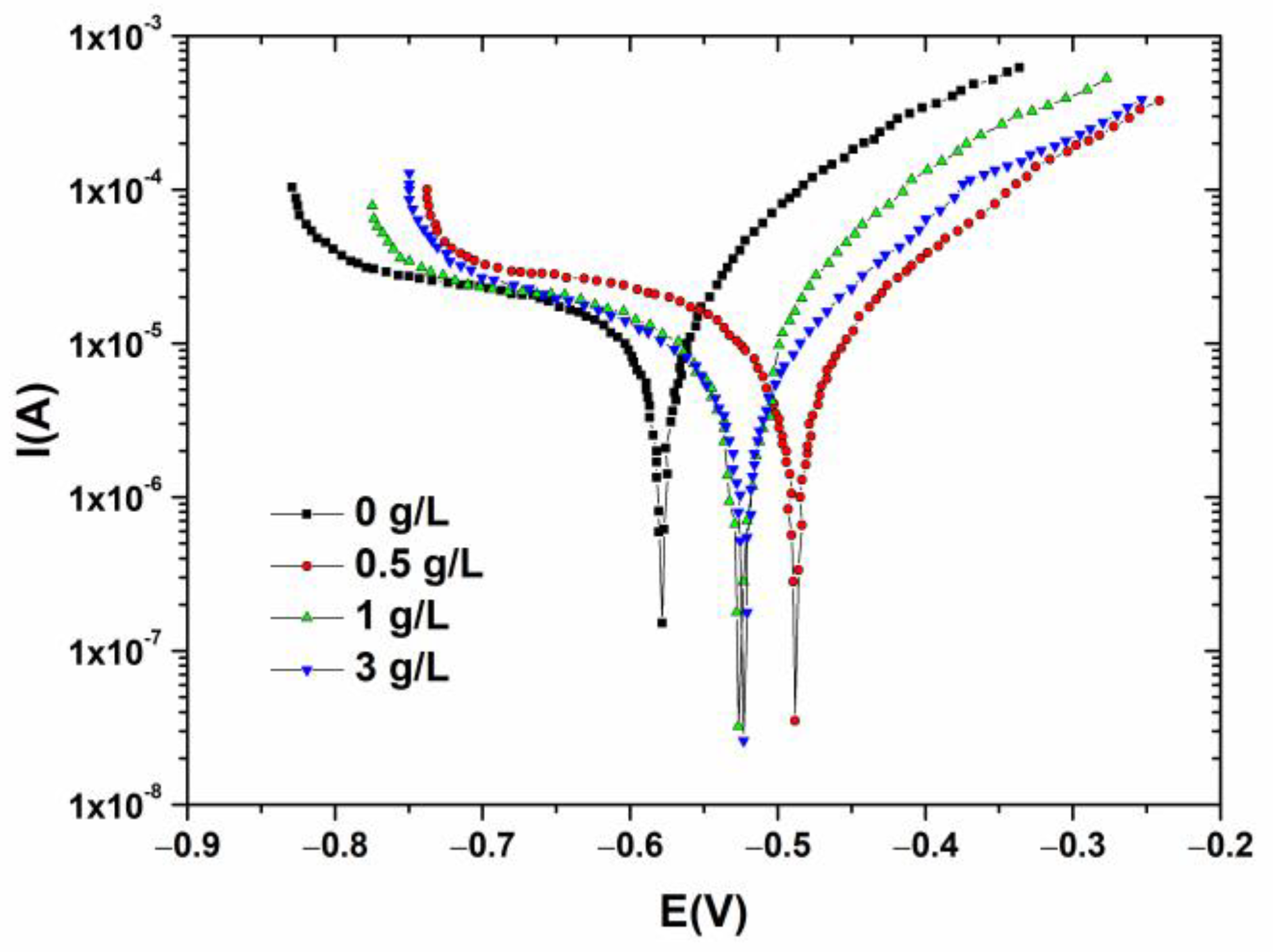
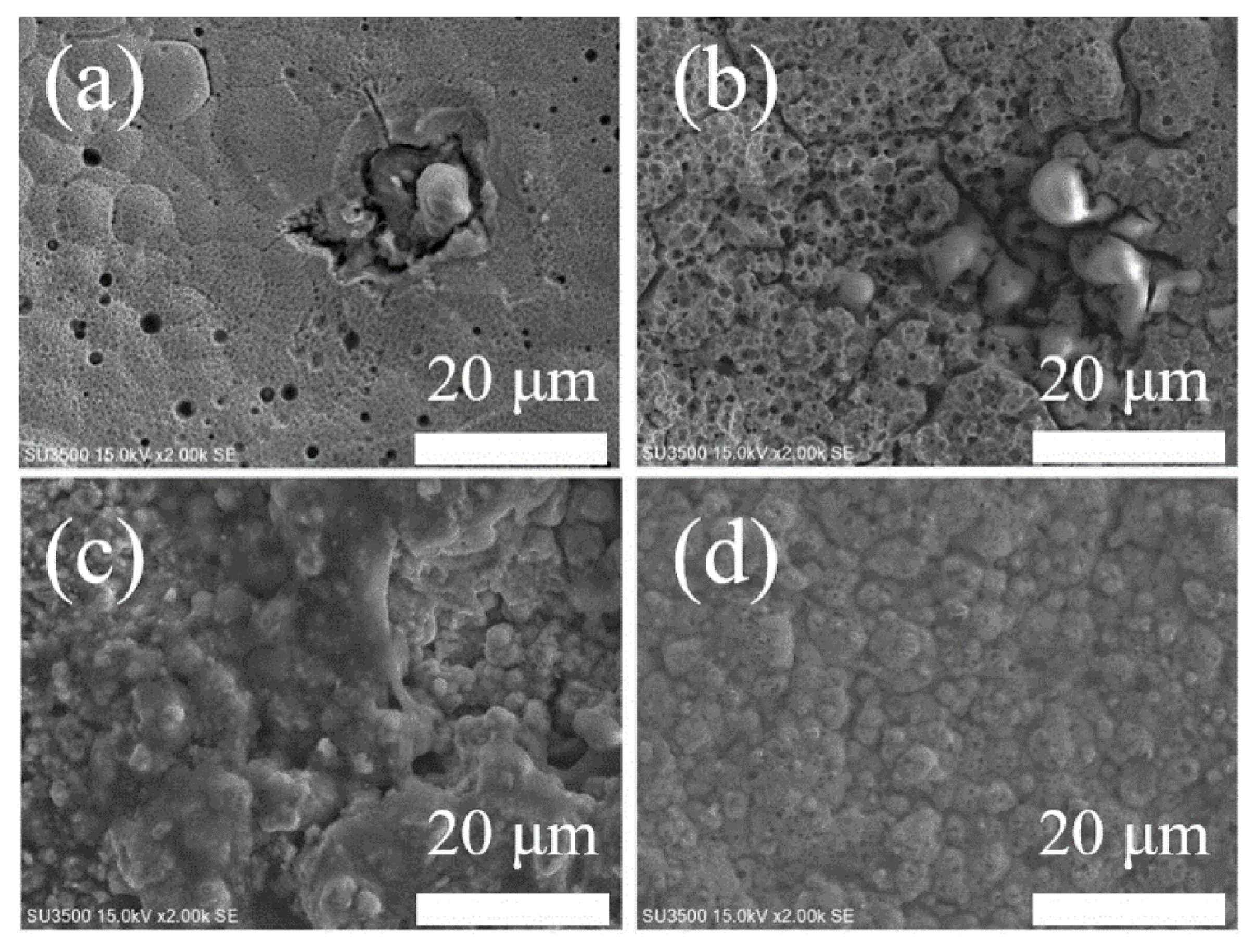

| Boron (g/L) | OCP vs. SCE(V) | Ecorr vs. SCE (V) | Icorr (µA/cm2) |
|---|---|---|---|
| 0 | −0.562 | −0.579 | 25.594 |
| 0.5 | −0.483 | −0.489 | 5.869 |
| 1 | −0.515 | −0.529 | 3.070 |
| 3 | −0.502 | −0.524 | 5.365 |
| Boron (g/L) | Initial Weight (g) | After Immersion (g) | Weight Loss (g) |
|---|---|---|---|
| 0 | 4.9038 | 4.8925 | 0.0113 |
| 0.5 | 4.8663 | 4.8652 | 0.0011 |
| 1 | 4.8962 | 4.8943 | 0.0019 |
| 3 | 4.6225 | 4.6188 | 0.0037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiatwisarnkij, N.; Mahattanatawee, S.; Lothongkum, G.; Qin, J. Effect of Amorphous Boron on the Microstructure and Corrosion Properties of Ni-W Coatings. Coatings 2023, 13, 377. https://doi.org/10.3390/coatings13020377
Kiatwisarnkij N, Mahattanatawee S, Lothongkum G, Qin J. Effect of Amorphous Boron on the Microstructure and Corrosion Properties of Ni-W Coatings. Coatings. 2023; 13(2):377. https://doi.org/10.3390/coatings13020377
Chicago/Turabian StyleKiatwisarnkij, Napat, Suchet Mahattanatawee, Gobboon Lothongkum, and Jiaqian Qin. 2023. "Effect of Amorphous Boron on the Microstructure and Corrosion Properties of Ni-W Coatings" Coatings 13, no. 2: 377. https://doi.org/10.3390/coatings13020377




