Fabrication of MnCoS Thin Films Deposited by the SILAR Method with the Assistance of Surfactants and Supercapacitor Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Deposition of MnCoS Thin Films
2.3. Characterizations
2.4. Electrochemical Measurements
3. Results and Discussion
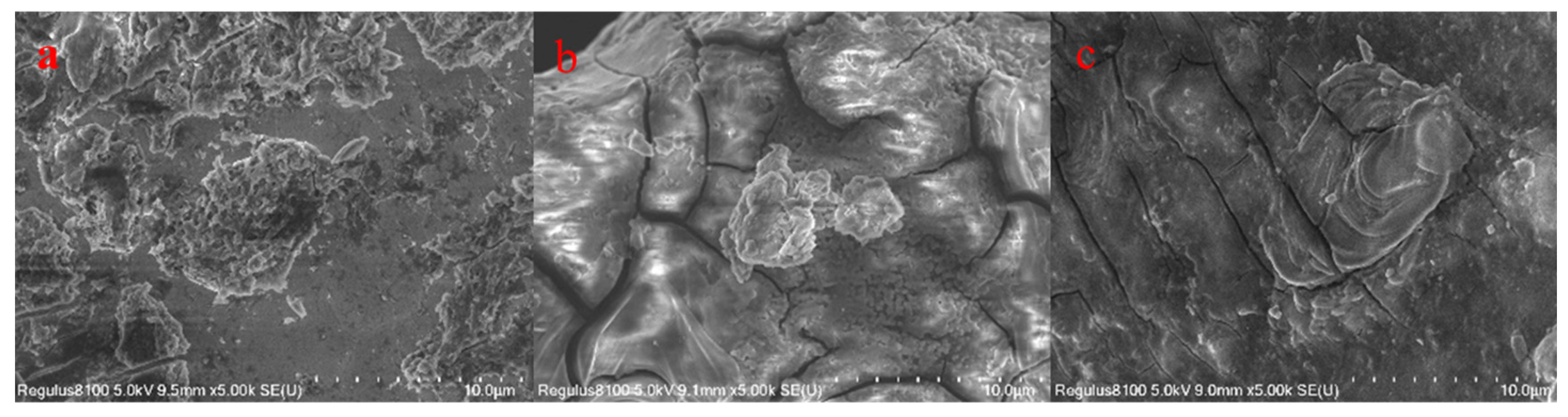
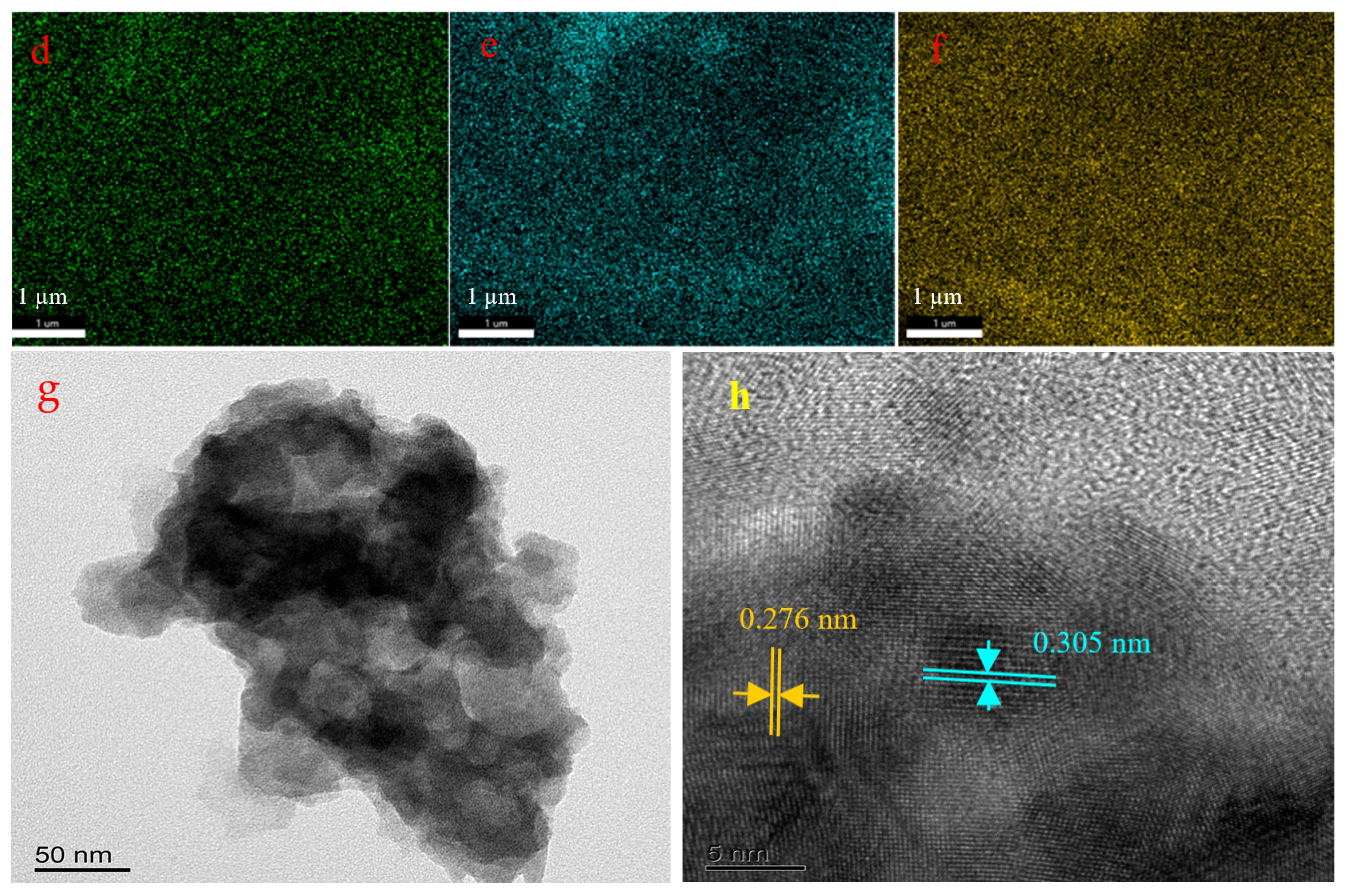
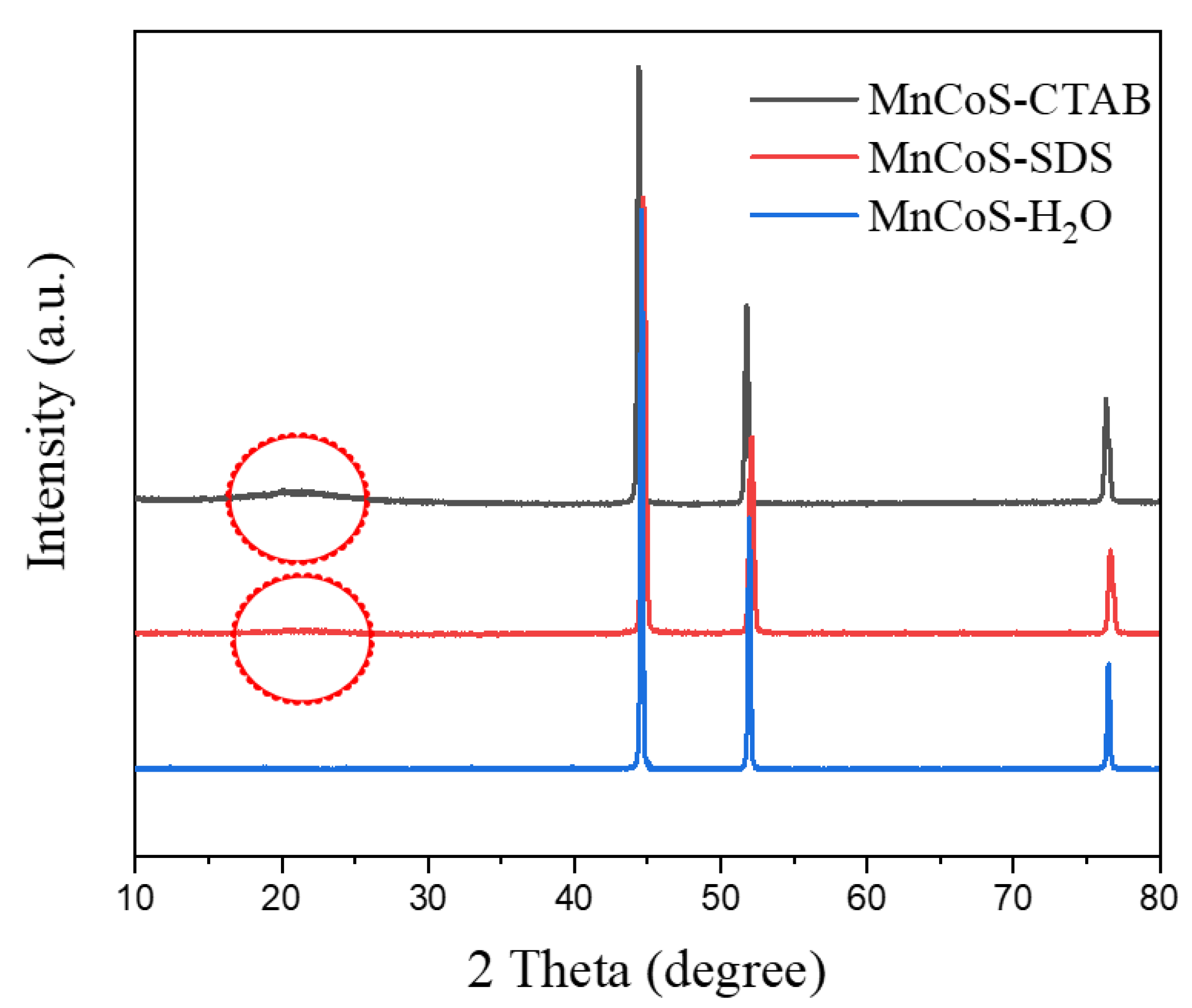
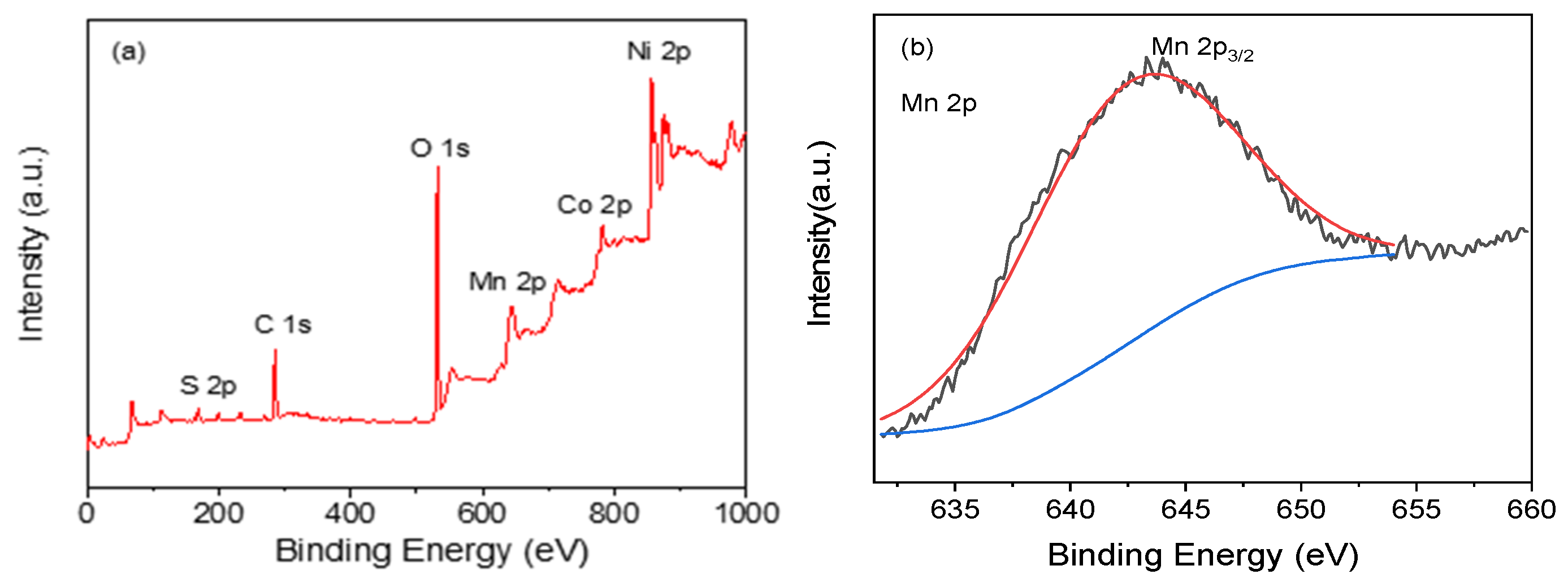
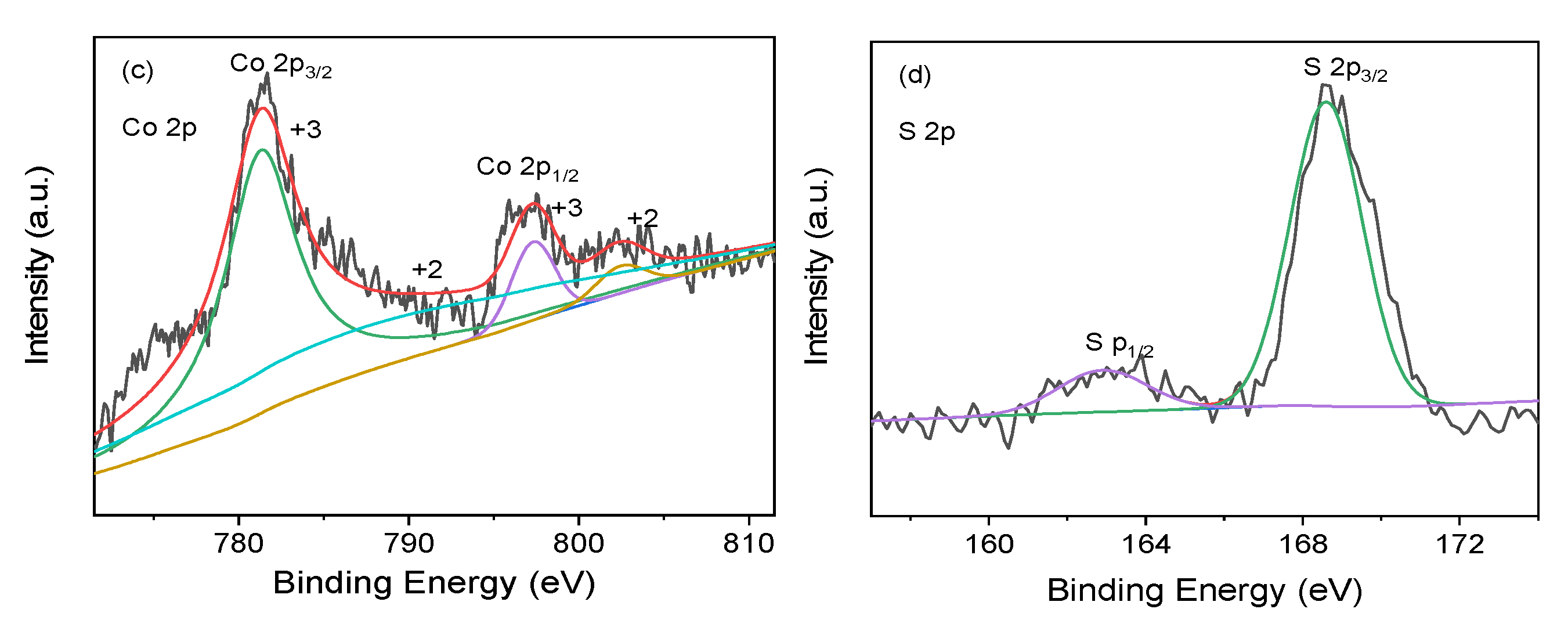
3.1. Characterization
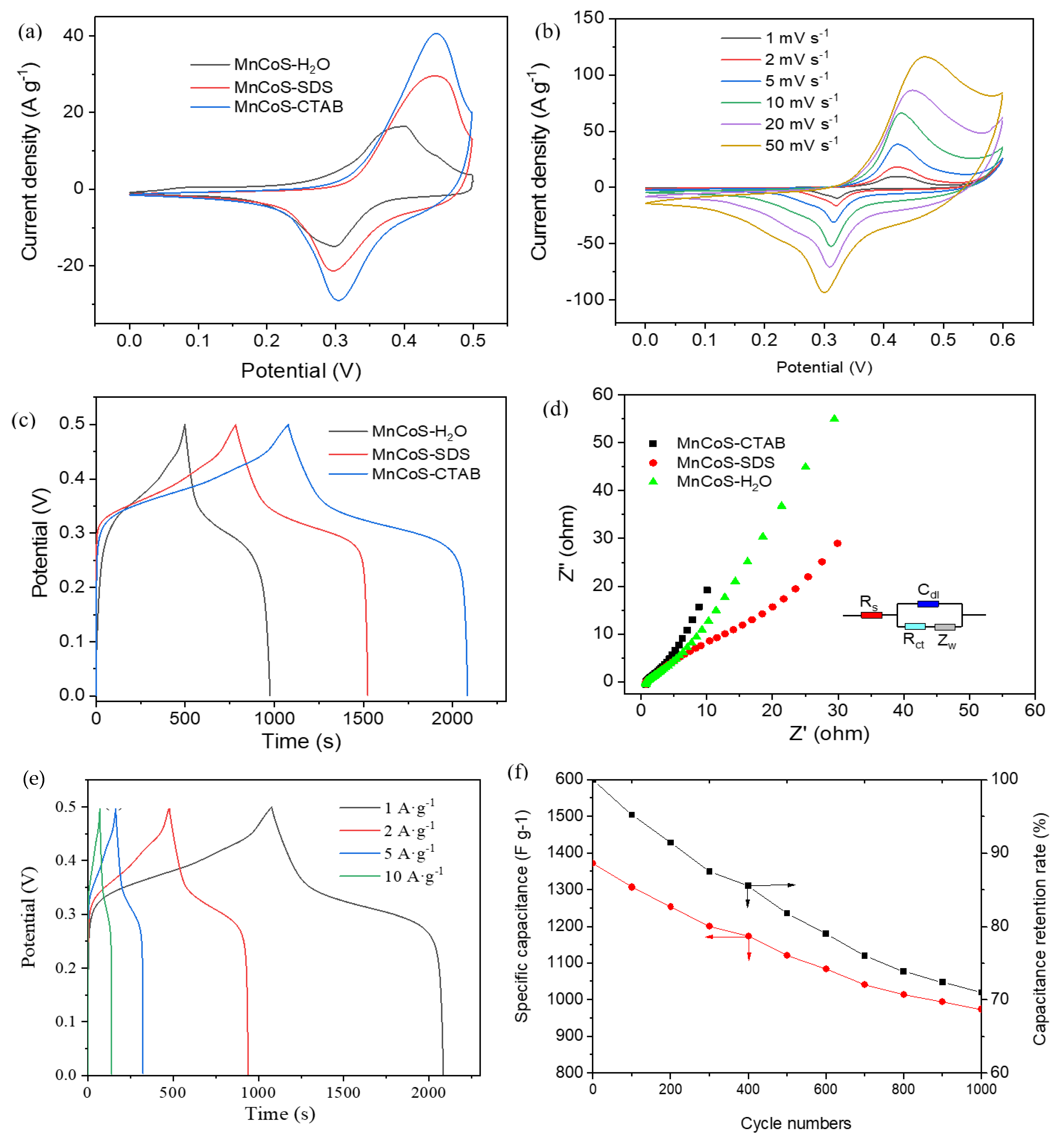
3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.T.N.; Ahmed, F.; Houda, S.; Manzoor, S.; Hasnain, K.; Zahra, M.; Hussain, R.; Ansari, M.Z.; Hegazy, H.H.; Ashiq, M.N. Facile synthesis of novel Ag@cerium zirconate heterostructure for efficient oxygen evolution reaction. Surf. Interfaces 2022, 35, 102410. [Google Scholar] [CrossRef]
- Alburaih, H.A.; Ansari, M.Z.; Abid, A.G.; Khosa, R.Y.; Ashiq, M.N.; Manzoor, S.; Aman, S.; Chaudhry, H.; Waheed, M.S.; Taha, T.A. Study on active sites of Mn-doped iron selenide on pencil electrode for electrocatalytic water splitting. J. Sol-Gel Sci. Technol. 2022, 22, 05961. [Google Scholar] [CrossRef]
- Owidah, Z.O.; Aman, S.; Abdullah, M.; Manzoor, S.; Fallatah, A.M.; Ibrahim, M.M.; Elnasr, T.A.; Ansari, M.Z. Metal oxide/carbon nanosheet arrays derivative of stacked metal organic frameworks for triggering oxygen evolution reaction. Ceram. Int. 2023, 49, 5936–5943. [Google Scholar] [CrossRef]
- Theerthagiria, J.; Senthilc, R.A.; Nithyadharseni, P.; Lee, S.J.; Durai, G.; Kuppusami, P.; Madhavan, J.; Choi, M.Y. Recent progress and emerging challenges of transition metal sulfides based composite electrodes for electrochemical supercapacitive energy storage. Ceram. Int. 2020, 46, 14317–14345. [Google Scholar]
- Kumar, S.; Saeed, G.; Zhu, L.; Hui, K.N.; Kim, N.H.; Lee, J.H. 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: A review. Chem. Eng. J. 2021, 403, 126352. [Google Scholar]
- Wang, Y.F.; Zhang, L.; Hou, H.Q.; Xu, W.H.; Duan, G.G.; He, S.J.; Liu, K.M.; Jiang, S.H. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar]
- Abdah, M.A.A.M.; Azman, N.H.N.; Kulandaivalu, S.; Sulaiman, Y. Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors. Mater. Design 2020, 186, 108199. [Google Scholar]
- Ray, A.; Roy, A.; Saha, S.; Ghosh, M.; Chowdhury, S.R.; Maiyalagan, T.; Bhattacharya, S.K.; Das, S. Electrochemical Energy Storage Properties of Ni-Mn-Oxide Electrodes for Advance Asymmetric Supercapacitor Application. Langmuir 2019, 35, 8257–8267. [Google Scholar] [CrossRef]
- Kandula, S.; RShrestha, K.; Kim, N.H.; Lee, J.H. Fabrication of a 3D Hierarchical Sandwich Co9S8 /alpha-MnS@N-C@MoS2 Nanowire Architectures as Advanced Electrode Material for High Performance Hybrid Supercapacitors. Small 2018, 14, e1800291. [Google Scholar] [CrossRef]
- Zou, J.; Xie, D.; Zhao, F.; Wu, H.L.; Niu, Y.; Li, Z.J.; Zou, Q.M.; Deng, F.; Zhang, Q.; Zeng, X.R. Microwave rapid synthesis of nickel cobalt sulfides/CNTs composites as superior cycling ability electrode materials for supercapacitors. J. Mater. Sci. 2020, 56, 1561–1576. [Google Scholar]
- Wang, Y.; Mayorga-Martinez, C.C.; Pumera, M. Polyaniline/MoSX Supercapacitor by Electrodeposition. Bull. Chem. Soc. Jpn. 2017, 90, 847–853. [Google Scholar] [CrossRef]
- Xin, B.; Zhao, Y.; Xu, C. A high mass loading electrode based on ultrathin Co3S4 nanosheets for high performance supercapacitor. J. Solid State Electrochem. 2016, 20, 2197–2205. [Google Scholar] [CrossRef]
- Wu, F.M.; Gao, J.P.; Zhai, X.G.; Xie, M.H.; Gao, C.J.; Sun, Y.; Liu, Y.; Yan, J. Hierarchical zinc cobalt sulfide flowers grown on nickel foam as binder-free electrodes for high-performance asymmetric supercapacitors. Electrochim. Acta 2019, 319, 859–868. [Google Scholar] [CrossRef]
- Zhang, G.H.; Xuan, H.C.; Wang, R.; Guan, Y.Y.; Li, H.S.; Liang, X.H.; Han, P.D.; Wu, Y.C. Enhanced supercapacitive performance in Ni3S2/MnS composites via an ion-exchange process for supercapacitor applications. Electrochim. Acta 2020, 353, 136517. [Google Scholar]
- Yu, X.Y.; David, L.; Xu, W. Mixed metal sulfides for electrochemical energy storage and conversion. Adv. Energy Mater. 2018, 8, 1701592. [Google Scholar]
- Yang, Q.; Liu, Y.; Yan, M.; Lei, Y.; Shi, W.D. MOF-derived hierarchical nanosheet arrays constructed by interconnected NiCo-alloy@NiCo-sulfide core-shell nanoparticles for high-performance asymmetric supercapacitors. Chem. Eng. J. 2019, 370, 666–676. [Google Scholar]
- Chen, Y.X.; Jing, C.; Fu, X.; Shen, M.; Li, K.L.; Li, K.L.; Liu, X.Y.; Yao, H.C.; Zhang, Y.X.; Yao, K.X. Synthesis of porous NiCoS nanosheets with Al leaching on ordered mesoporous carbon for high-performance supercapacitors. Chem. Eng. J. 2020, 384, 123367. [Google Scholar]
- Sangeetha, D.N.; Bhat, D.K.; Kumar, S.S.; Selvakumar, M. Improving hydrogen evolution reaction and capacitive properties on CoS/MoS2 decorated carbon fibers. Int. J. Hydrogen Energy 2020, 45, 7788–7800. [Google Scholar]
- Pan, Q.; Yang, X.; Yang, X.; Duan, L.F.; Zhao, L.J. Synthesis of a MnS/NixSy composite with nanoparticles coated on hexagonal sheet structures as an advanced electrode material for asymmetric supercapacitors. Rsc. Adv. 2018, 8, 17754–17763. [Google Scholar]
- Miao, Y.; Zhang, X.; Zhan, J.; Sui, Y.W.; Qi, J.Q.; Wei, F.X.; Meng, Q.K.; He, Y.Z.; Ren, Y.J.; Zhan, Z.Z. Hierarchical NiS@CoS with controllable core–shell structure by two–step strategy for supercapacitor electrodes. Adv. Mater. Interfaces 2019, 7, 1901618. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Khan, J. Optimization of cobalt-manganese binary sulfide for high performance supercapattery devices. Electrochim. Acta 2021, 368, 137529. [Google Scholar] [CrossRef]
- Zardkhoshoui, A.M.; Ameri, B.; Davarani, S.S.H. alpha-MnS@Co3S4 hollow nanospheres assembled from nanosheets for hybrid supercapacitors. Chem. Eng. J. 2021, 422, 129953. [Google Scholar] [CrossRef]
- Li, F.Z.; Zhen, C.; Dan, Z.; Sun, A.K.; Shi, P.; Liang, J.; He, Q.G. Neoteric hollow tubular MnS/Co3S4 hybrids as high-performance electrode materials for supercapacitors. Electrochim. Acta 2021, 390, 138893. [Google Scholar]
- Zhao, Y.; Shi, Z.; Li, H.; Wang, C.A. Designing pinecone-like and hierarchical manganese cobalt sulfides for advanced supercapacitor electrodes. J. Mater. Chem. A 2018, 6, 12782–12793. [Google Scholar] [CrossRef]
- Liu, H.M.; Li, Z.; Yao, Z.X.; Liu, Y.S.; Zhang, Q.F.; Sun, Y.J.; Li, Z. Designed MnS/Co9S8 micro-flowers composites with serrate edges as high-performance electrodes for asymmetric supercapacitor. J. Colloid Interf. Sci. 2019, 551, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, C.; Guo, X.; Kong, R.M.; Qu, F.L. A MnCo2S4 nanowire array as an earth-abundant electrocatalyst for an efficient oxygen evolution reaction under alkaline conditions. J. Mater. Chem. A 2017, 5, 17211–17215. [Google Scholar] [CrossRef]
- Saravanakumar, B.; Jayaseelan, S.S.; Seo, M.K.; Kim, H.Y.; Kim, B.S. NiCo2S4 nanosheet-decorated 3D, porous Ni film@Ni wire electrode materials for all solid-state asymmetric supercapacitor applications. Nanoscale 2017, 9, 18819–18834. [Google Scholar] [CrossRef]
- Soonmin, H. Recent advances in the growth and characterizations of SILAR-deposited thin films. Appl. Sci. 2022, 12, 8184. [Google Scholar] [CrossRef]
- Bagwade, P.P.; Malavekar, D.B.; Ubale, S.B.; Ghogare, T.T.; Bulakhe, R.N.; In, I.; Patil, U.M.; Lokhande, C.D. Characterization of Dy2S3 thin films deposited by successive ionic layer adsorption and reaction (SILAR) method. Solid State Sci. 2021, 119, 106693. [Google Scholar] [CrossRef]
- Khot, S.D.; Malavekar, D.B.; Nikam, R.P.; Ubale, S.B.; Bagwade, P.P.; Patil, D.J.; Lokhande, V.C.; Lokhande, C.D. SILAR synthesized dysprosium selenide (Dy2Se3) thin films for hybrid electrochemical capacitors. Synth. Met. 2022, 287, 117075. [Google Scholar] [CrossRef]
- Katkar, P.K.; Padalkar, N.S.; Padalkar, N.S.; Jeon, J.H.; Sheikh, Z.A.; Jerng, S.K.; Na, H.R.; Lee, S.; Chun, S.H. Development of amorphous Fe-doped nickel-cobalt phosphate (FexNiCo(PO4)(2)) nanostructure for enhanced performance of solid-state asymmetric supercapacitors. Int. J. Energy Res. 2022, 46, 12039–12056. [Google Scholar] [CrossRef]
- Patil, V.V.; Pujari, S.S.; Bhosale, S.B.; Kumbhar, S.S.; Parale, V.G.; Gunjakar, J.L.; Park, H.-H.; Lokhande, C.D.; Mali, M.G.; Mhamane, D.S.; et al. Hydrous and amorphous cobalt phosphate thin-film electrodes synthesized by the SILAR method for high-performing flexible hybrid energy storage devices. Energy Fuels 2022, 36, 12791–12806. [Google Scholar] [CrossRef]
- Patil, A.S.; Patil, M.D.; Lohar, G.M.; Jadhav, S.T.; Fulari, V.J. Supercapacitive properties of CuO thin films using modified SILAR method. Ionics 2017, 23, 1259–1266. [Google Scholar] [CrossRef]
- ElZein, B.; Abulikemu, M.; Barham, A.S.; Jabbour, G.E. In situ growth of PbS nanoparticles without organic linker on ZnO nanostructures via successive ionic layer adsorption and reaction (SILAR). Coatings 2022, 12, 1486. [Google Scholar] [CrossRef]
- Seong, C.; Mane, P.; Bae, H.; Lee, S.; Kang, S.H.; Ryu, S.W.; Ha, J.S. Simple fabrication of BiVO4 thin films synthesized by modified SILAR method: Effect of film thickness. J. Electrochem. Soc. 2022, 169, 016501. [Google Scholar] [CrossRef]
- Patil, D.J.; Malavekar, D.B.; Lokhande, C.D. Binder-free synthesis of mesoporous nickel tungstate for aqueous asymmetric supercapacitor applications: Effect of film thickness. Energy Technol. 2022, 10, 2600295. [Google Scholar] [CrossRef]
- Admuthe, A.; Kumbhar, S.S.; Chougule, S.K.; Padasare, G.N.; Tonape, M.M. Synthesis and characterization of MnS thin film at room temperature for supercapacitor application. Macromol. Symp. 2020, 392, 2000186. [Google Scholar] [CrossRef]
- Anish, R.N.; Sandhyarani, N. SILAR deposited nickel sulphide-nickel hydroxide nanocomposite for high performance asymmetric supercapacitor. Electrochim. Acta. 2020, 356, 136844. [Google Scholar]
- Xu, J.; Xiang, C.L.; Fang, S.W.; Zhu, L.P.; Xu, F.; Sun, L.X.; Zou, Y.J.; Zhang, J. Template strategy to synthesize porous Mn-Co-S nanospheres electrode for high-performance supercapacitors. Energy Storage 2021, 44, 103267. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Wu, Y.; Rui, M.C.; Zeng, H.B. Two-dimensional, porous nickel-cobalt sulfide for high-performance asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 22997–23005. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, B.; Gu, J.; Li, Y.; Ma, C.; Zhao, J.; Wang, T.Y.; Cheng, C.J. One-step synthesis of nickel cobalt sulphides particles: Tuning the composition for high performance supercapacitors. RSC Adv. 2016, 6, 58916–58924. [Google Scholar] [CrossRef]
- Liu, H.; Guo, Z.; Wang, X.; Hao, J.; Lian, J.S. CuS/MnS composite hexagonal nanosheet clusters: Synthesis and enhanced pseudocapacitive properties. Electrochem. Acta 2018, 271, 425–432. [Google Scholar] [CrossRef]
- Liu, S.; Jun, S.C. Hierarchical manganese cobalt sulfide core-shell nanostructures for high-performance asymmetric supercapacitors. J. Power Sources 2017, 342, 629–637. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Zakar, S.; Haider, S.S.; Afzal, A.M.; Iqbal, M.J.; Kamran, M.A.; Numan, A. Electrodeposited CuMnS and CoMnS electrodes for high performance asymmetric supercapacitor devices. Ceram. Int. 2020, 46, 21343–21350. [Google Scholar] [CrossRef]
- Lee, D.; Xia, Q.X.; Mane, R.S.; Yun, J.M.; Kim, K.H. Direct successive ionic layer adsorption and reaction (SILAR) synthesis of nickel and cobalt hydroxide composites for supercapacitor applications. J. Alloys Compd. 2017, 722, 809–817. [Google Scholar] [CrossRef]
- Ubale, S.B.; Kale, S.B.; Mane, V.J.; Yun, J.M.; Kim, K.H. SILAR synthesized nanostructured ytterbium sulfide thin film electrodes for symmetric supercapacitors. J. Solid State Electrochem. 2021, 25, 1753–1764. [Google Scholar] [CrossRef]
- Shinde, S.K.; Ramesh, S.; Yadav, H.M. Novel approach to synthesize NiCo2S4 composite for high performance supercapacitor application with different molar ratio of Ni and Co. Sci. Rep. 2019, 9, 13717. [Google Scholar] [CrossRef]
- Raut, S.S.; Dhobale, J.A.; Sankapal, B.R. SILAR deposited Bi2S3 thin film towards electrochemical supercapacitor. Phys.-Low-Dimens. Syst. Nanostruct. 2017, 87, 209–212. [Google Scholar] [CrossRef]

| Electrodes | Synthesis Method | Specific Capacity | Reference |
|---|---|---|---|
| CoS/MoS | hydrothermal | 733 F g−1 at 0.5 A g−1 | [18] |
| MnS/NixSy | hydrothermal | 1073.8 F g−1 at 1 A g−1 | [19] |
| NiS/CoS nanosheet | hydrothermal | 1210 F g−1 at 1 A g−1 | [20] |
| Co0.5Mn0.5S | sonochemical approach | 893.3 F g−1 at 1 A g−1 | [21] |
| NiCo2S4 | micelle-confined growth | 1304 F g−1 at 2 A g−1 | [40] |
| NiS2−CoS2 | hydrothermal | 954.3 F g−1 at 1 A g−1 | [41] |
| CuS/MnS composite | hydrothermal | 1144 F g−1 at 1 A g−1 | [42] |
| MnCo2S4 core/shell | hydrothermal | 2067 F g−1 at 1 A g−1 | [43] |
| CuMnS thin film CoMnS thin film | electrodeposited electrodeposited | 1691 F g−1 at 10 A g−1 2290 F g−1 at 10 A g−1 | [44] |
| MnS thin film | SILAR | 632 F g−1 at 0.2 A g−1 | [37] |
| NiCo(OH)(2)thin film | SILAR | 1113 F g−1 at 1 A g−1 | [45] |
| Yb2S3 thin film | SILAR | 181 F g−1 at 0.5 A g−1 | [46] |
| NiCo2S4 | SILAR | 1247 F g−1 at 1 A g−1 | [47] |
| Bi2S3 thin film | SILAR | 289 F g−1 at 5 A g−1 | [48] |
| MnCoS-CTAB thin film | SILAR | 2029.8 F·g−1 at 1 A g−1 1371.9 F·g−1 at 10 A g−1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Chen, Q.; Gong, F.; Li, Y. Fabrication of MnCoS Thin Films Deposited by the SILAR Method with the Assistance of Surfactants and Supercapacitor Properties. Coatings 2023, 13, 908. https://doi.org/10.3390/coatings13050908
Yang Q, Chen Q, Gong F, Li Y. Fabrication of MnCoS Thin Films Deposited by the SILAR Method with the Assistance of Surfactants and Supercapacitor Properties. Coatings. 2023; 13(5):908. https://doi.org/10.3390/coatings13050908
Chicago/Turabian StyleYang, Qifan, Qianhui Chen, Fuzhong Gong, and Yanlin Li. 2023. "Fabrication of MnCoS Thin Films Deposited by the SILAR Method with the Assistance of Surfactants and Supercapacitor Properties" Coatings 13, no. 5: 908. https://doi.org/10.3390/coatings13050908





