Sol–Gel Silica Coatings for Corrosion Protection of Aluminum Parts Manufactured by Selective Laser Melting (SLM) Technology
Abstract
:1. Introduction
2. Materials and Methods
- Adhesion, according to ASTM d3359-17
- Thickness analysis, according to UNI EN ISO 2808:2019
- Neutral salt-spray test, according to MIL-STD-810G w/change 1 Method 509.6
- (a)
- 24 h of salt spray (T 35 °C, pH 7.0);
- (b)
- 24 h under drying conditions at 23 °C +/−5 °C and humidity of 50%UR +/−10%UR;
- (c)
- 24 h salt spray (T 35 °C, pH 7.0);
- (d)
- 24 h under drying conditions at 23 °C +/−5 °C and humidity of 50%UR +/−10%UR.
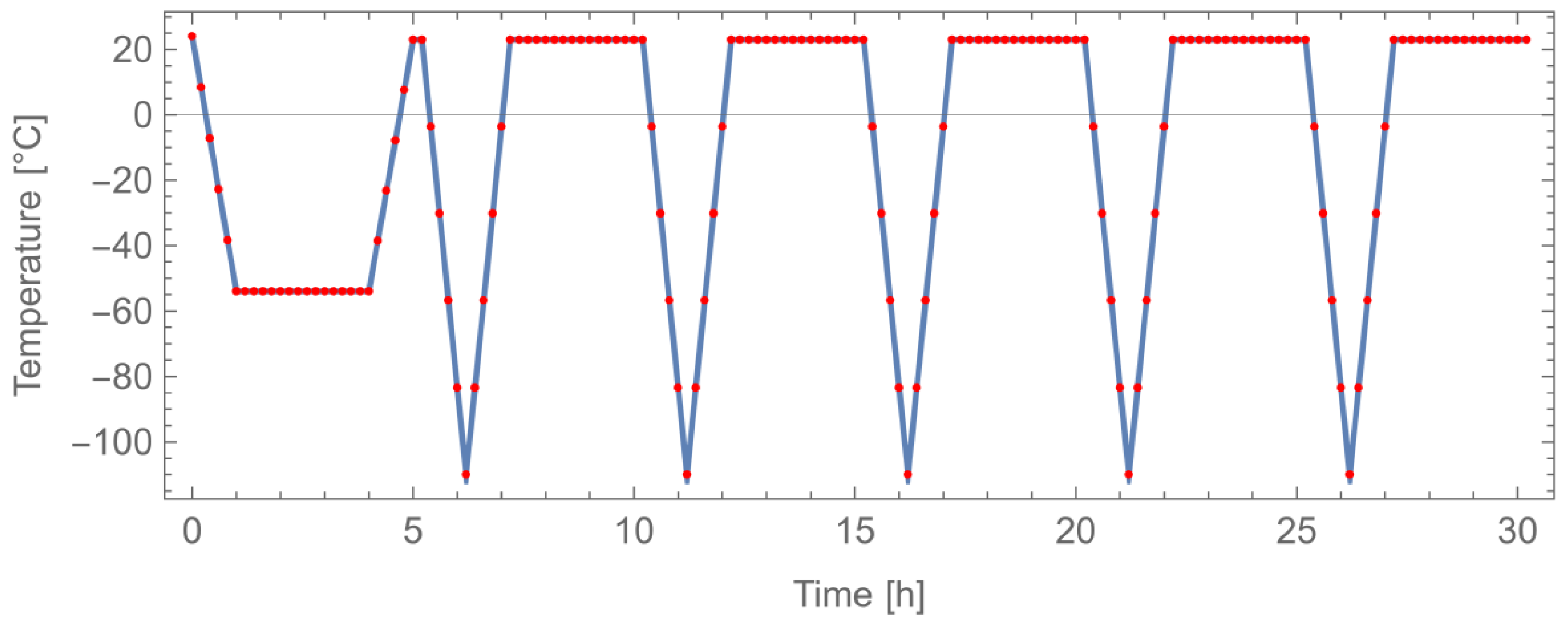
- Determination of thermal-cycling resistance
- Ramp of 1 h down to the temperature of −54 °C;
- Maintain the temperature of −54 °C for 3 h;
- Ramp of 1 h up to the temperature of 23 °C;
- Maintain the temperature of 23 °C for 10 min.
- Ramp of 1 h down to the temperature of −110 °C;
- Maintain the temperature of −110 °C for 3 min;
- Ramp of 1 h up to the temperature of 23 °C;
- Maintain the temperature of 23 °C for 3 h.
3. Results and Discussion
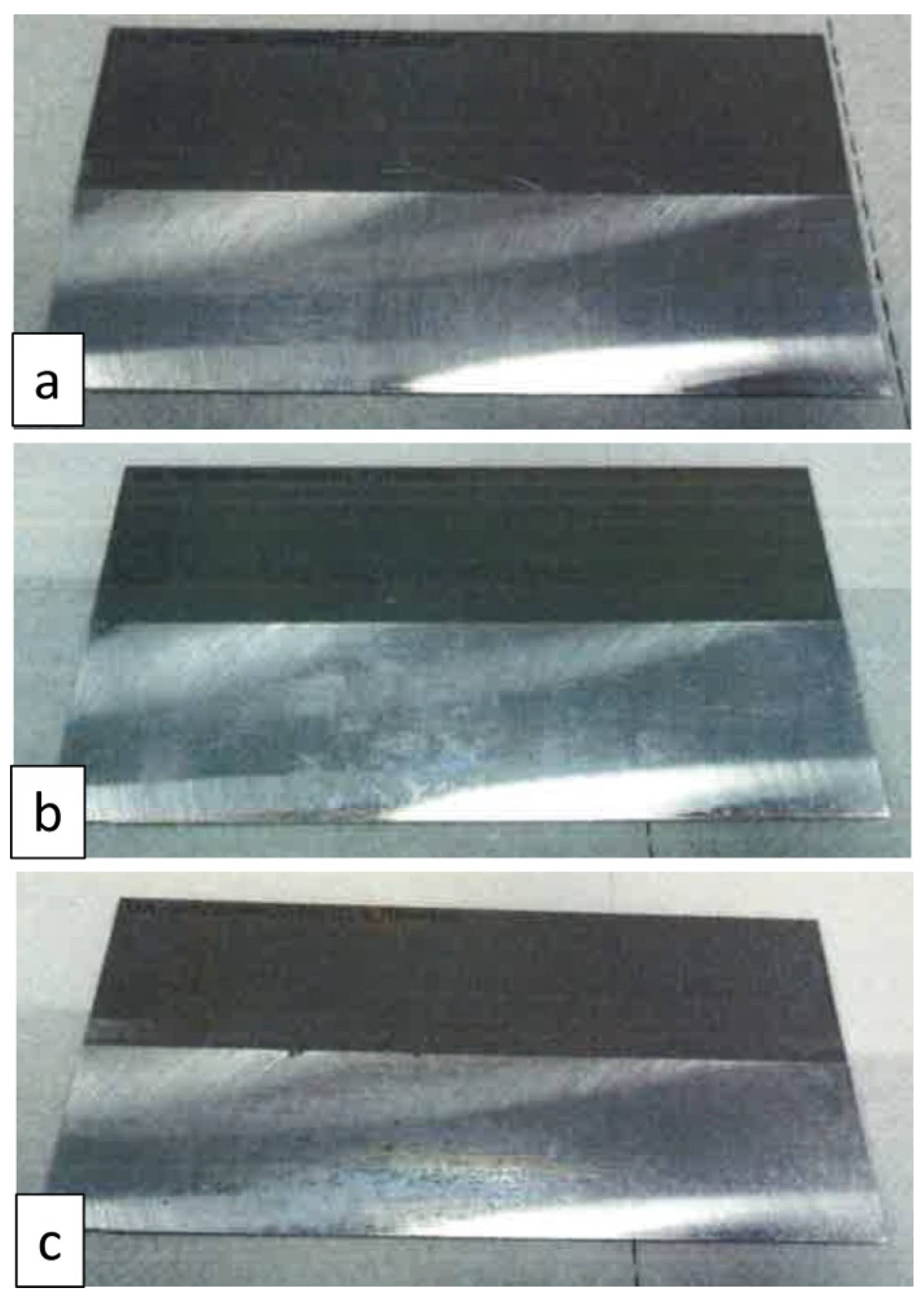
3.1. Salt-Spray Test
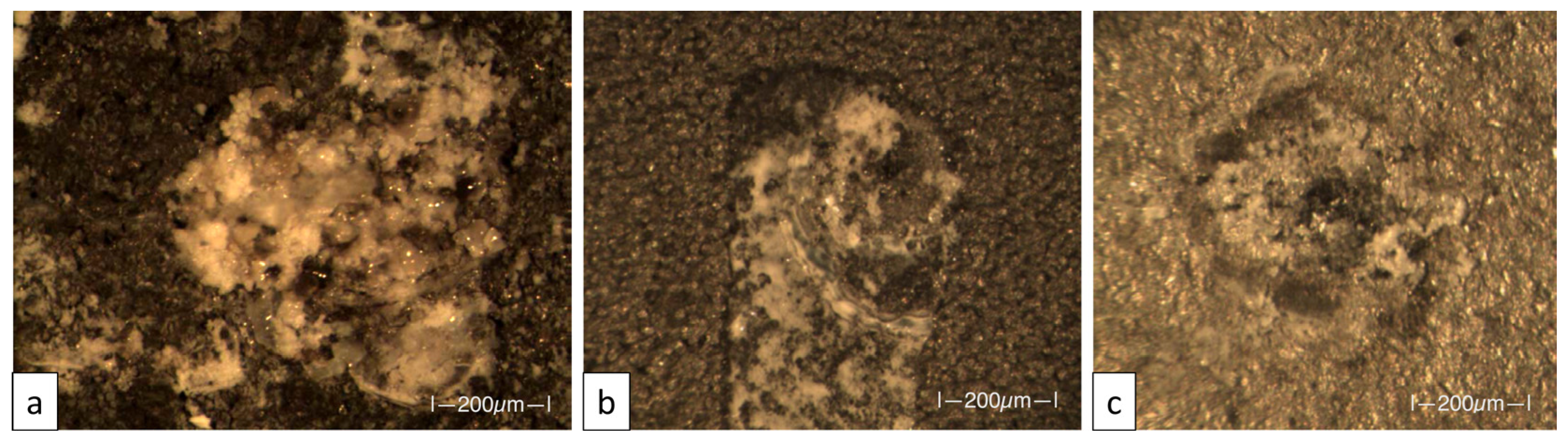
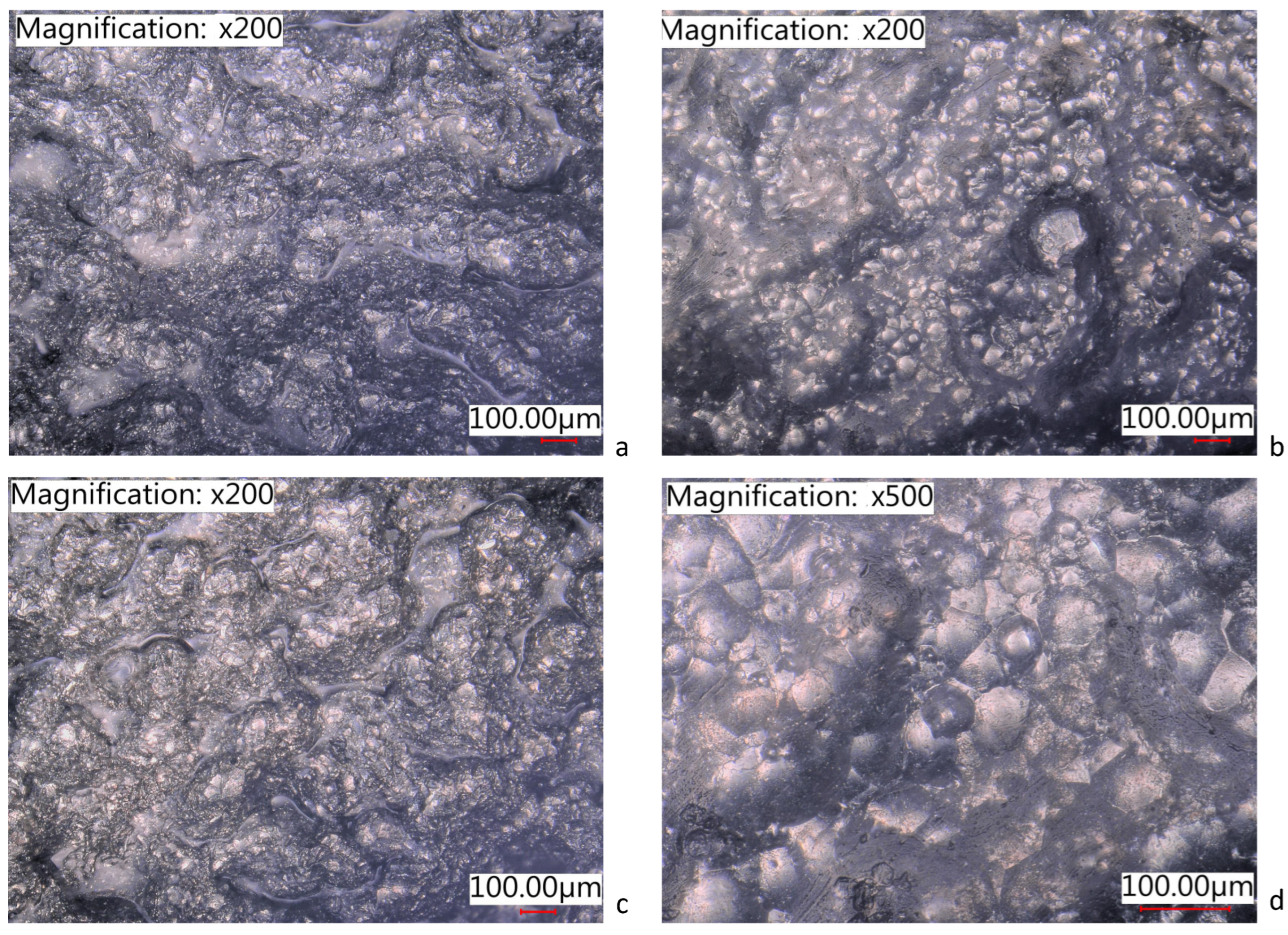
3.2. Adhesion Test
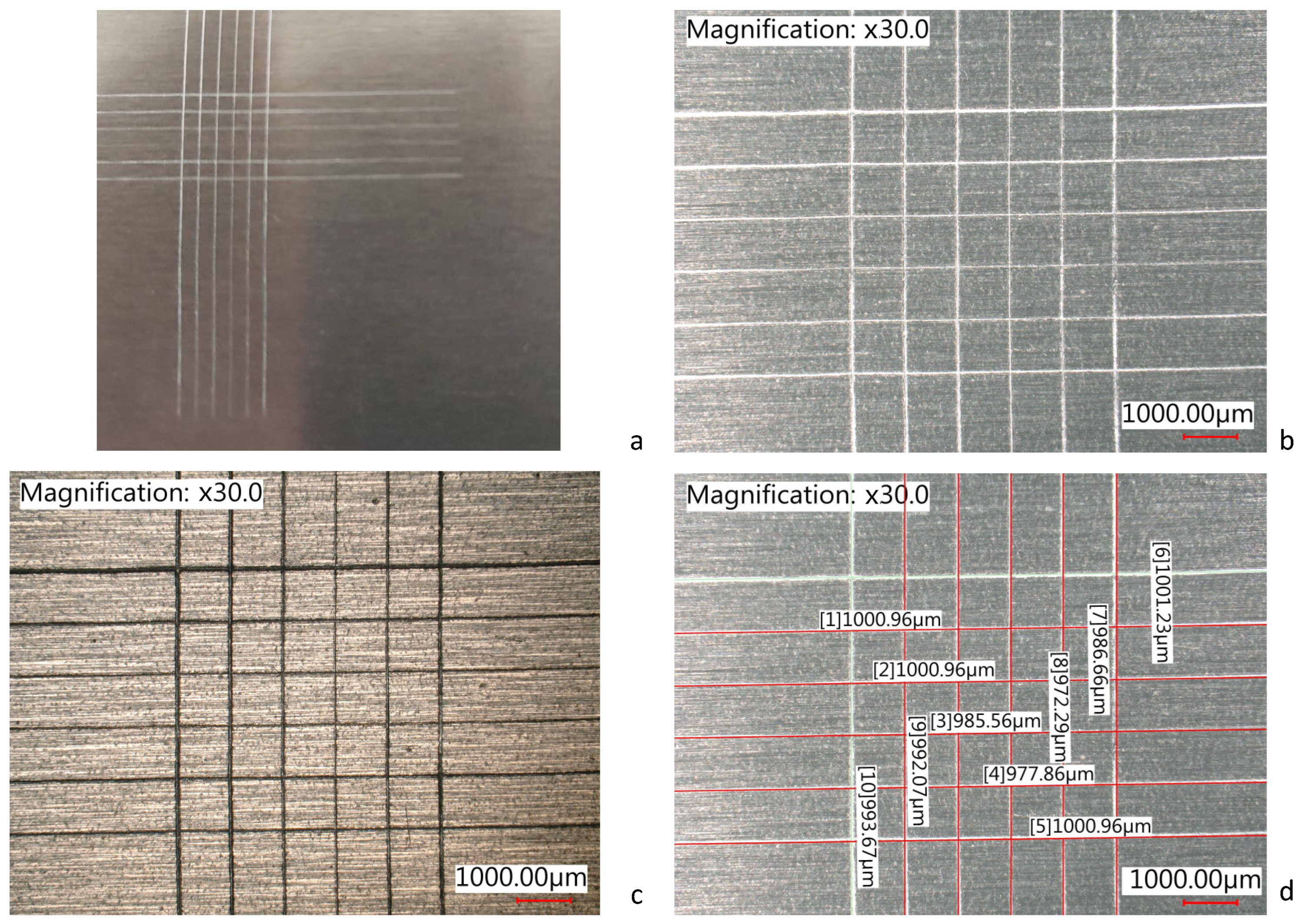
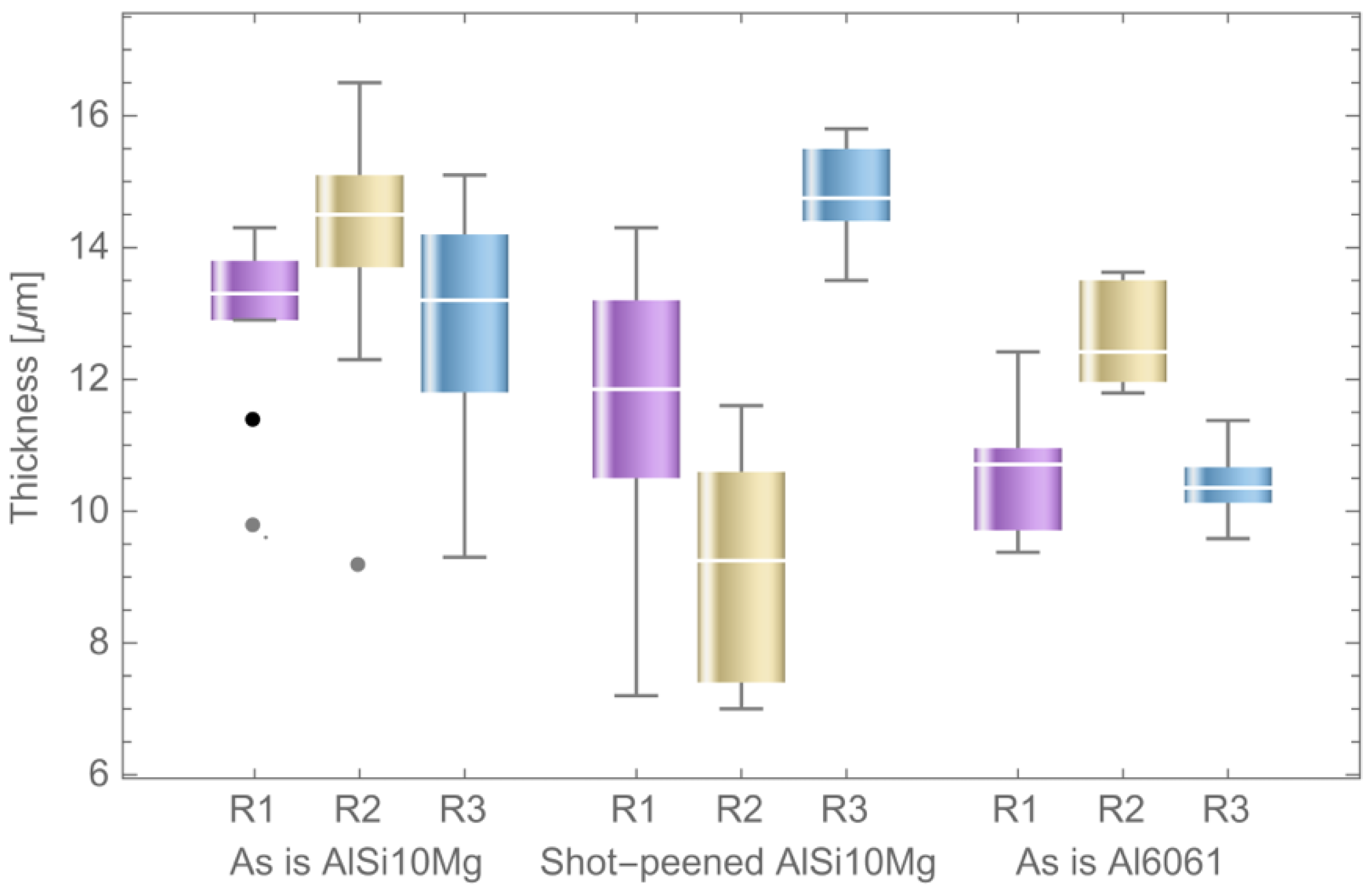
3.3. Thickness Analysis
3.4. Neutral Salt-Spray Test
3.5. Determination of Thermal Cycling Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, X.; Zhou, J.; Lin, Y.; Li, Y.; Yu, H.; Liu, Y. Smart manufacturing based on cyber-physical systems and beyond. J. Intell. Manuf. 2019, 30, 2805–2817. [Google Scholar] [CrossRef] [Green Version]
- Levitt, T. The Globalization of Markets. Harvard Business Review, May–June 1983; 92–102. [Google Scholar]
- Elshkaki, A. The implications of material and energy efficiencies for the climate change mitigation potential of global energy transition scenarios. Energy 2023, 267, 126596. [Google Scholar] [CrossRef]
- Yang, M.; Chen, L.; Wang, J.; Msigwa, G.; Osman, A.I.; Fawzy, S.; Rooney, D.W.; Yap, P.-S. Circular economy strategies for combating climate change and other environmental issues. Environ. Chem. Lett. 2023, 21, 55–80. [Google Scholar] [CrossRef]
- Wang, C.; Tang, J.; Yu, H.; Wang, Y.; Li, H.; Xu, S.; Li, G.; Zhou, Q. Microplastic Pollution in the Soil Environment: Characteristics, Influencing Factors, and Risks. Sustainability 2022, 14, 13405. [Google Scholar] [CrossRef]
- Sajjad, M.; Huang, Q.; Khan, S.; Khan, M.A.; Liu, Y.; Wang, J.; Lian, F.; Wang, Q.; Guo, G. Microplastics in the soil environment: A critical review. Environ. Technol. Innov. 2022, 27, 102408. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.W.; Stucker, B.; Khorasani, M. Additive Manufacturing Technologies; Springer: New York, NY, USA, 2021; ISBN 978-3-030-56127-7. [Google Scholar] [CrossRef]
- Westerweel, B.; Basten, R.J.; Van Houtum, G.J. Traditional or Additive Manufacturing? Assessing Component Design Options through Lifecycle Cost Analysis. Eur. J. Oper. Res. 2018, 2270, 570–585. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Tang, Y.; Zhao, Y.F. A new part consolidation method to embrace the design freedom of additive manufacturing. J. Manuf. Process. 2015, 20, 444–449. [Google Scholar] [CrossRef] [Green Version]
- Debnath, B.; Shakur, M.S.; Tanjum, F.; Rahman, M.A.; Adnan, Z.H. Impact of Additive Manufacturing on the Supply Chain of Aerospace Spare Parts Industry—A Review. Logistics 2022, 6, 28. [Google Scholar] [CrossRef]
- Peng, T.; Kellens, K.; Tang, R.; Chen, C.; Chen, G. Sustainability of additive manufacturing: An overview on its energy demand and environmental impact. Addit. Manuf. 2018, 21, 694–704. [Google Scholar] [CrossRef]
- Colorado, H.; Velásquez, E.; Monteiro, S. Sustainability of additive manufacturing: The circular economy of materials and environmental perspectives. J. Mater. Res. Technol. 2020, 9, 8221–8234. [Google Scholar] [CrossRef]
- Green, J.A. Aluminum Recycling and Processing for Energy Conservation and Sustainability; ASM International: Materials Park, OH, USA, 2007. [Google Scholar]
- Nordheim, E.; Barrasso, G. Sustainable development indicators of the European aluminium industry. J. Clean. Prod. 2007, 15, 275–279. [Google Scholar] [CrossRef]
- Fontaras, G.; Samaras, Z. On the way to 130gCO2/km—Estimating the future characteristics of the average European passenger car. Energy Policy 2010, 38, 1826–1833. [Google Scholar] [CrossRef]
- Yan, Q.; Song, B.; Shi, Y. Comparative study of performance comparison of AlSi10Mg alloy prepared by selective laser melting and casting. J. Mater. Sci. Technol. 2020, 41, 199–208. [Google Scholar] [CrossRef]
- Trevisan, F.; Calignano, F.; Lorusso, M.; Pakkanen, J.; Aversa, A.; Ambrosio, E.P.; Lombardi, M.; Fino, P.; Manfredi, D. On the Selective Laser Melting (SLM) of the AlSi10Mg Alloy: Process, Microstructure, and Mechanical Properties. Materials 2017, 10, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneller, W.; Leitner, M.; Springer, S.; Grün, F.; Taschauer, M. Effect of HIP Treatment on Microstructure and Fatigue Strength of Selectively Laser Melted AlSi10Mg. J. Manuf. Mater. Process. 2019, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Tommasi, A.; Maillol, N.; Bertinetti, A.; Penchev, P.; Bajolet, J.; Gili, F.; Pullini, D.; Mataix, D.B. Influence of Surface Preparation and Heat Treatment on Mechanical Behavior of Hybrid Aluminum Parts Manufactured by a Combination of Laser Powder Bed Fusion and Conventional Manufacturing Processes. Metals 2021, 11, 522. [Google Scholar] [CrossRef]
- Osborne, J.H. Observations on chromate conversion coatings from a sol–gel perspective. Prog. Org. Coat. 2001, 41, 280–286. [Google Scholar] [CrossRef]
- Zhang, X.; van den Bos, C.; Sloof, W.G.; Hovestad, A.; Terryn, H.; de Wit, J. Comparison of the morphology and corrosion performance of Cr(VI)- and Cr(III)-based conversion coatings on zinc. Surf. Coat. Technol. 2005, 199, 92–104. [Google Scholar] [CrossRef]
- Apte, A.; Tare, V.; Bose, P. Extent of oxidation of Cr(III) to Cr(VI) under various conditions pertaining to natural environment. J. Hazard. Mater. 2006, 128, 164–174. [Google Scholar] [CrossRef]
- Asgari, H.; Baxter, C.; Hosseinkhani, K.; Mohammadi, M. On microstructure and mechanical properties of additively manufactured AlSi10Mg_200C using recycled powder. Mater. Sci. Eng. A 2017, 707, 148–158. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Boston, MA, USA, 1990. [Google Scholar] [CrossRef]
- Mutin, P.H.; Vioux, A. Nonhydrolytic processing of oxide-based materials: Simple routes to control homogeneity, morphology, and nanostructure. Chem. Mater. 2009, 21, 582–596. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, D.Z.; You, H.; Chun, J.S. Corrosion resistance properties of organic-inorganic hybrid coatings on 2024 aluminum alloy. Appl. Surf. Sci. 2005, 246, 82–89. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Serra, R.; Montemor, M.F.; Salvado, I.M.M.; Ferreira, M.G.S. Corrosion protective properties of nanostructured sol–gel hybrid coatings to AA2024-T3. Surf. Coat. Technol. 2006, 200, 3084–3094. [Google Scholar] [CrossRef]
- Vreugdenhil, A.J.; Balbyshev, V.N.; Donley, M.S. Nanostructured silicon sol-gel surface treatments for Al 2024-T3 protection. J. Coat. Technol. 2001, 73, 35–43. [Google Scholar] [CrossRef]
- Voevodin, N.N.; Kurdziel, J.W.; Mantz, R. Corrosion protection for aerospace aluminum alloys by Modified Self-assembled NAnophase Particle (MSNAP) sol–gel. Surf. Coat. Technol. 2006, 201, 1080–1084. [Google Scholar] [CrossRef]
- ASTM B117-19; Standard Practice for Operating Salt Spray (Fog) Apparatus; Book of Standards Volume: 03.02. American National Standards Institute: Washington, DC, USA, 2019. [CrossRef]
- ISO 9227:2017; Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. ISO: Geneva, Switzerland, 2017.
- ASTM D3322-82(2017); Standard Practice for Testing Primers and Primer Surfacers Over Preformed Metal; Book of Standards Volume: 06.02. ASTM: West Conshohocken, PA, USA, 2017. [CrossRef]
- Yang, T.; Liu, T.; Liao, W.; MacDonald, E.; Wei, H.; Chen, X.; Jiang, L. The influence of process parameters on vertical surface roughness of the AlSi10Mg parts fabricated by selective laser melting. J. Mater. Process. Technol. 2019, 266, 26–36. [Google Scholar] [CrossRef]
- Whitehouse, D.J. Handbook of Surface and Nanometrology; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Wu, L.; Baghdachi, J. Functional Polymer Coatings: Principles, Methods, and Applications; John Wiley & Sons, Inc.: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Tan, M.Y. Localized Corrosion in Complex Environments; John Wiley & Sons: New York, NY, USA, 2023. [Google Scholar]
- Giovagnoli, M.; Silvi, G.; Merlin, M.; Di Giovanni, M.T. Optimisation of process parameters for an additively manufactured AlSi10Mg alloy: Limitations of the energy density-based approach on porosity and mechanical properties estimation. Mater. Sci. Eng. A 2021, 802, 140613. [Google Scholar] [CrossRef]
- Gu, D. Laser Additive Manufacturing of High-Performance Materials; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Vargel, C. Corrosion of Aluminium, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- During, E.D.D. Corrosion Atlas—A Collection of Illustrated Case Histories, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- ASTM D3359-23; Standard Test Methods for Rating Adhesion by Tape Test. ASTM: West Conshohocken, PA, USA, 2023. [CrossRef]
- Uzan, N.E.; Ramati, S.; Shneck, R.; Frage, N.; Yeheskel, O. On the effect of shot-peening on fatigue resistance of AlSi10Mg specimens fabricated by additive manufacturing using selective laser melting (AM-SLM). Addit. Manuf. 2018, 21, 458–464. [Google Scholar] [CrossRef]
- Maamoun, A.H.; Elbestawi, M.A.; Veldhuis, S.C. Influence of Shot Peening on AlSi10Mg Parts Fabricated by Additive Manufacturing. J. Manuf. Mater. Process. 2018, 2, 40. [Google Scholar] [CrossRef] [Green Version]
- Doerner, M.F.; Nix, W.D. Stresses and deformation processes in thin films on substrates. Crit. Rev. Solid State Mater. Sci. 2006, 14, 225–268. [Google Scholar] [CrossRef]







| SLM Process Parameters | Energy Density (J/mm3) | ||||
|---|---|---|---|---|---|
| Laser Power (W) | Scan Speed (mm/s) | Hatch Distance (mm) | Layer Thickness (mm) | ||
| AlSi10Mg | 370 | 1300 | 0.19 | 0.03 | 49.9 |
| Al6061-RAM2 | 350 | 1400 | 0.11 | 0.03 | 75.8 |
| Specimen | ID | Machined | Classification | Percent Area Removed |
|---|---|---|---|---|
| As-is AlSi10Mg | 1 | No | 5B | 0%, none |
| As-is AlSi10Mg | 2 | No | 5B | 0%, none |
| As-is AlSi10Mg | 3 | No | 5B | 0%, none |
| Shot-peened AlSi10Mg | 4 | No | 5B | 0%, none |
| Shot-peened AlSi10Mg | 5 | No | 5B | 0%, none |
| Shot-peened AlSi10Mg | 6 | No | 2B | 15%–35% |
| Al6061 | 7 | No | 5B | 0%, none |
| Al6061 | 8 | No | 5B | 0%, none |
| Al6061 | 9 | No | 5B | 0%, none |
| As-is AlSi10Mg | 1 | Yes | 5B | 0%, none |
| As-is AlSi10Mg | 2 | Yes | 4B | 0%, none |
| As-is AlSi10Mg | 3 | Yes | 5B | 0%, none |
| Shot-peened AlSi10Mg | 4 | Yes | 5B | 0%, none |
| Shot-peened AlSi10Mg | 5 | Yes | 0B | Greater than 65% |
| Shot-peened AlSi10Mg | 6 | Yes | 4B | Less than 5% |
| Al6061 | 7 | Yes | 5B | 0%, none |
| Al6061 | 8 | Yes | 3B | 5%–15% |
| Al6061 | 9 | Yes | 5B | 0%, none |
| Specimen | ID | Thickness in the Rough Area (µm) | Thickness in the Machined Area (µm) |
|---|---|---|---|
| As-is AlSi10Mg | T1 | 28, 17.1, 50, 39.2, 84, 56.8, 40.8, 28.1, 32.9, 57.6 | 12.9, 11.4, 13.8, 13.5, 9.8, 13.9, 13.8, 13.1, 14.3, 12.9 |
| As-is AlSi10Mg | T2 | 51.5, 31.5, 29.8, 32.8, 42.9, 42.1, 37.7, 45.1, 57.9, 43 | 14.9, 14.3, 14.2, 15.5, 15.1, 13.7, 12.3, 14.7, 16.5, 9.2 |
| As-is AlSi10Mg | T3 | 31.9, 19.4, 32, 41.3, 39.9, 19.6, 60.2, 38. | 9.3, 11.3, 11.8, 14.7, 13.6, 14.2, 15.1, 12.9, 12.5, 13.5 |
| Shot-peened AlSi10Mg | T4 | 61.8, 53, 50.3, 53.7, 47.8, 50.6, 30.7, 35, 48.9, 46.9 | 10.5, 11.1, 11.9, 10.1, 13.2, 11.8, 12.2, 14.3, 13.8, 7.2 |
| Shot-peened AlSi10Mg | T5 | 55.2, 42.8, 56.9, 48.5, 50.6, 73.1, 69.2, 58.3, 41.2, 59.7 | 9.9, 8.6, 10.6, 8.3, 11.6, 7, 11, 7.2, 9.9, 7.4 |
| Shot-peened AlSi10Mg | T6 | 54.4, 59.9, 38.5, 42.1, 58.8, 43.7, 41.8, 54.3, 54.4, 50.9 | 14, 13.5, 14.8, 15.4, 14.4, 15.5, 14.7, 15.6, 15.8, 14.4 |
| Al6061 | T7 | 32, 26.5, 29.5, 45.5, 29.8, 26.4, 36.4, 34.5, 29.9, 28.7 | 12.4, 10.9, 11.1, 10.2, 10.7, 9.7, 10. 7, 9.4,10.7, 9.6 |
| Al6061 | T8 | 35.7, 35.1, 60.8, 31.4, 39, 33.9, 53.4, 30.5, 32.1, 33.4 | 13.6, 12.5, 13.5, 11.8, 13.6, 11.9, 13.0, 12.2, 12.3, 11.9 |
| Al6061 | T9 | 32.6, 26.2, 31.7, 31.3, 23.3, 29.8, 27.1, 33, 40.7, 19.8 | 10.4, 10.7, 10.9, 10.6, 10.2, 10.3, 9.6, 11.4, 10.1, 9.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macera, L.; Pullini, D.; Boschetto, A.; Bottini, L.; Mingazzini, C.; Falleti, G.L. Sol–Gel Silica Coatings for Corrosion Protection of Aluminum Parts Manufactured by Selective Laser Melting (SLM) Technology. Coatings 2023, 13, 1081. https://doi.org/10.3390/coatings13061081
Macera L, Pullini D, Boschetto A, Bottini L, Mingazzini C, Falleti GL. Sol–Gel Silica Coatings for Corrosion Protection of Aluminum Parts Manufactured by Selective Laser Melting (SLM) Technology. Coatings. 2023; 13(6):1081. https://doi.org/10.3390/coatings13061081
Chicago/Turabian StyleMacera, Luciano, Daniele Pullini, Alberto Boschetto, Luana Bottini, Claudio Mingazzini, and Gian Luca Falleti. 2023. "Sol–Gel Silica Coatings for Corrosion Protection of Aluminum Parts Manufactured by Selective Laser Melting (SLM) Technology" Coatings 13, no. 6: 1081. https://doi.org/10.3390/coatings13061081






