Durability of Slippery Liquid-Infused Surfaces: Challenges and Advances
Abstract
:1. Introduction
2. Liquid-Infused Surfaces
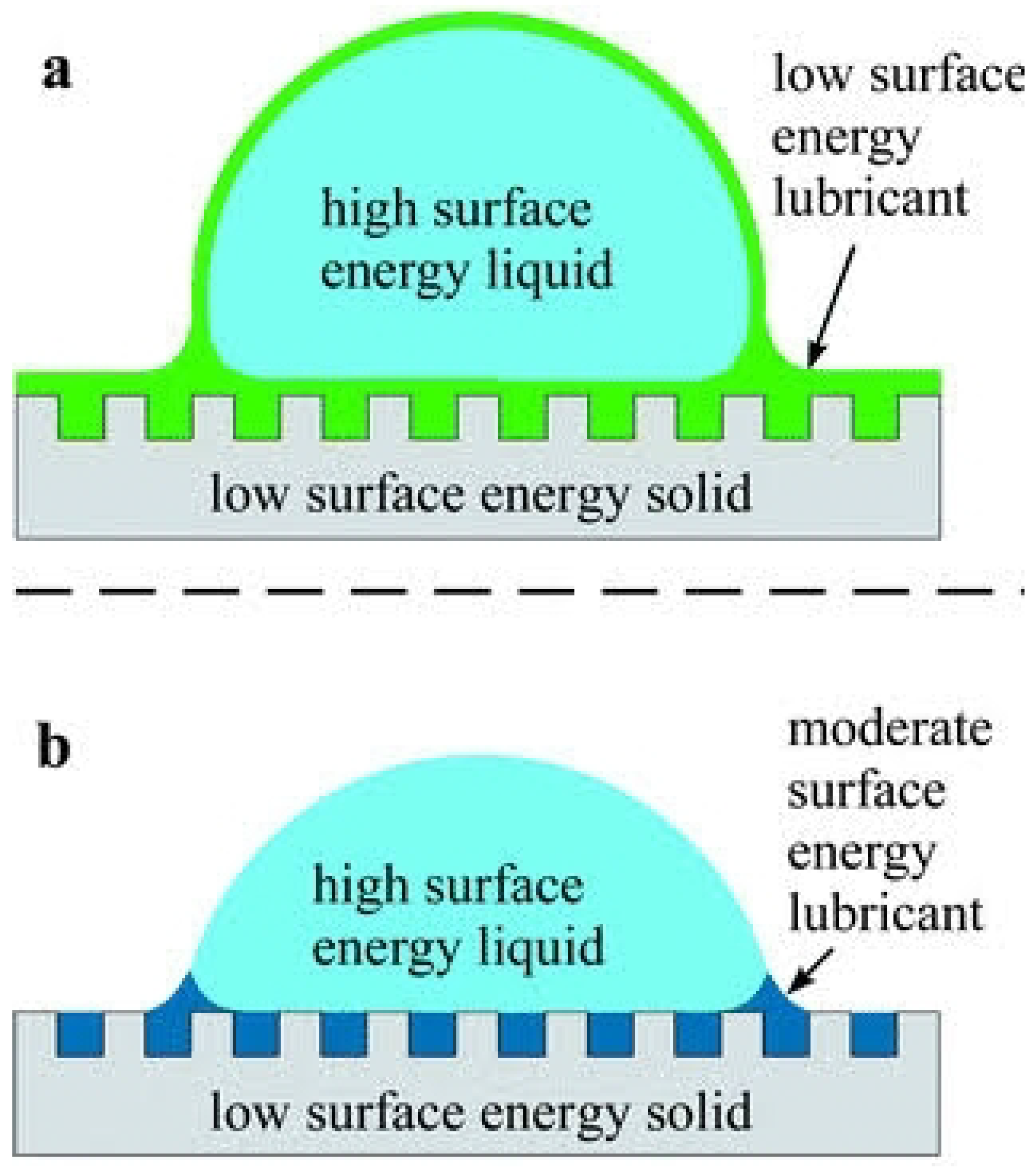
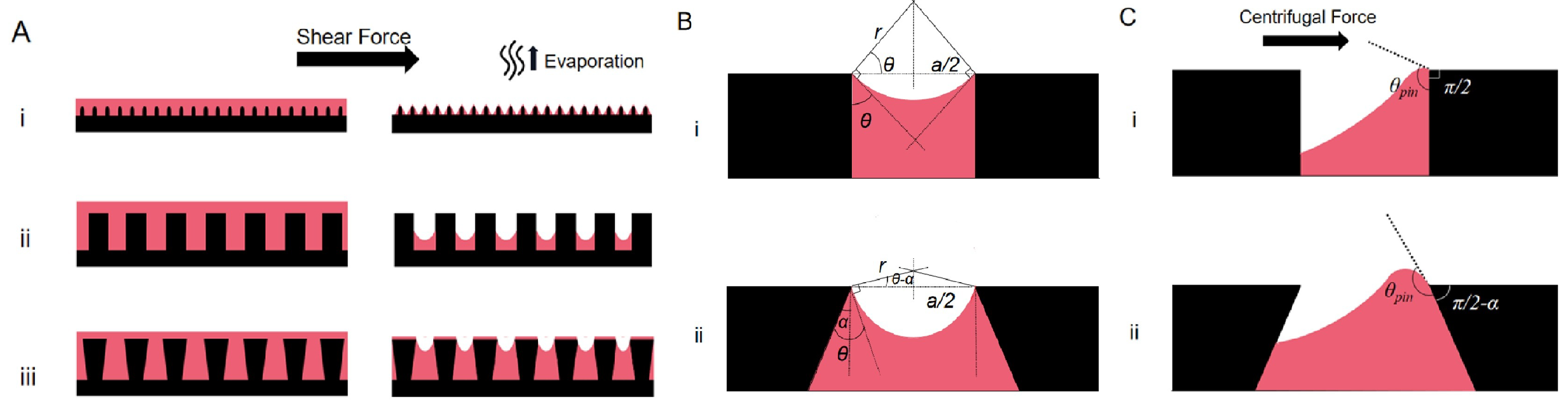
2.1. Wetting of a Solid Surface
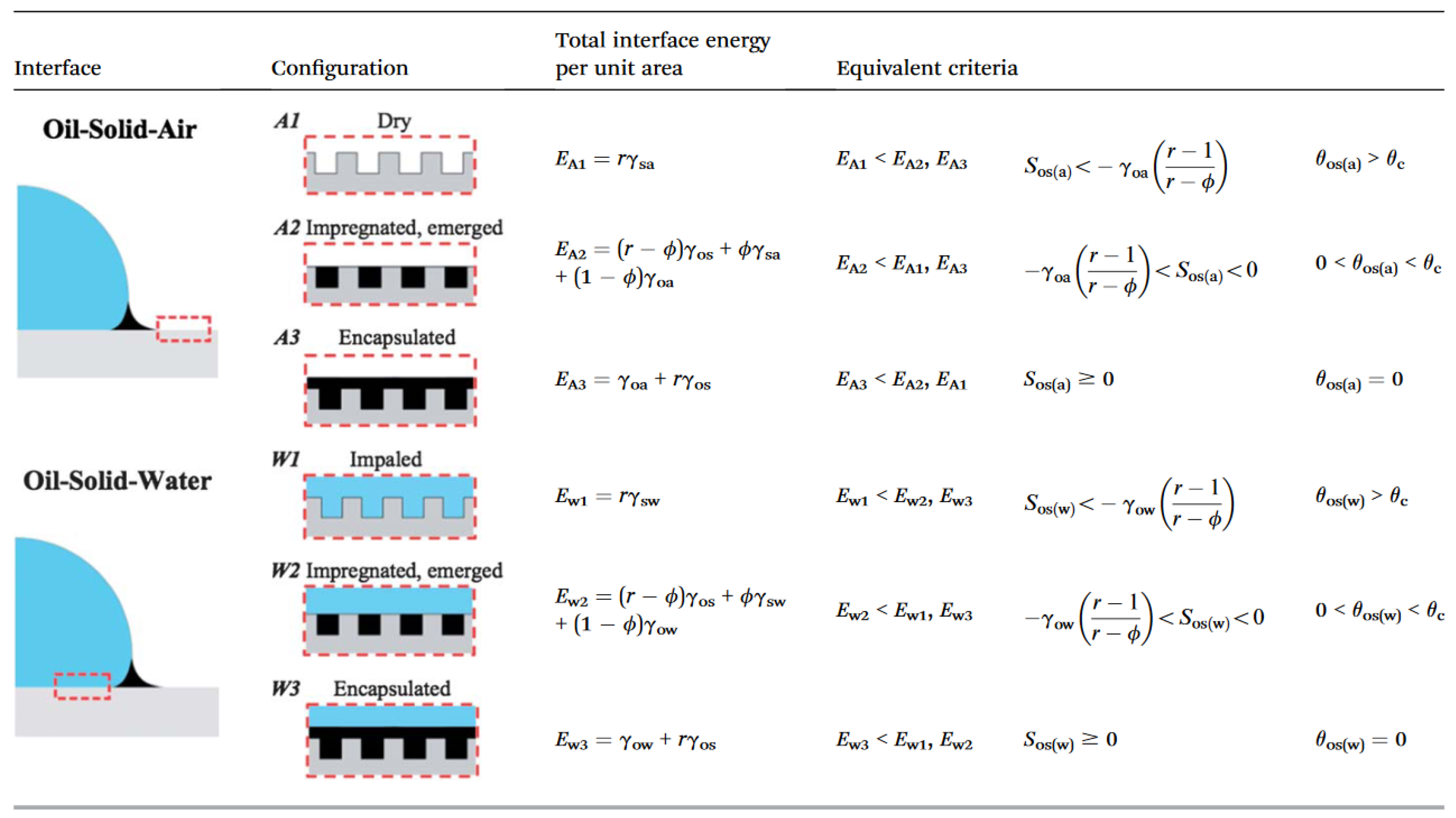
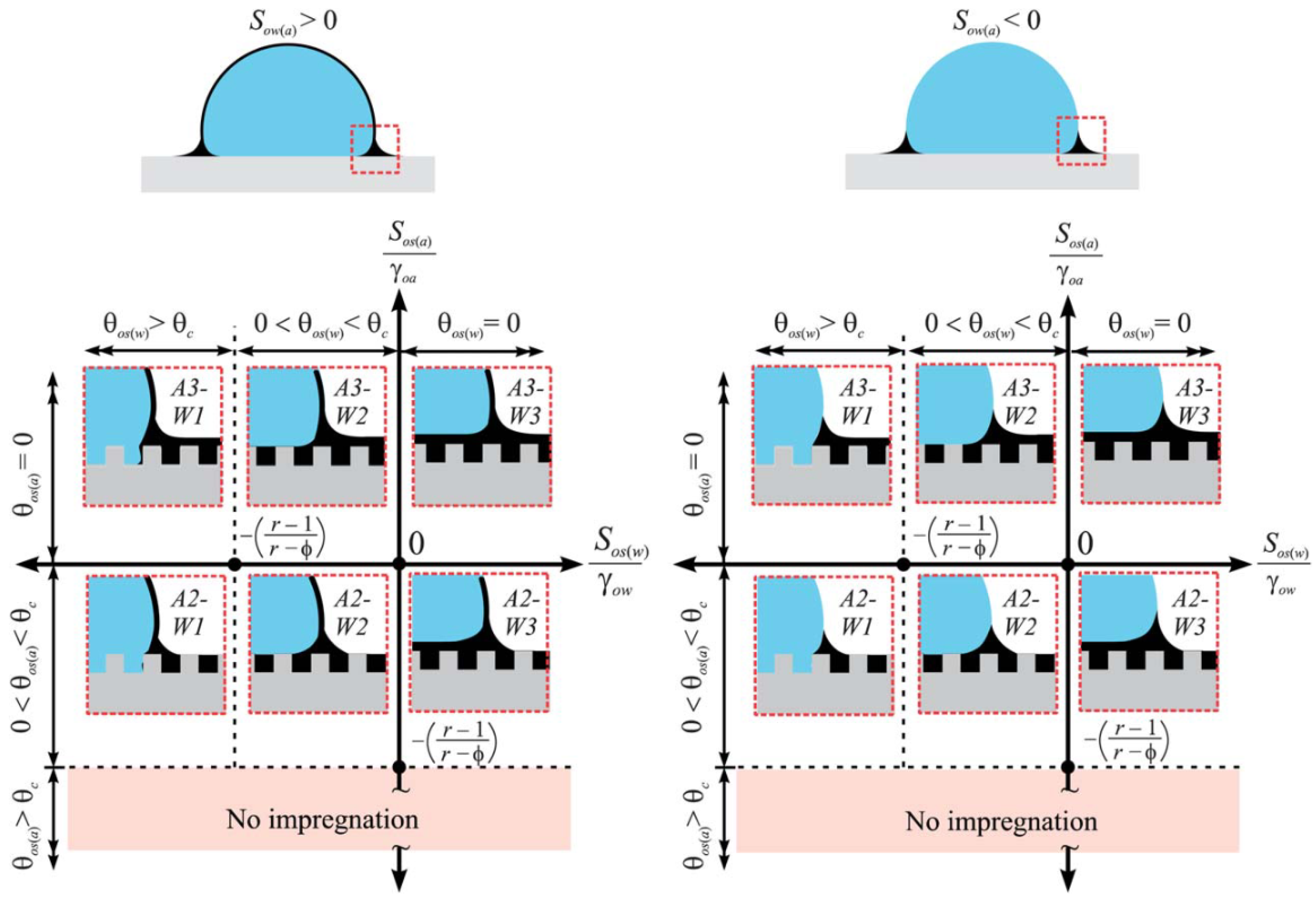
2.2. Wetting of Liquid-Infused Surfaces
3. Durability of Slippery Liquid-Infused Porous Surfaces (SLIPS)
- The interaction of acidic or alkaline contaminants with porous substrates can result in corrosion, leading to damage to the porous structure. Reactions with the lubricant lead to chemical degradation through reactive reactions [78].
- High temperatures can cause the lubricant to evaporate or decompose, which reduces the slipperiness of SLIPS [79].
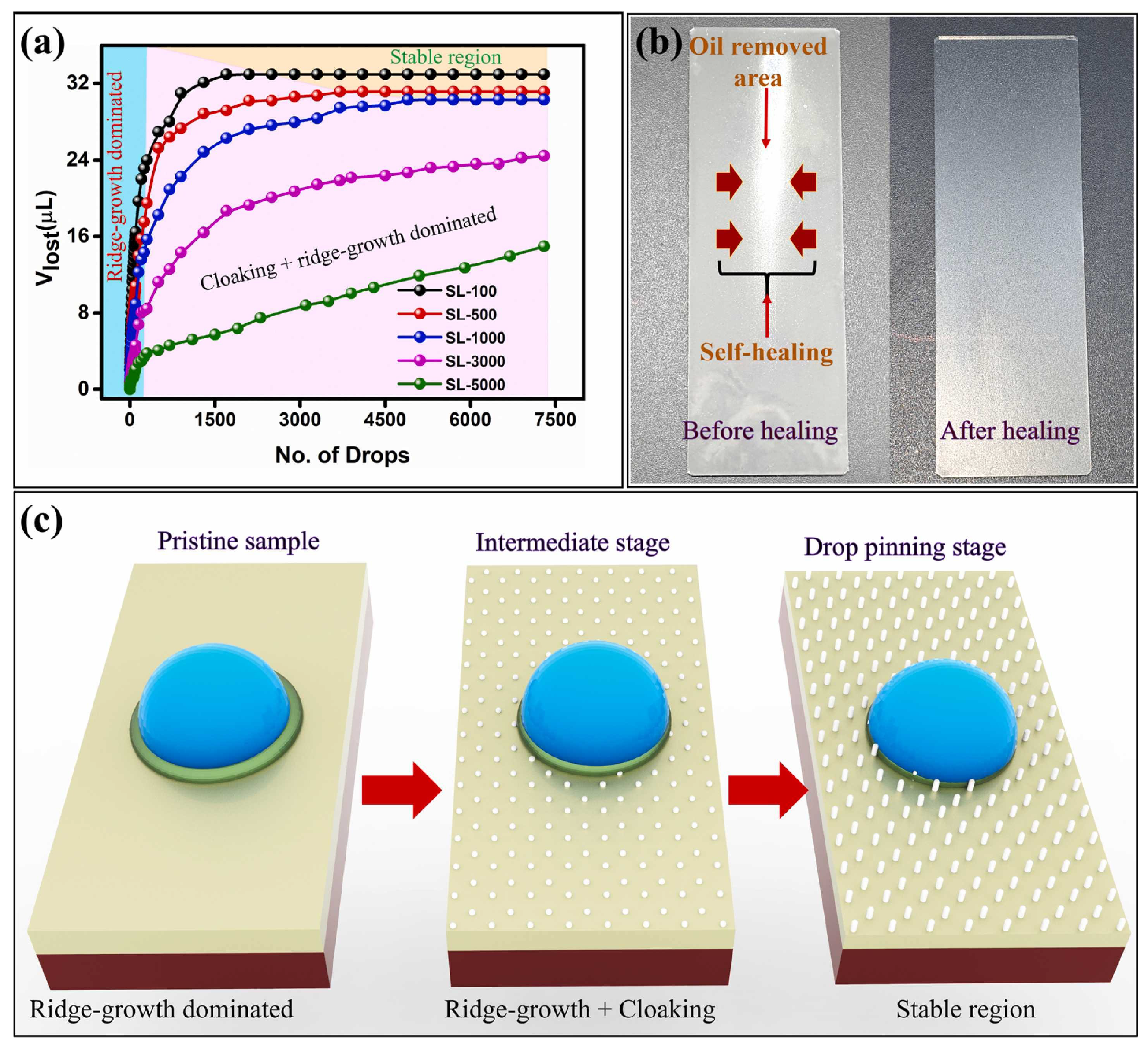
- The formation of wetting ridges around the external droplets can lead to the depletion of the lubricant during droplet motion on the surface [73].
3.1. Substrate Properties and Durability
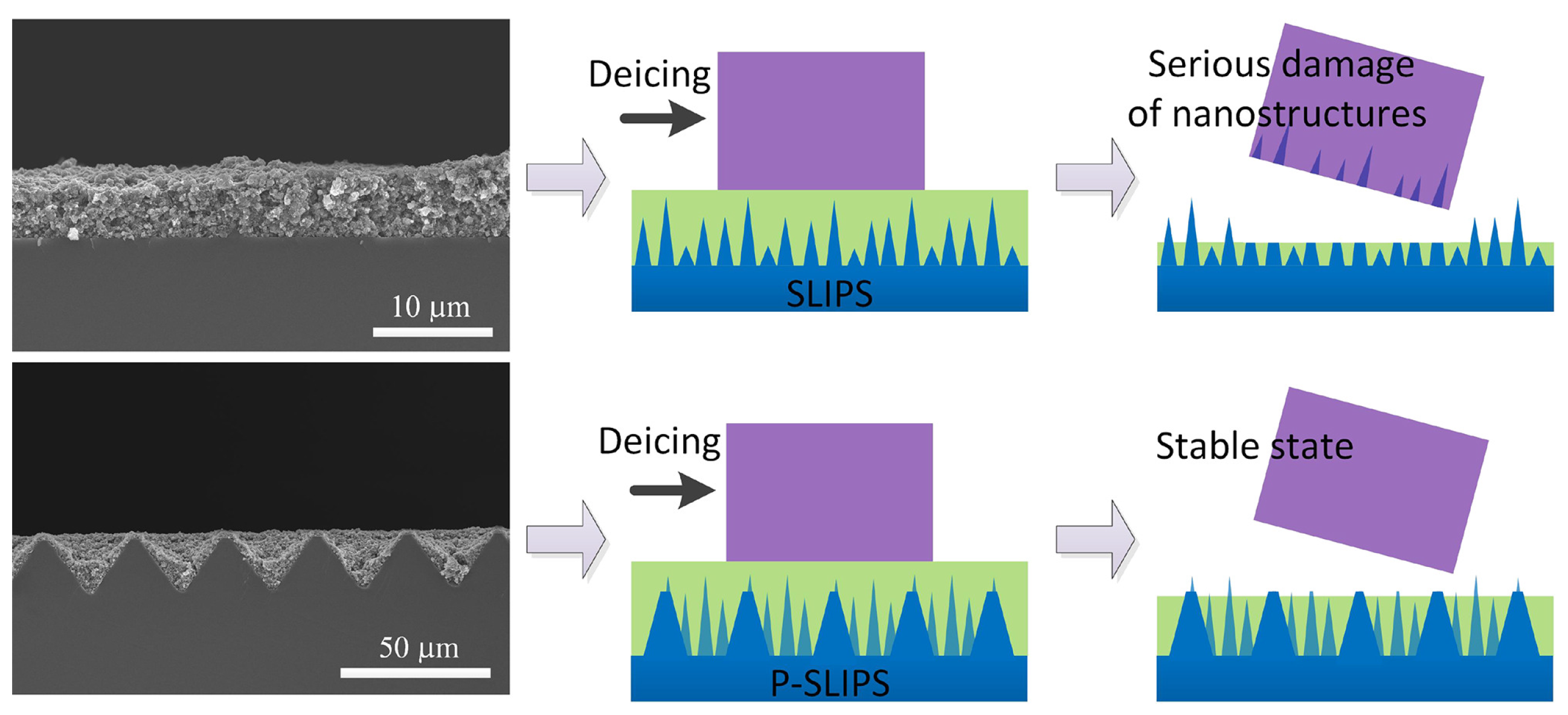
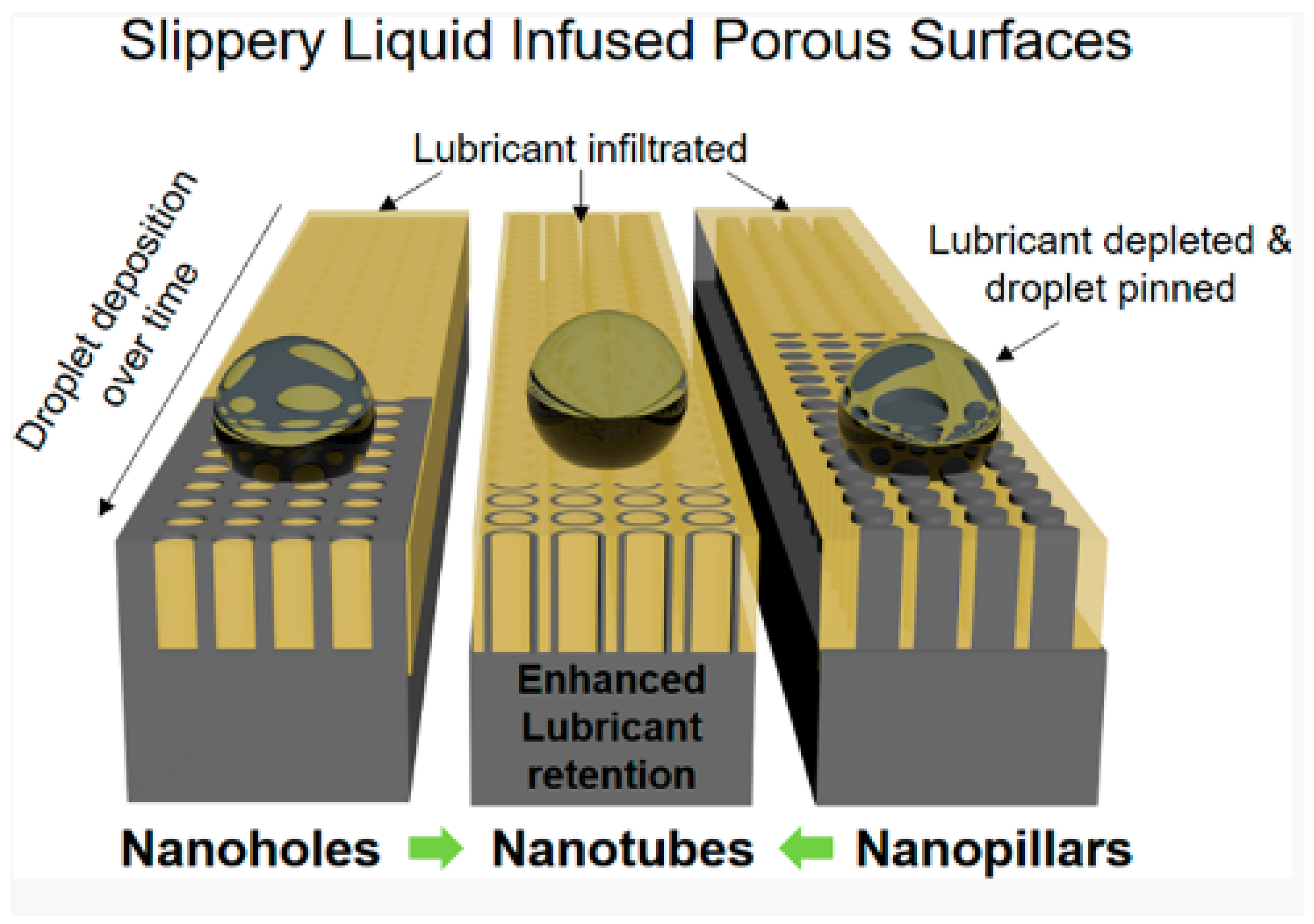
3.1.1. Surface Structure
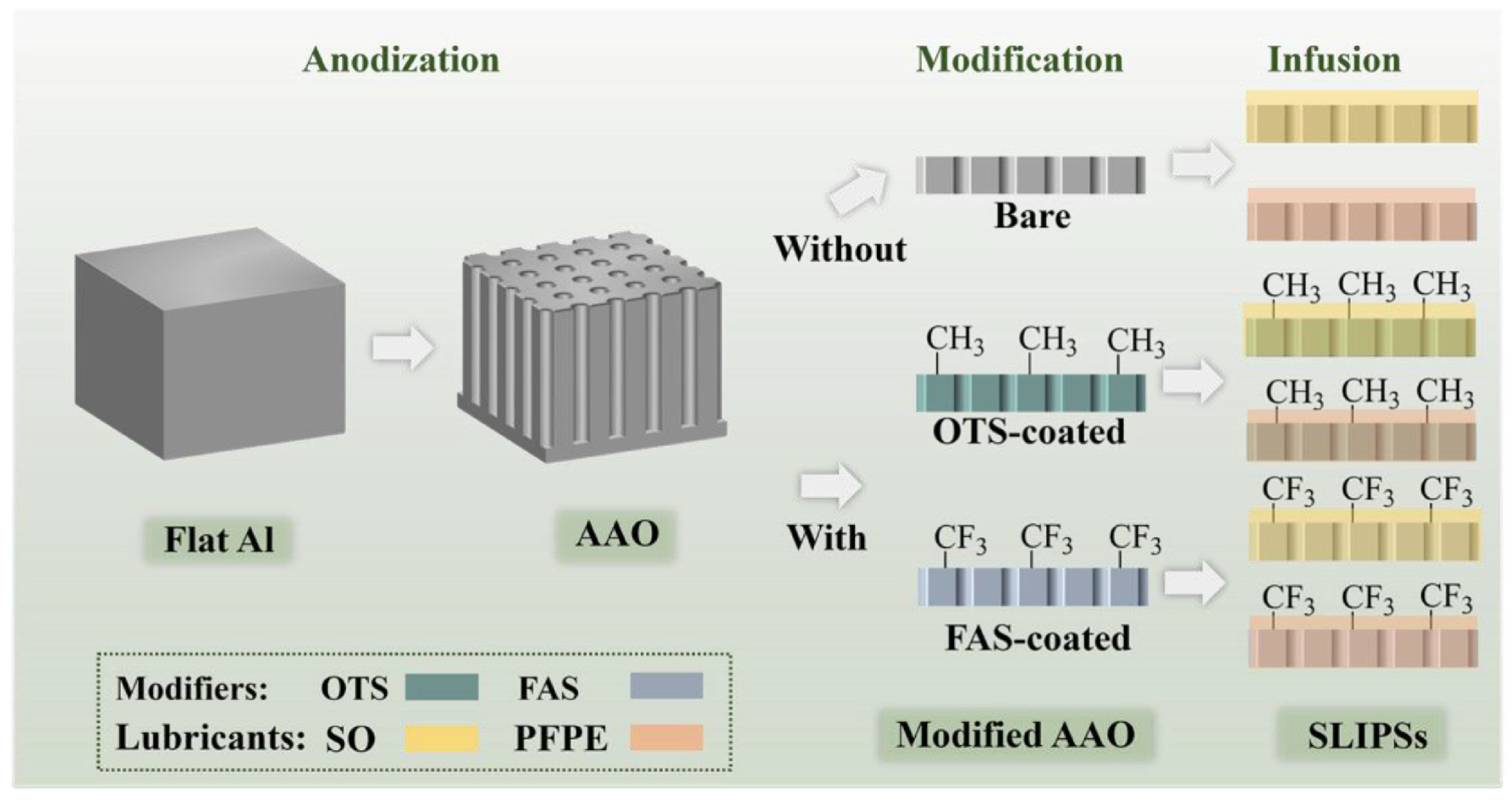
3.1.2. Substrate Coating and Durability
3.2. Lubricant and the Durability of SLIPS
- Low surface tension is important for a lubricant used on slippery liquid-infused surfaces, as it allows the lubricant to spread easily and thoroughly over the surface. This property ensures that the lubricant can reach all areas of the surface and provide complete coverage, achieving effective lubrication.
- Lubricants used should be less volatile; a low vapor pressure is desirable because it means that the lubricant is less likely to evaporate, and thus less likely to become depleted over time, leading to high thermal durability.
- The lubricant must be chemically inert so that its slippery property is not diminished when reacting with the other contaminants.
- Cloaking and wetting ridges are the primary sources of lubricant depletion. The viscosity of the lubricant should be carefully chosen so as to reduce the loss of lubricant due to moving along with the droplet.
3.2.1. Types of Lubricants
3.2.2. Effect of the Viscosity of the Lubricant
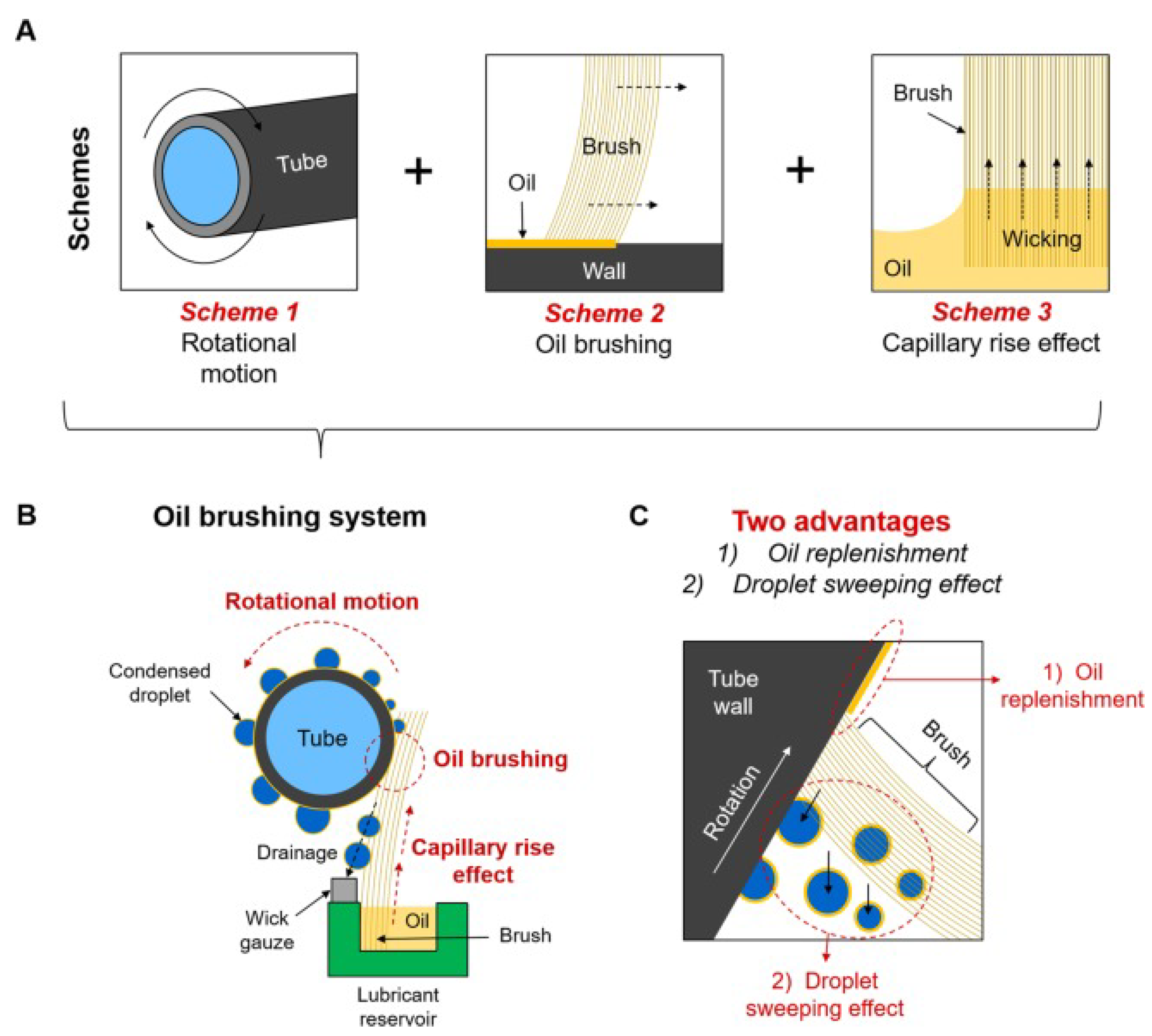
3.3. Replenishment of the Lubricant
3.3.1. Replenishment through External Reservoir
3.3.2. Replenishment through Built-In Reservoir
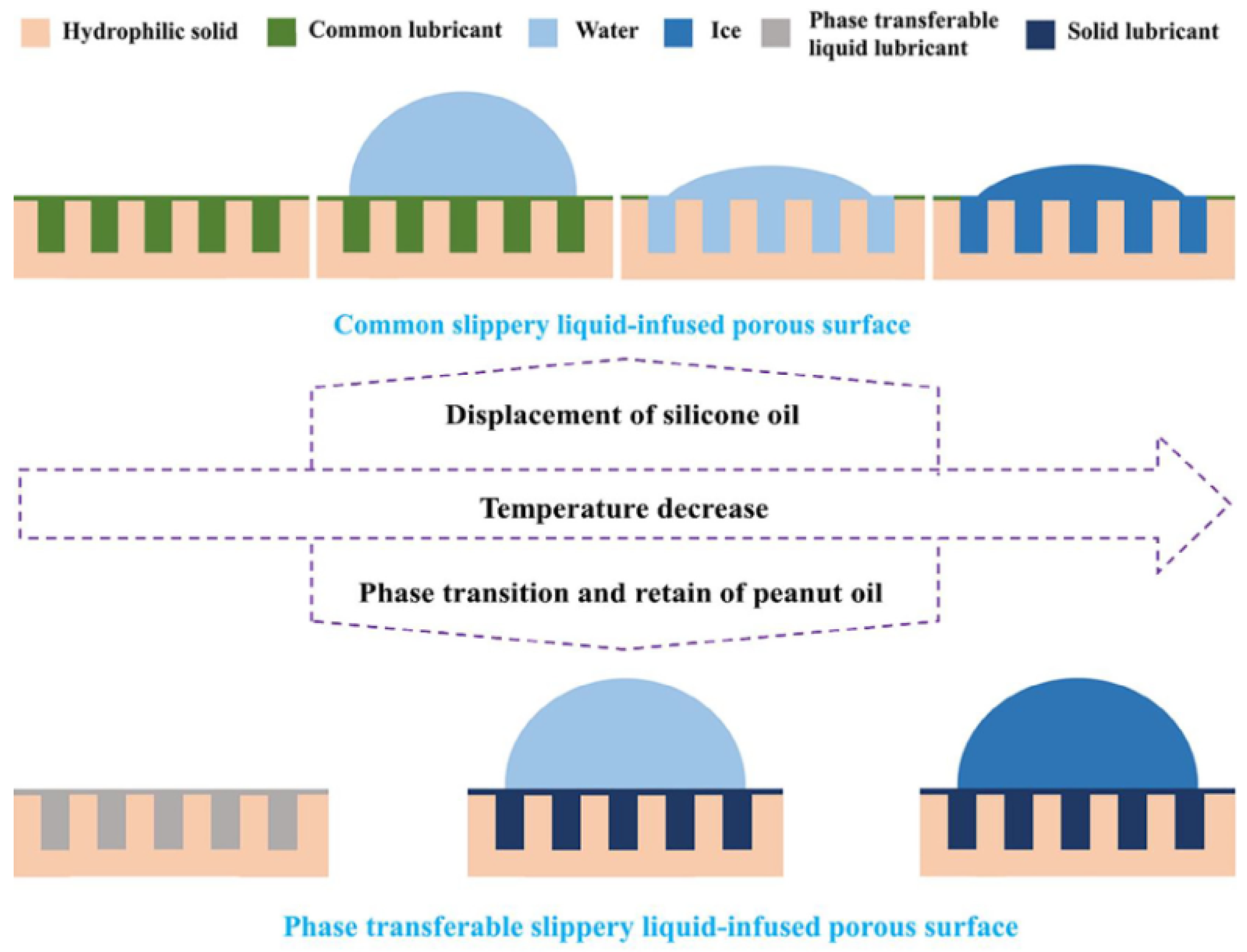
3.4. A Novel Liquid-Infused Surface: “Organogels”
4. Summary and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, C.K.; Bae, I.S.; Lee, S.B.; Cho, J.H.; Shin, E.S.; Choi, S.C.; Boo, J.H. Development of painting technology using plasma surface technology for automobile parts. Thin Solid Films 2006, 506–507, 316–322. [Google Scholar] [CrossRef]
- Singh, S.L.; Schimmele, L.; Dietrich, S. Cassie-Wenzel transition of a binary liquid mixture on a nanosculptured surface. Phys. Rev. E 2020, 101, 052115. [Google Scholar] [CrossRef]
- Manna, U.; Raman, N.; Welsh, M.A.; Zayas-Gonzalez, Y.M.; Blackwell, H.E.; Palecek, S.P.; Lynn, D.M. Slippery Liquid-Infused Porous Surfaces that Prevent Microbial Surface Fouling and Kill Non-Adherent Pathogens in Surrounding Media: A Controlled Release Approach. Adv. Funct. Mater. 2016, 26, 3599–3611. [Google Scholar] [CrossRef] [Green Version]
- Assefa, M.; Amare, A. Biofilm-Associated Multi-Drug Resistance in Hospital-Acquired Infections: A Review. Infect. Drug Resist. 2022, 15, 5061–5068. [Google Scholar] [CrossRef] [PubMed]
- Ruzi, M.; Celik, N.; Onses, M.S. Superhydrophobic coatings for food packaging applications: A review. Food Packag. Shelf Life 2022, 32, 100823. [Google Scholar] [CrossRef]
- Douterelo, I.; Sharpe, R.L.; Husband, S.; Fish, K.E.; Boxall, J.B. Understanding microbial ecology to improve management of drinking water distribution systems. Wires Water 2018, 6, e01325. [Google Scholar] [CrossRef] [Green Version]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Hakim, M.L.; Nugroho, B.; Nurrohman, M.N.; Suastika, I.K.; Utama, I.K.A.P. Investigation of fuel consumption on an operating ship due to biofouling growth and quality of anti-fouling coating. IOP Conf. Ser. Earth Environ. Sci. 2019, 339, 012037. [Google Scholar] [CrossRef]
- Deng, R.; Shen, T.; Chen, H.; Lu, J.; Yang, H.C.; Li, W. Slippery liquid-infused porous surfaces (SLIPSs): A perfect solution to both marine fouling and corrosion? J. Mater. Chem. A 2020, 8, 7536–7547. [Google Scholar] [CrossRef]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. NPJ Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Wang, S.; Cao, Q.; Li, Y.; Zhou, J.; Zhu, B.W. Functional food packaging for reducing residual liquid food: Thermo-resistant edible super-hydrophobic coating from coffee and beeswax. J. Colloid Interface Sci. 2019, 533, 742–749. [Google Scholar] [CrossRef]
- Shen, T.; Fan, S.; Li, Y.; Xu, G.; Fan, W. Preparation of Edible Non-wettable Coating with Soybean Wax for Repelling Liquid Foods with Little Residue. Materials 2020, 13, 3308. [Google Scholar] [CrossRef]
- Wei, T.; Dang-Sheng, X. Bioinspired Superhydrophobic Progress and Recent Advances of Its Functional Application. J. Inorg. Mater. 2019, 34, 5. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Wu, Z.; Su, Y.; Xu, Z. Aircraft flight characteristics in icing conditions. Prog. Aerosp. Sci. 2015, 74, 62–80. [Google Scholar] [CrossRef]
- Dijvejin, Z.A.; Jain, M.C.; Kozak, R.; Zarifi, M.H.; Golovin, K. Smart low interfacial toughness coatings for on-demand de-icing without melting. Nat. Commun. 2022, 13, 5119. [Google Scholar] [CrossRef]
- Xu, J.; Jia, L.; Dang, C.; Liu, X.; Ding, Y. Effects of solid–liquid interaction and mixture concentration on wettability of nano-droplets: Molecular dynamics simulations. AIP Adv. 2022, 12, 105313. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.; Lei, J.; Ma, Y.; Du, F. The wetting behavior of aqueous surfactant solutions on wheat (Triticum aestivum) leaf surfaces. Soft Matter 2017, 13, 503–513. [Google Scholar] [CrossRef]
- Metya, A.K.; Khan, S.; Singh, J.K. Wetting Transition of the Ethanol—Water Droplet on Smooth and Textured Surfaces. J. Phys. Chem. C 2014, 118, 4113–4121. [Google Scholar] [CrossRef]
- Ivanova, N.; Starov, V. Wetting of low free energy surfaces by aqueous surfactant solutions. Curr. Opin. Colloid Interface Sci. 2011, 16, 285–291. [Google Scholar] [CrossRef]
- Fu, M.K.; Arenas, I.; Leonardi, S.; Hultmark, M. Liquid-infused surfaces as a passive method of turbulent drag reduction. J. Fluid Mech. 2017, 824, 688–700. [Google Scholar] [CrossRef] [Green Version]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, W.; Han, L.; Tam, K.C. Superhydrophobic surfaces from sustainable colloidal systems. Curr. Opin. Colloid Interface Sci. 2022, 57, 101534. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, G.; Tong, Q.; Yang, W.; Hao, W. Fluorine-free superhydrophobic coatings from polydimethylsiloxane for sustainable chemical engineering: Preparation methods and applications. Chem. Eng. J. 2021, 426, 130829. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, L.; Mou, J.; Wu, D.; Xu, M.; Zhou, P.; Ren, Y. Research Strategies to Develop Environmentally Friendly Marine Antifouling Coatings. Mar. Drugs 2020, 18, 371. [Google Scholar] [CrossRef]
- Hooda, A.; Goyat, M.; Pandey, J.K.; Kumar, A.; Gupta, R. A review on fundamentals, constraints and fabrication techniques of superhydrophobic coatings. Prog. Org. Coatings 2020, 142, 105557. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, H.; Shao, Y.; Zhang, H.; Zhu, J. Recent Progresses of Superhydrophobic Coatings in Different Application Fields: An Overview. Coatings 2021, 11, 116. [Google Scholar] [CrossRef]
- Bayer, I.S. Superhydrophobic Coatings from Ecofriendly Materials and Processes: A Review. Adv. Mater. Interfaces 2020, 7, 2000095. [Google Scholar] [CrossRef]
- Kumar, R.; Sahani, A.K. Role of superhydrophobic coatings in biomedical applications. Mater. Today Proc. 2021, 45, 5655–5659. [Google Scholar] [CrossRef]
- Chu, Z.; Seeger, S. Superamphiphobic surfaces. Chem. Soc. Rev. 2014, 43, 2784–2798. [Google Scholar] [CrossRef] [PubMed]
- Bellanger, H.; Darmanin, T.; de Givenchy, E.T.; Guittard, F. Chemical and Physical Pathways for the Preparation of Superoleophobic Surfaces and Related Wetting Theories. Chem. Rev. 2014, 114, 2694–2716. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Robust omniphobic surfaces. Proc. Natl. Acad. Sci. USA 2008, 105, 18200–18205. [Google Scholar] [CrossRef] [Green Version]
- Giacomello, A.; Schimmele, L.; Dietrich, S.; Tasinkevych, M. Perpetual superhydrophobicity. Soft Matter 2016, 12, 8927–8934. [Google Scholar] [CrossRef] [Green Version]
- Giacomello, A.; Schimmele, L.; Dietrich, S.; Tasinkevych, M. Recovering superhydrophobicity in nanoscale and macroscale surface textures. Soft Matter 2019, 15, 7462–7471. [Google Scholar] [CrossRef] [Green Version]
- Nyankson, E.; Agbe, H.; Takyi, G.K.S.; Bensah, Y.D.; Sarkar, D.K. Recent advances in nanostructured superhydrophobic surfaces: Fabrication and long-term durability challenges. Curr. Opin. Chem. Eng. 2022, 36, 100790. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, D.; Sun, Z.; Song, J.; Deng, X. Robust superhydrophobicity: Mechanisms and strategies. Chem. Soc. Rev. 2021, 50, 4031–4061. [Google Scholar] [CrossRef] [PubMed]
- Salimi, E. Omniphobic surfaces: State-of-the-art and future perspectives. J. Adhes. Sci. Technol. 2019, 33, 1369–1379. [Google Scholar] [CrossRef]
- Scarratt, L.R.; Steiner, U.; Neto, C. A review on the mechanical and thermodynamic robustness of superhydrophobic surfaces. Adv. Colloid Interface Sci. 2017, 246, 133–152. [Google Scholar] [CrossRef]
- Milionis, A.; Loth, E.; Bayer, I.S. Recent advances in the mechanical durability of superhydrophobic materials. Adv. Colloid Interface Sci. 2016, 229, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Malavasi, I.; Bernagozzi, I.; Antonini, C.; Marengo, M. Assessing durability of superhydrophobic surfaces. Surf. Innov. 2015, 3, 49–60. [Google Scholar] [CrossRef]
- Quéré, D. Non-sticking drops. Rep. Prog. Phys. 2005, 68, 2495–2532. [Google Scholar] [CrossRef]
- Wong, T.S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef]
- Smith, J.D.; Dhiman, R.; Varanasi, K. Liquid-encapsulating surfaces: Overcoming the limitations of superhydrophobic surfaces for robust non-wetting and anti-icing surfaces. In APS Division of Fluid Dynamics Meeting Abstracts; APS Meeting Abstracts; ADS: Cambridge, MA, USA, 2011; Volume 64, p. S4.001. [Google Scholar]
- Yong, J.; Chen, F.; Yang, Q.; Fang, Y.; Huo, J.; Zhang, J.; Hou, X. Nepenthes Inspired Design of Self-Repairing Omniphobic Slippery Liquid Infused Porous Surface (SLIPS) by Femtosecond Laser Direct Writing. Adv. Mater. Interfaces 2017, 4, 1700552. [Google Scholar] [CrossRef]
- Solomon, B.R.; Subramanyam, S.B.; Farnham, T.A.; Khalil, K.S.; Anand, S.; Varanasi, K.K. Chapter 10. Lubricant-Impregnated Surfaces. In Soft Matter Series; Royal Society of Chemistry: London, UK, 2016; pp. 285–318. [Google Scholar] [CrossRef]
- Heydarian, S.; Jafari, R.; Momen, G. Recent progress in the anti-icing performance of slippery liquid-infused surfaces. Prog. Org. Coatings 2021, 151, 106096. [Google Scholar] [CrossRef]
- Sakuraba, K.; Kitano, S.; Kowalski, D.; Aoki, Y.; Habazaki, H. Slippery Liquid-Infused Porous Surfaces on Aluminum for Corrosion Protection with Improved Self-Healing Ability. ACS Appl. Mater. Interfaces 2021, 13, 45089–45096. [Google Scholar] [CrossRef]
- Yan, W.; Xue, S.; Xiang, B.; Zhao, X.; Zhang, W.; Mu, P.; Li, J. Recent advances of slippery liquid-infused porous surfaces with anti-corrosion. Chem. Commun. 2023, 59, 2182–2198. [Google Scholar] [CrossRef]
- He, W.; Liu, P.; Zhang, J.; Yao, X. Emerging Applications of Bioinspired Slippery Surfaces in Biomedical Fields. Chem.—A Eur. J. 2018, 24, 14864–14877. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Z.; Gao, L.; Liu, Y.; Hu, H. An exploratory study on using Slippery-Liquid-Infused-Porous-Surface (SLIPS) for wind turbine icing mitigation. Renew. Energy 2020, 162, 2344–2360. [Google Scholar] [CrossRef]
- Bohn, H.F.; Federle, W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc. Natl. Acad. Sci. USA 2004, 101, 14138–14143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafuma, A.; Quéré, D. Slippery pre-suffused surfaces. Europhys. Lett. 2011, 96, 56001. [Google Scholar] [CrossRef]
- Young, T., III. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Law, K.Y.; Zhao, H. Surface Wetting; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- de Gennes, P.G. Wetting: Statics and dynamics. Rev. Mod. Phys. 1985, 57, 827–863. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Bico, J.; Thiele, U.; Quéré, D. Wetting of textured surfaces. Colloids Surfaces A Physicochem. Eng. Asp. 2002, 206, 41–46. [Google Scholar] [CrossRef]
- Waugh, D.; Lawrence, J.; Langer, N.; Bidault, S. Mixed-State Wetting and Wetting Transitions on Laser Surface Engineered Polymeric Materials. Int. J. Wettability Sci. Technol. 2018, 1, 63–84. [Google Scholar]
- Singh, A.V.; Baylan, S.; Park, B.W.; Richter, G.; Sitti, M. Hydrophobic pinning with copper nanowhiskers leads to bactericidal properties. PLoS ONE 2017, 12, e0175428. [Google Scholar] [CrossRef] [Green Version]
- Maiti, S.K. Fracture Mechanics: Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Herminghaus, S. Roughness-induced non-wetting. Europhys. Lett. 2000, 52, 165–170. [Google Scholar] [CrossRef]
- Teisala, H.; Butt, H.J. Hierarchical Structures for Superhydrophobic and Superoleophobic Surfaces. Langmuir 2018, 35, 10689–10703. [Google Scholar] [CrossRef]
- Quan, Y.Y.; Chen, Z.; Lai, Y.; Huang, Z.S.; Li, H. Recent advances in fabricating durable superhydrophobic surfaces: A review in the aspects of structures and materials. Mater. Chem. Front. 2021, 5, 1655–1682. [Google Scholar] [CrossRef]
- Smith, J.D.; Dhiman, R.; Anand, S.; Reza-Garduno, E.; Cohen, R.E.; McKinley, G.H.; Varanasi, K.K. Droplet mobility on lubricant-impregnated surfaces. Soft Matter 2013, 9, 1772–1780. [Google Scholar] [CrossRef] [Green Version]
- Anand, S.; Rykaczewski, K.; Subramanyam, S.B.; Beysens, D.; Varanasi, K.K. How droplets nucleate and grow on liquids and liquid impregnated surfaces. Soft Matter 2015, 11, 69–80. [Google Scholar] [CrossRef]
- Hoque, M.J.; Sett, S.; Yan, X.; Liu, D.; Rabbi, K.F.; Qiu, H.; Qureshi, M.; Barac, G.; Bolton, L.; Miljkovic, N. Life Span of Slippery Lubricant Infused Surfaces. ACS Appl. Mater. Interfaces 2022, 14, 4598–4611. [Google Scholar] [CrossRef] [PubMed]
- Peppou-Chapman, S.; Hong, J.K.; Waterhouse, A.; Neto, C. Life and death of liquid-infused surfaces: A review on the choice, analysis and fate of the infused liquid layer. Chem. Soc. Rev. 2020, 49, 3688–3715. [Google Scholar] [CrossRef]
- Preston, D.J.; Song, Y.; Lu, Z.; Antao, D.S.; Wang, E.N. Design of Lubricant Infused Surfaces. ACS Appl. Mater. Interfaces 2017, 9, 42383–42392. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wen, G.; Guo, Z. What are the design principles, from the choice of lubricants and structures to the preparation method, for a stable slippery lubricant-infused porous surface? Mater. Horizons 2020, 7, 1697–1726. [Google Scholar] [CrossRef]
- Villegas, M.; Zhang, Y.; Jarad, N.A.; Soleymani, L.; Didar, T.F. Liquid-Infused Surfaces: A Review of Theory, Design, and Applications. ACS Nano 2019, 13, 8517–8536. [Google Scholar] [CrossRef]
- Semprebon, C.; McHale, G.; Kusumaatmaja, H. Apparent contact angle and contact angle hysteresis on liquid infused surfaces. Soft Matter 2017, 13, 101–110. [Google Scholar] [CrossRef] [Green Version]
- McHale, G.; Orme, B.V.; Wells, G.G.; Ledesma-Aguilar, R. Apparent Contact Angles on Lubricant-Impregnated Surfaces/SLIPS: From Superhydrophobicity to Electrowetting. Langmuir 2019, 35, 4197–4204. [Google Scholar] [CrossRef]
- Kreder, M.J.; Daniel, D.; Tetreault, A.; Cao, Z.; Lemaire, B.; Timonen, J.V.; Aizenberg, J. Film Dynamics and Lubricant Depletion by Droplets Moving on Lubricated Surfaces. Phys. Rev. X 2018, 8, 031053. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.H.; Lee, D.; Riassetto, D. Wetting ridges on slippery liquid-infused porous surfaces. Rep. Prog. Phys. 2023, 86, 066601. [Google Scholar] [CrossRef]
- Baumli, P.; DAcunzi, M.; Hegner, K.I.; Naga, A.; Wong, W.S.; Butt, H.; Vollmer, D. The challenge of lubricant-replenishment on lubricant-impregnated surfaces. Adv. Colloid Interface Sci. 2021, 287, 102329. [Google Scholar] [CrossRef]
- Goodband, S.J.; Armstrong, S.; Kusumaatmaja, H.; Voıïtchovsky, K. Effect of Ageing on the Structure and Properties of Model Liquid-Infused Surfaces. Langmuir 2020, 36, 3461–3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boinovich, L.B.; Chulkova, E.V.; Emelyanenko, K.A.; Domantovsky, A.G.; Emelyanenko, A.M. The mechanisms of anti-icing properties degradation for slippery liquid-infused porous surfaces under shear stresses. J. Colloid Interface Sci. 2022, 609, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Donadei, V.; Koivuluoto, H.; Sarlin, E.; Vuoristo, P. Durability of Lubricated Icephobic Coatings under Various Environmental Stresses. Polymers 2022, 14, 303. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Guarini, G.; Corozzi, A.; Raimondo, M. Evaluation of the Durability of Slippery, Liquid-Infused Porous Surfaces in Different Aggressive Environments: Influence of the Chemical-Physical Properties of Lubricants. Coatings 2021, 11, 1170. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, H.; Zhang, L.; Zhang, Y.; Zhang, D.; Jiang, L. Stable slippery liquid-infused anti-wetting surface at high temperatures. J. Mater. Chem. A 2016, 4, 12212–12220. [Google Scholar] [CrossRef]
- Emelyanenko, K.A.; Domantovsky, A.G.; Platonov, P.S.; Kochenkov, P.S.; Emelyanenko, A.M.; Boinovich, L.B. The durability of superhydrophobic and slippery liquid infused porous surface coatings under corona discharge characteristic of the operation of high voltage power transmission lines. Energy Rep. 2022, 8, 6837–6844. [Google Scholar] [CrossRef]
- Gunjan, M.R.; Kumar, A.; Raj, R. Cloaked Droplets on Lubricant-Infused Surfaces: Union of Constant Mean Curvature Interfaces Dictated by Thin-Film Tension. Langmuir 2021, 37, 6601–6612. [Google Scholar] [CrossRef]
- Feng, L.; He, X.Y.; Sun, H.; Ma, H.; Li, M.; Shi, W.Y. Cloaking effect on the thermocapillary motion of droplet on slippery liquid-infused porous surface. Int. J. Therm. Sci. 2023, 190, 108319. [Google Scholar] [CrossRef]
- Lu, Y.; He, G.; Carmalt, C.J.; Parkin, I.P. Synthesis and characterization of omniphobic surfaces with thermal, mechanical and chemical stability. RSC Adv. 2016, 6, 106491–106499. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Zhu, H.; Tu, Z.; Niu, D.; Liu, G.; Bei, Y.; Zhu, Q. Effect of the texture geometry on the slippery behavior of liquid-infused nanoporous surfaces. J. Mater. Sci. 2018, 54, 2729–2739. [Google Scholar] [CrossRef]
- Peppou-Chapman, S.; Neto, C. Mapping Depletion of Lubricant Films on Antibiofouling Wrinkled Slippery Surfaces. ACS Appl. Mater. Interfaces 2018, 10, 33669–33677. [Google Scholar] [CrossRef]
- Wong, W.S.Y.; Hegner, K.I.; Donadei, V.; Hauer, L.; Naga, A.; Vollmer, D. Capillary Balancing: Designing Frost-Resistant Lubricant-Infused Surfaces. Nano Lett. 2020, 20, 8508–8515. [Google Scholar] [CrossRef] [PubMed]
- Wexler, J.S.; Grosskopf, A.; Chow, M.; Fan, Y.; Jacobi, I.; Stone, H.A. Robust liquid-infused surfaces through patterned wettability. Soft Matter 2015, 11, 5023–5029. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Yuan, Y.; Zhang, C.; Dai, X.; Zhu, T.; Song, L.; Gai, Y.; Liao, R. Key Factors Affecting Durable Anti-Icing of Slippery Surfaces: Pore Size and Porosity. ACS Appl. Mater. Interfaces 2022, 15, 3599–3612. [Google Scholar] [CrossRef]
- Singh, S.L.; Schimmele, L.; Dietrich, S. Intrusion of liquids into liquid-infused surfaces with nanoscale roughness. Phys. Rev. E 2022, 105, 044803. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Ferri, M.; Tamplenizza, M.; Borghi, F.; Divitini, G.; Ducati, C.; Lenardi, C.; Piazzoni, C.; Merlini, M.; Podestà, A.; et al. Bottom-up engineering of the surface roughness of nanostructured cubic zirconia to control cell adhesion. Nanotechnology 2012, 23, 475101. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Guo, Z. Fabrications and Applications of Slippery Liquid-infused Porous Surfaces Inspired from Nature: A Review. J. Bionic Eng. 2019, 16, 769–793. [Google Scholar] [CrossRef]
- Wen, G.; Guo, Z.; Liu, W. Biomimetic polymeric superhydrophobic surfaces and nanostructures: From fabrication to applications. Nanoscale 2017, 9, 3338–3366. [Google Scholar] [CrossRef]
- Howell, C.; Grinthal, A.; Sunny, S.; Aizenberg, M.; Aizenberg, J. Designing Liquid-Infused Surfaces for Medical Applications: A Review. Adv. Mater. 2018, 30, 1802724. [Google Scholar] [CrossRef]
- Liu, R.; Chi, Z.; Cao, L.; Weng, Z.; Wang, L.; Li, L.; Saeed, S.; Lian, Z.; Wang, Z. Fabrication of biomimetic superhydrophobic and anti-icing Ti6Al4V alloy surfaces by direct laser interference lithography and hydrothermal treatment. Appl. Surf. Sci. 2020, 534, 147576. [Google Scholar] [CrossRef]
- Liu, M.; Li, M.T.; Xu, S.; Yang, H.; Sun, H.B. Bioinspired Superhydrophobic Surfaces via Laser-Structuring. Front. Chem. 2020, 8, 835. [Google Scholar] [CrossRef] [PubMed]
- Atthi, N.; Suwan, M.; Sangwong, N.; Pattamang, P.; Sripumkhai, W.; Meananeatra, R.; Saengdee, P.; Thongsook, O.; Ranron, N.; Pankong, K.; et al. Fabrication of slippery liquid-infused porous surfaces for anti-fouling applications. Jpn. J. Appl. Phys. 2021, 60, SCCJ04. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, P.; Li, J.; Wang, G. Water-repellent and corrosion resistance properties of epoxy-resin-based slippery liquid-infused porous surface. Prog. Org. Coatings 2022, 172, 107152. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, C.; Liu, R.; Zhang, Q.; Gong, J.; Tao, D.; Ji, Z. Fabrication of superhydrophobic surface with enhanced corrosion resistance on H62 brass substrate. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 589, 124475. [Google Scholar] [CrossRef]
- Yong, J.; Huo, J.; Yang, Q.; Chen, F.; Fang, Y.; Wu, X.; Liu, L.; Lu, X.; Zhang, J.; Hou, X. Femtosecond Laser Direct Writing of Porous Network Microstructures for Fabricating Super-Slippery Surfaces with Excellent Liquid Repellence and Anti-Cell Proliferation. Adv. Mater. Interfaces 2018, 5, 1701479. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Cheng, Y.; Yang, Q.; Lu, Y.; Zhang, C.; Li, M.; Du, B.; Hou, X.; Chen, F. Design of Metal-Based Slippery Liquid-Infused Porous Surfaces (SLIPSs) with Effective Liquid Repellency Achieved with a Femtosecond Laser. Micromachines 2022, 13, 1160. [Google Scholar] [CrossRef]
- Sunny, S.; Vogel, N.; Howell, C.; Vu, T.L.; Aizenberg, J. Lubricant-Infused Nanoparticulate Coatings Assembled by Layer-by-Layer Deposition. Adv. Funct. Mater. 2014, 24, 6658–6667. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Lee, Y.Y.; Chong, K.S.L.; He, C. Superhydrophobic and slippery liquid-infused porous surfaces formed by the self-assembly of a hybrid ABC triblock copolymer and their antifouling performance. J. Mater. Chem. B 2018, 6, 440–448. [Google Scholar] [CrossRef]
- Manabe, K.; Kyung, K.H.; Shiratori, S. Biocompatible Slippery Fluid-Infused Films Composed of Chitosan and Alginate via Layer-by-Layer Self-Assembly and Their Antithrombogenicity. ACS Appl. Mater. Interfaces 2015, 7, 4763–4771. [Google Scholar] [CrossRef]
- Zhu, G.H.; Cho, S.H.; Zhang, H.; Zhao, M.; Zacharia, N.S. Slippery Liquid-Infused Porous Surfaces (SLIPS) Using Layer-by-Layer Polyelectrolyte Assembly in Organic Solvent. Langmuir 2018, 34, 4722–4731. [Google Scholar] [CrossRef]
- Kasapgil, E.; Erbil, H.Y.; Sakir, I.A. Multi-liquid repellent, fluorine-free, heat stable SLIPS via layer-by-layer assembly. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 654, 130076. [Google Scholar] [CrossRef]
- Tesler, A.B.; Kim, P.; Kolle, S.; Howell, C.; Ahanotu, O.; Aizenberg, J. Extremely durable biofouling-resistant metallic surfaces based on electrodeposited nanoporous tungstite films on steel. Nat. Commun. 2015, 6, 8649. [Google Scholar] [CrossRef] [Green Version]
- Badv, M.; Jaffer, I.H.; Weitz, J.I.; Didar, T.F. An omniphobic lubricant-infused coating produced by chemical vapor deposition of hydrophobic organosilanes attenuates clotting on catheter surfaces. Sci. Rep. 2017, 7, 11639. [Google Scholar] [CrossRef] [Green Version]
- Khammas, R.; Koivuluoto, H. Durable Icephobic Slippery Liquid-Infused Porous Surfaces (SLIPS) Using Flame- and Cold-Spraying. Sustainability 2022, 14, 8422. [Google Scholar] [CrossRef]
- Li, Y.; Luo, B.; Guet, C.; Narasimalu, S.; Dong, Z. Preparation and Formula Analysis of Anti-Biofouling Titania—Polyurea Spray Coating with Nano/Micro-Structure. Coatings 2019, 9, 560. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Wang, L.; Liu, G.; Liao, R. Fabrication of Ultralow Ice-Adhesion Slippery Liquid Infused Porous Surfaces on Aluminum Alloy (7075-T651). Coatings 2020, 10, 1025. [Google Scholar] [CrossRef]
- Domagalski, J.T.; Xifre-Perez, E.; Marsal, L.F. Recent Advances in Nanoporous Anodic Alumina: Principles, Engineering, and Applications. Nanomaterials 2021, 11, 430. [Google Scholar] [CrossRef]
- Jeong, C.; Jung, J.; Sheppard, K.; Choi, C.H. Control of the Nanopore Architecture of Anodic Alumina via Stepwise Anodization with Voltage Modulation and Pore Widening. Nanomaterials 2023, 13, 342. [Google Scholar] [CrossRef]
- Li, B.; Bai, J.; Fan, L.; Mao, X.; Ding, Z.; Mu, H.; Liu, G.; Yuan, Y. Fabrication of Slippery Surfaces on Aluminum Alloy and Its Anti-Icing Performance in Glaze Ice. Coatings 2023, 13, 732. [Google Scholar] [CrossRef]
- Zhang, D.; Xia, Y.; Chen, X.; Shi, S.; Lei, L. PDMS-Infused Poly(High Internal Phase Emulsion) Templates for the Construction of Slippery Liquid-Infused Porous Surfaces with Self-cleaning and Self-repairing Properties. Langmuir 2019, 35, 8276–8284. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Kreder, M.J.; Alvarenga, J.; Aizenberg, J. Hierarchical or Not? Effect of the Length Scale and Hierarchy of the Surface Roughness on Omniphobicity of Lubricant-Infused Substrates. Nano Lett. 2013, 13, 1793–1799. [Google Scholar] [CrossRef]
- He, X.; Cao, P.; Tian, F.; Bai, X.; Yuan, C. Infused configurations induced by structures influence stability and antifouling performance of biomimetic lubricant-infused surfaces. Surf. Coatings Technol. 2019, 358, 159–166. [Google Scholar] [CrossRef]
- Karkantonis, T.; Gaddam, A.; See, T.L.; Joshi, S.S.; Dimov, S. Femtosecond laser-induced sub-micron and multi-scale topographies for durable lubricant impregnated surfaces for food packaging applications. Surf. Coatings Technol. 2020, 399, 126166. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Y.; Huang, M.; Zhou, Y.; Liu, Y.; Liang, X. Durability of a lubricant-infused Electrospray Silicon Rubber surface as an anti-icing coating. Appl. Surf. Sci. 2015, 346, 68–76. [Google Scholar] [CrossRef]
- Boveri, G.; Corozzi, A.; Veronesi, F.; Raimondo, M. Different Approaches to Low-Wettable Materials for Freezing Environments: Design, Performance and Durability. Coatings 2021, 11, 77. [Google Scholar] [CrossRef]
- Xi, S.; Zhang, Y.; Yang, Y.; Tan, X. Micrometric array integrated with slippery liquid-infused porous surface for improved anti-icing durability. J. Coatings Technol. Res. 2022, 19, 1211–1218. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Lu, C.; Liu, Y.; Feng, S.; Liu, Y. Robust Slippery Liquid-Infused Porous Network Surfaces for Enhanced Anti-icing/Deicing Performance. ACS Appl. Mater. Interfaces 2020, 12, 25471–25477. [Google Scholar] [CrossRef]
- Wei, X.; Zhong, Y.; Feng, Y.; Wei, J.; Wang, J. A Slippery Liquid-Infused Network-like Surface with Anti/De-icing Properties Constructed Based on the Phosphating Reaction. Langmuir 2022, 38, 14118–14128. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, Y.; Liu, X.; Xi, S.; Yan, Z.; Liu, Z.; Shi, T.; Liao, G. Employing micro pyramidal holes and porous nanostructures for enhancing the durability of lubricant-infused surfaces. Surf. Coatings Technol. 2021, 405, 126568. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, X.; Yang, X.; Liu, Q.; Liu, X.; Yang, Z.; Zhai, Y. Slippery surface with honeycomb structures for enhancing chemical durability of aluminum. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 648, 129187. [Google Scholar] [CrossRef]
- Cai, G.; Liu, F.; Wu, T. Slippery liquid-infused porous surfaces with inclined microstructures to enhance durable anti-biofouling performances. Colloids Surfaces B Biointerfaces 2021, 202, 111667. [Google Scholar] [CrossRef]
- Cai, G.; Zeng, Q.; Wu, T. Effect of Surface Microstructure on the Long-term Anti-bacterial Performance for Slippery Liquid Infused Porous Surfaces. In Proceedings of the 2020 IEEE SENSORS, Rotterdam, The Netherlands, 25–28 October 2020. [Google Scholar] [CrossRef]
- Han, X.; Tang, X.; Chen, R.; Li, W.; Zhu, P.; Wang, L. Citrus-peel-like durable slippery surfaces. Chem. Eng. J. 2021, 420, 129599. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, J.; Guo, Z.; Liu, W. Nanotree array textured lubricant-infused frame for efficient fog harvesting. Mater. Today Phys. 2022, 28, 100869. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, X.; Yang, X.; Xu, Q.; Chen, G.; Liu, X.; Yang, Z.; Zhai, Y. Slippery surface for enhancing surface robustness and chemical stability. J. Mater. Sci. 2023, 58, 5837–5847. [Google Scholar] [CrossRef]
- Huang, J.; Kang, J.; Zhang, J.; Huang, J.; Guo, Z. Slippery Surface with Petal-like Structure for Protecting Al Alloy: Anti-corrosion, Anti-fouling and Anti-icing. J. Bionic Eng. 2021, 19, 83–91. [Google Scholar] [CrossRef]
- Laney, S.K.; Michalska, M.; Li, T.; Ramirez, F.V.; Portnoi, M.; Oh, J.; Thayne, I.G.; Parkin, I.P.; Tiwari, M.K.; Papakonstantinou, I. Delayed Lubricant Depletion of Slippery Liquid Infused Porous Surfaces Using Precision Nanostructures. Langmuir 2021, 37, 10071–10078. [Google Scholar] [CrossRef]
- Shome, A.; Das, A.; Borbora, A.; Dhar, M.; Manna, U. Role of chemistry in bio-inspired liquid wettability. Chem. Soc. Rev. 2022, 51, 5452–5497. [Google Scholar] [CrossRef]
- Multanen, V.; Chaniel, G.; Grynyov, R.; Loew, R.Y.; Siany, N.K.; Bormashenko, E. Hydrophilization of liquid surfaces by plasma treatment. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 461, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.F.; Urata, C.; Masheder, B.; Hozumi, A. A Physical Approach To Specifically Improve the Mobility of Alkane Liquid Drops. J. Am. Chem. Soc. 2012, 134, 10191–10199. [Google Scholar] [CrossRef]
- Zhang, C.; Xia, Y.; Zhang, H.; Zacharia, N.S. Surface Functionalization for a Nontextured Liquid-Infused Surface with Enhanced Lifetime. ACS Appl. Mater. Interfaces 2018, 10, 5892–5901. [Google Scholar] [CrossRef]
- Jing, X.; Guo, Z. Fabrication of biocompatible super stable lubricant-immobilized slippery surfaces by grafting a polydimethylsiloxane brush: Excellent boiling water resistance, hot liquid repellency and long-term slippery stability. Nanoscale 2019, 11, 8870–8881. [Google Scholar] [CrossRef]
- Wooh, S.; Butt, H.J. A Photocatalytically Active Lubricant-Impregnated Surface. Angew. Chem. Int. Ed. 2017, 56, 4965–4969. [Google Scholar] [CrossRef]
- Xing, K.; Li, Z.; Wang, Z.; Qian, S.; Feng, J.; Gu, C.; Tu, J. Slippery coatings with mechanical robustness and self-replenishing properties as potential application on magnesium alloys. Chem. Eng. J. 2021, 418, 129079. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Z. An ionic liquid-infused slippery surface for temperature stability, shear resistance and corrosion resistance. J. Mater. Chem. A 2020, 8, 24075–24085. [Google Scholar] [CrossRef]
- Coady, M.J.; Wood, M.; Wallace, G.Q.; Nielsen, K.E.; Kietzig, A.M.; Lagugné-Labarthet, F.; Ragogna, P.J. Icephobic Behavior of UV-Cured Polymer Networks Incorporated into Slippery Lubricant-Infused Porous Surfaces: Improving SLIPS Durability. ACS Appl. Mater. Interfaces 2018, 10, 2890–2896. [Google Scholar] [CrossRef]
- Li, T.; Zhuo, Y.; Håkonsen, V.; Rønneberg, S.; He, J.; Zhang, Z. Epidermal Gland Inspired Self-Repairing Slippery Lubricant-Infused Porous Coatings with Durable Low Ice Adhesion. Coatings 2019, 9, 602. [Google Scholar] [CrossRef] [Green Version]
- Daneshmand, H.; Sazgar, A.; Araghchi, M. Fabrication of robust and versatile superhydrophobic coating by two-step spray method: An experimental and molecular dynamics simulation study. Appl. Surf. Sci. 2021, 567, 150825. [Google Scholar] [CrossRef]
- Zhu, Z.; Liang, H.; Sun, D.W. Infusing Silicone and Camellia Seed Oils into Micro-/Nanostructures for Developing Novel Anti-Icing/Frosting Surfaces for Food Freezing Applications. ACS Appl. Mater. Interfaces 2023, 15, 14874–14883. [Google Scholar] [CrossRef]
- Long, Y.; Yin, X.; Mu, P.; Wang, Q.; Hu, J.; Li, J. Slippery liquid-infused porous surface (SLIPS) with superior liquid repellency, anti-corrosion, anti-icing and intensified durability for protecting substrates. Chem. Eng. J. 2020, 401, 126137. [Google Scholar] [CrossRef]
- Togasawa, R.; Tenjimbayashi, M.; Matsubayashi, T.; Moriya, T.; Manabe, K.; Shiratori, S. A Fluorine-free Slippery Surface with Hot Water Repellency and Improved Stability against Boiling. ACS Appl. Mater. Interfaces 2018, 10, 4198–4205. [Google Scholar] [CrossRef]
- Tenjimbayashi, M.; Togasawa, R.; Manabe, K.; Matsubayashi, T.; Moriya, T.; Komine, M.; Shiratori, S. Liquid-Infused Smooth Coating with Transparency, Super-Durability, and Extraordinary Hydrophobicity. Adv. Funct. Mater. 2016, 26, 6693–6702. [Google Scholar] [CrossRef]
- Xiang, H.; Cheng, L.; Liu, G.; Zhu, T.; Dai, X.; Wei, Z.; Zhou, J.; Liao, R.; Yuan, Y. Proper matching of lubricants and modifiers: Another key factor for durable anti-icing performance of lubricated surfaces. Surfaces Interfaces 2023, 37, 102653. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiang, H.; Liu, G.; Wang, L.; Liu, H.; Liao, R. Self-Repairing Performance of Slippery Liquid Infused Porous Surfaces for Durable Anti-Icing. Adv. Mater. Interfaces 2022, 9, 2101968. [Google Scholar] [CrossRef]
- Song, T.; Liu, Q.; Liu, J.; Yang, W.; Chen, R.; Jing, X.; Takahashi, K.; Wang, J. Fabrication of super slippery sheet-layered and porous anodic aluminium oxide surfaces and its anticorrosion property. Appl. Surf. Sci. 2015, 355, 495–501. [Google Scholar] [CrossRef]
- Sett, S.; Yan, X.; Barac, G.; Bolton, L.W.; Miljkovic, N. Lubricant-Infused Surfaces for Low-Surface-Tension Fluids: Promise versus Reality. ACS Appl. Mater. Interfaces 2017, 9, 36400–36408. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Yin, S.; Huang, S.; Chen, F.; Tang, Q.; Li, X. Fabrication of slippery Zn surface with improved water-impellent, condensation and anti-icing properties. Appl. Surf. Sci. 2019, 470, 1139–1147. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Sun, S.; Li, T.; Sun, Y. Fabrication of Slippery Lubricant-Infused Porous Surface with High Underwater Transparency for the Control of Marine Biofouling. ACS Appl. Mater. Interfaces 2016, 9, 972–982. [Google Scholar] [CrossRef]
- Stamatopoulos, C.; Hemrle, J.; Wang, D.; Poulikakos, D. Exceptional Anti-Icing Performance of Self-Impregnating Slippery Surfaces. ACS Appl. Mater. Interfaces 2017, 9, 10233–10242. [Google Scholar] [CrossRef] [Green Version]
- Masripan, N.A.B.; Salim, M.A.; Omar, G.; Mansor, M.R.; Saad, A.M.; Hamid, N.A.; Syakir, M.I.; Dai, F. Vegetable Oil as Bio-Lubricant and Natural Additive in Lubrication: A Review. Int. J. Nanoelectron. Mater. 2020, 113, 161–176. [Google Scholar]
- Tulashie, S.K.; Kotoka, F. The potential of castor, palm kernel, and coconut oils as biolubricant base oil via chemical modification and formulation. Therm. Sci. Eng. Prog. 2020, 16, 100480. [Google Scholar] [CrossRef]
- Kohli, R. Applications of Ionic Liquids in Removal of Surface Contaminants. In Developments in Surface Contamination and Cleaning: Applications of Cleaning Techniques; Elsevier: Amsterdam, The Netherlands, 2019; pp. 619–680. [Google Scholar] [CrossRef]
- Zhou, F.; Liang, Y.; Liu, W. Ionic liquid lubricants: Designed chemistry for engineering applications. Chem. Soc. Rev. 2009, 38, 2590. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, C.; Wang, L.; Zhang, J.; Tu, J. Ionic liquids-infused slippery surfaces for condensation and hot water repellency. Chem. Eng. J. 2018, 343, 561–571. [Google Scholar] [CrossRef]
- Miranda, D.F.; Urata, C.; Masheder, B.; Dunderdale, G.J.; Yagihashi, M.; Hozumi, A. Physically and chemically stable ionic liquid-infused textured surfaces showing excellent dynamic omniphobicity. APL Mater. 2014, 2, 056108. [Google Scholar] [CrossRef] [Green Version]
- Bittner, R.W.; Bica, K.; Hoffmann, H. Fluorine-free, liquid-repellent surfaces made from ionic liquid-infused nanostructured silicon. Monatshefte Chem.—Chem. Mon. 2016, 148, 167–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, T.; Koyama, A.; Kowalski, D.; Zhu, C.; Aoki, Y.; Habazaki, H. Fluorine-Free Slippery Liquid-Infused Porous Surfaces Prepared Using Hierarchically Porous Aluminum. Phys. Status Solidi 2020, 217, 1900836. [Google Scholar] [CrossRef]
- Wang, F.; Ding, W.; He, J.; Zhang, Z. Phase transition enabled durable anti-icing surfaces and its DIY design. Chem. Eng. J. 2019, 360, 243–249. [Google Scholar] [CrossRef]
- Manabe, K.; Matsubayashi, T.; Tenjimbayashi, M.; Moriya, T.; Tsuge, Y.; Kyung, K.H.; Shiratori, S. Controllable Broadband Optical Transparency and Wettability Switching of Temperature-Activated Solid/Liquid-Infused Nanofibrous Membranes. ACS Nano 2016, 10, 9387–9396. [Google Scholar] [CrossRef]
- Wang, B.L.; Heng, L.; Jiang, L. Temperature-Responsive Anisotropic Slippery Surface for Smart Control of the Droplet Motion. ACS Appl. Mater. Interfaces 2018, 10, 7442–7450. [Google Scholar] [CrossRef]
- Gulfam, R.; Orejon, D.; Choi, C.H.; Zhang, P. Phase-Change Slippery Liquid-Infused Porous Surfaces with Thermo-Responsive Wetting and Shedding States. ACS Appl. Mater. Interfaces 2020, 12, 34306–34316. [Google Scholar] [CrossRef]
- Tonelli, M.; Peppou-Chapman, S.; Ridi, F.; Neto, C. Effect of Pore Size, Lubricant Viscosity, and Distribution on the Slippery Properties of Infused Cement Surfaces. J. Phys. Chem. C 2019, 123, 2987–2995. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Khare, S.; Bhandaru, N.; Mukherjee, R.; Chakraborty, S. High Temperature Durability of Oleoplaned Slippery Copper Surfaces. Langmuir 2020, 36, 4135–4143. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, R.; Nithyanandam, K.; Pitchumani, R. Fabrication and durability characterization of superhydrophobic and lubricant-infused surfaces. J. Colloid Interface Sci. 2022, 608, 662–672. [Google Scholar] [CrossRef]
- Xiang, H.; Yuan, Y.; Zhang, C.; Zhu, T.; Dai, X.; Liu, G.; Song, L. Effect of Lubricant Viscosity on Wetting Behaviors and Durability of Anti-icing Slippery Liquid-Infused Porous Surfaces. J. Phys. Conf. Ser. 2022, 2351, 012004. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Y.; Liao, R.; Wang, L.; Gao, X. Fabrication of a Porous Slippery Icephobic Surface and Effect of Lubricant Viscosity on Anti-Icing Properties and Durability. Coatings 2020, 10, 896. [Google Scholar] [CrossRef]
- Wang, Z.; Heng, L.; Jiang, L. Effect of lubricant viscosity on the self-healing properties and electrically driven sliding of droplets on anisotropic slippery surfaces. J. Mater. Chem. A 2018, 6, 3414–3421. [Google Scholar] [CrossRef]
- Sasidharanpillai, A.; Lee, Y.; Lee, S. Design of stable liquid infused surfaces: Influence of oil viscosity on stability. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 646, 128923. [Google Scholar] [CrossRef]
- Daniel, D.; Timonen, J.V.I.; Li, R.; Velling, S.J.; Aizenberg, J. Oleoplaning droplets on lubricated surfaces. Nat. Phys. 2017, 13, 1020–1025. [Google Scholar] [CrossRef] [Green Version]
- Schellenberger, F.; Xie, J.; Encinas, N.; Hardy, A.; Klapper, M.; Papadopoulos, P.; Butt, H.J.; Vollmer, D. Direct observation of drops on slippery lubricant-infused surfaces. Soft Matter 2015, 11, 7617–7626. [Google Scholar] [CrossRef] [Green Version]
- Varanasi, K.; Smith, J.D.; Yague, J.; Jordan, B.; Hibben, C.W.; Xu, J.; Cong, T. Spray Processes and Methods for Forming Liquid-Impregnated Surfaces. US Patent 0,273,518 A1, 1 October 2015. [Google Scholar]
- Aizenberg, J.; Hatton, B.; Ingber, D.; Super, M.; Wong, T.S. Slippery Liquid-Infused Porous Surfaces and Biological Applications Thereof. US Patent 9,932,484, 3 April 2018. [Google Scholar]
- Damle, V.G.; Uppal, A.; Sun, X.; Burgin, T.P.; Rykaczewski, K. Rapid and scalable lubrication and replenishment of liquid-infused materials. Surf. Innov. 2016, 4, 102–108. [Google Scholar] [CrossRef]
- Howell, C.; Vu, T.L.; Lin, J.J.; Kolle, S.; Juthani, N.; Watson, E.; Weaver, J.C.; Alvarenga, J.; Aizenberg, J. Self-Replenishing Vascularized Fouling-Release Surfaces. ACS Appl. Mater. Interfaces 2014, 6, 13299–13307. [Google Scholar] [CrossRef]
- Wu, W.; DeConinck, A.; Lewis, J.A. Omnidirectional Printing of 3D Microvascular Networks. Adv. Mater. 2011, 23, H178–H183. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.; Shim, J.; Lee, C.; Nam, Y. Brushed lubricant-impregnated surfaces (BLIS) for long-lasting high condensation heat transfer. Sci. Rep. 2020, 10, 2959. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Zhang, C.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Gao, L.; Sun, G.; Wang, J. Fast self-replenishing slippery surfaces with a 3D fibrous porous network for the healing of surface properties. J. Mater. Chem. A 2019, 7, 24900–24907. [Google Scholar] [CrossRef]
- Rao, Q.; Lu, Y.; Song, L.; Hou, Y.; Zhan, X.; Zhang, Q. Highly Efficient Self-Repairing Slippery Liquid-Infused Surface with Promising Anti-Icing and Anti-Fouling Performance. ACS Appl. Mater. Interfaces 2021, 13, 40032–40041. [Google Scholar] [CrossRef] [PubMed]
- Vogel, N.; Belisle, R.A.; Hatton, B.; Wong, T.S.; Aizenberg, J. Transparency and damage tolerance of patternable omniphobic lubricated surfaces based on inverse colloidal monolayers. Nat. Commun. 2013, 4, 2176. [Google Scholar] [CrossRef] [Green Version]
- Ruan, H.; Zhang, Y.; Wang, Q.; Wang, C.; Wang, T. A novel earthworm-inspired smart lubrication material with self-healing function. Tribol. Int. 2022, 165, 107303. [Google Scholar] [CrossRef]
- Yang, X.; Huang, Y.; Zhao, Y.; Zhang, X.; Wang, J.; Sann, E.E.; Mon, K.H.; Lou, X.; Xia, F. Bioinspired Slippery Lubricant-Infused Surfaces With External Stimuli Responsive Wettability: A Mini Review. Front. Chem. 2019, 7, 826. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Daniel, D.; Grinthal, A.; Lin, K.; Aizenberg, J. Dynamic polymer systems with self-regulated secretion for the control of surface properties and material healing. Nat. Mater. 2015, 14, 790–795. [Google Scholar] [CrossRef]
- Hao, Z.; Li, W. A Review of Smart Lubricant-Infused Surfaces for Droplet Manipulation. Nanomaterials 2021, 11, 801. [Google Scholar] [CrossRef]
- Lou, X.; Huang, Y.; Yang, X.; Zhu, H.; Heng, L.; Xia, F. External Stimuli Responsive Liquid-Infused Surfaces Switching between Slippery and Nonslippery States: Fabrications and Applications. Adv. Funct. Mater. 2020, 30, 1901130. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Zhang, D. A robust and anti-UV layered textured superhydrophobic surface based on water-glass interface enhancement. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 624, 126835. [Google Scholar] [CrossRef]
- Yao, X.; Dunn, S.S.; Kim, P.; Duffy, M.; Alvarenga, J.; Aizenberg, J. Fluorogel Elastomers with Tunable Transparency, Elasticity, Shape-Memory, and Antifouling Properties. Angew. Chem. Int. Ed. 2014, 53, 4418–4422. [Google Scholar] [CrossRef] [PubMed]
- Urata, C.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Self-lubricating organogels (SLUGs) with exceptional syneresis-induced anti-sticking properties against viscous emulsions and ices. J. Mater. Chem. A 2015, 3, 12626–12630. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, Q.; Deng, X.; Cui, J. Earthworm-Inspired Rough Polymer Coatings with Self-Replenishing Lubrication for Adaptive Friction-Reduction and Antifouling Surfaces. Adv. Mater. 2018, 30, 1802141. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, W.; Huang, J.; Guo, Z. Lubricant self-replenishing slippery surface with prolonged service life for fog harvesting. Friction 2021, 10, 1676–1692. [Google Scholar] [CrossRef]
- Zhuo, Y.; Wang, F.; Xiao, S.; He, J.; Zhang, Z. One-Step Fabrication of Bioinspired Lubricant-Regenerable Icephobic Slippery Liquid-Infused Porous Surfaces. ACS Omega 2018, 3, 10139–10144. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, X.; Wu, S.; Li, Q.; Lv, J.; Wang, J.; Jiang, L. Bioinspired Solid Organogel Materials with a Regenerable Sacrificial Alkane Surface Layer. Adv. Mater. 2017, 29, 1700865. [Google Scholar] [CrossRef]
- Mujawar, N.K.; Ghatage, S.L.; Yeligar, V.C. Organogel: Factors and its importance. Int. J. Pharm. 2014, 4, 758–773. [Google Scholar]
- Hinze, W.L.; Uemasu, I.; Dai, F.; Braun, J.M. Analytical and related applications of organogels. Curr. Opin. Colloid Interface Sci. 1996, 1, 502–513. [Google Scholar] [CrossRef]
- Yao, X.; Wu, S.; Chen, L.; Ju, J.; Gu, Z.; Liu, M.; Wang, J.; Jiang, L. Self-Replenishable Anti-Waxing Organogel Materials. Angew. Chem. 2015, 127, 9103–9107. [Google Scholar] [CrossRef] [Green Version]
- Sotiri, I.; Tajik, A.; Lai, Y.; Zhang, C.T.; Kovalenko, Y.; Nemr, C.R.; Ledoux, H.; Alvarenga, J.; Johnson, E.; Patanwala, H.S.; et al. Tunability of liquid-infused silicone materials for biointerfaces. Biointerphases 2018, 13, 06D401. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, P.; Liu, M.; Wang, S.; Jiang, L. Organogel-based Thin Films for Self-Cleaning on Various Surfaces. Adv. Mater. 2013, 25, 4477–4481. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, W.; Feng, K.; Wu, Y.; Yu, B.; Liu, S.; Zhou, F. Sebaceous gland-inspired self-lubricated de-icing coating by continuously secreting lubricants. Prog. Org. Coatings 2023, 174, 107311. [Google Scholar] [CrossRef]
- Basu, S.; Hanh, B.M.; Chua, J.I.; Daniel, D.; Ismail, M.H.; Marchioro, M.; Amini, S.; Rice, S.A.; Miserez, A. Green biolubricant infused slippery surfaces to combat marine biofouling. J. Colloid Interface Sci. 2020, 568, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Ju, J.; Yang, S.; Wang, J.; Jiang, L. Temperature-Driven Switching of Water Adhesion on Organogel Surface. Adv. Mater. 2013, 26, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, H.; Meng, J.; Yang, G.; Liu, X.; Wang, S.; Jiang, L. Grooved Organogel Surfaces towards Anisotropic Sliding of Water Droplets. Adv. Mater. 2014, 26, 3131–3135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, P.; Zhang, D. Designing a transparent organogel layer with self-repairing property for the inhibition of marine biofouling. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 538, 140–147. [Google Scholar] [CrossRef]
- Binh, N.T.; Hanh, V.T.H.; Ngoc, N.T.; Duc, N.B. Anti-icing efficiency on bio-inspired slippery elastomer surface. Mater. Chem. Phys. 2021, 265, 124502. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, X.; Chen, J.; He, Z.; Liu, J.; Li, Q.; Wang, J.; Jiang, L. Organogel as durable anti-icing coatings. Sci. China Mater. 2015, 58, 559–565. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.J.; Lee, B.; Chun, J.M.; Patil, V.; Kim, Y.S. Durable ice-lubricating surfaces based on polydimethylsiloxane embedded silicone oil infused silica aerogel. Appl. Surf. Sci. 2020, 512, 145728. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, Y.; Wang, P.; Zhang, D. Design of slippery organogel layer with room-temperature self-healing property for marine anti-fouling application. Prog. Org. Coatings 2019, 132, 132–138. [Google Scholar] [CrossRef]
- Urata, C.; Nagashima, H.; Hatton, B.D.; Hozumi, A. Transparent Organogel Films Showing Extremely Efficient and Durable Anti-Icing Performance. ACS Appl. Mater. Interfaces 2021, 13, 28925–28937. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jin, B.; Jamil, M.I.; Cheng, D.; Zhang, Q.; Zhan, X.; Chen, F. Highly Stable Amphiphilic Organogel with Exceptional Anti-icing Performance. ACS Appl. Mater. Interfaces 2019, 11, 12838–12845. [Google Scholar] [CrossRef] [PubMed]
- Buddingh, J.V.; Nakamura, S.; Liu, G.; Hozumi, A. Thermo-responsive Fluorinated Organogels Showing Anti-fouling and Long-Lasting/Repeatable Icephobic Properties. Langmuir 2022, 38, 11362–11371. [Google Scholar] [CrossRef]
- Zang, L.; Shang, H.; Wei, D.; Jiang, S. A multi-stimuli-responsive organogel based on salicylidene Schiff base. Sens. Actuators B Chem. 2013, 185, 389–397. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, T.; Miao, X.; Lu, J.; Zhu, X.; Song, Y.; Ren, G.; Jia, Y.; Li, X. A thermal-driven self-replenishing slippery coating. Surfaces Interfaces 2021, 24, 101022. [Google Scholar] [CrossRef]
- Singh, A.V.; Vyas, V.; Patil, R.; Sharma, V.; Scopelliti, P.E.; Bongiorno, G.; Podestà, A.; Lenardi, C.; Gade, W.N.; Milani, P. Quantitative Characterization of the Influence of the Nanoscale Morphology of Nanostructured Surfaces on Bacterial Adhesion and Biofilm Formation. PLoS ONE 2011, 6, e25029. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Bai, H.; Li, Z.; Cao, M. Fluid manipulation via multifunctional lubricant infused slippery surfaces: Principle, design and applications. Soft Matter 2023, 19, 588–608. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, W. Superhydrophobic, superamphiphobic and SLIPS materials as anti-corrosion and anti-biofouling barriers. New J. Chem. 2021, 45, 15170–15179. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Z. Liquid infused surfaces with anti-icing properties. Nanoscale 2019, 11, 22615–22635. [Google Scholar] [CrossRef]
- Regan, D.P.; Howell, C. Droplet manipulation with bioinspired liquid-infused surfaces: A review of recent progress and potential for integrated detection. Curr. Opin. Colloid Interface Sci. 2019, 39, 137–147. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Z.; Zhu, Q.; Narasimalu, S.; Dong, Z. Fabrication of Zinc Substrate Encapsulated by Fluoropolyurethane and Its Drag-Reduction Enhancement by Chemical Etching. Coatings 2020, 10, 377. [Google Scholar] [CrossRef] [Green Version]
- Prakash, C.J.; Prasanth, R. Recent trends in fabrication of nepenthes inspired SLIPs: Design strategies for self-healing efficient anti-icing surfaces. Surfaces Interfaces 2020, 21, 100678. [Google Scholar] [CrossRef]
- Jenner, E.; D’Urso, B. Wetting states on structured immiscible liquid coated surfaces. Appl. Phys. Lett. 2013, 103, 251606. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Z.; Hwang, D.J.; Colosqui, C.E.; Cubaud, T. Viscous liquid–liquid wetting and dewetting of surfaces. Soft Matter 2021, 17, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, M.; Kim, J.H.; Lee, J. Dynamic contact angle measurements on lubricant infused surfaces. J. Colloid Interface Sci. 2021, 586, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Tang, G.H.; Kumar, S. Droplet Morphology and Mobility on Lubricant-Impregnated Surfaces: A Molecular Dynamics Study. Langmuir 2019, 35, 16377–16387. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Bhatt, B.; Sharma, M.; Khare, K. Numerical and experimental investigation of static wetting morphologies of aqueous drops on lubricated slippery surfaces using a quasi-static approach. Soft Matter 2023, 19, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- McHale, G.; Afify, N.; Armstrong, S.; Wells, G.G.; Ledesma-Aguilar, R. The Liquid Young’s Law on SLIPS: Liquid–Liquid Interfacial Tensions and Zisman Plots. Langmuir 2022, 38, 10032–10042. [Google Scholar] [CrossRef]
- Semprebon, C.; Sadullah, M.S.; McHale, G.; Kusumaatmaja, H. Apparent contact angle of drops on liquid infused surfaces: Geometric interpretation. Soft Matter 2021, 17, 9553–9559. [Google Scholar] [CrossRef]
- Barrio-Zhang, H.; Ruiz-Gutiérrez, É.; Armstrong, S.; McHale, G.; Wells, G.G.; Ledesma-Aguilar, R. Contact-Angle Hysteresis and Contact-Line Friction on Slippery Liquid-like Surfaces. Langmuir 2020, 36, 15094–15101. [Google Scholar] [CrossRef] [PubMed]
- Sadullah, M.S.; Panter, J.R.; Kusumaatmaja, H. Factors controlling the pinning force of liquid droplets on liquid infused surfaces. Soft Matter 2020, 16, 8114–8121. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathi, D.; Ray, P.; Singh, A.V.; Kishore, V.; Singh, S.L. Durability of Slippery Liquid-Infused Surfaces: Challenges and Advances. Coatings 2023, 13, 1095. https://doi.org/10.3390/coatings13061095
Tripathi D, Ray P, Singh AV, Kishore V, Singh SL. Durability of Slippery Liquid-Infused Surfaces: Challenges and Advances. Coatings. 2023; 13(6):1095. https://doi.org/10.3390/coatings13061095
Chicago/Turabian StyleTripathi, Divyansh, Prauteeto Ray, Ajay Vikram Singh, Vimal Kishore, and Swarn Lata Singh. 2023. "Durability of Slippery Liquid-Infused Surfaces: Challenges and Advances" Coatings 13, no. 6: 1095. https://doi.org/10.3390/coatings13061095






