Characterization of Two Types of Polylactic Acid Coating Loaded with Gentamicin Sulphate Deposed on AZ31 Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Equipment
2.3. Procedures
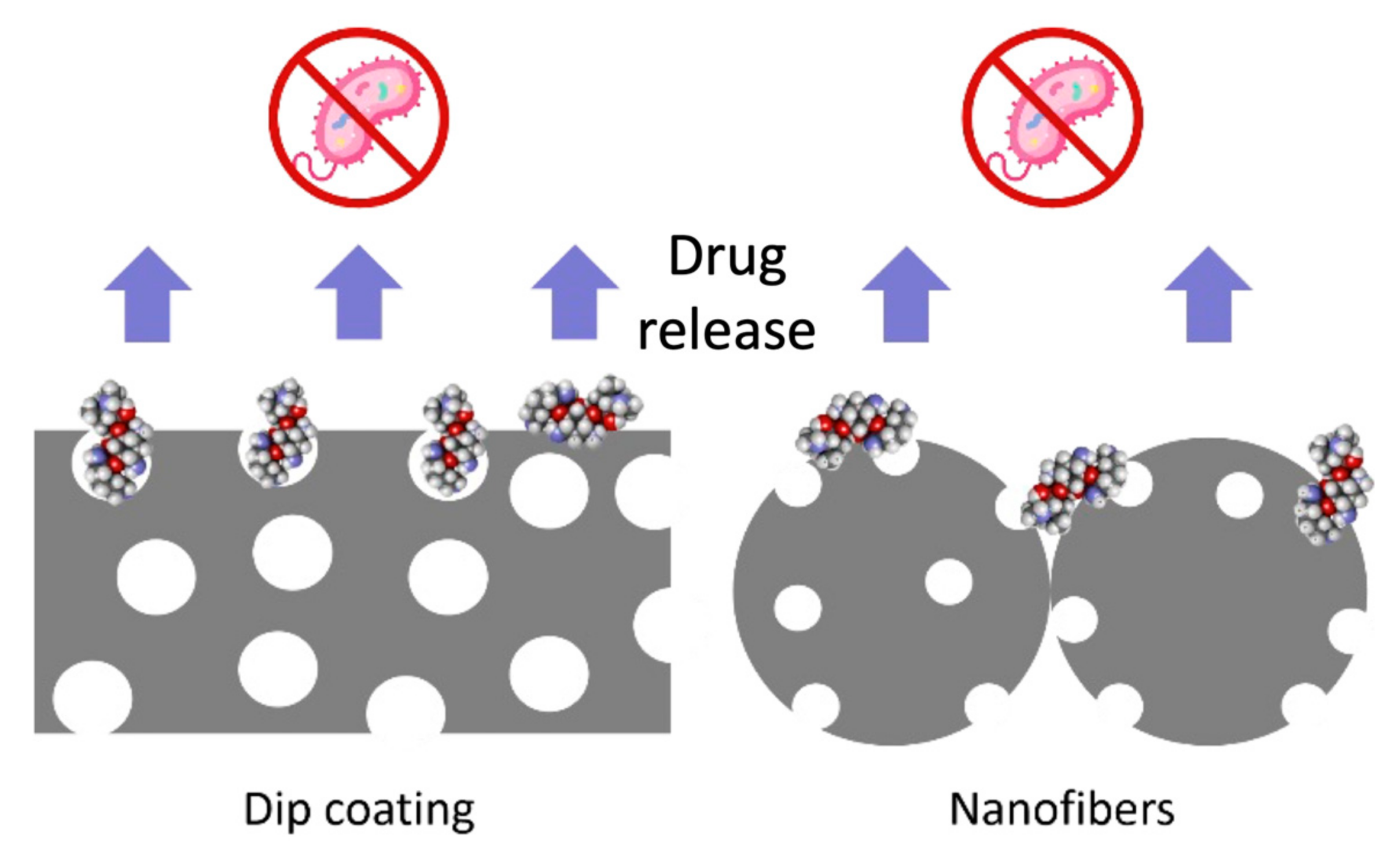
2.3.1. PLA Deposition on AZ31
2.3.2. Drug Loading–Release and Antibacterial Effect
3. Results and Discussion
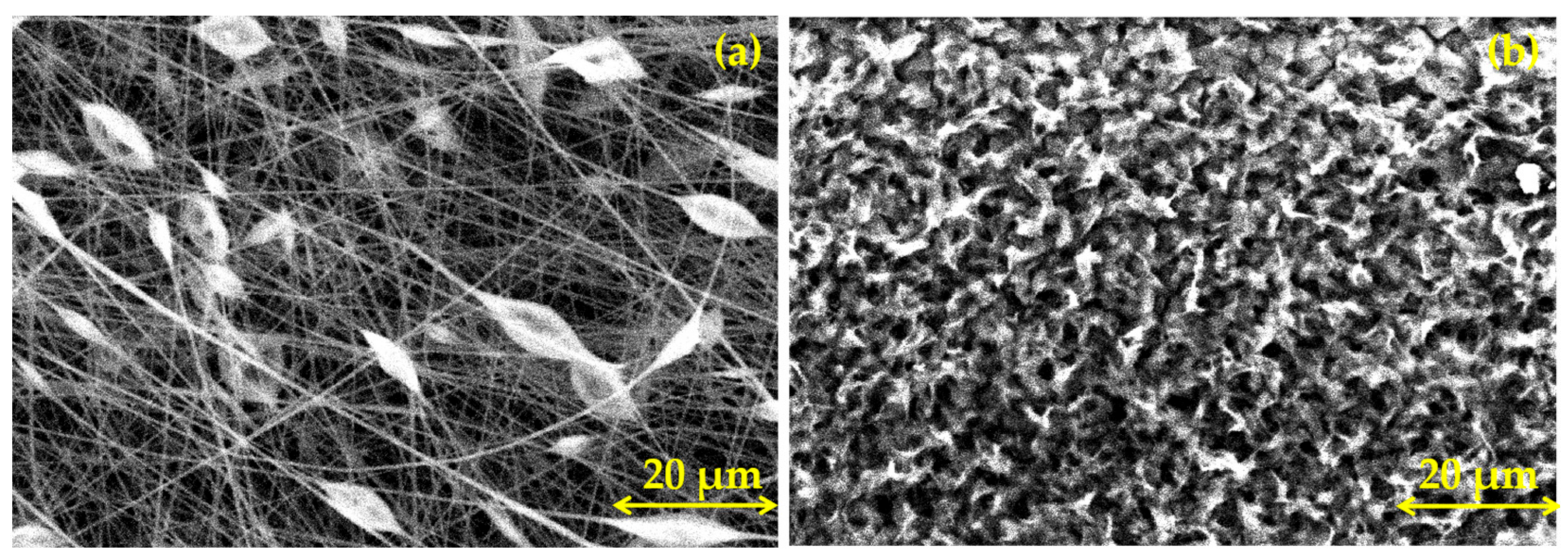
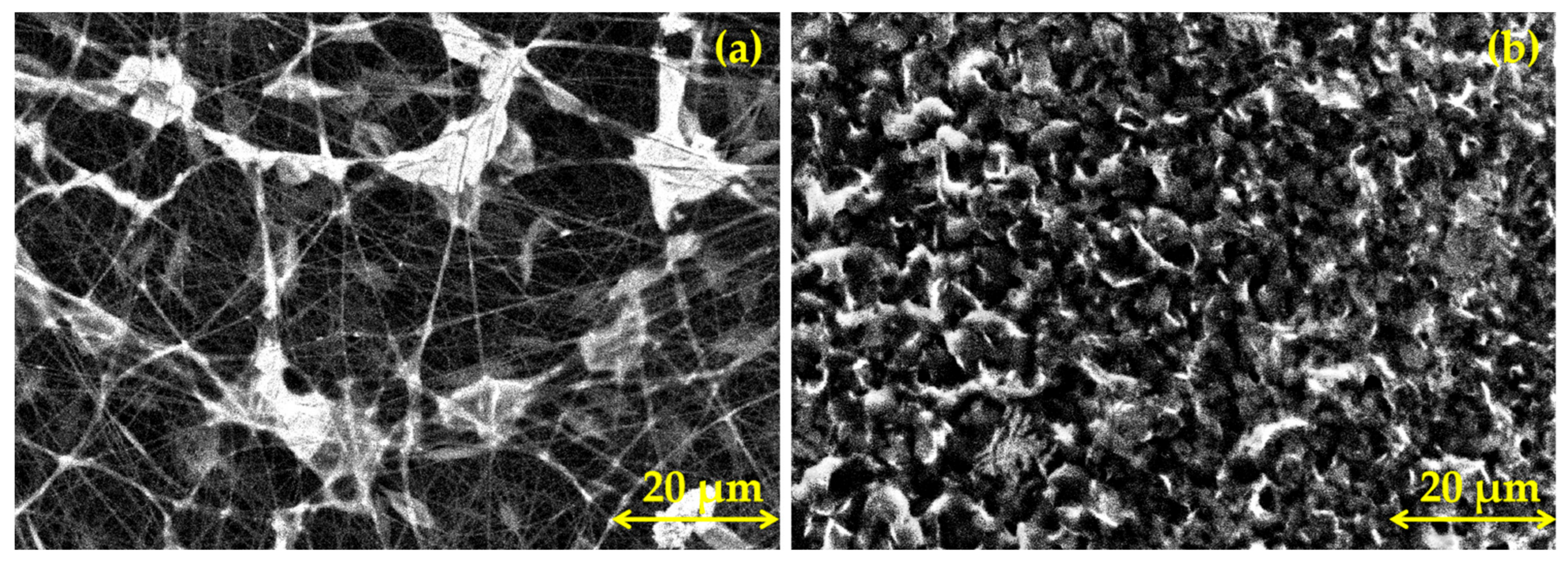
3.1. Morphological Characterization of PLA Coatings
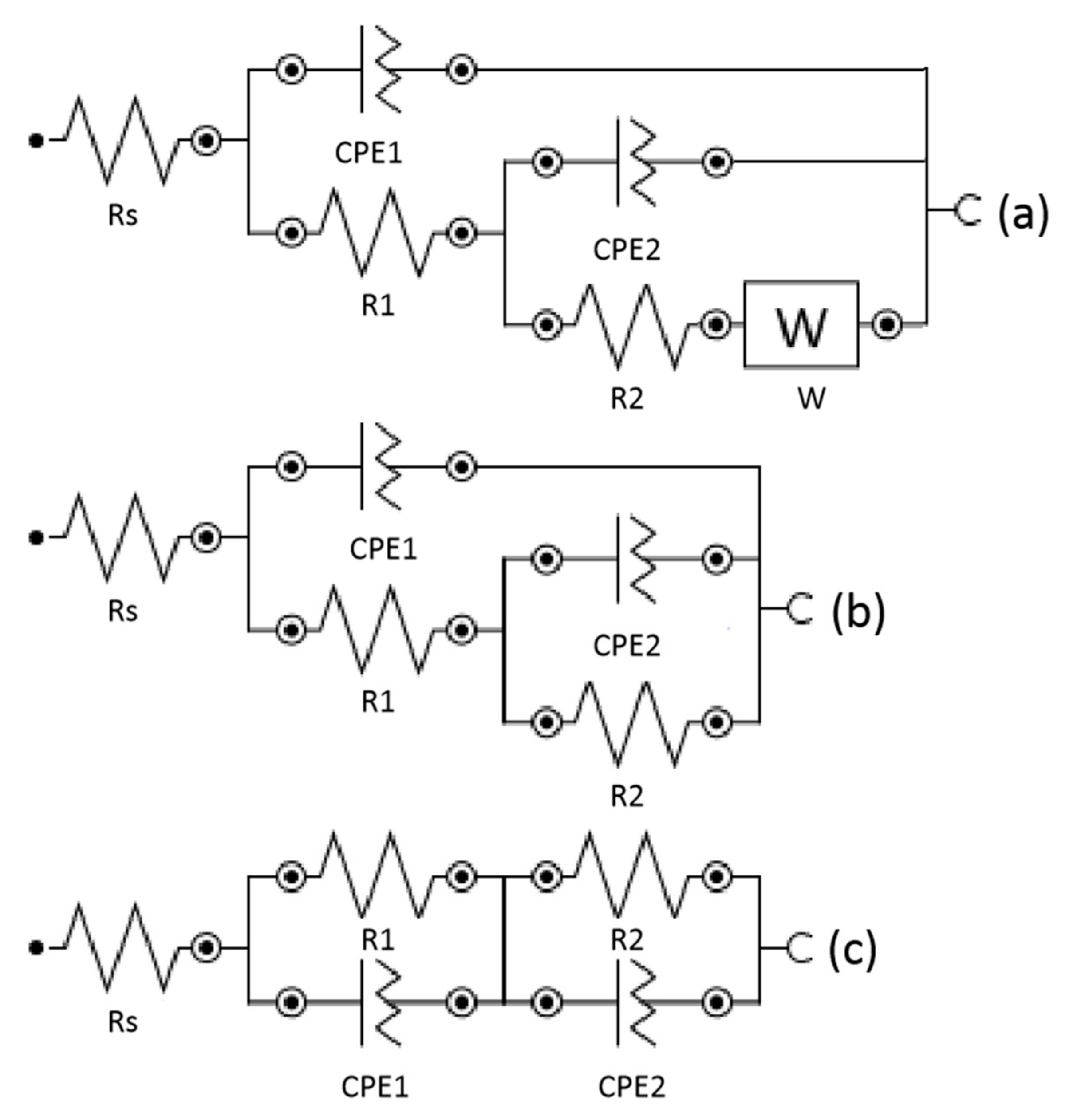
3.2. Electrochemical Characterization
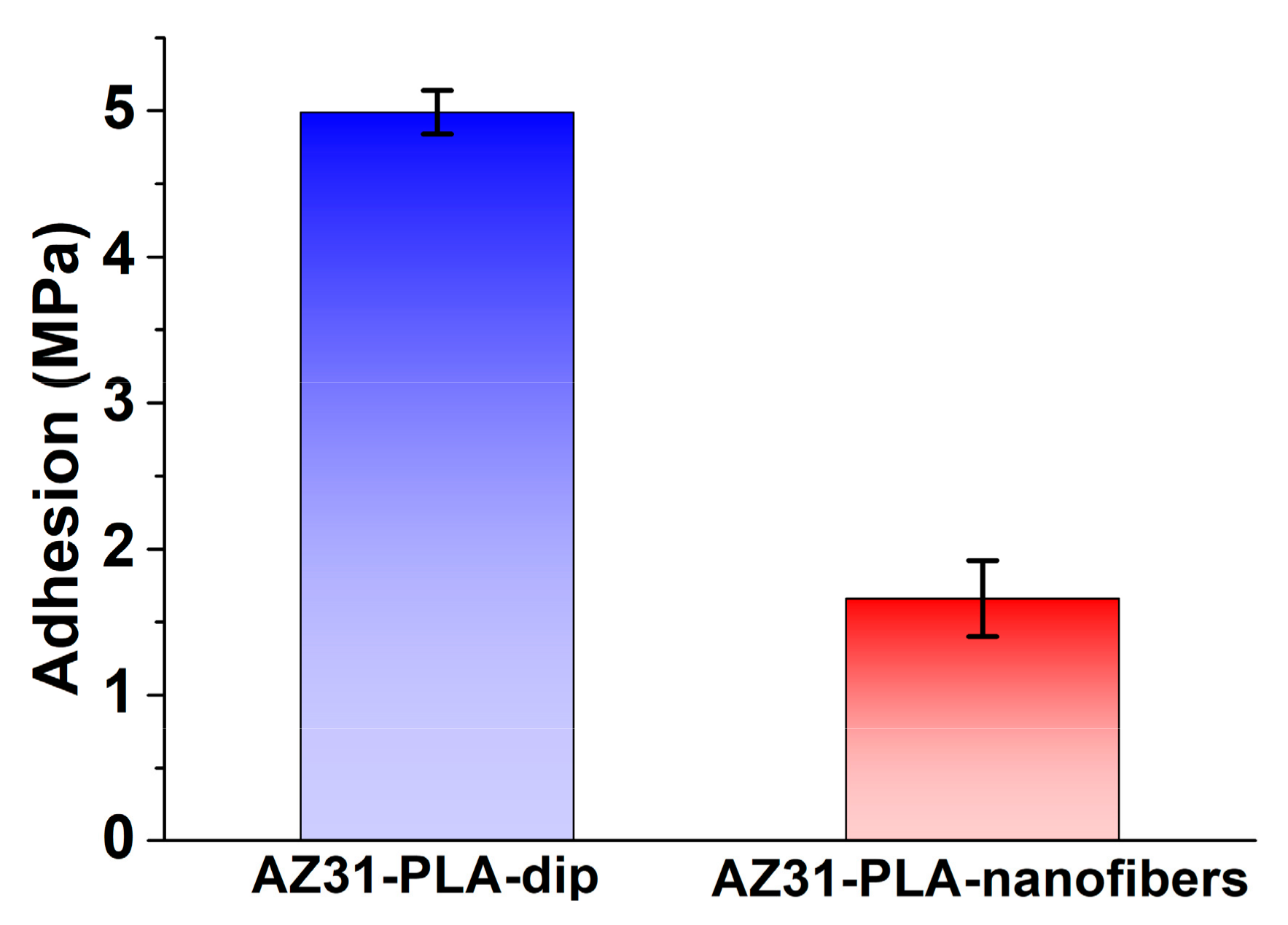
3.3. Adhesion of PLA Coatings
3.4. Determination of Surface Area by BET Method
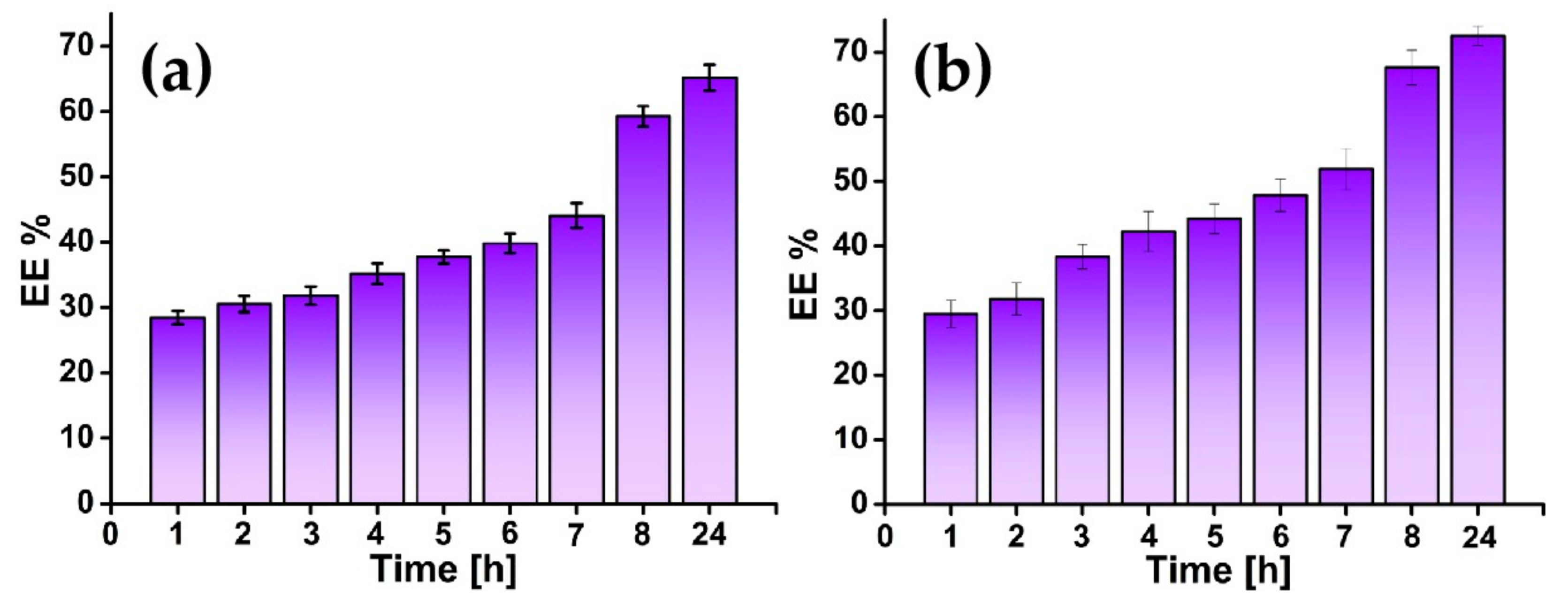
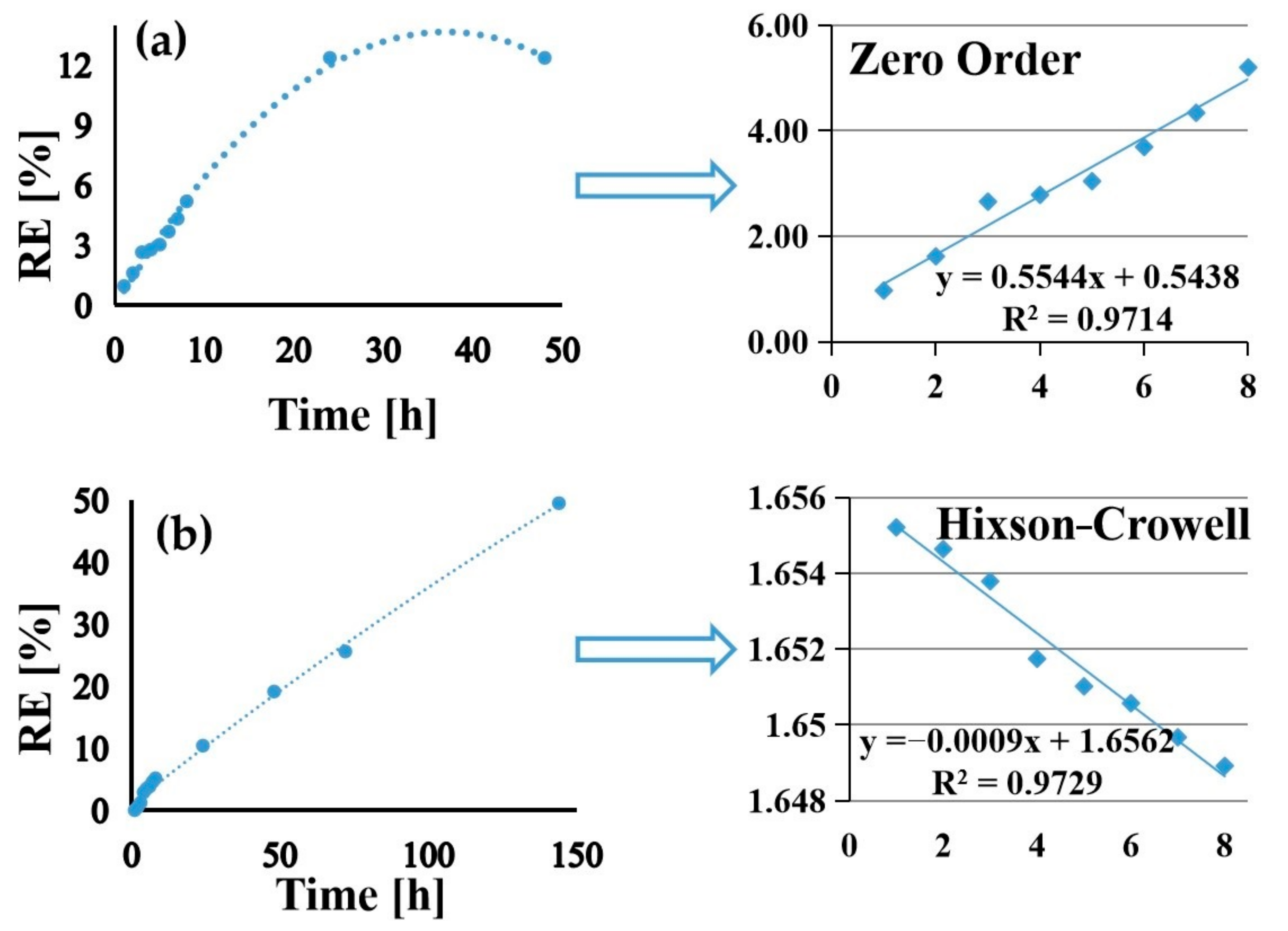
3.5. Drug Loading and Release
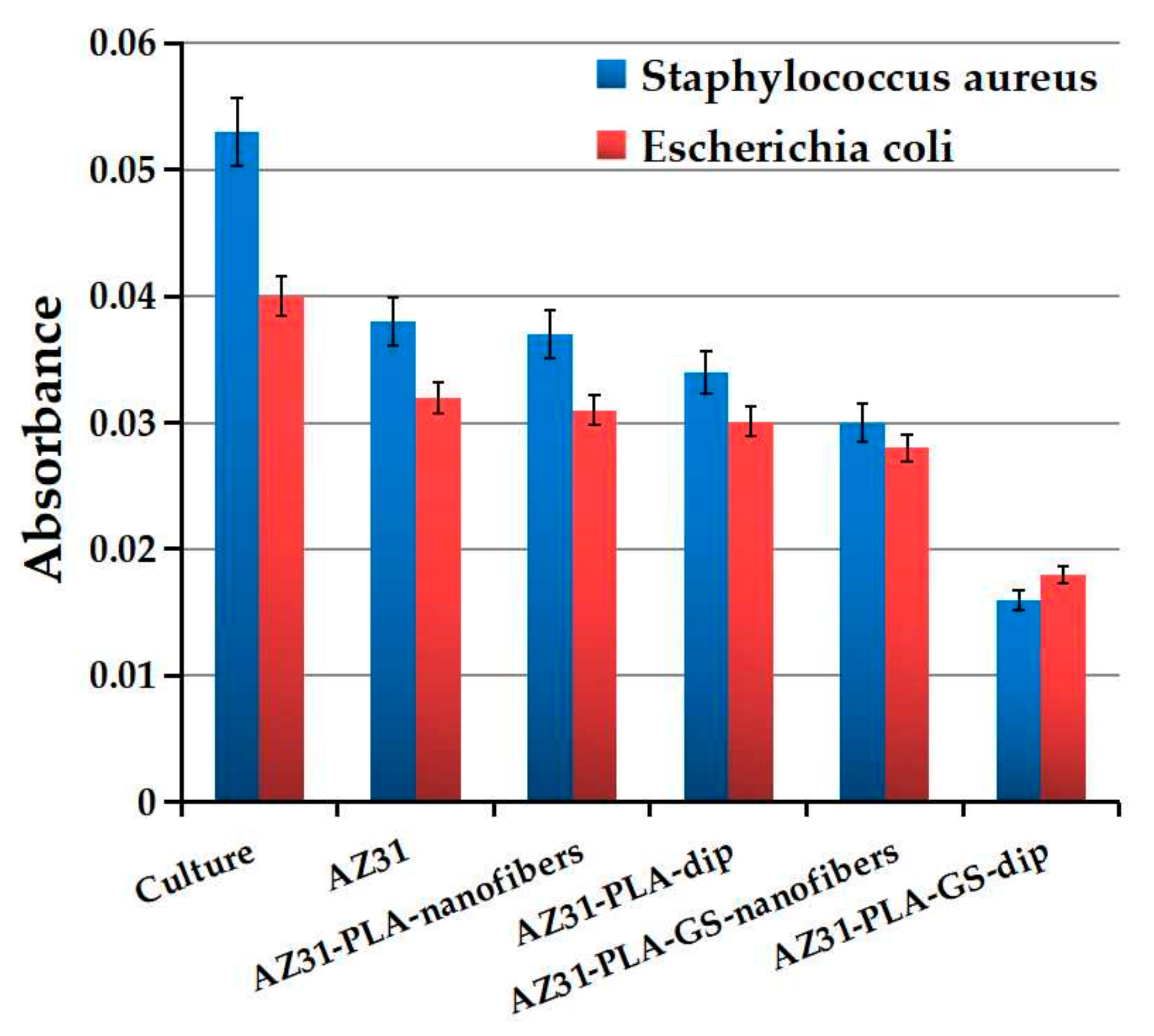
3.6. Antibacterial Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Ion, R.; Stoian, A.B.; Dumitriu, C.; Grigorescu, S.; Mazare, A.; Cimpean, A.; Demetrescu, I.; Schmuki, P. Nanochannels formed on TiZr alloy improve biological response. Acta Biomater. 2015, 24, 370–377. [Google Scholar] [CrossRef]
- Albrektsson, T.; Chrcanovic, B.; Jacobsson, M.; Wennerberg, A. Osseointegration of Implants—A Biological and Clinical Overview. JSM Dent. Surg. 2017, 2, 1022. [Google Scholar]
- Popa, M.; Demetrescu, I.; Vasilescu, E.; Drob, P.; Ionita, D.; Vasilescu, C. Stability of some dental implant materials in oral biofluids. Rev. Roum. Chim. 2005, 50, 399–406. [Google Scholar]
- Amini, A.R.; Wallace, J.S.; Nukavarapu, S.P. Short-Term and Long-Term Effects of Orthopedic Biodegradable Implants. J. Long. Term. Eff. Med. Implant. 2011, 21, 93–122. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.-Z.; Qi, W.-C.; Zeng, R.-C.; Chen, X.-B.; Gu, C.-D.; Guan, S.-K.; Zheng, Y.-F. Advances in coatings on biodegradable magnesium alloys. J. Magnes. Alloy. 2020, 8, 42–65. [Google Scholar] [CrossRef]
- Bender, S.; Goellner, J.; Heyn, A.; Boese, E. Corrosion and corrosion testing of magnesium alloys. Mater. Corros. 2007, 58, 977–982. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ibrahim, H.; Amerinatanzi, A.; Elahinia, M. Biodegradable magnesium alloys. In Metals for Biomedical Devices; Elsevier: Amsterdam, The Netherlands, 2019; pp. 265–289. [Google Scholar]
- Voicu, M.-E.; Golgovici, F. A biointerface growth at immersion of a biodegradable magnesium alloy in simulated body fluid. U.P.B. Sci. Bull. Ser. B 2020, 82, 57–68. [Google Scholar]
- Parau, A.C.; Dinu, M.; Cotrut, C.M.; Pana, I.; Vranceanu, D.M.; Constantin, L.R.; Serratore, G.; Marinescu, I.M.; Vitelaru, C.; Ambrogio, G.; et al. Effect of Deposition Temperature on the Structure, Mechanical, Electrochemical Evaluation, Degradation Rate and Peptides Adhesion of Mg and Si-Doped Hydroxyapatite Deposited on AZ31B Alloy. Coatings 2023, 13, 591. [Google Scholar] [CrossRef]
- Xu, L.; Liu, X.; Sun, K.; Fu, R.; Wang, G. Corrosion Behavior in Magnesium-Based Alloys for Biomedical Applications. Materials 2022, 15, 2613. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Ivanova, A.A.; Surmeneva, M.A.; Braic, M.; Tyurin, A.I.; Braic, V.; Surmenev, R.A.; Vladescu, A. Tribological behaviour of RF-magnetron sputter deposited hydroxyapatite coatings in physiological solution. Ceram. Int. 2017, 43, 6858–6867. [Google Scholar] [CrossRef]
- Li, L.-Y.; Cui, L.-Y.; Zeng, R.-C.; Li, S.-Q.; Chen, X.-B.; Zheng, Y.; Kannan, M.B. Advances in functionalized polymer coatings on biodegradable magnesium alloys—A review. Acta Biomater. 2018, 79, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, X.; Li, S.; Zeng, R.; Zhang, F.; Wang, Z.; Guan, S. Corrosion resistance and drug release profile of gentamicin-loaded polyelectrolyte multilayers on magnesium alloys: Effects of heat treatment. J. Colloid Interface Sci. 2019, 547, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.-Y.; Gao, S.-D.; Li, P.-P.; Zeng, R.-C.; Zhang, F.; Li, S.-Q.; Han, E.-H. Corrosion resistance of a self-healing micro-arc oxidation/polymethyltrimethoxysilane composite coating on magnesium alloy AZ31. Corros. Sci. 2017, 118, 84–95. [Google Scholar] [CrossRef]
- Shadanbaz, S.; Dias, G.J. Calcium phosphate coatings on magnesium alloys for biomedical applications: A review. Acta Biomater. 2012, 8, 20–30. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Yang, Y.-X.; Li, J.-A.; Zeng, R.-C.; Guan, S.-K. Advances in coatings on magnesium alloys for cardiovascular stents—A review. Bioact. Mater. 2021, 6, 4729–4757. [Google Scholar] [CrossRef]
- Voicu, M.E.; Demetrescu, I.; Dorobantu, A.; Enachescu, M.; Buica, G.-O.; Ionita, D. Interaction of Mg Alloy with PLA Electrospun Nanofibers Coating in Understanding Changes of Corrosion, Wettability, and pH. Nanomaterials 2022, 12, 1369. [Google Scholar] [CrossRef]
- Lakalayeh, G.A.; Rahvar, M.; Nazeri, N.; Ghanbari, H. Evaluation of drug-eluting nanoparticle coating on magnesium alloy for development of next generation bioabsorbable cardiovascular stents. Med. Eng. Phys. 2022, 108, 103878. [Google Scholar] [CrossRef]
- Gao, F.; Hu, Y.; Gong, Z.; Liu, T.; Gong, T.; Liu, S.; Zhang, C.; Quan, L.; Kaveendran, B.; Pan, C. Fabrication of chitosan/heparinized graphene oxide multilayer coating to improve corrosion resistance and biocompatibility of magnesium alloys. Mater. Sci. Eng. C 2019, 104, 109947. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.-J.; Gao, L.; Liu, J.-C.; Wang, J.; Cheng, Q.; Li, J.-P.; Li, S.-Q.; Zhi, K.-Q.; Zeng, R.-C.; Wang, Z.-L. Corrosion resistance and antibacterial properties of hydroxyapatite coating induced by gentamicin-loaded polymeric multilayers on magnesium alloys. Colloids Surf. B Biointerfaces 2019, 179, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Gallardo-Moreno, A.M.; González-Martín, M.L. Influence of Solvent and Substrate on Hydrophobicity of PLA Films. Polymers 2021, 13, 4289. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Miyazaki, T.; Kokubo, T.; Nakamura, T. Revised Simulated Body Fluid. Key Eng. Mater. 2000, 192–195, 47–50. [Google Scholar] [CrossRef]
- Schwartz, A.; Kossenko, A.; Zinigrad, M.; Gofer, Y.; Borodianskiy, K.; Sobolev, A. Hydroxyapatite Coating on Ti-6Al-7Nb Alloy by Plasma Electrolytic Oxidation in Salt-Based Electrolyte. Materials 2022, 15, 7374. [Google Scholar] [CrossRef]
- Gemeinder, J.L.P.; de Barros, N.R.; Pegorin, G.S.; Singulani, J.d.L.; Borges, F.A.; Del Arco, M.C.G.; Giannini, M.J.S.M.; Almeida, A.M.F.; Salvador, S.L.d.S.; Herculano, R.D. Gentamicin encapsulated within a biopolymer for the treatment of Staphylococcus aureus and Escherichia coli infected skin ulcers. J. Biomater. Sci. Polym. Ed. 2021, 32, 93–111. [Google Scholar] [CrossRef]
- Voicu, M.E.; Draganescu, D.; Anuta, V.; Stoian, A.B.; Ionita, D.; Demetrescu, I. The interrelationship between the drug embedded TiZr surface properties and antibacterial effect. Farmacia 2022, 70, 1064–1071. [Google Scholar] [CrossRef]
- Eren Boncu, T.; Ozdemir, N. Electrospinning of ampicillin trihydrate loaded electrospun PLA nanofibers I: Effect of polymer concentration and PCL addition on its morphology, drug delivery and mechanical properties. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 669–676. [Google Scholar] [CrossRef]
- Jira, J.; Rezek, B.; Kriha, V.; Artemenko, A.; Matolínová, I.; Skakalova, V.; Stenclova, P.; Kromka, A. Inhibition of E. coli Growth by Nanodiamond and Graphene Oxide Enhanced by Luria-Bertani Medium. Nanomaterials 2018, 8, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaiswal, S.; Duffy, B.; Jaiswal, A.K.; Stobie, N.; McHale, P. Enhancement of the antibacterial properties of silver nanoparticles using β-cyclodextrin as a capping agent. Int. J. Antimicrob. Agents 2010, 36, 280–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vardaki, M.; Ionita, D.; Stoian, A.B.; Demetrescu, I. Increasing corrosion resistance of a ZrTi alloy with a bioinspired coating with low porosity. Mater. Corros. 2017, 68, 988–994. [Google Scholar] [CrossRef]
- Alizadeh-Osgouei, M.; Li, Y.; Vahid, A.; Ataee, A.; Wen, C. High strength porous PLA gyroid scaffolds manufactured via fused deposition modeling for tissue-engineering applications. Smart Mater. Med. 2021, 2, 15–25. [Google Scholar] [CrossRef]
- Shi, P.; Niu, B.; Shanshan, E.; Chen, Y.; Li, Q. Preparation and characterization of PLA coating and PLA/MAO composite coatings on AZ31 magnesium alloy for improvement of corrosion resistance. Surf. Coat. Technol. 2015, 262, 26–32. [Google Scholar] [CrossRef]
- Gungor, B.; Esen, Ş.; Gök, A.; Yılmaz, H.; Malazgirt, Z.; Leblebicioğlu, H. Comparison of the adherence of E. coli and S. aureus to ten different prosthetic mesh grafts: In vitro experimental study. Indian J. Surg. 2010, 72, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Cavalu, S.; Roiu, G.; Pop, O.; Heredea, D.A.P.; Costea, T.O.; Costea, C.F. Nano-Scale Modifications of Amniotic Membrane Induced by UV and Antibiotic Treatment: Histological, AFM and FTIR Spectroscopy Evidence. Materials 2021, 14, 863. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Torres, B.; Mohedano, M.; Matykina, E.; Arrabal, R.; López, A.J.; Rams, J. PLA deposition on surface treated magnesium alloy: Adhesion, toughness and corrosion behaviour. Surf. Coat. Technol. 2020, 388, 125593. [Google Scholar] [CrossRef]
- Parent, M.; Magnaudeix, A.; Delebassée, S.; Sarre, E.; Champion, E.; Viana Trecant, M.; Damia, C. Hydroxyapatite microporous bioceramics as vancomycin reservoir: Antibacterial efficiency and biocompatibility investigation. J. Biomater. Appl. 2016, 31, 488–498. [Google Scholar] [CrossRef]
- Li, J.; Xie, S.; Ahmed, S.; Wang, F.; Gu, Y.; Zhang, C.; Chai, X.; Wu, Y.; Cai, J.; Cheng, G. Antimicrobial Activity and Resistance: Influencing Factors. Front. Pharmacol. 2017, 8, 364. [Google Scholar] [CrossRef] [Green Version]

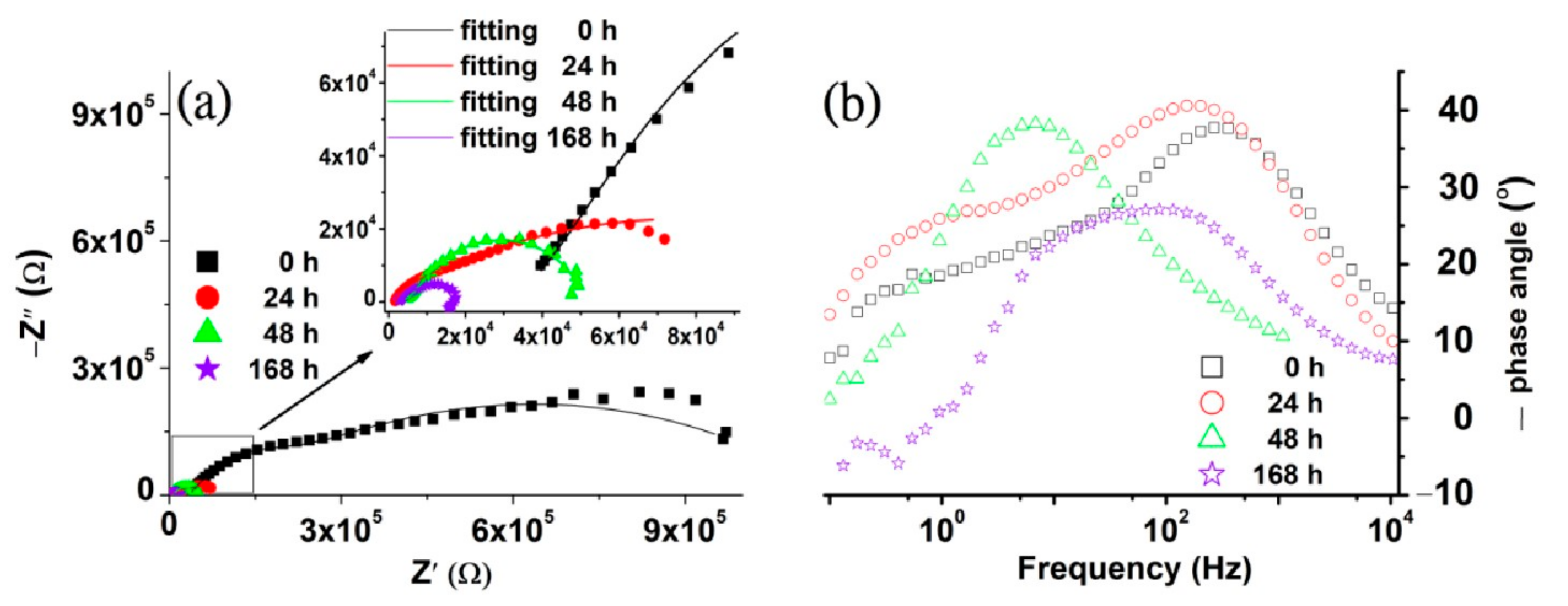

| Time (h) | Ecorr (V) | Icorr (µA) | Vcorr (µm/Year) | Rp (Ω) | Βa (V/Decade) | Βc (V/Decade) |
|---|---|---|---|---|---|---|
| 0 | −1.50 | 0.072 | 5.68 | 1.25 × 106 | 0.42 | 0.41 |
| 24 | −1.44 | 0.527 | 41.6 | 0.11 × 106 | 0.20 | 0.37 |
| 48 | −1.24 | 0.149 | 11.78 | 0.34 × 106 | 0.19 | 0.29 |
| 168 | −1.06 | 0.259 | 20.39 | 0.36 × 106 | 0.66 | 0.32 |
| Time (h) | Rs (kΩ) | R1 (kΩ) | CPE1 | R2 (kΩ) | CPE2 | ||
|---|---|---|---|---|---|---|---|
| YO (µMho) | N1 | YO (µMho) | N2 | ||||
| 0 | 32.3 | 1020 | 5.46 | 0.88 | 150 | 0.037 | 0.821 |
| 24 | 1.53 | 152 | 9.48 | 0.925 | 15.1 | 1.65 | 0.66 |
| 48 | 2.61 | 206 | 10.4 | 0.805 | 42 | 3.35 | 0.733 |
| 168 | 3.58 | 240 | 14.50 | 0.842 | 30.8 | 3.00 | 0.545 |
| Sample | Surface Area (BJH Method) (m2/g) | Total Pore Volume (cm3/g) | Pore Diameter (nm) | Microporous Surface (m2/g) | Volume of Micropores (cm3/g) |
|---|---|---|---|---|---|
| PLA dip coating | 5.43 | 0.0066 | 3.30 | 13.71 | 0.0049 |
| PLA nanofibers | 12.09 | 0.0012 | 3.67 | 3.43 | 0.0012 |
| Sample | Zero-Order | First-Order | Higuchi | Hixson–Crowell | Peppas–Korsmeyer | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | k0 | R2 | k1 | R2 | k2 | R2 | k3 | R2 | k4 | n | |
| AZ31-PLA-GS- nanofibers | 0.9714 | 0.5544 | 0.9711 | 0.0057 | 0.9562 | 2.1441 | 0.9413 | 0.094 | 0.9397 | 1.07 | 0.6 |
| AZ31-PLA-GS- dip | 0.9714 | 0.771 | 0.9727 | 0.0079 | 0.9701 | 3.003 | 0.9729 | 0.0009 | 0.757 | 1.719 | 1.85 |
| Sample | I % | |
|---|---|---|
| Staphylococcus aureus | Escherichia coli | |
| AZ31 | 28.30 ± 0.02 | 20 ± 1.22 |
| AZ31-PLA-nanofibers | 30.18 ± 0.03 | 22.5 ± 0.68 |
| AZ31-PLA-dip | 35.84 ± 1.02 | 24.75 ± 1.16 |
| AZ31-PLA-GS-nanofibers | 43.39 ± 1.32 | 30 ± 2.2 |
| AZ31-PLA-GS-dip | 69.81 ± 1.83 | 55 ± 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voicu, M.E.; Ionita, D.; Buica, G.-O.; Draganescu, D.; Anuta, V.; Raduly, F.M.; Demetrescu, I. Characterization of Two Types of Polylactic Acid Coating Loaded with Gentamicin Sulphate Deposed on AZ31 Alloy. Coatings 2023, 13, 1105. https://doi.org/10.3390/coatings13061105
Voicu ME, Ionita D, Buica G-O, Draganescu D, Anuta V, Raduly FM, Demetrescu I. Characterization of Two Types of Polylactic Acid Coating Loaded with Gentamicin Sulphate Deposed on AZ31 Alloy. Coatings. 2023; 13(6):1105. https://doi.org/10.3390/coatings13061105
Chicago/Turabian StyleVoicu, Manuela Elena, Daniela Ionita, George-Octavian Buica, Doina Draganescu, Valentina Anuta, Florentina Monica Raduly, and Ioana Demetrescu. 2023. "Characterization of Two Types of Polylactic Acid Coating Loaded with Gentamicin Sulphate Deposed on AZ31 Alloy" Coatings 13, no. 6: 1105. https://doi.org/10.3390/coatings13061105





