Effect of Polyethyleneimine Cationic Surfactant on Morphology and Corrosion Resistance of Electrodeposited Ni-Co-SiC Composite Coatings
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
3.1. Effect of PEI Concentration on Ni-Co-SiC Composite Coatings
3.1.1. Effect of PEI Concentration on Ni-Co-SiC Co-Deposition Process
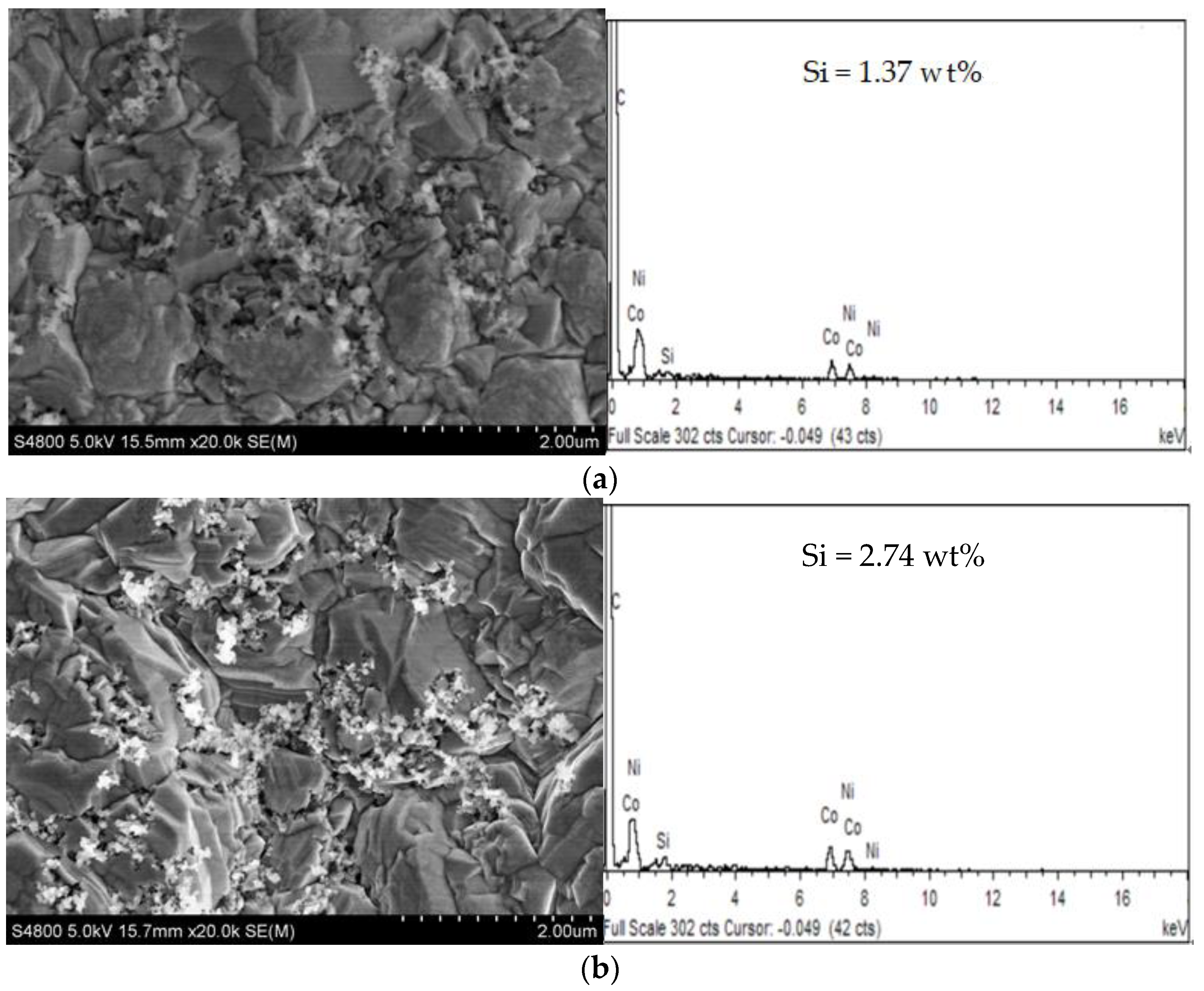
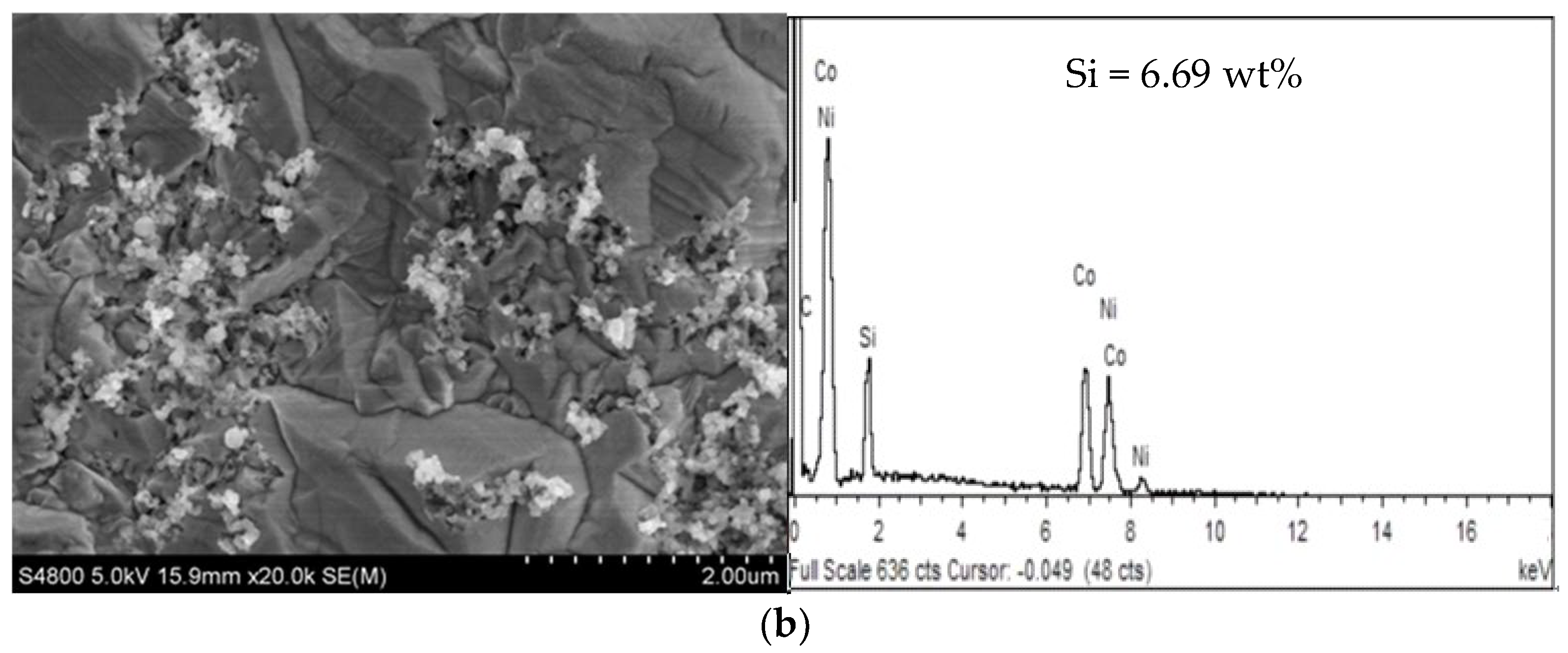
3.1.2. Effect of PEI Concentration on Ni-Co-SiC Composite Coatings
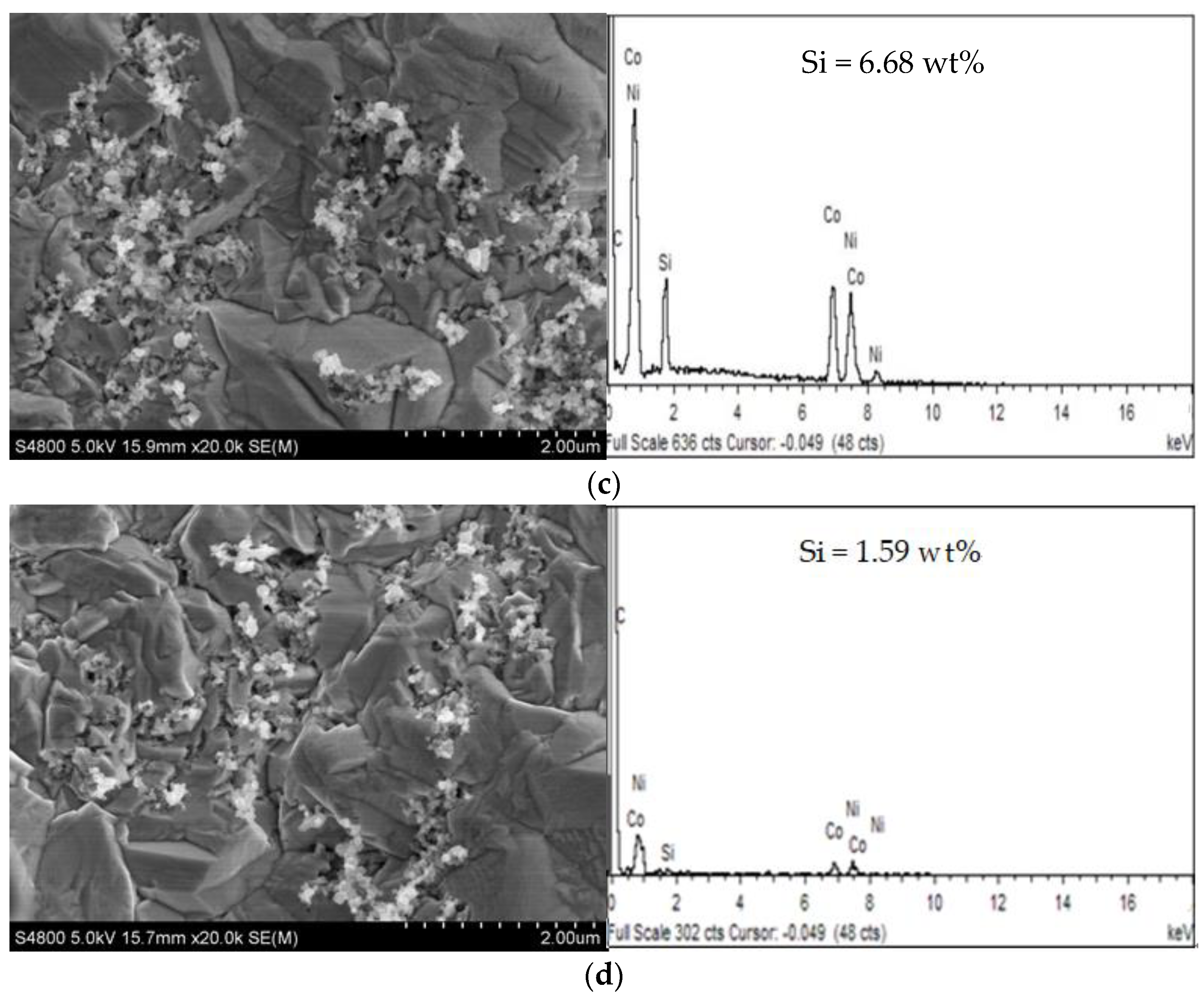
3.1.3. The Effect of Higher Concentration PEI on Ni-Co-SiC Composite Coatings
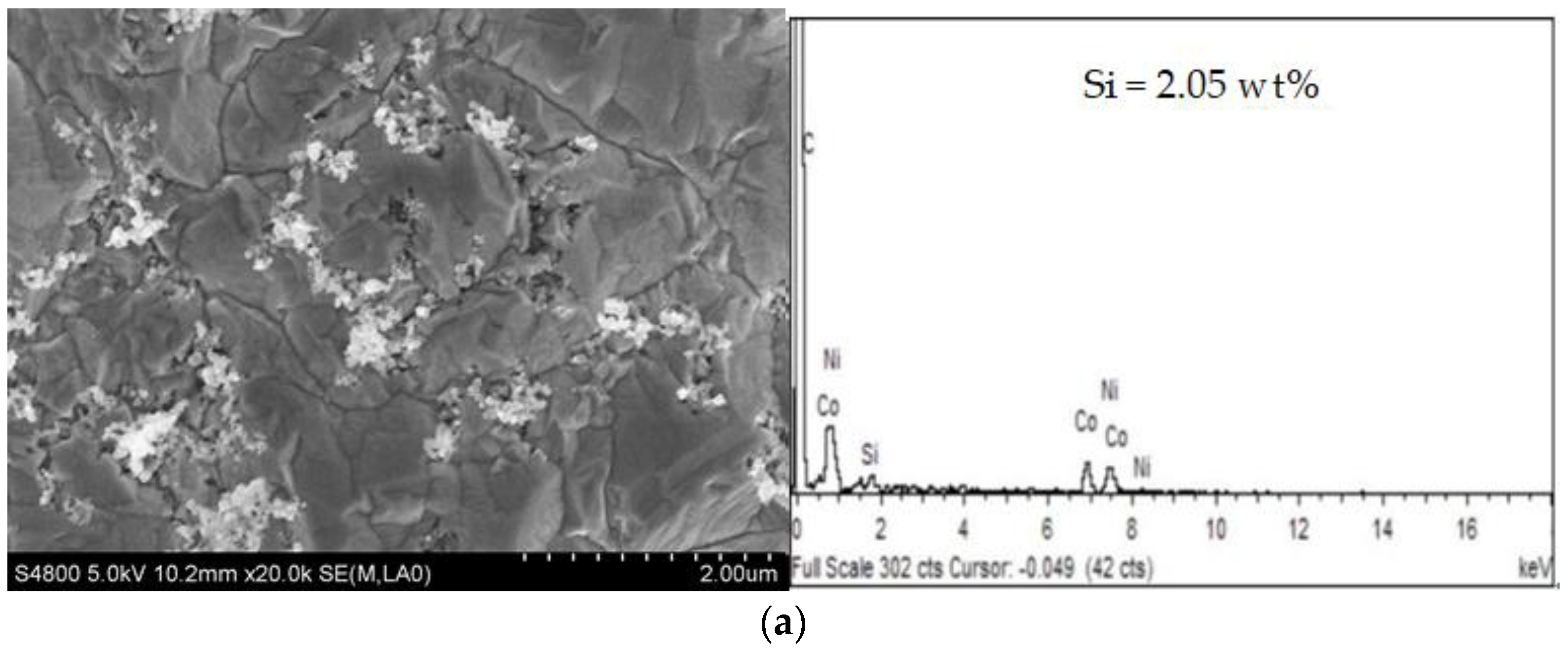
3.2. Effect of Pretreatment on Ni-Co-SiC Composite Coatings
3.3. Effect of PEI on Hardness of Ni-Co-SiC Composite Coatings
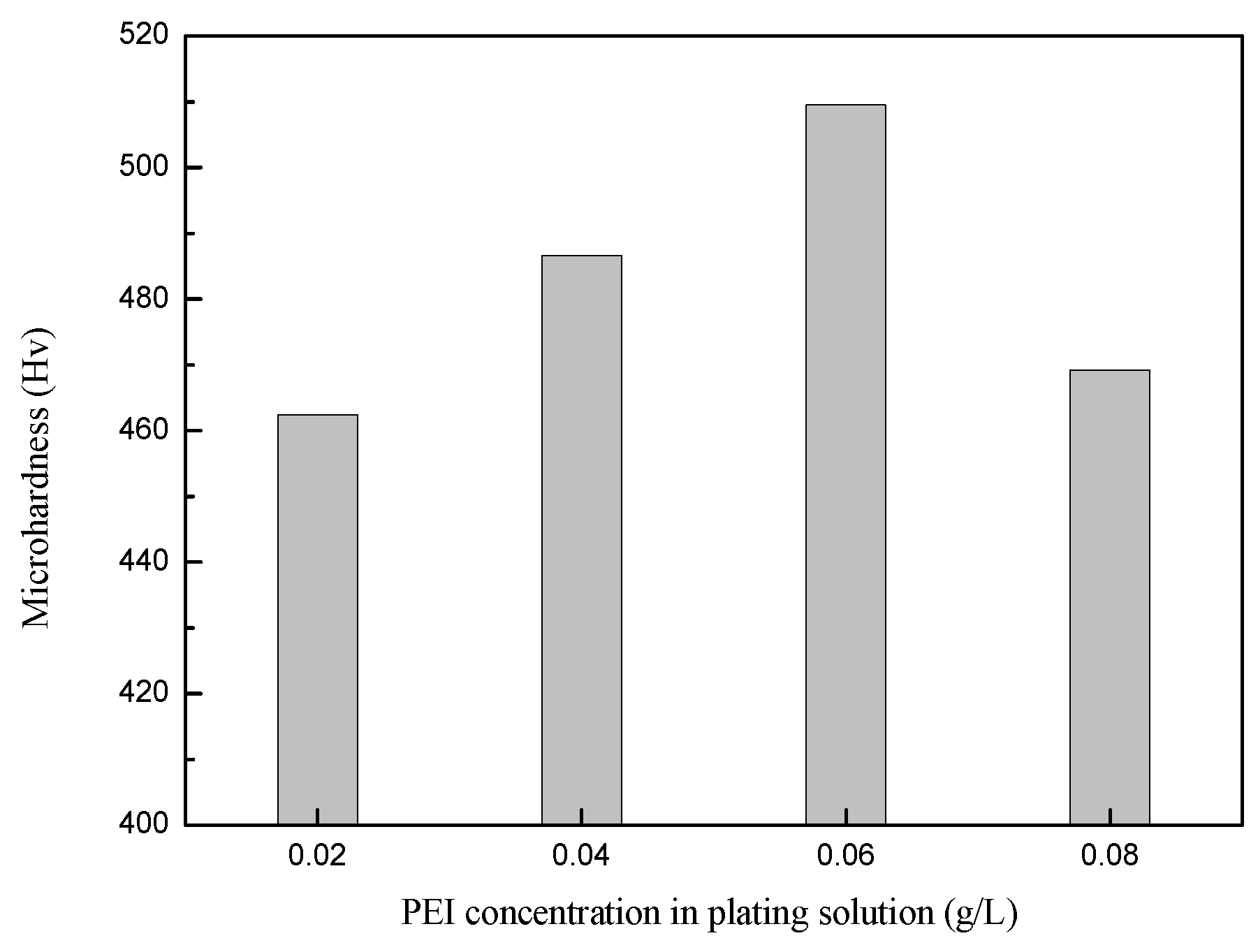
3.3.1. Effect of PEI Concentration on Hardness of Ni-Co-SiC Composite Coatings
3.3.2. The Effect of Higher PEI Concentration on Hardness of Ni-Co-SiC Composite Coatings
3.3.3. Effect of Pretreatment on Hardness of Ni-Co-SiC Composite Coatings
3.4. Effect of PEI on Corrosion Resistance of Ni-Co-SiC Composite Coatings
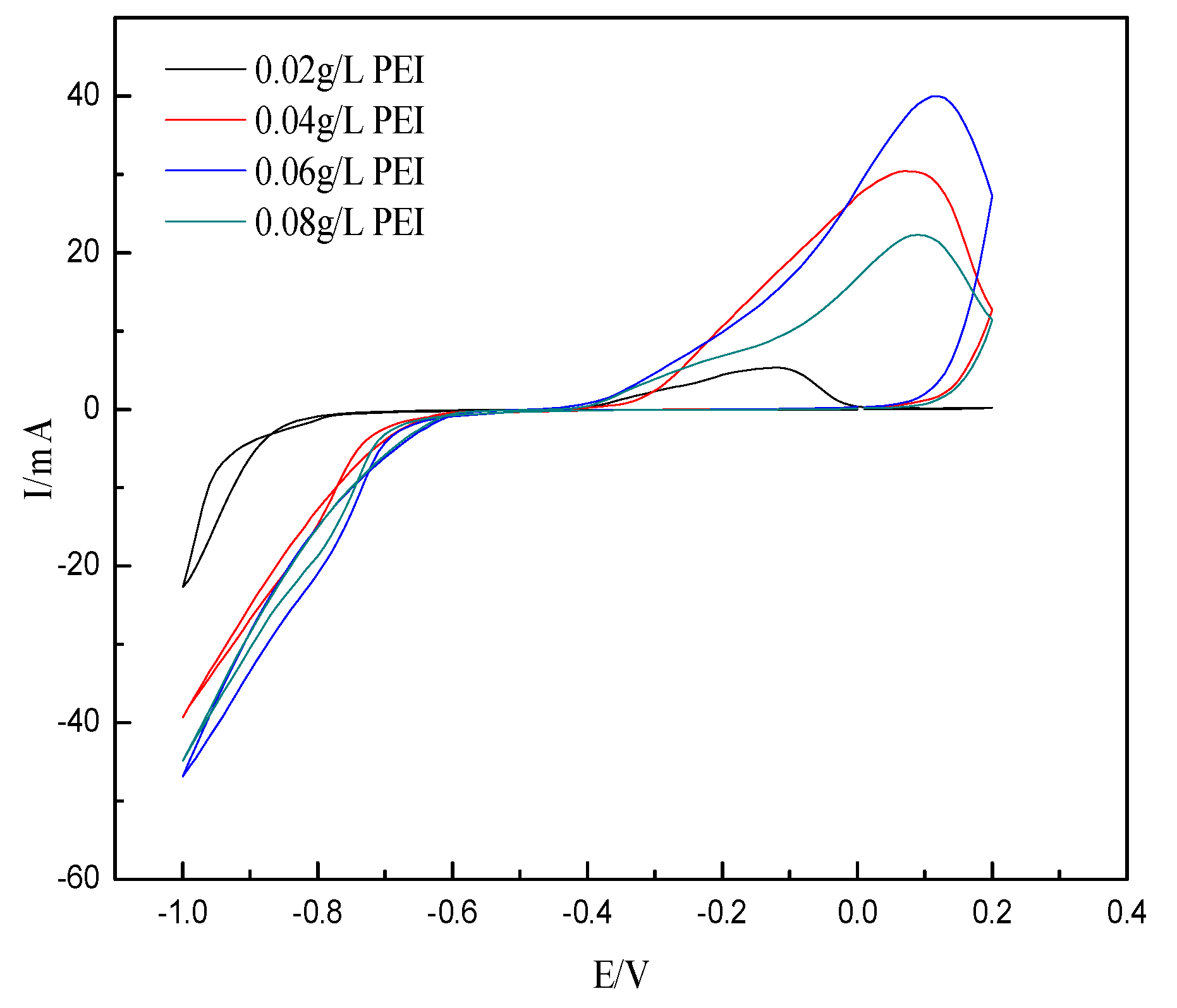
3.4.1. Effect of PEI Concentration on Corrosion Resistance of Ni-Co-SiC Composite Coatings
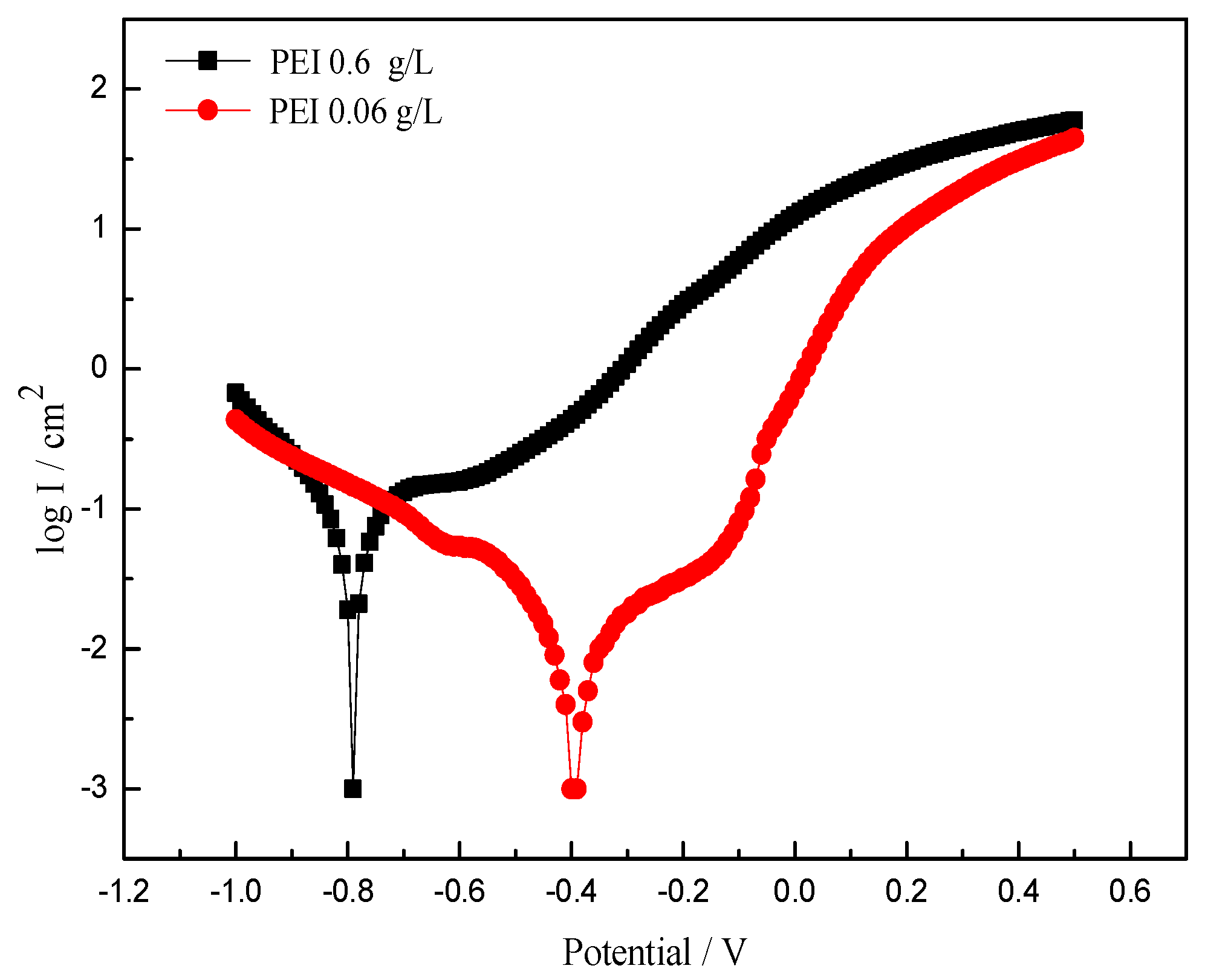
3.4.2. The Effect of Higher PEI Concentration on Corrosion Resistance of Ni-Co-SiC Composite Coatings
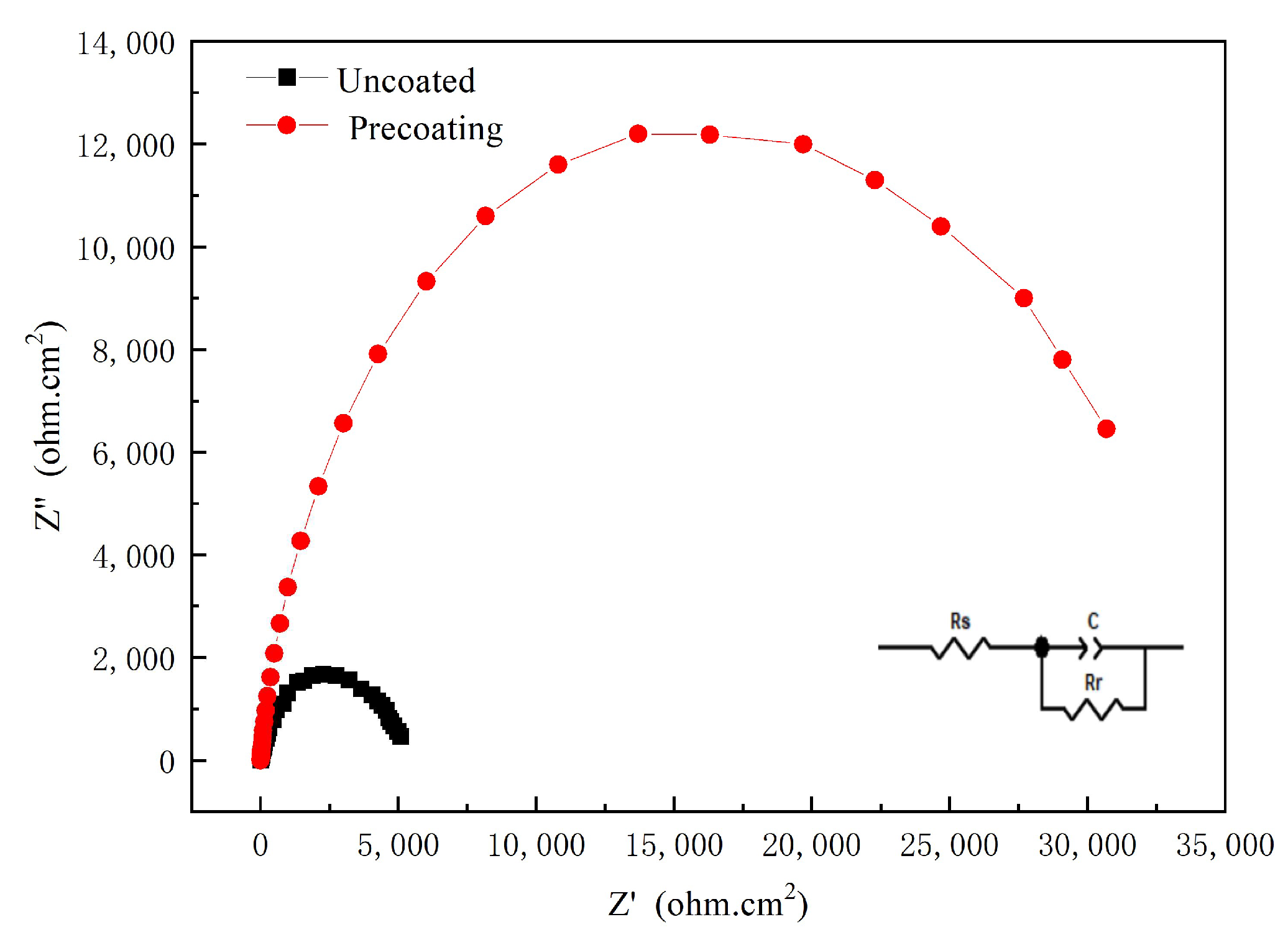
3.4.3. Effect of Pretreatment on Corrosion Resistance of Ni-Co-SiC Composite Coatings
4. Conclusions
- When the PEI concentration was 0.06 g/L, the coating was flat and dense. The dispersion of SiC nanoparticles in the coating was the best, the grain size in the Ni-Co-SiC composite coating was smaller.
- With the increase in PEI concentration, the hardness and corrosion resistance of Ni-Co-SiC composite coatings showed a trend of first increasing and then decreasing. When the PEI concentration was 0.06 g/L, the hardness and corrosion resistance of Ni-Co-SiC composite coating was the best.
- When the PEI concentration was increased by an order of magnitude to 0.6 g/L, the microscopic morphology of the Ni-Co-SiC composite coating changed from the original cone to an ellipsoid with unclear edges, and obvious cracks occurred on the surface of the coating. The grain in the coating became larger, and the agglomeration of nano-SiC particles was obvious. The hardness and corrosion resistance of Ni-Co-SiC composite coatings were not improved with the increase of PEI concentration by an order of magnitude.
- Compared with the Ni-Co-SiC composite coating prepared without pretreatment, the Ni-Co-SiC composite coating prepared by pretreatment was denser and uniform, the hardness of the composite coating was increased by 119.26 Hv, and the corrosion resistance also improved.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mousavi, S.M.A.; Pitchumani, R. A study of corrosion on electrodeposited superhydrophobic copper surfaces. Corros. Sci. 2021, 186, 109420. [Google Scholar] [CrossRef]
- Wang, L.; Lu, J.L.; Liu, K.; He, L.; Zheng, Q.; Luo, X. Effect of Electrodeposition Method on Properties of Ni-SiC Nanocomposite Coatings. Surf. Technol. 2017, 46, 180–184. [Google Scholar]
- Raghavendra, C.R.; Basavarajappa, S.; Sogalad, I.; Naik, K.; Karthik, K.Y. Study on Ni composite coating on Al6061 substrate material with different nano particle reinforcement by electrodeposition process. Mater. Today Proc. 2020, 24, 1680–1685. [Google Scholar] [CrossRef]
- Noorbakhsh Nezhad, A.H.; Mohammadi Zahrani, E.; Alfantazi, A.M. Erosion-corrosion of electrodeposited superhydrophobic Ni-Al2O3 nanocomposite coatings under jet saline-sand slurry impingement. Corros. Sci. 2022, 197, 110095. [Google Scholar] [CrossRef]
- Masoumi Balashadehi, M.; Nourpour, P.; Sabour Rouh Aghdam, A.; Allahyarzadeha, M.H.; Heydarzadeh, A.; Hamdi, M. The formation, microstructure and hot corrosion behaviour of slurry aluminide coating modified by Ni/Ni-Co electrodeposited layer on Ni-base superalloy. Surf. Coat. Technol. 2020, 402, 126–283. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Gao, Y.; Dong, C.; Wang, S. Microstructure and Properties of Ni-Co Composite Cladding Coating on Mould Copper Plate. Materials 2019, 12, 2782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimzadeh, A.; Aliofkhazraei, M.; Walsh, F. A review of electrodeposited Ni-Co alloy and composite coatings: Microstructure, properties and applications. Surf. Coat. Technol. 2019, 372, 463–498. [Google Scholar] [CrossRef]
- Gorospe, A.E.; Balela, M.D.L. Ni-Co nanocomposites deposited on carbon fiber paper as an electrocatalyst towards hydrogen evolution reaction. Mater. Today Proc. 2020, 22, 255–261. [Google Scholar] [CrossRef]
- Feng, J.W.; Zhou, Q. Effect of Different Co contents on Structure of Nanoporous Ni-Co and Catalytic Performance of Hydrogen Evolution. Chin. J. Inorg. Chem. 2019, 35, 1746–1754. [Google Scholar]
- Huang, P.C.; Hou, K.H.; Hong, J.J.; Lin, M.H.; Wang, G.L. Study of fabrication and wear properties of Ni–SiC composite coatings on A356 aluminum alloy. Wear 2021, 477, 203772. [Google Scholar] [CrossRef]
- Bakhit, B.; Akbari, A.; Nasirpouri, F.; Hosseini, M.G. Corrosion resistance of Ni–Co alloy and Ni–Co/SiC nanocomposite coatings electrodeposited by sediment codeposition technique. Appl. Surf. Sci. 2014, 307, 351–359. [Google Scholar] [CrossRef]
- Dheeraj, P.R.; Patra, A.; Sengupta, S.; Das, S.; Das, K. Synergistic effect of peak current density and nature of surfactant on microstructure, mechanical and electrochemical properties of pulsed electrodeposited Ni-Co-SiC nanocomposites. J. Alloys Compd. 2017, 729, 1093–1107. [Google Scholar] [CrossRef]
- Babu, C.V.; Ramji, K. Influence of SiC Nano Particles on Microhardness and Corrosion Resistance of Electroless Ni-P Coatings. J. Bio Tribo Corros. 2018, 4, 1–8. [Google Scholar]
- Liu, J.H.; Liu, Y.D.; Pei, Z.L.; Li, W.H.; Shi, W.B.; Gong, J.; Sun, C. Influence of particle size and content on the friction and wear behaviors of as-annealed Ni–Mo/diamond composite coatings. Wear 2020, 452–453, 203300. [Google Scholar] [CrossRef]
- Walsh, F.C.; Low CT, J.; Bello, J.O. Influence of surfactants on electrodeposition of a Ni-nanoparticulate SiC composite coating. Trans. IMF 2015, 93, 147–156. [Google Scholar] [CrossRef]
- Song, Y.T.; Min, M.; Yi, Y.; Zhao, T.; Zhang, X.; Li, Z.L. Effect of Surfactant on Properties of Electrodeposited Ni-P-Al2O3 Coatings. Mater. Prot. 2018, 51, 66–69. [Google Scholar]
- Rouhollahi, A.; Fazlolahzadeh, O.; Dolati, A.; Ghahramanifard, F. Effects of different surfactants on the silica content and characterization of Ni–SiO2 nanocomposites. J. Nanostruct. Chem. 2018, 8, 139–152. [Google Scholar] [CrossRef] [Green Version]
- Baghal, S.L.; Amadeh, A.; Sohi, M.H.; Hadavi, S.M.M. The effect of SDS surfactant on tensile properties of electrodeposited Ni–Co/SiC nanocomposites. Mater. Sci. Eng. A 2013, 559, 583–590. [Google Scholar] [CrossRef]
- Kan, H.M.; Meng, Y.Y.; Ramana, G.R.J. Influence of particle size and surfactants on uniformity and quantity of silicon carbide particles in electrodeposited nickel-silicon carbide coatings. Cent. South Univ. 2021, 28, 1627–1636. [Google Scholar] [CrossRef]
- Zhang, W.L.; Wang, Y.; Sun, D.B.; Li, H.Q. Study on dispersion effect of some surfactants upon Ni-P-SiC amorphous composite electroplating. Electroplat. Finish. 2006, 25, 4–7. [Google Scholar]


| Electrolyte Ingredients | Concentration (g/L) |
|---|---|
| NiSO4·7H2O | 250 |
| NiCl2·6H2O | 40 |
| CoSO4·7H2O | 50 |
| H3BO3 | 40 |
| SiC | 3 |
| Deposition parameters | Amount |
| SiC particle size | 40 nm |
| Type of current | DC |
| Current density (A/dm2) | 3 |
| Temperature (°C) | 50 |
| Solution pH | 4.3 |
| Magnetic agitation rate (rpm) | 500 |
| electrodeposition cell voltage (V) | 1.1 |
| deposition time (min) | 75 |
| bath volume (mL) | 100 |
| Coating Conditions | 1 | 2 | 3 | 4 | 5 | Average Value |
|---|---|---|---|---|---|---|
| pretreatment | 472.45 Hv | 519.22 Hv | 504.71 Hv | 528.12 Hv | 523.02 Hv | 509.50 Hv |
| without pretreatment | 437.96 Hv | 327.45 Hv | 407.89 Hv | 472.63 Hv | 305.26 Hv | 390.24 Hv |
| PEI Concentration (g/L) | Ecorr (V) | Icorr (A/cm2) | Corr.Rate (mm/y) |
|---|---|---|---|
| 0.02 | −0.4085 | 5.04 × 10−6 | 5.854 × 10−2 |
| 0.04 | −0.3817 | 2.13 × 10−6 | 2.476 × 10−2 |
| 0.06 | −0.2943 | 2.62 × 10−7 | 3.045 × 10−3 |
| 0.08 | −0.4616 | 8.20 × 10−7 | 9.527 × 10−3 |
| PEI Concentration (g/L) | Ecorr (V) | Icorr (A/cm2) | Corr.Rate (mm/y) |
|---|---|---|---|
| 0.06 | −0.2943 | 2.62 × 10−7 | 3.045 × 10−3 |
| 0.6 | −0.7406 | 2.57 × 10−5 | 2.986 × 10−1 |
| Coating Conditions | RP (ohm) | Cd (10−5 F) |
|---|---|---|
| Without pretreatment | 5126 | 5.120 |
| pretreatment | 21,558 | 4.624 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kan, H.; Wang, W.; Meng, Y. Effect of Polyethyleneimine Cationic Surfactant on Morphology and Corrosion Resistance of Electrodeposited Ni-Co-SiC Composite Coatings. Coatings 2023, 13, 1232. https://doi.org/10.3390/coatings13071232
Kan H, Wang W, Meng Y. Effect of Polyethyleneimine Cationic Surfactant on Morphology and Corrosion Resistance of Electrodeposited Ni-Co-SiC Composite Coatings. Coatings. 2023; 13(7):1232. https://doi.org/10.3390/coatings13071232
Chicago/Turabian StyleKan, Hongmin, Wenxin Wang, and Yuanyuan Meng. 2023. "Effect of Polyethyleneimine Cationic Surfactant on Morphology and Corrosion Resistance of Electrodeposited Ni-Co-SiC Composite Coatings" Coatings 13, no. 7: 1232. https://doi.org/10.3390/coatings13071232






