Synthesis, Properties, and Applications of Metal Organic Frameworks Supported on Graphene Oxide
Abstract
:1. Introduction
2. The Properties of Metal-Organic Frameworks/Graphene Oxide (MOFs/GO)
3. Synthesis Methods of Metal-Organic Frameworks/Graphene Oxide (MOFs/GO)
3.1. Post-Synthesis Method
3.2. Hydrothermal and Solvothermal Methods
3.3. In Situ Method
3.4. Co-Precipitation Method
3.5. Mixing Method
3.6. Ultrasonication Method
| Methods | Advantages | Challenges | Application | Ref. |
|---|---|---|---|---|
| Post-Synthesis |
|
|
| [59,60,83,84] |
| Hydrothermal or Solvothermal |
|
|
| [63,64,85,86] |
| In situ |
|
|
| [68,69,70,87,88] |
| Co-Precipitation |
|
|
| [72,73,74] |
| Mixing |
|
|
| [75,76,77] |
| Ultrasonication |
|
|
| [78,79,80,81,82] |
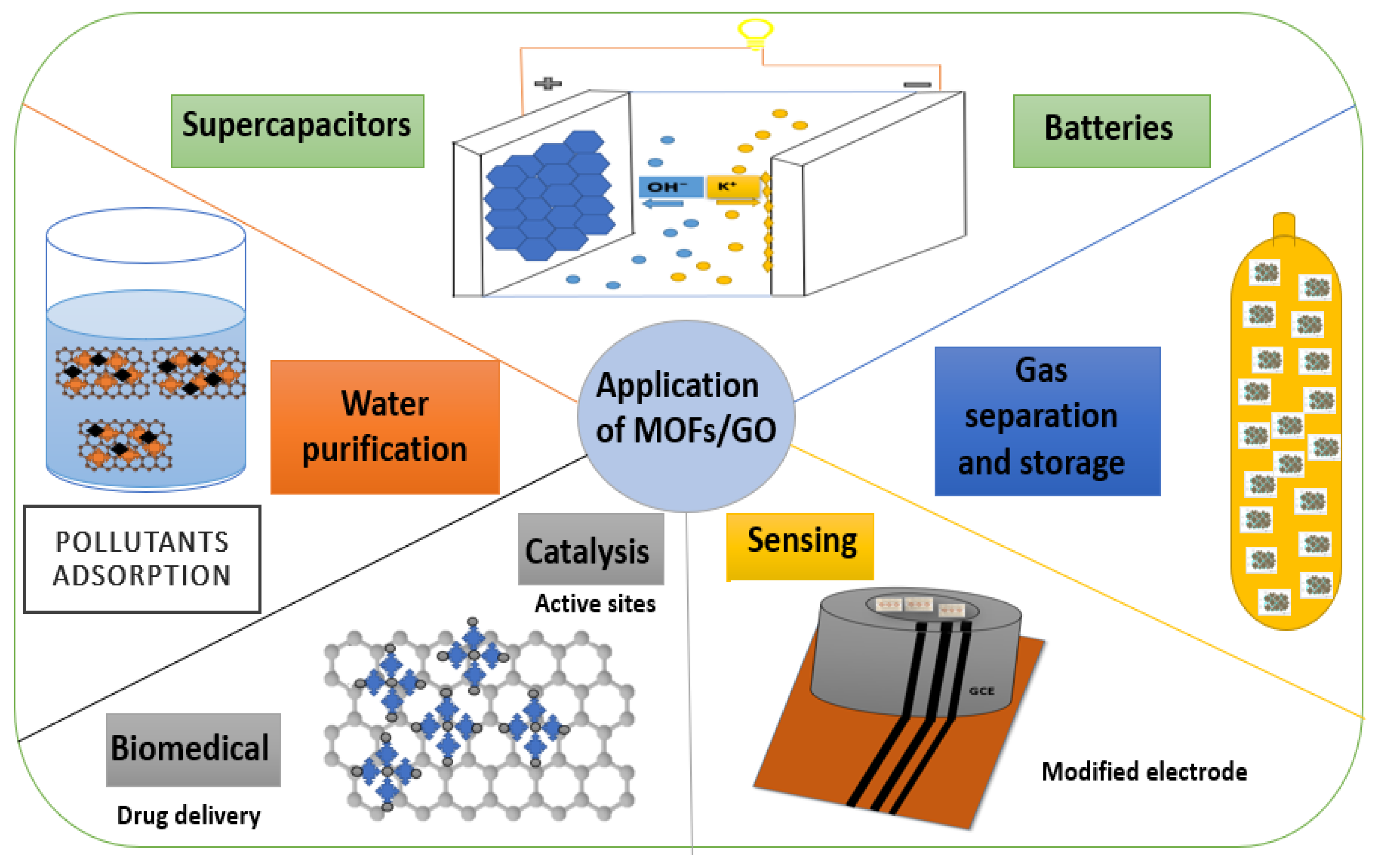
4. Applications of Metal-Organic Frameworks/Graphene Oxide (MOFs/GO)
4.1. Supercapacitors
Specific Features of MOF-GO as Supercapacitors
4.2. Gas Separation and Storage
4.3. Water Purification
4.4. Sensors
4.5. Catalysis
4.5.1. Electrocatalysts
4.5.2. Photocatalysts
4.6. Batteries
4.7. Other Applications (Biomedical)
5. The Advantages and Challenges of Metal-Organic Frameworks/Graphene Oxide (MOFs/GO)
6. Conclusion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| Metal-organic frameworks | MOFs |
| Graphene oxide | GO |
| Carbon nanotubes | CNTs |
| Activated carbon | AC |
| Unsaturated metal sites | CUSs |
| Nanofiltration | NF |
| Solid-phase microextraction | SPME |
| Gas chromatography | GC |
| Reduced graphene oxide | rGO |
| Nonsteroidal anti-inflammatory drugs | NSAIDs |
| Post-synthetic modification | PSM |
| Oxygen evolution reaction | OER |
| Catalytic wet peroxide oxidation | CWPO |
| Zeolitic imidazolate frameworks | ZIFs |
| Polyether sulfone | PES |
| Ultrafiltration | UF |
| Triethylamine | TEA |
| Zirconium-porphyrin MOF | PCN-222 |
| 1,3,5-Benzenetricarboxylic acid | BTC |
| Epoxy resin | EP |
| Limited oxygen index | LOI |
| Peak heat release rate | pHRR |
| Total heat release | THR |
| Maximum value of smoke density | Ds,max |
| Glassy carbon electrode | GCE |
| Mixed-matrix membranes | MMMs |
| Ethyl cellulose | EC |
| Supercapacitors | SCs |
| Electrical energy storage | EES |
| Asymmetric supercapacitor | ASC |
| Graphene nanoplatelets | GNP |
| Graphene | G |
| Polysulfone | PSF |
| DIRECT RED 16 | DR16 |
| Chitosan | CTS |
| Rhodamine B | RhB |
| Polyethersulfone | PES |
| p-Chloronitrobenzene | p-CNB |
| Cetyltrimethylammonium bromide | CTAB |
| Bisphenol A | BPA |
| Hydrogen evolution reaction | HER |
| Oxygen reduction reaction | ORR |
| Direct methanol fuel cell | DMFC |
| Carbamazepine | CBZ |
| Lithium-ion batteries | LIBs |
| Sodium-ion batteries | SIBs |
| Potassium-ion batteries | PIBs |
| Lithium-sulfur batteries | LSBs |
| Nanoparticles | NPs |
| Persulfate | PS |
| Trichlorophenol | TCP |
| Peroxymonosulfate | PMS |
| Tetracycline | TC |
| Density functional theory | DFT |
References
- Gautam, S.; Agrawal, H.; Thakur, M.; Akbari, A.; Sharda, H.; Kaur, R.; Amini, M. Metal oxides and metal organic frameworks for the photocatalytic degradation: A review. J. Environ. Chem. Eng. 2020, 8, 103726. [Google Scholar] [CrossRef]
- Čitaković, N.M. Physical properties of nanomaterials. Vojnoteh. Glas. Mil. Tech. Cour. 2019, 67, 159–171. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Zawadzka-Knefel, A.; Lubojański, A.; Dobrzyński, W.; Janecki, M.; Kurek, K.; Szymonowicz, M.; Wiglusz, R.J.; Rybak, Z. Nanomaterials application in endodontics. Materials 2021, 14, 5296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, W.; Wu, Z.H.; Xu, H. Nanoscale metal− organic frameworks and their nanomedicine applications. Front. Chem. 2022, 9, 1243. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M. Nanomaterials: Types, properties, recent advances, and toxicity concerns. Curr. Opin. Environ. Sci. Health 2022, 25, 100319. [Google Scholar] [CrossRef]
- Naghdi, S.; Shahrestani, M.M.; Zendehbad, M.; Djahaniani, H.; Kazemian, H.; Eder, D. Recent advances in application of metal-organic frameworks (MOFs) as adsorbent and catalyst in removal of persistent organic pollutants (POPs). J. Hazard. Mater. 2023, 442, 130127. [Google Scholar] [CrossRef] [PubMed]
- Dutt, S.; Kumar, A.; Singh, S. Synthesis of Metal Organic Frameworks (MOFs) and Their Derived Materials for Energy Storage Applications. Clean Technol. 2023, 5, 140–166. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Yang, L.; Lan, T.; Wang, H.; Hu, C.; Han, X.; Liu, Q.; Chen, J.; Feng, Z.; et al. Porous framework materials for energy & environment relevant applications: A systematic review. Green Energy Environ. 2023, in press.
- Razavi, S.A.; Morsali, A. Linker functionalized metal-organic frameworks. Coord. Chem. Rev. 2019, 399, 213023. [Google Scholar] [CrossRef]
- Rai, S.R.; Sarkar, A.; Pandey, A.; Dasgupta, S.; Majumder, I.; Das, D.; Bose, D.; Mukhopadhyay, M. Photometric study of the interaction of zinc (II) complexes of Schiff bases with cetyltrimethyl ammonium bromide. Macromol. Symp. 2019, 388, 1900030. [Google Scholar] [CrossRef]
- Yusuf, V.F.; Malek, N.I.; Kailasa, S.K. Review on Metal–Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment. ACS Omega 2022, 7, 44507–44531. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Ajiwokewu, B.; Bankole, A.A.; Ismail, O.; Al-Amodi, H.; Kumar, N. MOF based composites with engineering aspects and morphological developments for photocatalytic CO2 reduction and hydrogen production: A comprehensive review. J. Environ. Chem. Eng. 2023, 1, 109408. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-organic framework functionalization and design strategies for advanced electrochemical energy storage devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Y.; Liu, S.; Xu, M. The applications of metal–Organic frameworks in electrochemical sensors. ChemElectroChem 2018, 5, 6–19. [Google Scholar] [CrossRef]
- Ghorbani-Choghamarani, A.; Taherinia, Z.; Mohammadi, M. Facile synthesis of Fe3O4@ GlcA@ Ni-MOF composites as environmentally green catalyst in organic reactions. Environ. Technol. Innov. 2021, 24, 102050. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Lin, R.B.; Zhou, W.; Zhang, Z.; Xiang, S.; Chen, B. Porous metal-organic frameworks for gas storage and separation: Status and challenges. EnergyChem 2019, 1, 100006. [Google Scholar] [CrossRef]
- Lu, K.; Aung, T.; Guo, N.; Weichselbaum, R.; Lin, W. Nanoscale metal–organic frameworks for therapeutic, imaging, and sensing applications. Adv. Mater. 2018, 30, 1707634. [Google Scholar] [CrossRef]
- Daglar, H.; Altintas, C.; Erucar, I.; Heidari, G.; Zare, E.N.; Moradi, O.; Srivastava, V.; Iftekhar, S.; Keskin, S.; Sillanpää, M. Metal-organic framework-based materials for the abatement of air pollution and decontamination of wastewater. Chemosphere 2022, 23, 135082. [Google Scholar] [CrossRef]
- Cirujano, F.G.; Luque, R.; Dhakshinamoorthy, A. Metal-organic frameworks as versatile heterogeneous solid catalysts for henry reactions. Molecules 2021, 26, 1445. [Google Scholar] [CrossRef]
- Li, D.; Xu, H.Q.; Jiao, L.; Jiang, H.L. Metal-organic frameworks for catalysis: State of the art, challenges, and opportunities. EnergyChem 2019, 1, 100005. [Google Scholar] [CrossRef]
- Raptopoulou, C.P. Metal-organic frameworks: Synthetic methods and potential applications. Materials 2021, 14, 310. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.Y.; Skinner, W.S.; Wang, Z.U.; Jiang, H.L. Metal–organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Xie, Y.; Lyu, S.; Zhang, Y.; Cai, C. Adsorption and Degradation of Volatile Organic Compounds by Metal–Organic Frameworks (MOFs): A Review. Materials 2022, 15, 7727. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Abazari, R.; Chen, J.; Tahir, M.; Kumar, A.; Ikreedeegh, R.R.; Rani, E.; Singh, H.; Kirillov, A.M. Bimetallic metal–organic frameworks and MOF-derived composites: Recent progress on electro-and photoelectrocatalytic applications. Coord. Chem. Rev. 2022, 451, 214264. [Google Scholar] [CrossRef]
- Zhong, L.; Ding, J.; Qian, J.; Hong, M. Unconventional inorganic precursors determine the growth of metal-organic frameworks. Coord. Chem. Rev. 2021, 434, 213804. [Google Scholar] [CrossRef]
- Joseph, J.; Iftekhar, S.; Srivastava, V.; Fallah, Z.; Zare, E.N.; Sillanpää, M. Iron-based metal-organic framework: Synthesis, structure and current technologies for water reclamation with deep insight into framework integrity. Chemosphere 2021, 284, 131171. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.J.; Li, J.R. An overview of metal–organic frameworks for green chemical engineering. Engineering 2021, 7, 1115–1139. [Google Scholar] [CrossRef]
- Wu, H.; Almalki, M.; Xu, X.; Lei, Y.; Ming, F.; Mallick, A.; Roddatis, V.; Lopatin, S.; Shekhah, O.; Eddaoudi, M.; et al. MXene derived metal–organic frameworks. J. Am. Chem. Soc. 2019, 141, 20037–20042. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, H.; Xia, B.Y.; Davey, K.; Zheng, Y.; Qiao, S.Z. Molecular cleavage of metal-organic frameworks and application to energy storage and conversion. Adv. Mater. 2021, 33, 2104341. [Google Scholar] [CrossRef]
- Karimzadeh, Z.; Javanbakht, S.; Namazi, H. Carboxymethylcellulose/MOF-5/Graphene oxide bio-nanocomposite as antibacterial drug nanocarrier agent. BioImpacts BI 2019, 9, 5. [Google Scholar] [CrossRef]
- Nivetha, R.; Sharma, S.; Jana, J.; Chung, J.S.; Choi, W.M.; Hur, S.H. Recent Advances and New Challenges: Two-Dimensional Metal–Organic Framework and Their Composites/Derivatives for Electrochemical Energy Conversion and Storage. Int. J. Energy Res. 2023, 2023, 8711034. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, S.; Xue, H.; Pang, H. Metal-organic frameworks/graphene-based materials: Preparations and applications. Adv. Funct. Mater. 2018, 28, 1804950. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Q. Metal-organic framework composites for catalysis. Matter 2019, 1, 57–89. [Google Scholar] [CrossRef]
- Annamalai, J.; Murugan, P.; Ganapathy, D.; Nallaswamy, D.; Atchudan, R.; Arya, S.; Khosla, A.; Barathi, S.; Sundramoorthy, A.K. Synthesis of various dimensional metal organic frameworks (MOFs) and their hybrid composites for emerging applications–a review. Chemosphere 2022, 298, 134184. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Chen, M.; Wang, H.; Zeng, G.; Huang, D.; Cheng, M.; Liu, Y.; Xue, W.; Wang, Z. The application of different typological and structural MOFs-based materials for the dyes adsorption. Coord. Chem. Rev. 2019, 380, 471–483. [Google Scholar] [CrossRef]
- Kumari, V.; Singh, P.P.; Kaushal, S. Synthesis and applications of metal-organic frameworks and graphene-based composites: A review. Polyhedron 2022, 214, 115645. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Mukherjee, S.; Morales, D.M.; Dubal, D.P.; Nanjundan, A.K.; Schneemann, A.; Masa, J.; Kment, S.; Schuhmann, W.; Otyepka, M.; et al. Graphene-Based Metal–Organic Framework Hybrids for Applications in Catalysis, Environmental, and Energy Technologies. Chem. Rev. 2022, 122, 17241–17338. [Google Scholar] [CrossRef] [PubMed]
- Van Ngo, T.; Moussa, M.; Tung, T.T.; Coghlan, C.; Losic, D. Hybridization of MOFs and graphene: A new strategy for the synthesis of porous 3D carbon composites for high performing supercapacitors. Electrochim. Acta. 2020, 329, 135104. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Abbas, Q.; Mouselly, M.; Alawadhi, H.; Olabi, A.G. High-performance effective metal-organic frameworks for electrochemical applications. J. Sci. Adv. Mater. Devices 2022, 7, 100465. [Google Scholar] [CrossRef]
- Javed, N.; Noor, T.; Iqbal, N.; Naqvi, S.R. A review on development of metal–organic framework-derived bifunctional electrocatalysts for oxygen electrodes in metal–air batteries. RSC Adv. 2023, 13, 1137–1161. [Google Scholar] [CrossRef]
- Rasheed, M.; Shihab, S.; Sabah, O.W. An investigation of the structural, electrical and optical properties of graphene-oxide thin films using different solvents. Journal Phys. Conf. Ser. 2021, 1795, 012052. [Google Scholar] [CrossRef]
- Islam, S.; Ashraf, S.S. Point and space groups of graphene. Resonance 2019, 24, 445–457. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- McCoy, T.M.; Turpin, G.; Teo, B.M.; Tabor, R.F. Graphene oxide: A surfactant or particle? Curr. Opin. Colloid Interface Sci. 2019, 39, 98–109. [Google Scholar] [CrossRef]
- Ghulam, A.N.; Dos Santos, O.A.; Hazeem, L.; Pizzorno Backx, B.; Bououdina, M.; Bellucci, S. Graphene Oxide (GO) Materials—Applications and Toxicity on Living Organisms and Environment. J. Funct. Biomater. 2022, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.; Gaur, A.; Duchaniya, R.K. Synthesis, properties and applications of graphene oxide: An overview. World Sci. News 2020, 143, 17–27. [Google Scholar]
- Ibrahim, A.; Klopocinska, A.; Horvat, K.; Abdel Hamid, Z. Graphene-based nanocomposites: Synthesis, mechanical properties, and characterizations. Polymers 2021, 13, 2869. [Google Scholar] [CrossRef]
- Sun, L. Structure and synthesis of graphene oxide. Chin. J. Chem. Eng. 2019, 27, 2251–2260. [Google Scholar] [CrossRef]
- Huang, B.; Li, Y.; Zeng, W. Application of Metal-Organic Framework-Based Composites for Gas Sensing and Effects of Synthesis Strategies on Gas-Sensitive Performance. Chemosensors 2021, 9, 226. [Google Scholar] [CrossRef]
- Qu, H.J.; Huang, L.J.; Han, Z.Y.; Wang, Y.X.; Zhang, Z.J.; Wang, Y.; Chang, Q.R.; Wei, N.; Kipper, M.J.; Tang, J.G. A review of graphene-oxide/metal–organic framework composites materials: Characteristics, preparation and applications. J. Porous Mater. 2021, 28, 1837–1865. [Google Scholar] [CrossRef]
- Hong, J.; Park, S.J.; Kim, S. Synthesis and electrochemical characterization of nanostructured Ni-Co-MOF/graphene oxide composites as capacitor electrodes. Electrochim. Acta 2019, 311, 62–71. [Google Scholar] [CrossRef]
- Kumar, G.; Masram, D.T. Sustainable synthesis of MOF-5@ GO nanocomposites for efficient removal of rhodamine B from water. ACS Omega 2021, 6, 9587–9599. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zou, Q.; Wang, T.; Zhang, L. Effects of GO and MOF@ GO on the permeation and antifouling properties of cellulose acetate ultrafiltration membrane. J. Membr. Sci. 2019, 569, 48–59. [Google Scholar] [CrossRef]
- Heu, R.; Ateia, M.; Yoshimura, C. Photocatalytic nanofiltration membrane using Zr-MOF/GO nanocomposite with high-flux and anti-fouling properties. Catalysts 2020, 10, 711. [Google Scholar] [CrossRef]
- Liu, H.; Fan, H.; Dang, S.; Li, M.; Yu, H. A Zr-MOF@ GO-coated fiber with high specific surface areas for efficient, green, long-life solid-phase microextraction of nonsteroidal anti-inflammatory drugs in water. Chromatographia 2020, 83, 1065–1073. [Google Scholar] [CrossRef]
- Shinde, S.K.; Kim, D.Y.; Kumar, M.; Murugadoss, G.; Ramesh, S.; Tamboli, A.M.; Yadav, H.M. MOFs-graphene composites synthesis and application for electrochemical supercapacitor: A review. Polymers 2022, 14, 511. [Google Scholar] [CrossRef]
- Jagódka, P.; Matus, K.; Łamacz, A. On the HKUST-1/GO and HKUST-1/rGO composites: The impact of synthesis method on physicochemical properties. Molecules 2022, 27, 7082. [Google Scholar] [CrossRef]
- Muthukumaraswamy Rangaraj, V.; Wahab, M.A.; Reddy, K.S.; Kakosimos, G.; Abdalla, O.; Favvas, E.P.; Reinalda, D.; Geuzebroek, F.; Abdala, A.; Karanikolos, G.N. Metal organic framework—based mixed matrix membranes for carbon dioxide separation: Recent advances and future directions. Front. Chem. 2020, 8, 534. [Google Scholar] [CrossRef]
- Haeri, Z.; Ramezanzadeh, B.; Ramezanzadeh, M. Recent progress on the metal-organic frameworks decorated graphene oxide (MOFs-GO) nano-building application for epoxy coating mechanical-thermal/flame-retardant and anti-corrosion features improvement. Prog. Org. Coat. 2022, 163, 106645. [Google Scholar] [CrossRef]
- Karamipour, M.; Fathi, S.; Safari, M. Removal of phenol from aqueous solution using MOF/GO: Synthesis, characteristic, adsorption performance and mechanism. Int. J. Environ. Anal. Chem. 2021, 6, 1–2. [Google Scholar] [CrossRef]
- Ullah, S.; Bustam, M.A.; Al-Sehemi, A.G.; Assiri, M.A.; Kareem, F.A.; Mukhtar, A.; Ayoub, M.; Gonfa, G. Influence of post-synthetic graphene oxide (GO) functionalization on the selective CO2/CH4 adsorption behavior of MOF-200 at different temperatures; an experimental and adsorption isotherms study. Microporous Mesoporous Mater. 2020, 296, 110002. [Google Scholar] [CrossRef]
- Mokhtar, N.A.; Zawawi, R.M.; Khairul, W.M.; Yusof, N.A. Electrochemical and optical sensors made of composites of metal–organic frameworks and carbon-based materials. A review. Environ. Chem. Lett. 2022, 20, 3099–3131. [Google Scholar] [CrossRef]
- Nandihalli, N.; Gregory, D.H.; Mori, T. Energy-Saving Pathways for Thermoelectric Nanomaterial Synthesis: Hydrother-mal/Solvothermal, Microwave-Assisted, Solution-Based, and Powder Processing. Adv. Sci. 2022, 9, 2106052. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Malik, W.M.; Afaq, S.; Mahmood, A.; Niu, L.; Yousaf ur Rehman, M.; Ibrahim, M.; Mohyuddin, A.; Qureshi, A.M.; Ashiq, M.N.; Chughtai, A.H. A facile synthesis of CeO2 from the GO@ Ce-MOF precursor and its efficient performance in the oxygen evolution reaction. Front. Chem. 2022, 10, 996560. [Google Scholar] [CrossRef] [PubMed]
- Fallatah, A.M.; Shah, H.U.; Ahmad, K.; Ashfaq, M.; Rauf, A.; Muneer, M.; Ibrahim, M.M.; El-Bahy, Z.M.; Shahzad, A.; Babras, A. Rational synthesis and characterization of highly water stable MOF@ GO composite for efficient removal of mercury (Hg2+) from water. Heliyon 2022, 8, e10936. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Xu, Y. Enhancing the catalytic behaviour of HKUST-1 by graphene oxide for phenol oxidation. Environ. Technol. 2021, 42, 694–704. [Google Scholar] [CrossRef]
- Liu, K.G.; Sharifzadeh, Z.; Rouhani, F.; Ghorbanloo, M.; Morsali, A. Metal-organic framework composites as green/sustainable catalysts. Coord. Chem. Rev. 2021, 436, 213827. [Google Scholar] [CrossRef]
- Makhetha, T.A.; Moutloali, R.M. Stable zeolitic imidazolate framework-8 supported onto graphene oxide hybrid ultrafiltration 1124 membranes with improved fouling resistance and water flux. Chem. Eng. J. Adv. 2020, 1, 100005. [Google Scholar] [CrossRef]
- Li, H.K.; Ye, H.L.; Zhao, X.X.; Sun, X.L.; Zhu, Q.Q.; Han, Z.Y.; Yuan, R.; He, H. Artful union of a zirconium-porphyrin MOF/GO composite for fabricating an aptamer-based electrochemical sensor with superb detecting performance. Chin. Chem. Lett. 2021, 32, 2851–2855. [Google Scholar] [CrossRef]
- Chen, T.; Shen, T.; Wang, Y.; Yu, Z.; Zhang, W.; Zhang, Y.; Ouyang, Z.; Cai, Q.; Ji, Y.; Wang, S. In Situ Synthesis of Ni-BTC Metal–Organic Framework@ Graphene Oxide Composites for High-Performance Supercapacitor Electrodes. ACS Omega 2023, 8, 10888–10898. [Google Scholar] [CrossRef]
- Zaman, N.; Iqbal, N.; Noor, T. Advances and challenges of MOF derived carbon-based electrocatalysts and photocatalyst for water splitting: A review. Arab. J. Chem. 2022, 14, 103906. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Wu, Y.; Li, W.; Chen, C. Functionalized graphene with Co-ZIF adsorbed borate ions as an effective flame retardant and smoke suppression agent for epoxy resin. J. Hazard. Mater. 2019, 363, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Beka, L.G.; Bu, X.; Li, X.; Wang, X.; Han, C.; Liu, W. A 2D metal–organic framework/reduced graphene oxide heterostructure for supercapacitor application. RSC Adv. 2019, 9, 36123–36135. [Google Scholar] [CrossRef]
- Wang, K.; Hui, K.N.; San Hui, K.; Peng, S.; Xu, Y. Recent progress in metal–organic framework/graphene-derived materials for energy storage and conversion: Design, preparation, and application. Chem. Sci. 2021, 12, 5737–5766. [Google Scholar] [CrossRef] [PubMed]
- Haroon, H.; Wahid, M.; Majid, K. Structure-Activity Relationships of a Ni-MOF, a Ni-MOF-rGO, and pyrolyzed Ni/C@rGO Structures for Sodium-ion Batteries. ChemistrySelect 2022, 7, e202202011. [Google Scholar] [CrossRef]
- Munir, A.; Ahmad, A.; Sadiq, M.T.; Sarosh, A.; Abbas, G.; Ali, A. Synthesis and Characterization of Carbon-Based Composites for Hydrogen Storage Application. Eng. Proc. 2021, 12, 52. [Google Scholar]
- Arul, P.; Gowthaman, N.S.; John, S.A.; Lim, H.N. Ultrasonic assisted synthesis of size-controlled Cu-metal–organic framework decorated graphene oxide composite: Sustainable electrocatalyst for the trace-level determination of nitrite in environmental water samples. ACS Omega 2020, 5, 14242–14253. [Google Scholar] [CrossRef]
- Kasula, M.; Le, T.; Thomsen, A.; Esfahani, M.R. Silver metal organic frameworks and copper metal organic frameworks immobilized on graphene oxide for enhanced adsorption in water treatment. Chem. Eng. J. 2022, 439, 135542. [Google Scholar] [CrossRef]
- Cui, H.; Cui, S.; Tian, Q.; Zhang, S.; Wang, M.; Zhang, P.; Liu, Y.; Zhang, J.; Li, X. Electrochemical Sensor for the Detection of 1-Hydroxypyrene Based on Composites of PAMAM-Regulated Chromium-Centered Metal–Organic Framework Nanoparticles and Graphene Oxide. ACS Omega 2021, 6, 31184–31195. [Google Scholar] [CrossRef]
- Firouzjaei, M.D.; Afkhami, F.A.; Esfahani, M.R.; Turner, C.H.; Nejati, S. Experimental and molecular dynamics study on dye removal from water by a graphene oxide-copper-metal organic framework nanocomposite. J. Water Process Eng. 2020, 34, 101180. [Google Scholar] [CrossRef]
- Yang, K.; Dai, Y.; Zheng, W.; Ruan, X.; Li, H.; He, G. ZIFs-modified GO plates for enhanced CO2 separation performance of ethyl cellulose based mixed matrix membranesf. Sep. Purif. Technol. 2019, 214, 87–94. [Google Scholar] [CrossRef]
- Goh, P.S.; Wong, K.C.; Ismail, A.F. Nanocomposite membranes for liquid and gas separations from the perspective of nanostruc-1157 ture dimensions. Membranes 2020, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Metal-organic frameworks for flame retardant polymers application: A critical re-1159 view. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106113. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, R.; Wang, Y.; Cao, Y.; Shen, Y.; Huang, Y.; Chen, Y. Recent advances in graphene aerogels as absorption-dominated electromagnetic interference shielding materials. Carbon 2023, 205, 112–137. [Google Scholar] [CrossRef]
- Ndlwana, L.; Raleie, N.; Dimpe, K.M.; Ogutu, H.F.; Oseghe, E.O.; Motsa, M.M.; Msagati, T.A.; Mamba, B.B. Sustainable hydrothermal and solvothermal synthesis of advanced carbon materials in multidimensional applications: A review. Materials 2021, 14, 5094. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Bahamon, D.; Khaleel, M.; Vega, L.F. Insights into the performance of hybrid graphene oxide/MOFs for CO2 capture at process conditions by molecular simulations. Chem. Eng. J. 2022, 449, 137884. [Google Scholar] [CrossRef]
- Choudhary, P.; Ola, S.K.; Chopra, I.; Dhayal, V.; Shekhawat, D.S. Metal–organic framework (MOF)/graphene–oxide (GO) nano-composites materials: A potential formulation for anti-corrosive coatings—A review. Mater. Today Proc. 2022, 1162, 19. [Google Scholar]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Muschi, M.; Serre, C. Progress and challenges of graphene oxide/metal-organic composites. Coord. Chem. Rev. 2019, 387, 262–272. [Google Scholar] [CrossRef]
- Zhu, L.; Meng, L.; Shi, J.; Li, J.; Zhang, X.; Feng, M. Metal-organic frameworks/carbon-based materials for environmental remediation: A state-of-the-art mini-review. J. Environ. Manag. 2019, 232, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, X.; Li, Y.; Zhu, R.; Pang, H. Applications of metal–organic-framework-derived carbon materials. Adv. Mater. 2019, 31, 1804740. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.G.; Liang, Z.; Gao, S.; Qu, C.; Zou, R. Metal-organic framework-based materials for hybrid supercapacitor application. Coord. Chem. Rev. 2020, 404, 213093. [Google Scholar] [CrossRef]
- Huang, S.; Shi, X.R.; Sun, C.; Duan, Z.; Ma, P.; Xu, S. The application of metal–organic frameworks and their derivatives for supercapacitors. Nanomaterials 2020, 10, 2268. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.; Ryu, Y.K.; Boscá, A.; Ladrón-de-Guevara, A.; Hunt, E.; Zuo, J.; Pedrós, J.; Calle, F.; Martinez, J. Recent trends in graphene supercapacitors: From large area to microsupercapacitors. Sustain. Energy Fuels 2021, 5, 1235–1254. [Google Scholar] [CrossRef]
- Fang, H.; Yang, D.; Su, Z.; Sun, X.; Ren, J.; Li, L.; Wang, K. Preparation and Application of Graphene and Derived Carbon Materials in Supercapacitors: A Review. Coatings 2022, 12, 1312. [Google Scholar] [CrossRef]
- Li, S.; Shi, C.; Pan, Y.; Wang, Y. 2D/2D NiCo-MOFs/GO hybrid nanosheets for high-performance asymmetrical supercapacitor. Diam. Relat. Mater. 2021, 115, 108358. [Google Scholar] [CrossRef]
- Chen, T.; Yang, A.; Zhang, W.; Nie, J.; Wang, T.; Gong, J.; Wang, Y.; Ji, Y. Architecting Nanostructured Co-BTC@ GO Composites for Supercapacitor Electrode Application. Nanomaterials 2022, 12, 3234. [Google Scholar] [CrossRef]
- Sundriyal, S.; Kaur, H.; Bhardwaj, S.K.; Mishra, S.; Kim, K.H.; Deep, A. Metal-organic frameworks and their composites as efficient electrodes for supercapacitor applications. Coord. Chem. Rev. 2018, 369, 15–38. [Google Scholar] [CrossRef]
- Ibrahim, I.; Zheng, S.; Foo, C.Y.; Huang, N.M.; Lim, H.N. Hierarchical nickel-based metal-organic framework/graphene oxide incorporated graphene nanoplatelet electrode with exceptional cycling stability for coin cell and pouch cell supercapacitors. J. Energy Storage 2021, 43, 103304. [Google Scholar] [CrossRef]
- Ramachandran, R.; Xuan, W.; Zhao, C.; Leng, X.; Sun, D.; Luo, D.; Wang, F. Enhanced electrochemical properties of cerium metal–organic framework based composite electrodes for high-performance supercapacitor application. RSC Adv. 2018, 8, 3462–3469. [Google Scholar] [CrossRef] [PubMed]
- Azadfalah, M.; Sedghi, A.; Hosseini, H.; Kashani, H. Cobalt based metal organic framework/graphene nanocomposite as high performance battery-type electrode materials for asymmetric supercapacitors. J. Energy Storage 2021, 33, 101925. [Google Scholar] [CrossRef]
- Roohollahi, H.; Zeinalzadeh, H.; Kazemian, H. Recent Advances in Adsorption and Separation of Methane and Carbon Dioxide Greenhouse Gases Using Metal–Organic Framework-Based Composites. Ind. Eng. Chem. Res. 2022, 61, 10555–10586. [Google Scholar] [CrossRef]
- Ventura, K.; Arrieta, R.A.; Marcos-Hernández, M.; Jabbari, V.; Powell, C.D.; Turley, R.; Lounsbury, A.W.; Zimmerman, J.B.; Gardea-Torresdey, J.; Wong, M.S.; et al. Superparamagnetic MOF@ GO Ni and Co based hybrid nanocomposites as efficient water pollutant adsorbents. Sci. Total Environ. 2020, 738, 139213. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.A.; Mullangi, D.; Deng, Z.; Wang, Y.; Peh, S.B.; Wei, F.; Wang, J.; Brown, C.M.; Zhao, D.; Canepa, P.; et al. Aluminum formate, Al (HCOO)3: An earth-abundant, scalable, and highly selective material for CO2 capture. Sci. Adv. 2022, 8, eade1473. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ge, H.; Miao, Y.; Chen, J.; Cai, W. CO2 capture ability of Cu-based metal-organic frameworks synergized with amino acid-functionalized layered materials. Catal. Today 2020, 356, 604–612. [Google Scholar] [CrossRef]
- Azizi, A.; Feijani, E.A.; Ghorbani, Z.; Tavasoli, A. Fabrication and characterization of highly efficient three component CuBTC/graphene oxide/PSF membrane for gas separation application. Int. J. Hydrogen Energy 2021, 46, 2244–2254. [Google Scholar] [CrossRef]
- Hooriabad Saboor, F.; Nasirpour, N.; Shahsavari, S.; Kazemian, H. The effectiveness of MOFs for the removal of pharmaceuticals from aquatic environments: A review focused on antibiotics removal. Chem. Asian J. 2022, 17, e202101105. [Google Scholar] [CrossRef]
- Chen, J.Q.; Sharifzadeh, Z.; Bigdeli, F.; Gholizadeh, S.; Li, Z.; Hu, M.L.; Morsali, A. MOF Composites as high Potential Materials for Hazardous Organic Contaminants Removal in Aqueous Environments. J. Environ. Chem. Eng. 2023, 11, 109469. [Google Scholar] [CrossRef]
- Yang, F.; Du, M.; Yin, K.; Qiu, Z.; Zhao, J.; Liu, C.; Zhang, G.; Gao, Y.; Pang, H. Applications of Metal-Organic Frameworks in Water Treatment: A Review. Small 2022, 18, 2105715. [Google Scholar] [CrossRef]
- Jun, B.M.; Al-Hamadani, Y.A.; Son, A.; Park, C.M.; Jang, M.; Jang, A.; Kim, N.C.; Yoon, Y. Applications of metal-organic framework based membranes in water purification: A review. Sep. Purif. Technol. 2020, 247, 116947. [Google Scholar] [CrossRef]
- Jafarian, H.; Firouzjaei, M.D.; Aktij, S.A.; Aghaei, A.; Khomami, M.P.; Elliott, M.; Wujcik, E.K.; Sadrzadeh, M.; Rahimpour, A. Synthesis of heterogeneous metal organic Framework-Graphene oxide nanocomposite membranes for water treatment. Chem. Eng. J. 2023, 455, 140851. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, D.; Zhu, G.; Zhou, T.; Song, M.; Qu, L.; Xiong, K.; Li, H. A Sm-MOF/GO nanocomposite membrane for efficient organic dye removal from wastewater. RSC Adv. 2020, 10, 8540–8547. [Google Scholar] [CrossRef] [PubMed]
- Venkadesh, A.; Mathiyarasu, J.; Radhakrishnan, S. Voltammetric Sensing of Caffeine in Food Sample Using Cu-MOF and Graphene. Electroanalysis 2021, 33, 1007–1013. [Google Scholar] [CrossRef]
- Chang, Y.; Lou, J.; Yang, L.; Liu, M.; Xia, N.; Liu, L. Design and Application of Electrochemical Sensors with Metal–Organic Frameworks as the Electrode Materials or Signal Tags. Nanomaterials 2022, 12, 3248. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; He, P.; Yang, T.; Wang, X.; Zhou, L.; He, Q.; Jia, L.; Deng, H.; Zhang, H.; Jia, B.; et al. Short rod-like Ni-MOF anchored on graphene oxide nanosheets: A promising voltammetric platform for highly sensitive determination of p-chloronitrobenzene. J. Electroanal. Chem. 2020, 861, 113954. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Y.; Shan, J.; Zhou, H.; Li, M. Electrochemical sensor for determination of bisphenol A based on MOF-reduced graphene oxide composites coupled with cetyltrimethylammonium bromide signal amplification. Ionics 2020, 26, 3135–3146. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, M.; Jiang, H.; Chen, R. Controllable synthesis of Pd-ZIF-L-GO: The role of drying temperature. Ind. Eng. Chem. Res. 2021, 60, 4847–4859. [Google Scholar] [CrossRef]
- Miceli, M.; Frontera, P.; Macario, A.; Malara, A. Recovery/reuse of heterogeneous supported spent catalysts. Catalysts 2021, 11, 591. [Google Scholar] [CrossRef]
- Andrade, M.A.; Martins, L.M. Sustainability in catalytic cyclohexane oxidation: The contribution of porous support materials. Catalysts 2019, 10, 2. [Google Scholar] [CrossRef]
- Alamgholiloo, H.; Rostamnia, S.; Zhang, K.; Lee, T.H.; Lee, Y.S.; Varma, R.S.; Jang, H.W.; Shokouhimehr, M. Boosting aerobic oxidation of alcohols via synergistic effect between TEMPO and a composite Fe3O4/Cu-BDC/GO nanocatalyst. ACS Omega 2020, 5, 5182–5191. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Wang, B.; Li, S.; Wie, Y.; Wang, Q.; Li, D. Fabrication of Pd@ ZnNi-MOF/GO Nanocomposite and Its Application for H2O2 Detection and Catalytic Degradation of Methylene Blue Dyes. ChemistrySelect 2021, 6, 8480–8489. [Google Scholar] [CrossRef]
- Hoseinzadeh, H.; Hayati, B.; Ghaheh, F.S.; Seifpanahi-Shabani, K.; Mahmoodi, N.M. Development of room temperature synthesized and functionalized metal-organic framework/graphene oxide composite and pollutant adsorption ability. Mater. Res. Bull. 2021, 142, 111408. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Zhang, R.; Jiao, L.; Jiang, H.L. Metal–organic framework-derived porous materials for catalysis. Coord. Chem. Rev. 2018, 362, 1–23. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, N.; Liang, Y. Preparation of CeO2/Cu-MOF/GO composite for efficient electrocatalytic oxygen evolution reaction. Ionics 2021, 27, 4347–4360. [Google Scholar] [CrossRef]
- Tang, B.; Wang, S.; Li, R.; Gou, X.; Long, J. Urea treated metal organic frameworks-graphene oxide composites derived N-doped Co-based materials as efficient catalyst for enhanced oxygen reduction. J. Power Sources 2019, 425, 76–86. [Google Scholar] [CrossRef]
- Radwan, A.; Jin, H.; He, D.; Mu, S. Design engineering, synthesis protocols, and energy applications of MOF-derived electrocatalysts. Nano-Micro Lett. 2021, 13, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; Panda, A.; Ramu, A.G.; Theerthagiri, J.; Kim, H.; Yun, K. Bifunctional electrocatalysts for water splitting from a bimetallic (V doped-NixFey) Metal–Organic framework MOF@ Graphene oxide composite. Int. J. Hydrogen Energy 2022, 47, 42122–42135. [Google Scholar] [CrossRef]
- Noor, T.; Ammad, M.; Zaman, N.; Iqbal, N.; Yaqoob, L.; Nasir, H. A highly efficient and stable copper BTC metal organic framework derived electrocatalyst for oxidation of methanol in DMFC application. Catal. Lett. 2019, 149, 3312–3327. [Google Scholar] [CrossRef]
- Bagherzadeh, S.B.; Kazemeini, M.; Mahmoodi, N.M. Preparation of novel and highly active magnetic ternary structures (metal-organic framework/cobalt ferrite/graphene oxide) for effective visible-light-driven photocatalytic and photo-Fenton-like degradation of organic contaminants. J. Colloid Interface Sci. 2021, 602, 73–94. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, F.; Pang, H. A review of MOFs and their composites-based photocatalysts: Synthesis and applications. Adv. Funct. Mater. 2021, 31, 2104231. [Google Scholar] [CrossRef]
- Jin, J.; Kim, J.P.; Wan, S.; Kim, K.H.; Choi, Y.; Li, P.; Kang, J.; Ma, Z.; Lee, J.H.; Kwon, O.; et al. Hierarchical pore enhanced adsorption and photocatalytic performance of graphene oxide/Ti-based metal-organic framework hybrid for toluene removal. Appl. Catal. B Environ. 2022, 317, 121751. [Google Scholar] [CrossRef]
- Heu, R.; Ateia, M.; Awfa, D.; Punyapalakul, P.; Yoshimura, C. Photocatalytic degradation of organic micropollutants in water by Zr-MOF/GO composites. J. Compos. Sci. 2020, 4, 54. [Google Scholar] [CrossRef]
- Xiao, X.; Zou, L.; Pang, H.; Xu, Q. Synthesis of micro/nanoscaled metal–organic frameworks and their direct electrochemical applications. Chem. Soc. Rev. 2020, 49, 301–331. [Google Scholar] [CrossRef] [PubMed]
- Teffu, D.M.; Ramoroka, M.E.; Makhafola, M.D.; Makgopa, K.; Maponya, T.C.; Seerane, O.A.; Hato, M.J.; Iwuoha, E.I.; Modibane, K.D. High-performance supercabattery based on reduced graphene oxide/metal organic framework nanocomposite decorated with palladium nanoparticles. Electrochim. Acta 2022, 412, 140136. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Shi, W.; Xu, X.; Mao, J.; Li, P.; Ye, C.; Yin, R.; Ye, S.; Liu, X.; et al. Boosting lithium storage properties of MOF derivatives through a wet-spinning assembled fiber strategy. Chem. A Eur. J. 2018, 24, 13792–13799. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Xu, Y. Three-dimensional graphene/metal–organic framework composites for electrochemical energy storage and conversion. Chem. Commun. 2023, 59, 6475–6494. [Google Scholar] [CrossRef]
- Du, Y.; Wang, M.; Ye, X.; Liu, B.; Han, L.; Jafri, S.H.; Liu, W.; Zheng, X.; Ning, Y.; Li, H. Advances in the Field of Graphene-Based Composites for Energy–Storage Applications. Crystals 2023, 13, 912. [Google Scholar] [CrossRef]
- Zhang, M.; Shan, Y.; Kong, Q.; Pang, H. Applications of metal–organic framework–graphene composite materials in electrochemical energy storage. FlatChem 2022, 32, 100332. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Q.; Zhang, H.; Zhang, Q.; Zhang, K.; Mei, H.; Xu, B.; Sun, D. Self-Assembly of Metal–Organic Frameworks on Graphene Oxide Nanosheets and In Situ Conversion into a Nickel Hydroxide/Graphene Oxide Battery-Type Electrode. Inorg. Chem. 2022, 61, 12129–12137. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, H.; Ke, F.S.; Deng, H. Three-Dimensional Hierarchical Constructs of MOF-on-Reduced Graphene Oxide for Lithium–Sulfur Batteries. Chem. Asian J. 2019, 14, 3577–3582. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, D.; Zhu, N.; Wu, Y.; Li, G. Antibacterial mechanisms and applications of metal-organic frameworks and their derived nanomaterials. Trends Food Sci. Technol. 2021, 109, 413–434. [Google Scholar] [CrossRef]
- Ge, X.; Wong, R.; Anisa, A.; Ma, S. Recent development of metal-organic framework nanocomposites for biomedical applications. Biomaterials 2022, 281, 121322. [Google Scholar] [CrossRef] [PubMed]
- Sontakke, A.D.; Tiwari, S.; Purkait, M.K. A comprehensive review on graphene oxide-based nanocarriers: Synthesis, functionalization and biomedical applications. FlatChem 2023, 38, 100484. [Google Scholar] [CrossRef]
- Asl, E.A.; Pooresmaeil, M.; Namazi, H. Chitosan coated MOF/GO nanohybrid as a co-anticancer drug delivery vehicle: Synthesis, characterization, and drug delivery application. Mater. Chem. Phys. 2023, 293, 126933. [Google Scholar]
- Pooresmaeil, M.; Asl, E.A.; Namazi, H. A new pH-sensitive CS/Zn-MOF@ GO ternary hybrid compound as a biofriendly and implantable platform for prolonged 5-Fluorouracil delivery to human breast cancer cells. J. Alloys Compd. 2021, 885, 160992. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, D.; Ji, N.; Lee, S.; Wang, G.; Zheng, Y.; Zhang, X.; Yang, L.; Qin, Z.; Yang, Y. Bioinspired design of sericin/chitosan/Ag@ MOF/GO hydrogels for efficiently combating resistant bacteria, rapid hemostasis, and wound healing. Polymers 2021, 13, 2812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, S.; Tang, Y.; Huang, X.; Pang, H. Recent advances and challenges of metal–organic framework/graphene-based composites. Compos. Part B Eng. 2022, 230, 109532. [Google Scholar] [CrossRef]
- Wang, S.; Ye, B.; An, C.; Wang, J.; Li, Q.; Guo, H.; Zhang, J. Exploring the coordination effect of GO@ MOF-5 as catalyst on thermal decomposition of ammonium perchlorate. Nanoscale Res. Lett. 2019, 14, 1–11. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, Y. Single-atom engineering of metal-organic frameworks toward healthcare. Chem 2021, 7, 2635–2671. [Google Scholar] [CrossRef]
- Li, C.; Ji, Y.; Wang, Y.; Liu, C.; Chen, Z.; Tang, J.; Hong, Y.; Li, X.; Zheng, T.; Jiang, Q.; et al. Applications of Metal–Organic Frameworks and Their Derivatives in Electrochemical CO2 Reduction. Nano-Micro Lett. 2023, 15, 113. [Google Scholar] [CrossRef]
- Zadehahmadi, F.; Eden, N.T.; Mahdavi, H.; Konstas, K.; Mardel, J.I.; Shaibani, M.; Banerjee, P.C.; Hill, M.R. Removal of metals from water using MOF-based composite adsorbents. Environ. Sci. Water Res. Technol. 2023, 9, 1305–1330. [Google Scholar] [CrossRef]
- Mazlan, N.A.; Butt, F.S.; Lewis, A.; Yang, Y.; Yang, S.; Huang, Y. The Growth of Metal–Organic Frameworks in the Presence of Graphene Oxide: A Mini Review. Membranes 2022, 12, 501. [Google Scholar] [CrossRef] [PubMed]
- Uflyand, I.E.; Naumkina, V.N.; Zhinzhilo, V.A. Nanocomposites of Graphene Oxide and Metal-Organic Frameworks. Russ. J. Appl. Chem. 2021, 94, 1453–1468. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Qi, X.L.; Wang, D.Y. Recent progress on metal–organic framework and its derivatives as novel fire retardants to polymeric materials. Nano-Micro Lett. 2020, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Yang, A.; Jiang, C.; Zhang, G.; Mao, J.; Meng, Q. Polyphenol etched ZIF-8 modified graphene oxide nanofiltration membrane for efficient removal of salts and organic molecules. J. Membr. Sci. 2021, 635, 119521. [Google Scholar] [CrossRef]
- Shao, Q.; Lu, F.; Yu, L.; Xu, X.; Huang, X.; Huang, Y.; Hu, Z. Facile immobilization of graphene nanosheets onto PBO fibers via MOF-mediated coagulation strategy: Multifunctional interface with self-healing and ultraviolet-resistance performance. J. Colloid Interface Sci. 2021, 587, 661–671. [Google Scholar] [CrossRef]

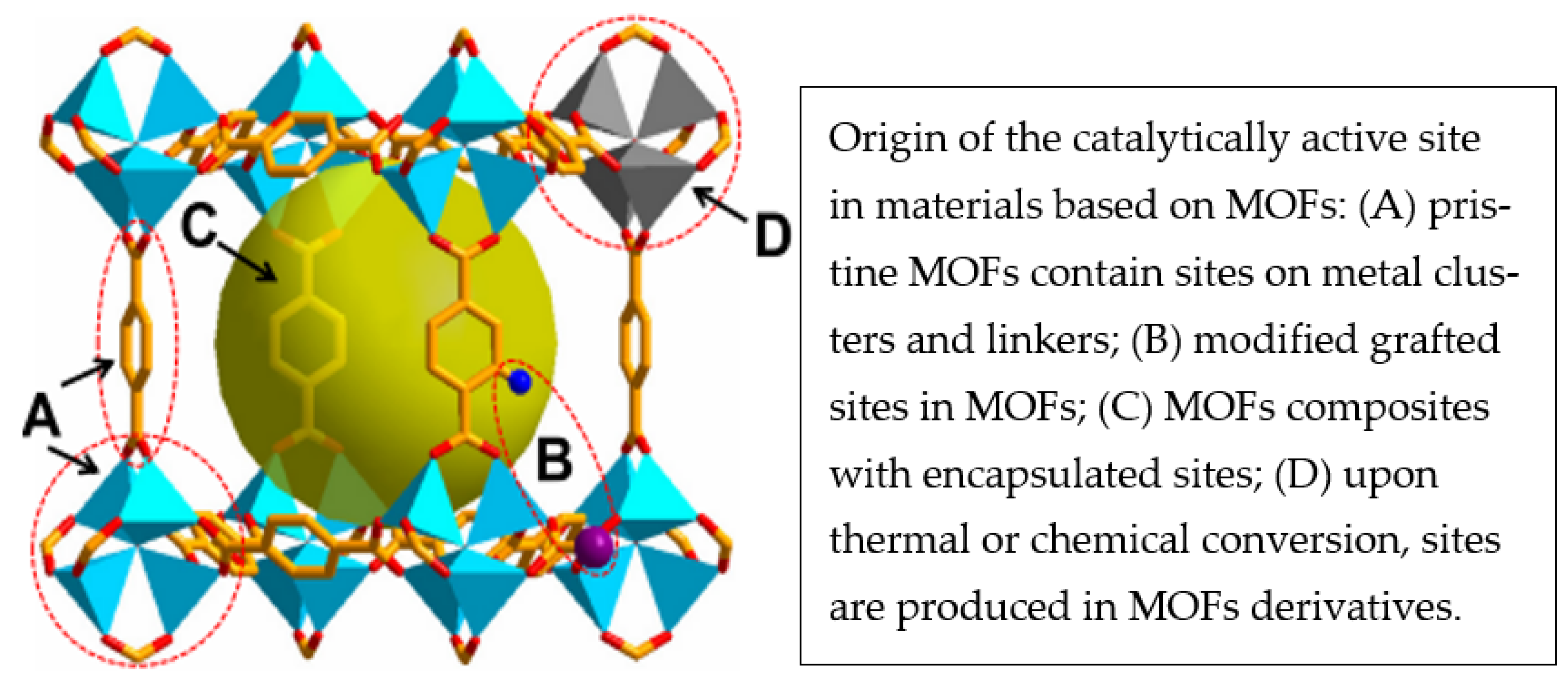

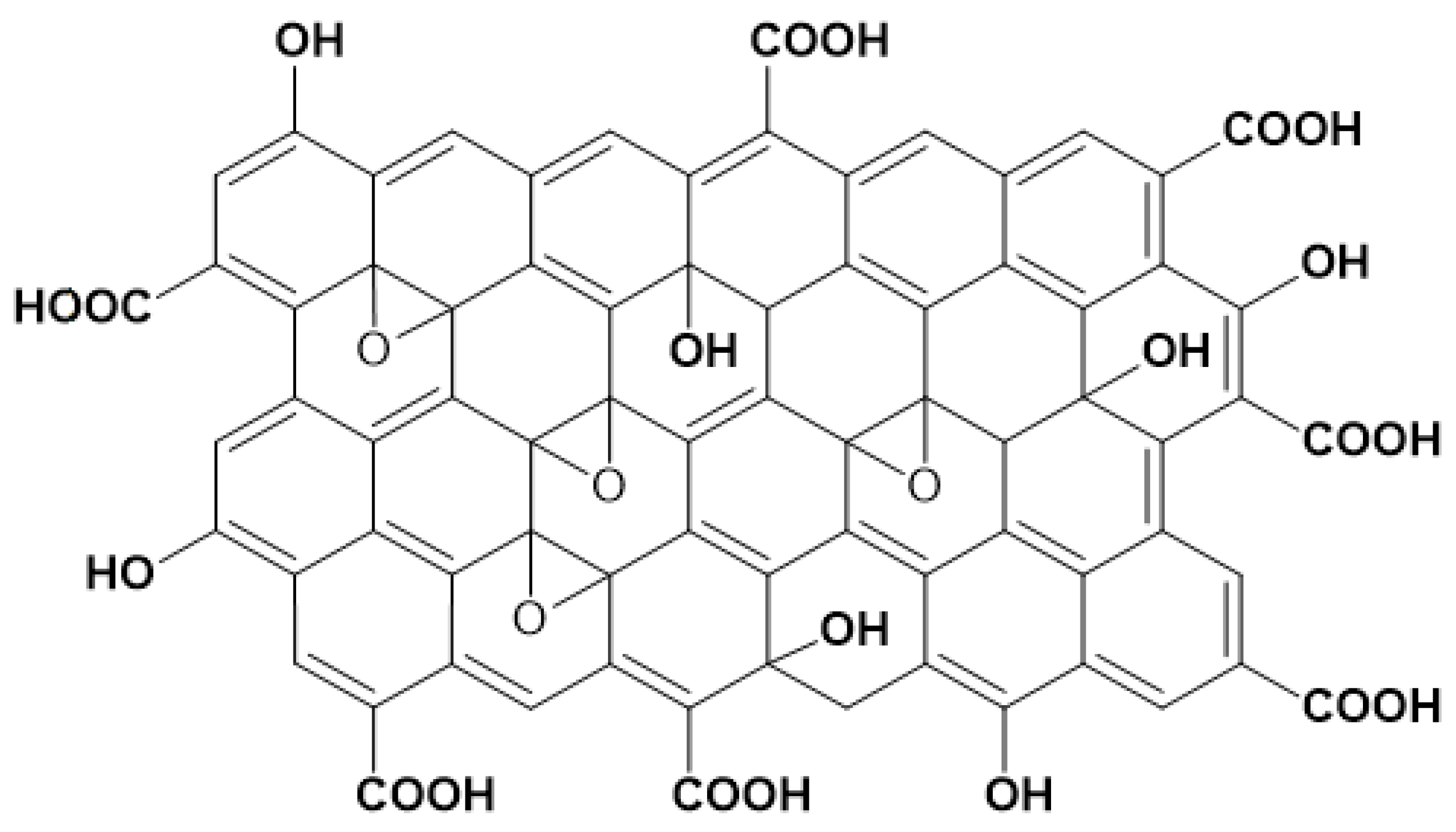

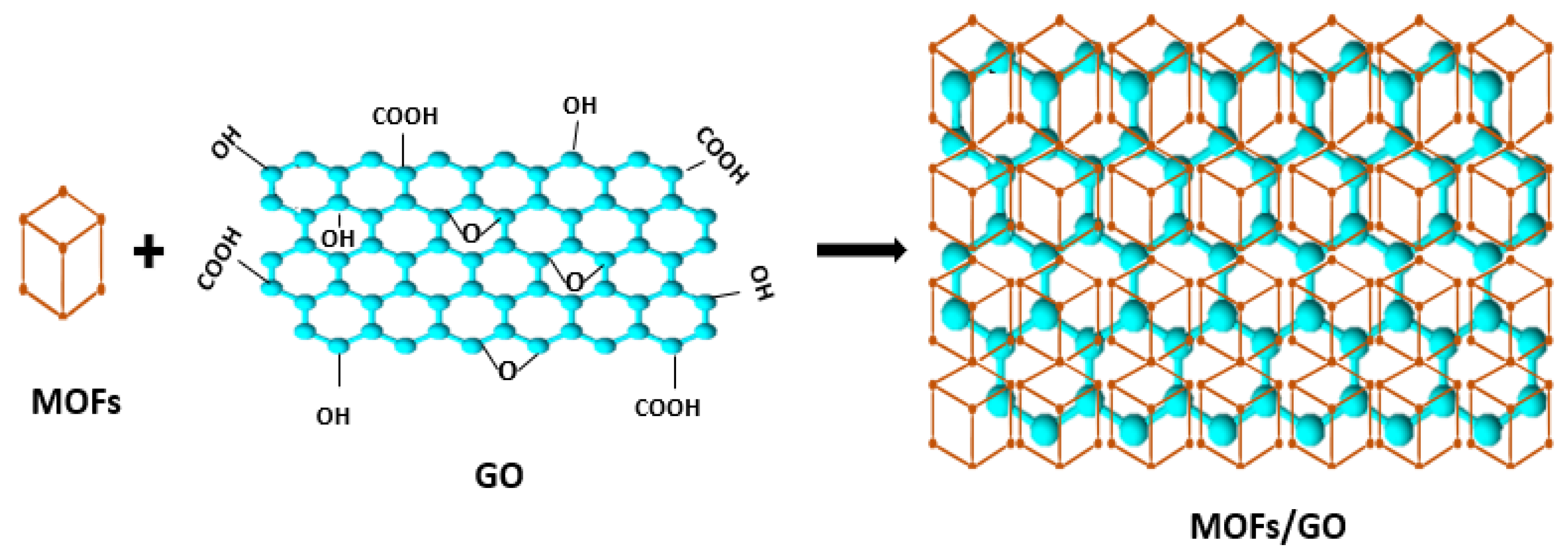
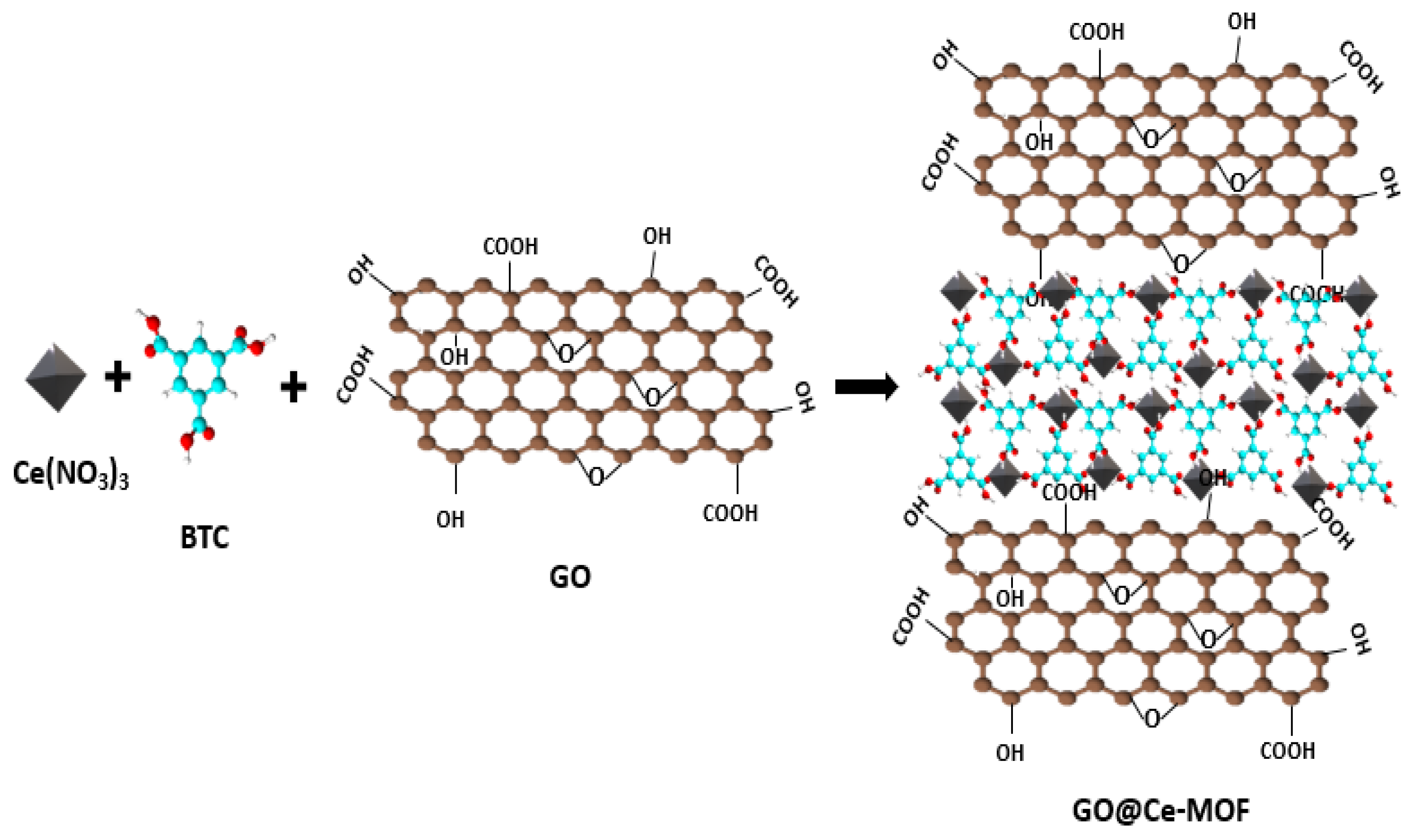

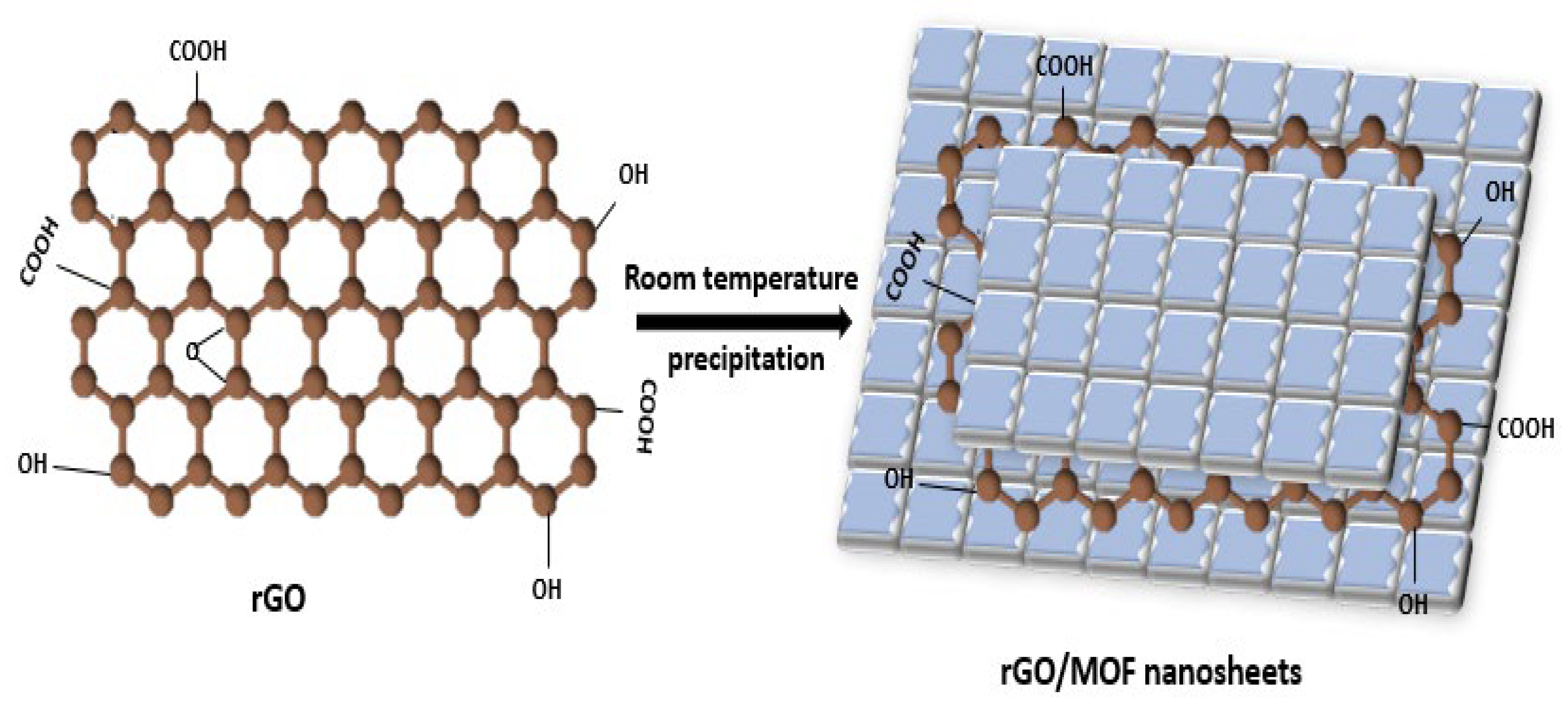
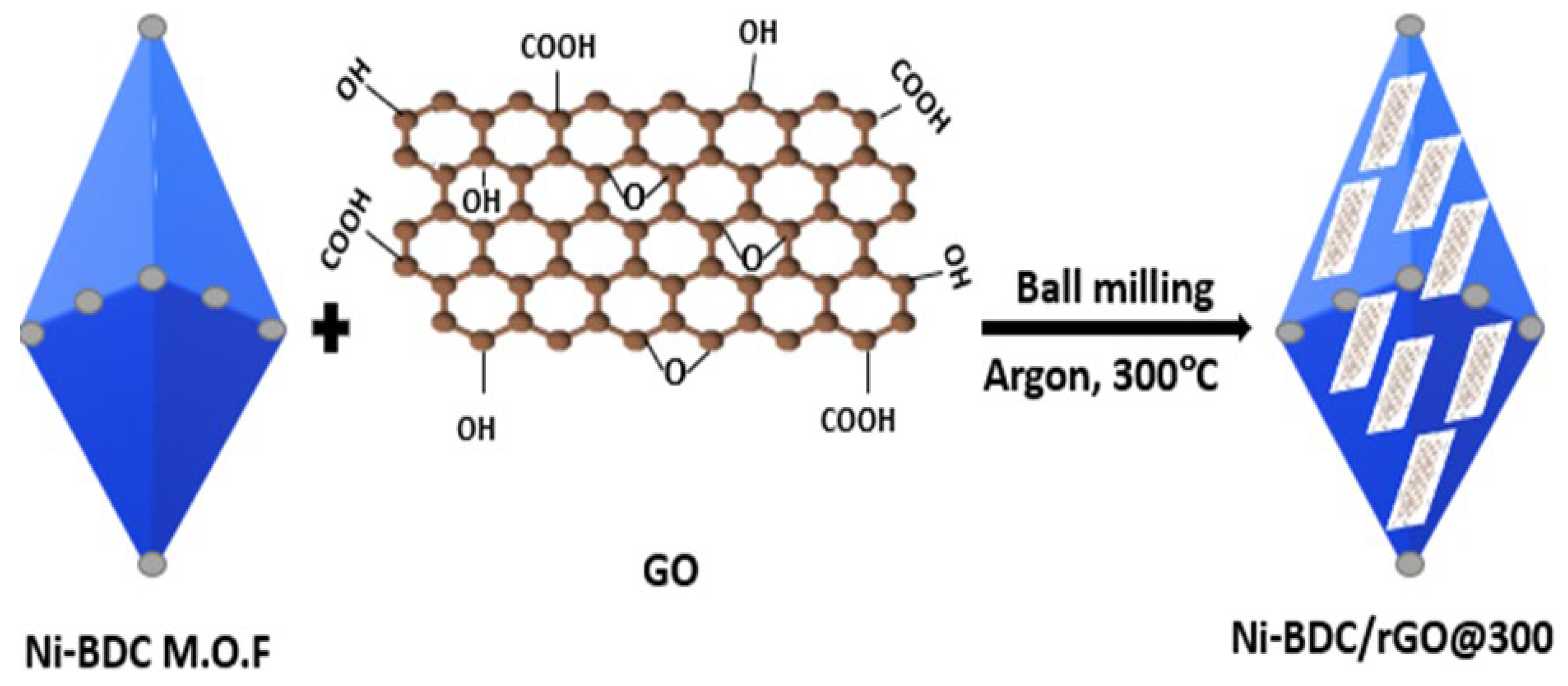

| Applications | Composites | Output | Synthesis Methods | Ref. |
|---|---|---|---|---|
| Supercapacitors |
|
|
| [97] |
| Gas separation and Storage |
|
|
| [107] |
| Water Purification |
|
|
| [113] |
| Sensor |
|
|
| [116] |
| Electrocatalysts |
|
|
| [129] |
| Photocatalysts |
|
|
| [133] |
| Batteries |
|
|
| [140] |
| Biomedical |
|
|
| [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naser, S.A.E.; Badmus, K.O.; Khotseng, L. Synthesis, Properties, and Applications of Metal Organic Frameworks Supported on Graphene Oxide. Coatings 2023, 13, 1456. https://doi.org/10.3390/coatings13081456
Naser SAE, Badmus KO, Khotseng L. Synthesis, Properties, and Applications of Metal Organic Frameworks Supported on Graphene Oxide. Coatings. 2023; 13(8):1456. https://doi.org/10.3390/coatings13081456
Chicago/Turabian StyleNaser, Sahar Altegani Ebrahim, Kassim O. Badmus, and Lindiwe Khotseng. 2023. "Synthesis, Properties, and Applications of Metal Organic Frameworks Supported on Graphene Oxide" Coatings 13, no. 8: 1456. https://doi.org/10.3390/coatings13081456





